Abstract
Approximately 15% of preterm infants may develop postnatal cytomegalovirus (CMV) infection from seropositive mothers via breast milk and are at risk for neurological sequelae in childhood. The aims of this study were to assess the effects and outcomes on growth, neurodevelopmental status, and hearing in very low birth weight (VLBW) premature infants with postnatal CMV infection via breast milk at the corrected age of 12 and 24 months.
The prospective follow-up study population comprised all living preterm children (n = 55) with a birth weight ≤1500 g and gestational age of ≤35 weeks, who had been participated in our “postnatal CMV infection via breast milk” studies in 2000 and 2009, respectively. The cohort of children was assessed at 12 and 24 months. Clinical outcomes were documented during hospitalization and after discharge. Long-term outcomes included anthropometry, audiologic tests, gross motor quotient, Infant International Battery, and neurodevelopmental outcomes; all were assessed at postcorrected age in 12 and 24 months during follow-up visits.
Of the 55 infants enrolled in the study (4 noninfected infants were excluded because their parents did not join this follow-up program later), 14 infants postnatally acquired CMV infection through breast-feeding (infected group) and were compared with 41 infants without CMV infection (control group). No significant differences were observed between the groups with regard to baseline characteristics, clinical outcomes, anthropometry, or psychomotor and mental development on the Bayley scale of infant development. None of the infants had CMV-related death or permanent sensorineural hearing loss.
Transmission of CMV from seropositive mother via breast milk to preterm infants does not appear at this time to have major adverse effects on clinical outcomes, growth, neurodevelopmental status, and hearing function at 12 and 24 months corrected age.
INTRODUCTION
Cytomegalovirus (CMV) infection is widespread throughout the world, and can be transmitted to newborns in the congenital, perinatal, or postnatal period. Breastfeeding is the most common route for CMV transmission from seropositive mothers to babies postnatally. Maternal CMV-seropositive rates can range from 51.6% to 100%,1 and over 96% of these pregnant women may have viral reactivation during lactation with CMV shedding into breast milk.2–4
Full-term infants with postnatal CMV infection are often asymptomatic, without long-term sequelae or hearing problems. However, preterm infants, born at birth weight less than 1 kg or gestational age less than 32 weeks, are at highest risk for postnatal CMV acquisition of CMV via breast milk.2,5–9
Transmission rates of CMV from infected breast milk to preterm infants ranges from 5.7% to 58.6%. Symptomatic postnatal CMV infection rates showed a wide variation, ranging from 0 to 75%, and was directly dependent on the maturity of preterm infants.1,2,10,11 Preterm infants exposed to CMV via breast milk might have symptoms such as neutropenia (<1500 cells/mm3), lymphocytosis, thrombocytopenia (<150,000/mm3), petechiae, hepatitis, cholestasis, pneumonitis, hepatosplenomegaly, and sepsis-like symptoms (SLS).1,2,12 Preterm infants with CMV infection via breast milk might be at higher risk for developmental delay, cognitive impairment, motor function dysfunction, and hearing problems.13–16 However, the literature on long-term follow-up and neurodevelopmental outcome of postnatal CMV infection in preterm infants is sparse, and those available data are impeded by small numbers. Thus, the aims of this prospective follow-up study are to assess outcome during hospital stay and at discharge, long-term follows anthropometry, neurodevelopmental outcomes, and auditory function of very low birth weight (VLBW) infants with postnatal CMV infection via breast milk at 12 and 24 months of corrected age.
METHODS
This study was approved by the Ethics Committee of MacKay Memorial Hospital (no. 10MMHIS182), and informed consent was obtained from all the parents before study initiation.
All surviving VLBW preterm infants, who had been managed in a neonatal intensive care unit of MacKay Memorial Hospital and participated in our postnatal CMV infection via breast milk studies,4,9 following by the 2 periods, from 2000 to 2002 and 2009 to 2011, were enrolled for this postnatal acquired CMV infection 2 years follow-up study. These infants were born with body weight of less than 1500 g and at a gestational age of less than 35 weeks. Postnatal CMV infection was defined as PCR-positive viral DNA in urine (DNAuria) and culture-positive urine (viruria) samples 3 weeks after birth. Infants were excluded if they were not fed with their own mother's milk, or if they had evidence of congenital CMV infection, defined as a positive DNAuria or viruria within the first 3 weeks of life or a positive serum immunoglobulin M (IgM) during the first postpartum week. Specific procedures for the laboratory methods to detect postnatal CMV infection via breast milk have been published previously.4,9 Fifty-nine preterm infants were eligible for this prospective 2 years follow-up study; however, 4 non-infected infants were excluded because their parents did not join this follow-up program later. Fourteen infants in the infected group were compared with 41 infants without CMV infection (control group). All of these premature infants were followed up after discharge. The follow-up program examined clinical outcome of preterm infants during hospitalization and discharge. Long-term outcome studies included anthropometry, neurodevelopmental assessment, and audiometric tests at 12 and 24 months of corrected age (corrected for gestational age at birth using the mother's expected date of confinement).
Exclusion criteria included major congenital abnormality, congenital CMV infection, brain malformation, metabolic diseases, chromosomal anomaly, nonbreast milk fed infants, and infants lost to follow-up.
Preterm Infant Follow-Up Program
The baseline characteristics of all participants, included gestational age, birth body weight, gender ratio, birth weight ratio or neonatal therapeutic intervention scoring system, and Apgar scores at 1 and 5 minutes, were reviewed and recorded after admission and before discharge from neonatal units.
Clinical Outcomes
Outcome measures for preterm infants during hospitalization and after discharge included survival, length of stay, presence and grade of intraventricular hemorrhage (IVH), SLS, retinopathy of prematurity (ROP), bronchopulmonary dysplasia (BPD defined as oxygen requirement of more than 21% at 36 weeks’ postmenstrual age),17 periventricular leukomalacia (PVL), cerebral palsy (CP), and need for rehabilitation. Definition of CP was based on Surveillance of Cerebral Palsy in Europe criteria.18
Long-Term Outcomes
During outpatient clinic visits, anthropometric measurements, a mental development index (MDI) and psychomotor development index (PDI) on the Bayley scales of infant development II, Gross motor quotient, Infant International Battery, and hearing function of infants were conducted by neonatologists, pediatric neurologist, and clinical examiners. Examiners were blinded to the infants’ CMV infection status. A threshold of MDI < 70 or PDI < 70 was used, representing 2 standard deviations below the mean and corresponding to significant developmental delay.
Anthropometry
Body length, body weight, and head circumference were measured at follow-up visits for each patient by a trained nurse. Standard growth and weight charts were based on Hsieh et al study.19 Normal growth was defined as within 10th to 90th percentile, and abnormal below the 10th or over 90th percentile for corrected age of life. Microcephaly was defined as a head circumference below 2 standard deviations of the mean.
Hearing Test
All infants underwent audiometric testing and otoscopic examination at follow-up. Auditory brainstem responses were measured using a Medelec Synergy T-EP system (NY) and interpreted by pediatric neurologists. Hearing impairment was graded as follows: mild (26–40 dB), moderate (41–55 dB), moderate to severe (56–70 dB), severe (71–90 dB), and profound (more than 90 dB).20
Statistical Analysis
Infant baseline characteristics, anthropometric measurements, clinical, and long-term outcome data were analyzed using SPSS for Windows, version 17 (SPSS Inc, Chicago, IL). The categorical and continuous variables of infants were compared between 2 groups by Chi-square test, Fisher exact test, and 2-tailed unpaired Student's t-test. Statistical significance was defined as a P value of less than 0.05.
RESULTS
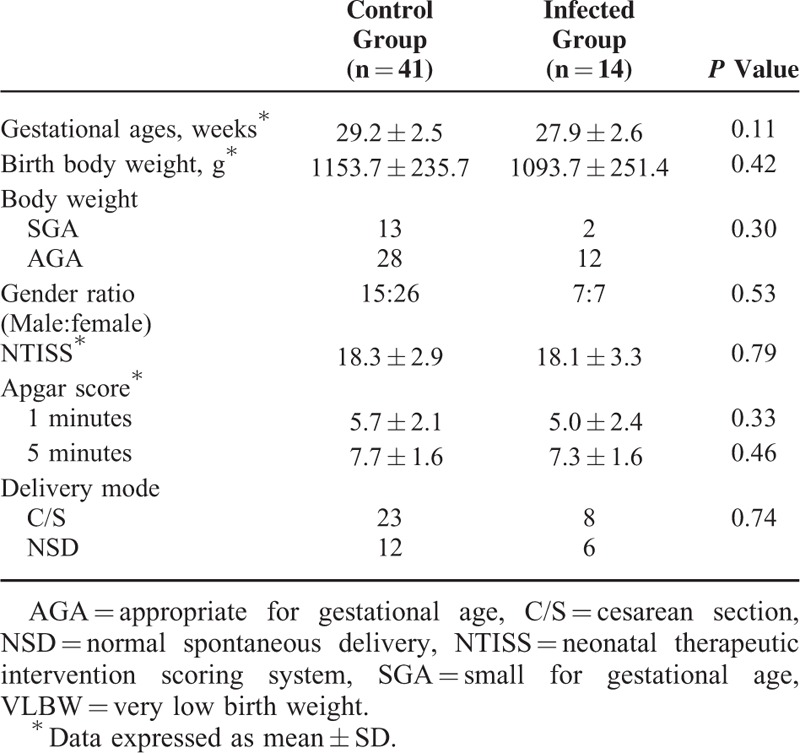
Fifty-five preterm infants were enrolled in the prospective follow-up study. Fourteen of these infants acquired CMV infection through breast feeding (infected group) and were compared with 41 children without CMV infection (control group). Gestational age of preterm infant was greater, and birth weight was heavier in control group compared to the infected group; however, there were no statistical differences between the 2 groups. Other characteristics such as gender ratio, birth weight ratio or neonatal therapeutic intervention scoring system, and Apgar scores showed no significant difference between the infected and control groups (Table 1).
TABLE 1.
Baseline Characteristics of VLBW Infants With or Without CMV Infection
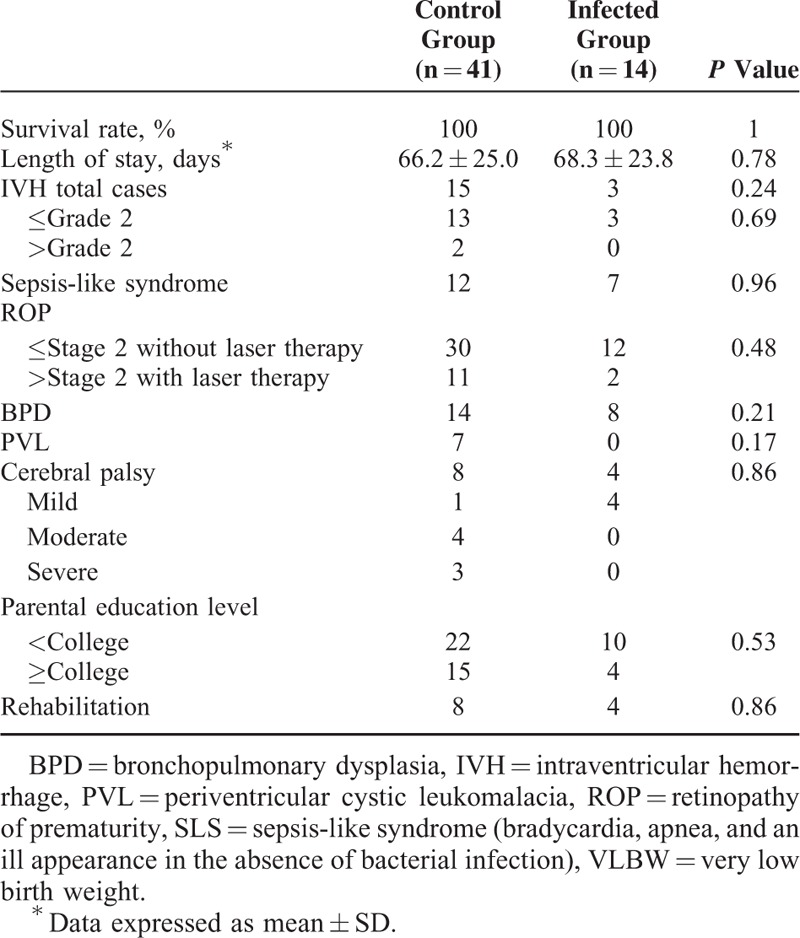
There was no infant death in this study related to CMV-infection. Seven of 14 infected infants had SLS and 8 of the 14 were diagnosed with BPD, but no statistically significant difference was observed between the infected and control groups. Other variables measured also did not show statistical significance, including length of stay, IVH, ROP requiring or not requiring laser therapy, and parental education level. Notably, 7 of the 41 infants in the control group developed PVL and presented with moderate to severe CP in follow-up visits (Table 2). All infants with the diagnosis of CP were transferred to the department of rehabilitation for early intervention.
TABLE 2.
Clinical Outcome of VLBW Preterm Infants With or Without CMV During Hospital Stay and After Discharge
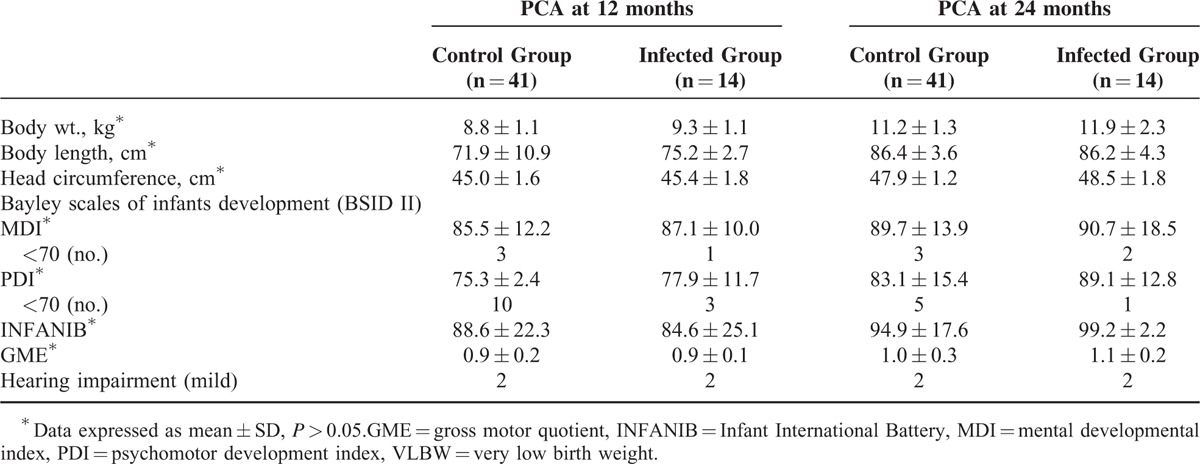
During the follow-up visits, body length was higher and body weight was heavier in the infected group than the control group by anthropometric measurements at corrected age of 12 months, but no significant differences were observed at the 24-month visit. Microcephaly was not found in either infected or control groups. The PDI and MDI scores of CMV-infected infants were higher than scores in the control group. Of the 41 infants in the control group, 10 infants had PDI < 70 at the 12-month visit and only 5 at 24-months after early intervention. Three infants had MDI < 70 at 12- and again at their 24-month visits. No statistical differences were observed for PDI and MDI scores between the groups. Two infants with mild peripheral hearing impairment were identified in both groups; however, neither of them presented permanent sensorineural hearing loss or delayed speech in follow-up visits. The raw data of anthropometry, neurodevelopment assessment, and results of hearing tests for long-term outcome are given in Table 3.
TABLE 3.
Long-Term Outcome of VLBW Preterm Infants Included Anthropometry, Neurodevelopmental Outcome, and Hearing Impairment of Preterm Infants at 12 and 24 months of Corrected Age
DISCUSSION
Our prospective follow-up study indicates that preterm infants with postnatal CMV-infection acquired through breast milk undergo no major adverse effects in clinical outcomes, anthropometry, neurodevelopmental status, and hearing function at up to 24-months. Breast milk is the optimal food for infants, providing many advantages for preterm infants, such as improved neurological development, lower risk of ROP, mitigate infections, and risk for necrotizing enterocolitis.21 Based on our results, we can support the breastfeeding recommendations of the American Academy of Pediatrics,21 in spite of the mothers being CMV seropositive.
Risk factors associated with CMV-seropositive mothers infecting their preterm infants included early onset of DNAlactia, virolactia, high viral load and prolonged excretion of virus in breast milk, lack of transplacental maternal CMV-specific antibody, and infant prematurity (gestational age <30 weeks) and low birth weight (<1 kg).2,5,7,9 Approximately 13.7% to 15% of these infected infants have been reported to develop SLS,1,2,22,23 and this increases to 80% frequency of SLS in extremely low birth weight (gestational age <26 weeks) infants.23 Although SLS, ROP, and BPD were frequently observed in infected infants, no statistically significant difference was found between the infected and control groups. Moreover, none of them died related to CMV infection nor need antiviral treatment.
Interestingly, perventricular leukomalacia (PVL) is the most common ischemic brain injury in premature infants with a multifactorial etiology and pathogenesis.24,25 Premature infants are especially vulnerable to PVL which can resulted in CP later in life. Thus, VLBW infants are predisposed to develop CP after discharge from neonatal units. VLBW preterm infants whose gestational age is less than 28 weeks are more than 20% more likely to have CP than term infants.26 Advances in care have resulted in increased survival (50%–70%) of VLBW infants in recent years, resulting in more than 50% of surviving preterm infants with PVL. PVL patients may go on to develop disabilities such as CP, subsequent occurrence of cognitive, behavioral, attentional, or socialization deficits, in 25% to 50% of patients later in life.27,28
In this study, 7 infants with PVL were identified by serial neonatal cranial ultrasound in the control group within 4–6 weeks of life. PVL was diagnosed prior to CMV infection and moderate to severe CP was diagnosed at follow-up. Therefore, we were not surprized that infected infants had higher PDI and MDI scores on the Bayley scales of infant development II than noninfected control group. In follow-up, infants with PVL showed improved neurological function later in life as a result of early intervention. Likewise, hearing impairments resolved without delay in speech.
Follow-up duration in the published literature varies from 6 months to 10.7 years of life. Long-term outcome in VLBW preterm infants after CMV-infection transmitted through CMV-seropositive mothers is still controversial. In the 1980s, Paryani and Yeager13 first reported that early onset of CMV excretion and infection in infants with birth weight less than 2 kg had significant risks of neurological impairments at 3 years of age.14 Other follow-up studies showed that preterm infants might not have increased risk for neurological, cognitive sequelae, and sensorineural hearing problems after CMV postnatal infection.1,29–32 In contrast, recent long-term follow-up studies reported neuropsychiological sequalae and poor cognitive function in adolescents born preterm with early postnatal CMV infection.16,33 In our study, there were no significant differences in the outcome of infected infants with postnatal CMV infection via breast milk between study groups, included mortality, length of hospital stay, BPD, IVH, PVL, or ROP, CP, head circumference, and body weight followed for up to 2 years of corrected age. As feeding intolerance was less frequent in infected infants during hospitalization and after discharge, we expect this resulted in higher anthropometric measures for body weight and height than noninfected babies at follow-up visits.
The available literature that has conducted long-term and large-scale follow-up of the cases is limited. Our patient number is also not large and we only follow up until 12 to 24 months of age. Some confounding factors, such as comorbidities, different treatment regimens, socioeconomic status, parental education level, that might affect neurodevelopmental and neuropsychological outcomes of VLBW infants, were not recorded or analyzed in this study. We, therefore, recommend large prospective, well-controlled, long-term cohort studies are needed to further examine the issues.
In summary, postnatal symptomatic CMV infection transmitted through breastfeeding in preterm infants is not uncommon. Considering the advantages and benefits of breast milk for preterm infants, preventing and minimizing the possibility of postnatal CMV infection in preterm infants is critical. Our results suggest that the benefits of breastfeeding outweigh the risk of acquiring CMV infection, and there is no evidence for increased risk of clinical sequelae at 2 years of age.
Acknowledgements
The authors thank R.H. Davis, PhD. and N.Y. Yim, Msc. for their critical revision of the manuscript and FJ Sun, Msc., Department of Medical Research, MacKay Memorial Hospital, for assistance with statistical analysis.
Footnotes
Abbreviations: BPD = bronchopulmonary dysplasia, CMV = cytomegalovirus, CP = cerebral palsy, IVH = intraventricular hemorrhage, MDI = mental development index, PDIp = sychomotor development index, PVL = periventricular leukomalacia, ROP = retinopathy of prematurity, SLS = sepsis-like symptoms, VLBW = very low birth weight.
NCC and C-SH contributed equally to this work.
The sources of funding of this research were from the Premature Baby Foundation of Taiwan and MacKay Children's Hospital, Taipei, Taiwan (program no.: 8903 and 9503).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kurath S, Halwachs-Baumann H, Müller W, et al. Transmission of cytomegalovirus via breast milk to the prematurely born infant: a systematic review. Clin Microbiol Infect 2010; 16:1172–1178. [DOI] [PubMed] [Google Scholar]
- 2.Hamprecht K, Maschmann J, Vochem M, et al. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet 2001; 357:513–518. [DOI] [PubMed] [Google Scholar]
- 3.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007; 17:253–276. [DOI] [PubMed] [Google Scholar]
- 4.Jim WT, Shu CH, Chiu NC, et al. Transmission of cytomegalovirus from mothers to preterm infants by breast milk. Pediatr Infect Dis J 2004; 23:845–851. [DOI] [PubMed] [Google Scholar]
- 5.Maschmann J, Hamprecht K, Dietz K, et al. Cytomegalovirus Infection of extremely low birth weight infants via breast milk. CID 2001; 33:1998–2003. [DOI] [PubMed] [Google Scholar]
- 6.Hamprecht K, Goeiz R, Maschmann J. Breast milk and cytomegalovirus infection in preterm infants. Early Hum Dev 2005; 81:989–996. [DOI] [PubMed] [Google Scholar]
- 7.Schleiss MR. Acquisition of human cytomegalovirus infection in infants via breast milk: natural immunization or cause for concern? Rev Med Virol 2006; 16:73–82. [DOI] [PubMed] [Google Scholar]
- 8.Hamprecht K, Maschmann J, Jahn G, et al. Cytomegalovirus transmission to preterm infants during lactation. J Clin Virol 2008; 41:198–205. [DOI] [PubMed] [Google Scholar]
- 9.Jim WT, Shu CH, Chiu NC, et al. High cytomegalovirus load and prolonged virus excretion in breast milk increase risk for viral acquisition by very low birth weight infants. Pediatr Infect Dis J 2009; 28:891–894. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda A, Kimura H, Hayakawa M, et al. Evaluation of cytomegalovirus infections transmitted via breast milk in preterm infants with a real-time polymerase chain reaction assay. Pediatrics 2003; 111:1333–1336. [DOI] [PubMed] [Google Scholar]
- 11.Miron D, Brosilow S, Felszer K, et al. Incidence and clinical manifestations of breast milk-acquired cytomegalovirus infection in low birth weight infants. J Perinatol 2005; 25:299–303. [DOI] [PubMed] [Google Scholar]
- 12.Lanzieri TM, Dollard SC, Josephson CD, et al. Breast milk-acquired cytomegalovirus infection and disease in VLBW and premature Infants. Pediatrics 2013; 131:e1937–e1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeager AS, Palumbo PE, Malachowski N, et al. Sequelae of maternally derived cytomegalovirus infections in premature infants. J Pediatr 1983; 102:918–922. [DOI] [PubMed] [Google Scholar]
- 14.Paryani S, Yeager AS, Hosford-Dunn H, et al. Seqielae of acquired cytomegalovirus infecion in premature and sick term infants. J Pediatr 1985; 107:451–456. [DOI] [PubMed] [Google Scholar]
- 15.Johnson S, Hosford-Dunn H, Paryani S, et al. Prevalence of sensorineural hearing loss in premature and sick term infants with perinatally acquired cytomegalovirus infection. Ear Hear 1986; 7:325–327. [DOI] [PubMed] [Google Scholar]
- 16.Bevot A, Hamprecht K, Krägeloh-Mann I, et al. Long-term outcome in preterm children with human cytomegalovirus infection transmitted via breast milk. Acta Paediatric 2012; 101:e167–e172. [DOI] [PubMed] [Google Scholar]
- 17.Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the national institutes of health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005; 116:1353–1360. [DOI] [PubMed] [Google Scholar]
- 18.Surveillance of Cerebral Palsy in Europe (SCPE). Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol 2000; 42:816–824. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh WS, Wu HC, Jeng SF, et al. Nationwide singleton birth Weight percentiles by gestational age in Taiwan, 1998–2002. Acta Paediatr Tw 2006; 47:25–33. [PubMed] [Google Scholar]
- 20.Yoshinaga-Itano C, Sedey AL, Coulter DK, et al. Language of early- and later-identified children with hearing loss. Pediatrics 1998; 102:1161–1171. [DOI] [PubMed] [Google Scholar]
- 21.American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012; 129:e827–e841. [DOI] [PubMed] [Google Scholar]
- 22.Dworsky M, Yow M, Stagno S, et al. Cytomegalovirus infection of breast milk and transmission in infancy. Pediatrics 1983; 72:295–299. [PubMed] [Google Scholar]
- 23.Vochem M, Hamprecht K, Jahn G, et al. Transmission of cytomegalovirus to preterm infants through breast milk. Pediatr Infect Dis J 1998; 17:53–58. [DOI] [PubMed] [Google Scholar]
- 24.Perlman JM, Risser R, Broyles RS. Bilateral cystic periventricular leukomalacia in the premature infant: associated risk factors. Pediatrics 1996; 97:822–827. [PubMed] [Google Scholar]
- 25.Blumenthal I. Periventricular leucomalacia: a review. Eur J Pediatr 2004; 163:435–442. [DOI] [PubMed] [Google Scholar]
- 26.Wu YW, Xing G, Fuentes-Afflick E, et al. Racial ethnic and socioeconomic disparities in the prevalence of cerebral palsy. Pediatrics 2011; 127:e674–e681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res 2001; 50:553–562. [DOI] [PubMed] [Google Scholar]
- 28.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 2009; 8:110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar M, Nankervis GA, Jacobs IB. Congenitally and postnatally acquired cytomegalovirus infections: long-term follow-up. J Pediatr 1984; 104:674–679. [DOI] [PubMed] [Google Scholar]
- 30.Vollmer B, Seibold-Weiger K, Schmitz-Salue C, et al. Postnatally acquired cytomegalovirus infection via breast milk: effects on hearing and development in preterm infants. Pediatr Infect Dis J 2004; 23:322–327. [DOI] [PubMed] [Google Scholar]
- 31.Capretti MG, Lanari M, Lazzarotto T, et al. Very low birth weight infants born to cytomegalovirus-seropositive mothers fed with their mother's milk: a prospective study. J Pediatr 2009; 154:842–848. [DOI] [PubMed] [Google Scholar]
- 32.Luck S, Sharland M. Postnatal cytomegalovirus: innocent bystander or hidden problem? Arch Dis Child Fetal Neonatal Ed 2009; 94:F58–F64. [DOI] [PubMed] [Google Scholar]
- 33.Brecht KF, Goelz R, Bevot A, et al. Postnatal human cytomegalovirus infection in preterm infants has long term neuropsychological sequelae. J Pediatr 2015; 166:834–839. [DOI] [PubMed] [Google Scholar]


