Supplemental Digital Content is available in the text
Abstract
To assess left ventricular ejection fraction (LVEF) accurately, cardiac magnetic resonance (CMR) can be indicated and lays on the evaluation of multiple slices of the left ventricle in short axis (CMRSAX). The objective of this study was to assess another method consisting of the evaluation of 2 long-axis slices (CMRLAX) for LVEF determination in acute myocardial infarction.
One hundred patients underwent CMR 2 to 4 days after acute myocardial infarction. LVEF was computed by the area-length method on horizontal and vertical CMRLAX images. Those results were compared to reference values obtained on contiguous CMRSAX images in one hand, and to values obtained from transthoracic echocardiography (TTE) in the other hand. For CMRSAX and TTE, LVEF was computed with Simpson method. Reproducibility of LVEF measurements was additionally determined. The accuracy of volume measurements was assessed against reference aortic stroke volumes obtained by phase-contrast MR imaging.
LVEF from CMRLAX had a mean value of 47 ± 8% and were on average 5% higher than reference LVEF from CMRSAX (42 ± 8%), closer to routine values from TTELAX (49 ± 8%), much better correlated with the reference LVEF from CMRSAX (R = 0.88) than that from TTE (R = 0.58), obtained with a higher reproducibility than with the 2 other techniques (% of interobserver variability: CMRLAX 5%, CMRSAX 11%, and TTE 13%), and obtained with 4-fold lower recording and calculation times than for CMRSAX. Apart from this, CMRLAX stroke volume was well correlated with phase-contrast values (R = 0.81).
In patients with predominantly regional contractility abnormalities, the determination of LVEF by CMRLAX is twice more reproducible than the reference CMRSAX method, even though the LVEF is consistently overestimated compared with CMRSAX. However, the CMRLAX LVEF determination provides values closer to TTE measurements, the most available and commonly used method in clinical practice, clinical trials, and guidelines in ischemic cardiomyopathy. Moreover, LVEF determination by CMRLAX allows a 63% gain of acquisition/reading time compared with CMRSAX. Thus, despite the fact that LVEF obtained from CMRSAX remains the gold standard, CMRLAX should be considered to shorten the overall imaging acquisition and reading time as a putative replacement.
INTRODUCTION
Left ventricular ejection fraction (LVEF) with left ventricle (LV) volume estimation remains the main imaging prognostic marker after a ST-segment elevation myocardial infarction (STEMI).1,2
Cardiac magnetic resonance (CMR) imaging with a cardiac short-axis stack acquisition (CMRSAX) is the gold standard for LVEF estimation because of its volumetric approach for nonsymmetric ventricles with wall motion abnormalities.3 After a STEMI, CMR is increasingly used for coronary microvascular status assessment and quantification of infarct size and viability. However, as it involves multiple short-axis acquisitions, CMRSAX is time-consuming and requires multiple breath-holds. Therefore, the utilization of CMRSAX is often difficult in unstable patients such as those in the acute phase of STEMI. Within days of their admission, STEMI patients are indeed likely to have dyspnea, to require oxygen therapy or continuous intravenous infusions, and can suffer from recurring ischemia or experience atrial and ventricular arrhythmia. As a consequence, the “classical” CMRSAX strategy, owing to the time needed for its acquisition and the need for multiple breath-holds required for its implementation, is unlikely to be routinely used during the initial hospitalization for a STEMI.
In routine clinical practice and in clinical trials, transthoracic echocardiography (TTE) LV volume and LVEF evaluation by the Simpson method of disks summation is the most commonly used and is the recommended method.4
Nevertheless, measurements of cardiac volumes and LVEF can be performed with CMR long-axis views (CMRLAX) using the area-length method. Since this method necessitates shorter acquisition and data analysis time, this approach may be of interest in the setting of the index hospitalization for a STEMI. Despite the above noteworthy features of long-axis CMR measurements, very limited data have been reported with regard to comparison of LV volume calculation by long and short-axis CMR methods, with no available data in the setting of the acute phase of STEMI.
The aim of the present study was hence to compare LV volume quantification and LVEF measurements using CMRSAX, CMRLAX, and TTE.
METHODS
Population
Hundred patients (55.4 ± 11.1 years) over 145 were taken out from a prospective monocentric cohort study (REMI study, Remodeling after Myocardial Infarction) performed in our university hospital between April 2010 and December 2012. Those patients presented successfully reperfused first acute STEMI and were referred for both CMR and TTE after the revascularization. The measurements for this ancillary study were performed before the end of the REMI study in the first 100 consecutive patients to validate readers and anticipate the final reading.
The study protocol was approved by the local Ethics Committee and released on the ClinicalTrials.gov site under the identifier NCT01109225.
Contrast-enhanced Cardiac Magnetic Resonance Imaging
Imaging was performed on a 3T system (General Electric Signa HDxt, Milwaukee, USA) with an 8-phased array cardiac coil, ECG triggering, and breath-holding in expiration. After a series of scout images to determine the position and orientation of the LV within the thorax, cine CMR sequences for cardiac function were performed with steady-state free precession technique in 10 to 15 parallel short-axis, 1 horizontal long-axis, and 1 vertical long-axis, at 30 phases/cardiac cycle. Each slice (slice thickness: 8 mm, gap: 0 mm) was obtained during 1 breath-hold of 10 to 15 seconds. Phase-contrast (PC) images were also acquired to compute the stroke volume (SV).
SAX Analysis
On short-axis view, the outline of the endocardial border of the LV was traced manually on all slices of each phase (Fig. 1A) by 1 experienced cardiologist or 1 radiologist using standard software (Mass Research software, version V2013-EXP, Leiden University Medical Center). Volumes were computed by Simpson method of disks summation, whereby the sum of cross-sectional areas was multiplied by slice thickness (8 mm). We followed the Task Force for Postprocessing developed by the Society for Cardiovascular MR (SCMR).5 The LV outflow tract is included as part of the LV blood volume. Papillary muscles and trabeculation were included as part of the LV volume.
FIGURE 1.
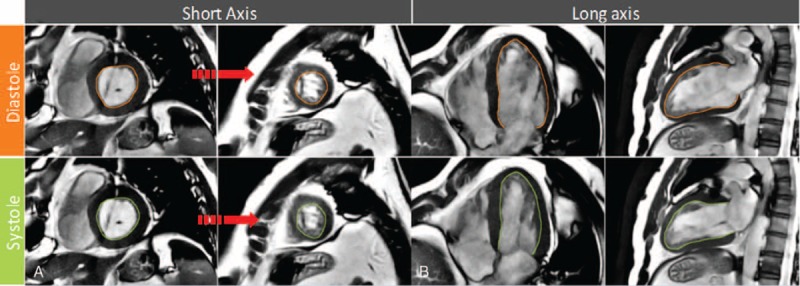
A, Basal and midventricular short-axis view with diastolic and systolic contours. B, Long-axis view in a VLAX and HLAX with diastolic and systolic contours. Noted that LV trabecular tissue and connected papillary muscles included. The LV outflow tract is included as part of the LV blood volume. Papillary muscles and trabeculations were included as part of the LV volume. HLAX = horizontal long axis; LV = left ventricle; VLAX = vertical long axis.
LAX Analysis
Endocardial borders were manually traced using the same software on a vertical long-axis view (VLAX; Fig. 1B) and on a horizontal long-axis view (HLAX) for each phase. Volumes were determined using the long-axis area-length method. End-diastolic volume (EDV), end-systolic volume (ESV), and LVEF associated with VLAX and HLAX were obtained. LAX estimates were obtained by calculating the mean of estimates from the 2 long-axis planes.
Phase-contrast Analysis
On PC images, the lumen of the ascending aorta was segmented automatically and corrected manually throughout the cardiac cycle by the same operator. SV (mL) was obtained by dividing cardiac output (L/min) by heart rate (bpm). PC imaging was considered as the reference method for SV estimation.6
Echocardiography
Echocardiographic examinations were performed by an experienced sonographer who acquired a complete 2-dimensional (2D) TTE including 4 and 2-chamber apical LV views. (Vivid E9, General Electric- Vingmed Horten Norway). All acquired images were evaluated offline by a cardiologist, using the standard biplane method. The LV volume was calculated from the summation of a stack of discs where the height of each disc is calculated as a fraction of LV length (Fig. 2).4
FIGURE 2.
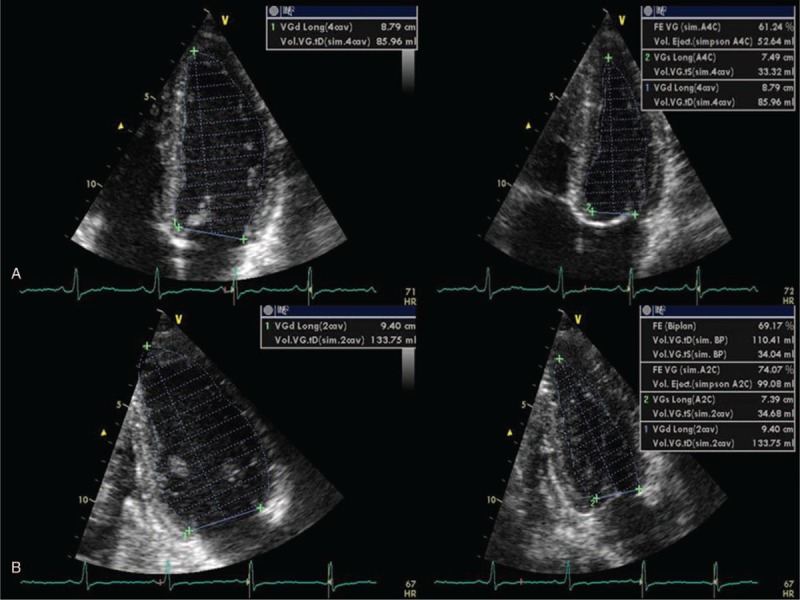
LV end-diastolic and end-systolic volume by Simpson 4-chamber (A) and LV end-diastolic and end-systolic volume by Biplane Simpson 2-chamber method (B). LV = left ventricle.
LVEF Estimation Method
Cardiac magnetic resonance and TTE LVEF were based on endocardial tracing of the LV chamber from the images obtained on different axis views. The quantitative determination of EF was calculated using LV end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV) estimates as follows: LVEF = (LVEDV − LVESV)/LVEDV according to guidelines.7 Both the Simpson method and the area-length method were applied to CMR.
Reproducibility Analysis
For both SAX and LAX measurements, a randomly chosen subgroup (30 patients) was processed twice for intraobserver analysis. For interobserver variability analysis, SAX volumes were measured separately by 1 experienced cardiologist and 1 radiologist. LAX volumes were computed by 1 cardiologist and 1 magnetic resonance imaging (MRI) technologist.
Statistical Analysis
Statistical analysis was performed using SPSS version 17 (Chicago, IL). Quantitative values were expressed as the mean value ± SD. Correlation between imaging strategies was summarized using standard (Pearson) correlation. Limits of agreement between imaging methods and between readings were estimated as mean difference of the differences, as described by Bland and Altman. Percentage variability was calculated as the absolute difference divided by the average of the measurements (bias) ± 2 SD. A P value <0.05 was considered to indicate statistical significance.
RESULTS
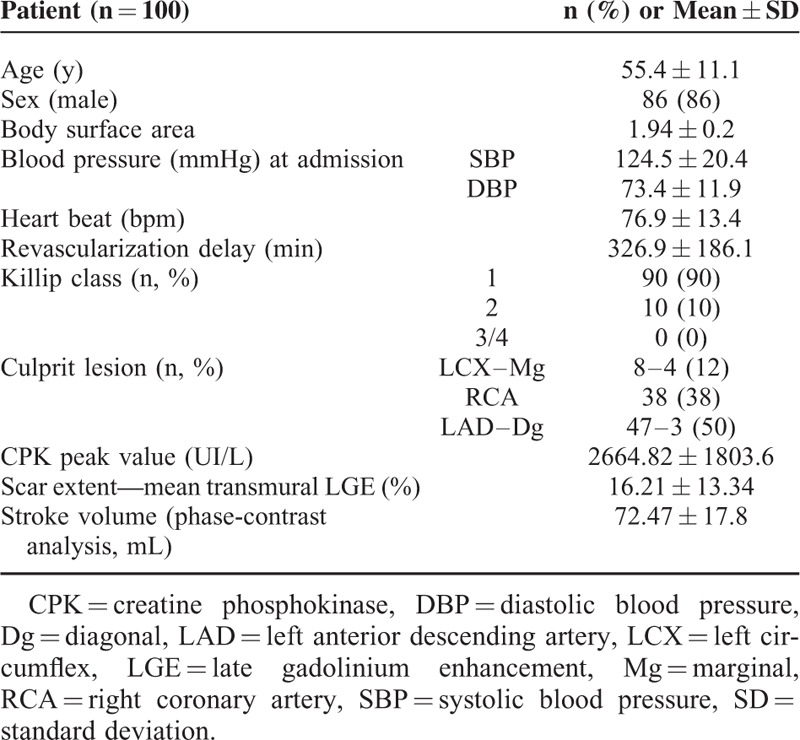
A total of 100 patients with first STEMI were enrolled in the study and completed the MRI and echo studies. Time from hospital admission to CMR was 2.38 ± 0.76 days. Echocardiography was performed in less than 48 hours on the same day as CMR in most patients (mean interval delay between CMR and TTE = 20.0 hours ± 21.2). Patients’ characteristics are summarized in Table 1. Patient characteristics of the whole REMI population (145 patients) are summarized in Supplemental Table 1 (http://links.lww.com/MD/A483).
TABLE 1.
Patient Characteristics
Comparison of LVEF Measurements According to the Different Techniques
The mean LVEF measured with CMRSAX (42.1 ± 7.9%) was lower than that measured with CMRLAX (47.1 ± 7.9%) and TTE (49.4 ± 8.3%): LVEF values obtained with CMRLAX were closer to TTE ones than with CMRSAX (Table 2). LVEF assessed with CMRLAX and CMRSAX showed good correlation (R = 0.88). Apart from this, the correlations between TTE and CMRSAX or CMRLAX were lower, but similar (R = 0.58 and 0.66, respectively). Bland–Altman analysis showed that LVEF determined from CMRLAX was almost systematically higher than LVEF determined from CMRSAX (mean absolute difference of 4.95 ± 3.9%; Fig. 3).
TABLE 2.
Echocardiographic and CMR Characteristics of LV Systolic Function
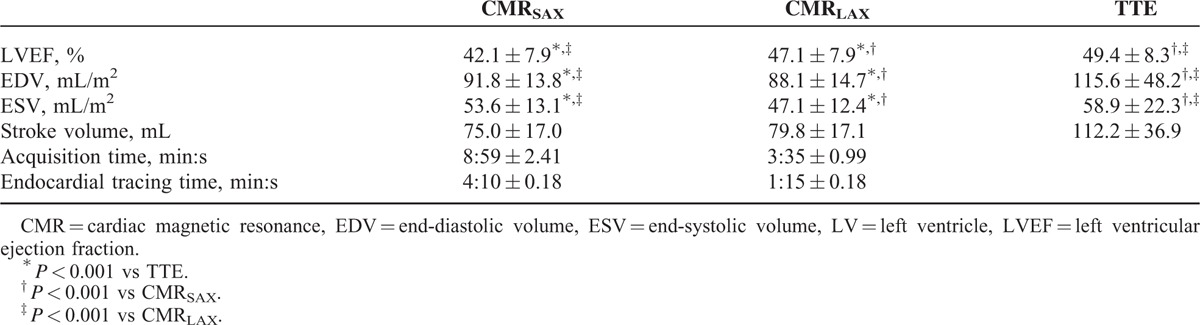
FIGURE 3.
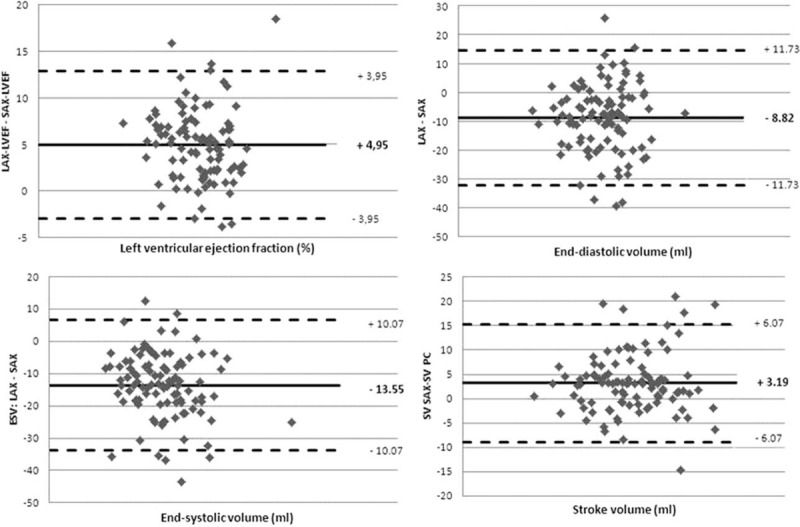
Bland–Altman analysis of intermethod agreement for global left ventricular function. The mean CMR difference and index tests are represented by the solid line with their limits of agreement (95% confidence intervals, 1.96 SD). CMR = cardiac magnetic resonance, SD = standard deviation.
Comparison of LV Volume Measurements According to the Different Techniques
End-diastolic volumes were very similar in CMRSAX and CMRLAX (Table 2), whereas TTEEDV was markedly higher. Bland–Altman analysis (Fig. 3) showed that LVEDV and LVESV estimated from CMRLAX were almost systematically lower than those estimated from CMRSAX.
Comparison of Stroke Volume Measurements According to the Different Techniques
Stroke volumes were very similar in CMRSAX and CMRLAX, whereas they were markedly higher in TTE. PC SV measurements were marginally closer to CMRSAX measurements than CMRLAX measurements (mean SVPC 72.5 ± 17.8 mL, range 30.9–138.0 mL). Linear comparison showed that SV estimation with the PC method was better correlated with CMRSAX (R = 0.89) than with CMRLAX (R = 0.81).
Comparison of the Reproducibility of the Different Imaging Techniques
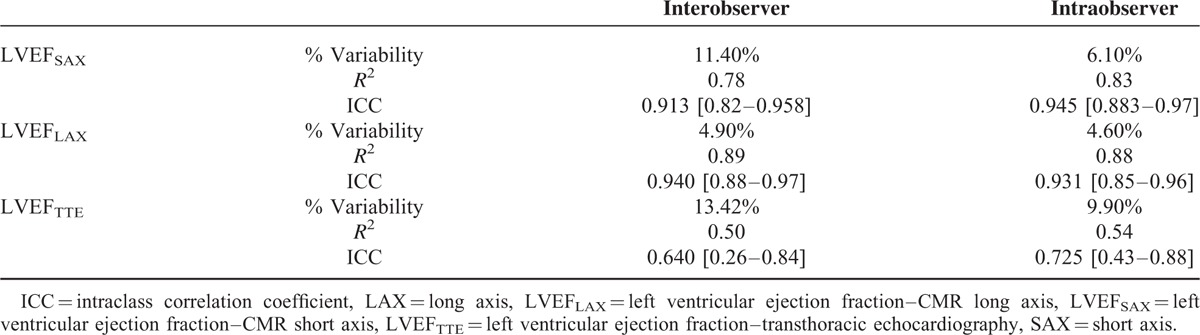
The interobserver limits of agreement with CMRSAX were −3.1 to 14.7 mL for EDV and −12.3% to 3.7% for EF. The interobserver limits of agreement with CMRLAX were broader with regard to EDV (−22.5 to 9.3 mL), but narrower with regard to EF (−4.5% to 6.9%). In contrast, the interobserver limits of agreement were much broader with TTE than that observed with both CMR methods (−35.03 to 31.89 mL for EDV and −17.01% to 9.71% for EF). The intraobserver limits of agreement were much wider for CMRSAX (−16.6 to 17.6 mL for EDV and −8.5% to 7.3% for EF) than that observed for CMRLAX (−10.1 to 7.7 mL for EDV and −3.8% to 5.7% for EF). The intraobserver limits of agreement were much wider for TTE than for the 2 CMR methods (−35.10 to 24.12 mL for EDV and −11.89% to 9.98% for EF). When focusing solely on LVEF, CMRLAX had a lower % of variability and higher correlation for both inter and intraobserver reproducibility than that observed with CMRSAX and TTE (Table 3). The intraclass correlation coefficient (ICC) for both inter and intraobserver reproducibility observed with CMRLAX was marginally better than that observed for CMRSAX.
TABLE 3.
Interobserver and Intraobserver Variability for Left Ventricular Ejection Fraction Measurement
Comparison of the Time Needed to Perform the 2 CMR Techniques
Acquisition time for CMRSAX and CMRLAX measurement was, respectively (in minute:second), 8:59 ± 2.41 and 3:35 ± 0.99, whereas the time needed for endocardial border tracing was 4:10 ± 0.18 for CMRSAX and 1:15 ± 0.18 for CMRLAX. The global time saving was 8 minutes and 19 seconds per study (Table 2).
DISCUSSION
In this study, CMRLAX proved to be an accurate method to measure global LV parameters, including LVEF, compared with CMRSAX. However, CMRLAX was associated with a systematic underestimation of LV volume compared with CMRSAX. LVEF measurements using CMRLAX and TTE were very similar, which could be the consequence of similar volume calculations based on long axis in both techniques. Our data also indicate that CMRLAX measurements were much faster and had an overall better reproducibility than CMRSAX, which would suggest its particular relevance in an acute clinical setting such as in patients within days of acute myocardial infarction (AMI).
Limits of SAX Evaluation
Functional analysis with cardiac MRI using short axis (SAX) images of the ventricles is independent of geometric assumption and is now accepted as the gold standard for LV volume calculation.8,9 Nevertheless, CMRSAX requires regular and sometimes long breath-holds,3,10 which leads to an uncomfortable and long acquisition. Time is thus the main limitations of CMRSAX and restricts its widespread implementation for patients in AMI. Indeed, this method can be challenging in patients with impaired cardiopulmonary status, who are unable to hold their breath, or in those with severe arrhythmia. If nearly all currently implemented volumetric methods, whether CMR or echocardiography-based, require multiple cross-sectional views of the heart, CMRSAX is the technique which requires the most time-intensive acquisition and analysis. In our study, SV estimated by LV volume calculation from the SAX has the best correlation with the SV calculated by PC and reinforce that this method remains a reference to compare several imaging strategies for LV systolic function quantification. Pearson correlation coefficients were 0.89 between PC and SAX measurement, and 0.81 between PC and LAX methods. Compared with standard invasive measurements, velocity-encoded phase-difference CMR can accurately and rapidly determine cardiac output. Several studies have already demonstrated the interest of cardiac output—PC guidance to validate the LVEF.11
Correlation of SAX and LAX Methods
The CMRLAX measurements have been already proposed as an alternative for LVEF calculation.12 Thus, biplane method is attractive, requiring only 2 views and minimal data acquisition and analysis times. Apart from this, biplane assessment cine CMR imaging does not rely on geometric assumptions or calculations based on incomplete sampling of the cardiac volumes.8
Only a few studies have compared SAX and LAX measurements by CMR imaging.13 The authors concluded that there were no significant advantages of simplified MRI techniques over modified Simpson method echocardiography. In our study, we found good correlation and agreement between CMRLAX and volumetric CMRSAX measurement, and better correlation between LVEFLAX versus LVEFTTE compared with LVEFSAX versus LVEFTTE. Biplane CMR is of particular interest, given that, in contrast to TTE, MRI is not dependent on good acoustic windows, and, in principle, allows reliable acquisition of the required 2 orthogonal standard planes of the heart. One of the reasons why CMRLAX measurements exhibit a better correlation with the biplane Simpson method than the CMRSAX measurement may be related to the fact that in CMRLAX, similar walls are taken into account for calculating global cardiac function.
Agreement Between SAX and LAX Methods
In our study, LAX tended to overestimate LVEF compared with the CMRSAX measurement. The results herein confirm that the bias is not due to the choice of the imaging modality (echocardiography or CMR), but rather to the method of measurement (SAX vs LAX). Compared with TTE measurements, CMRLAX showed a modest correlation, but good agreement (−2.4%). In the key comparative substudy published by Chuang et al,14 the authors found a poor agreement between biplane and volumetric methods, and worse agreement between biplane methods, with a greater impact on the results than the actual choice of imaging technique. Childs et al also showed that LVEF can be adequately estimated using the single and biplane ellipsoid models, whereas SV tends to be overestimated using LAX models compared to SAX.15 In their study, Dulce et al16 used nonbreath-hold cine MRI using a variety of monoplane and biplane geometric formulas to determine LV volume and EF, which they then compared with SAX stack volumetric results. The hemisphere-cylinder model (5/6 area-length) overestimated LV volumes while underestimating EF.16
These aforementioned studies suggest that aggregate volumetric and biplane MRI measurements of LV volume and LVEF differ only moderately. Hence, CMRLAX slightly overestimates LVEF compared with the CMRSAX measurement, mainly due to an underestimation of ESVLAX.
Reproducibility and Time-saving of LAX Measurements
One advantage of LAX views is the low susceptibility to errors induced by misinterpretation of slices in proximity to the mitral valve. Interobserver variability was smaller with LAX measurements as previously described.15 It should be emphasized that the through-out plane motion of the basal SAX image represents an important source of error, both for LV and RV SAX evaluation. In a study by Bloomer et al,17 high-quality images were obtained for analysis, and the measured volumes with LAX SSFP (Steady State Free Precession) sequence correlated well with SAX volumes (r > 0.98). They concluded that this combination of sequence and scan orientation displays intrinsic advantages for image analysis due to the improved contrast and the avoidance of errors associated with the basal slice in the SAX orientation.17 Given the greater reproducibility of CMRLAX in measuring LVEF over CMRSAX, the potential of detecting small changes in myocardial volume over time or as a result of LV remodeling or therapy is therefore enhanced.
Long-axis measurement is attractive, as it only requires 2 views and is completely transferable to the most commonly utilized biplane method used in clinical practice after an AMI. This method requires less imaging and data analysis time. The saving time to obtain LV function data (acquisition and off-line post-treatment) was approximately 63% (8 minutes and 19 seconds) per patient. Therefore, modified Simpson rule and biplane ellipsoid models, with their shorter acquisition and processing times, may increase the clinical availability of cine MRI.16 However, biplane MRI is still subject to the limitations of geometric assumptions. To improve the accuracy, a third-chamber LAX, similar to the case for triplane measurement by echocardiography, may therefore be added.
Limitations of the Study
One of the limitations of the study is the high number of patient Killip class 1 (90%). Only few patients had large MI with low EF; thus the range of LV dysfunction was limited, which might have contributed to the lower associations between LV function evaluation methods than expected. Nevertheless, we demonstrated good agreement in patients with relative preserved ejection fraction. Moreover, these small infarcts are the consequences of the currently very effective revascularization strategies. Yet, risk stratification for remodeling in these patients remains challenging.
In addition, our cohort participants were overall quite young. Our findings should consequently be verified in older populations and also in patients with higher Killip class score/signs of heart failure.
Clinical Implications
After a STEMI, CMR should increasingly be used for coronary microvascular status assessment and quantification of infarct size. Unfortunately, MRI is rarely performed in patients with MI in most countries and hospitals due primarily to lack of machine availability.18 One of the main limitations of CMRSAX is the time-consuming nature of data acquisition which restricts the widespread implementation for all the patients in AMI.
On top of the LV function information, CMR could provide precious information about microvascular damage, infarct size, and viability. The sole TTE is unable to provide this information in acute phase of an acute event. Considering the important clinical value of CMR, even when performed very early after MI, a practical approach would be to perform CMR at the time of the initial hospitalization for AMI. In this study, we show that the use of CMRLAX would decrease the total examination time by approximately 10 minutes compared with CMRSAX. Decreasing the duration of the CMR by 10 minutes would have an important impact on its availability within the initial hospital stay for an AMI; we would thus suggest the utility of a faster AMI protocol based on CMRLAX. Moreover, by decreasing the duration of the study and the number of apnea, it may be safer to realize CMR in patients with impaired cardiopulmonary status or Killip ≥2.
CONCLUSIONS
In a particular population composed mostly of patients with no clinical signs of heart failure and low mortality risk, CMR is useful to accurately quantify patients’ LVEF. In this case, CMRLAX measurements of LV volumes and ejection fraction allow accurate, fast, and reliable assessment of global LV function and exhibit good correlation with CMRSAX measurements. Despite the fact that CMR LVEFSAX remains the gold standard, a biplane LAX MRI study should nonetheless be considered to shorten the overall imaging acquisition and reading time as a putative replacement. Moreover, CMRLAX methods provide values closer to TTE ejection fraction measurements. Even if TTE is the most commonly used and available method in clinical practice, as well as for clinical trial and guidelines in ischemic cardiomyopathy because of its great availability and its cost, CMR has the advantage of allowing the adjunction of other specific useful sequences to evaluate scar expansion.
Acknowledgments
This REMI study was supported by PHRC (Programme Hospitalier de Recherche Clinique).
Footnotes
Abbreviations: AMI = acute myocardial infraction, CMR = cardiac magnetic resonance, EDV = end-diastolic volume, ESV = end-systolic volume, HLAX = horizontal long axis, LAX = long axis, LEVF = left ventricular ejection fraction, LV = left ventricular, MR = magnetic resonance, MRI = magnetic resonance imaging, PC = phase contrast, SAX = short axis, SCMR = SOCIETY of Cardiovascular Magnetic Resonance, STEMI = ST-segment elevation myocardial infarction, SV = stroke volume, TTE = transthoracic echocardiography, VLAX = vertical long axis.
Clinical Trial Registration http://clinicaltrials.gov/show/NCT01109225; NCT01109225.
The authors declare that they have no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Sanz G, Castaner A, Betriu A, et al. Determinants of prognosis in survivors of myocardial infarction: a prospective clinical angiographic study. N Engl J Med 1982; 306:1065–1070. [DOI] [PubMed] [Google Scholar]
- 2.Curtis JP, Sokol SI, Wang Y, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol 2003; 42:736–742. [DOI] [PubMed] [Google Scholar]
- 3.Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J 2000; 21:1387–1396. [DOI] [PubMed] [Google Scholar]
- 4.Lang RM, Bierig M, Devereux RB, et al. , Chamber Quantification Writing G, American Society of Echocardiography's G, Standards C, European Association of E. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18(12):1440–1463. [DOI] [PubMed] [Google Scholar]
- 5.Schulz-Menger J, Bluemke DA, Bremerich J, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. Journal of cardiovascular magnetic resonance: official journal of the Society for Cardiovascular. Magn Resonance 2013; 15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Firmin DN, Nayler GL, Kilner PJ, et al. The application of phase shifts in NMR for flow measurement. Magn Resonance Med 1990; 14:230–241. [DOI] [PubMed] [Google Scholar]
- 7.American College of R, Society of Cardiovascular Computed T, Society for Cardiovascular Magnetic R, American Society of Nuclear C, North American Society for Cardiac I, Society for Cardiovascular A, Interventions, Society of Interventional R. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging. A report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group. J Am Coll Radiol 2006; 3(10):751–771. [DOI] [PubMed] [Google Scholar]
- 8.Semelka RC, Tomei E, Wagner S, et al. Interstudy reproducibility of dimensional and functional measurements between cine magnetic resonance studies in the morphologically abnormal left ventricle. Am Heart J 1990; 119:1367–1373. [DOI] [PubMed] [Google Scholar]
- 9.Kleijn SA, Brouwer WP, Aly MF, et al. Comparison between three-dimensional speckle-tracking echocardiography and cardiac magnetic resonance imaging for quantification of left ventricular volumes and function. Eur Heart J Cardiovasc Imaging 2012; 13:834–839. [DOI] [PubMed] [Google Scholar]
- 10.Sakuma H, Fujita N, Foo TK, et al. Evaluation of left ventricular volume and mass with breath-hold cine MR imaging. Radiology 1993; 188:377–380. [DOI] [PubMed] [Google Scholar]
- 11.Hundley WG, Li HF, Hillis LD, et al. Quantitation of cardiac output with velocity-encoded, phase-difference magnetic resonance imaging. Am J Cardiol 1995; 75:1250–1255. [DOI] [PubMed] [Google Scholar]
- 12.Bloomgarden DC, Fayad ZA, Ferrari VA, et al. Global cardiac function using fast breath-hold MRI: validation of new acquisition and analysis techniques. Magn Resonance Med 1997; 37:683–692. [DOI] [PubMed] [Google Scholar]
- 13.Hazirolan T, Tasbas B, Dagoglu MG, et al. Comparison of short and long axis methods in cardiac MR imaging and echocardiography for left ventricular function. Diagn Interven Radiol 2007; 13:33–38. [PubMed] [Google Scholar]
- 14.Chuang ML, Hibberd MG, Salton CJ, et al. Importance of imaging method over imaging modality in noninvasive determination of left ventricular volumes and ejection fraction: assessment by two- and three-dimensional echocardiography and magnetic resonance imaging. J Am Coll Cardiol 2000; 35:477–484. [DOI] [PubMed] [Google Scholar]
- 15.Childs H, Ma L, Ma M, et al. Comparison of long and short axis quantification of left ventricular volume parameters by cardiovascular magnetic resonance, with ex-vivo validation. J Cardiovasc Magn Resonance 2011; 13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dulce MC, Mostbeck GH, Friese KK, et al. Quantification of the left ventricular volumes and function with cine MR imaging: comparison of geometric models with three-dimensional data. Radiology 1993; 188:371–376. [DOI] [PubMed] [Google Scholar]
- 17.Bloomer TN, Plein S, Radjenovic A, et al. Cine MRI using steady state free precession in the radial long axis orientation is a fast accurate method for obtaining volumetric data of the left ventricle. J Magn Resonance Imaging 2001; 14:685–692. [DOI] [PubMed] [Google Scholar]
- 18.Products - Health United States - Tables - 2011 Complete List [Internet]. Available from: http://www.cdc.gov/nchs/hus/contents2011.htm#123 2011. Cited July 6, 2014].


