Abstract
Tuberculosis (TB) disease may be transmitted to close contacts of index cases, causing physical illness. No studies have investigated the risk of developing depressive disorder among TB contacts in a TB-endemic area.
Adult participants with a new diagnosis of TB contact (ICD-9-CM codes V01.1 plus chest radiographic order) since January 1, 2008, were identified from the National Health Insurance Research Database in Taiwan. A control cohort matched for age (±5 y), sex, enrolled years, and income level was selected. These 2 cohorts were followed until December 31, 2012, and observed for the development of depressive disorder. The Kaplan-Meier method and the log-rank test were used to examine the difference in cumulative incidences of depressive disorder between groups. Cox proportional-hazard models were used to calculate adjusted hazard ratios (aHRs) for depressive disorder.
The TB contact cohort consisted of 9046 patients and matched controls of 36,184 ones. The mean age of TB contacts was 44.7 years, and 56.0% of them were women. During a mean follow-up period of 2.5 years, 127 (1.40%) TB contacts and 521 (1.44%) matched controls developed depressive disorder. TB exposure was found to be an independent risk factor of depressive disorder in women (aHR 1.34, 95% confidence interval [CI] 1.07–1.68), but not in men (aHR 0.71, 95% CI 0.48–1.06) after adjusting for age, comorbidities, and income levels. The risk of depression was significantly higher for female TB contacts than for matched controls in the first and second years (aHR 1.49, 95% CI 1.03–2.14; and aHR 1.53, 95% CI 1.05–2.23, respectively), but not thereafter. Of note, 67 (0.74%) TB contacts and 88 (0.24%) matched controls developed active TB, but none of them had subsequent depressive disorder during follow-up periods.
Female TB contacts had an increased risk of depression within the first 2 years after exposure. Clinicians should consider conducting depression evaluations in addition to routine TB contact investigations in this subgroup population.
INTRODUCTION
Patients infected with Mycobacterium tuberculosis have tuberculosis (TB) disease and are at high risk of morbidity and mortality. Although TB incidence rates have been falling worldwide, the rate of decline remains slow, and the rate of poor outcomes is unacceptably high.1 TB affects not only the physical health but also the psychosocial well being of patients.2,3 For TB patients, depression is common, and the presence of depressive disorder at baseline is associated with a negative outcome of TB treatment.4 In addition, the risk of developing depressive disorder is higher in TB patients than in the general population.5,6 More importantly, since TB is an airborne disease, an index case in a community can transmit infection to more than 10 people over a 1-year period, and this is a major public health issue.7
People exposed to TB cases have a higher risk of acquiring TB infection than the general population.8 They may have active TB or only latent TB infection (LTBI).9 Importantly, a person with LTBI still has a 5% to 10% lifetime risk of developing active TB disease, and half of this risk occurs in the first 2 years after exposure.10 Fears of infection and the perception of TB stigma are associated with serious socioeconomic consequences.11 Currently, close contacts of TB cases are investigated regularly to rule out active TB disease and, especially for risk groups, to detect LTBI in TB-endemic areas.12 However, no mental health surveys on TB contacts have been conducted in the past. Thus, whether TB contacts are at risk of psychological stress remains unclear.
We hypothesized that TB contacts may develop depressive disorder because of worrying about the risk of TB infection in themselves and potential socioeconomic stress if the index cases of TB are their family members or live with them. To test this hypothesis, we conducted a population-based cohort study to investigate the risk of depressive disorder among TB contacts.
METHODS
Study Design, Setting and Data Source
This research was a retrospective, population-based cohort study using data obtained from the National Health Insurance Research Database (NHIRD) in Taiwan. In Taiwan, an area with intermediate TB incidence, either an 8-hour exposure to index TB cases within 1 day or a 40-hour cumulative exposure is used to define contacts.13,14 TB contacts received chest radiographic imaging (CXR) to rule out active TB at outpatient clinics and had a documented International Classification of Disease, Nine Revision, Clinical Modification (ICD-9-CM) diagnostic code of V01.1. Since 2008, a postexposure LTBI survey using the tuberculin skin test has also been provided to TB contacts in high-risk groups under the diagnosis code of V01.1.12 As previously reported, the medical data of more than 99% of the population of Taiwan, including diagnosis codes, are collected in the NHIRD.15 In addition, the accuracy of diagnoses of major diseases such as diabetes mellitus and cerebrovascular disease in the NHIRD has been well validated.16,17 The data source we used was the Longitudinal Health Insurance Database 2005 (LHID 2005), which is a subset of NHIRD and included the data of one million NHI beneficiaries randomly sampled from the NHIRD. Hence, this study analyzed medical data of TB contacts retrieved from the LHID 2005. This study was approved by the institutional review board of Taipei Veterans General Hospital (IRB: 2015-04-004AC).
Participants and Validation
In this cohort study, we enrolled patients who were aged 20 years or older and were newly diagnosed with TB contact between January 1, 2008 and December 31, 2012. TB contact was defined as those with an ICD-9-CM code of V01.1 plus a CXR order in the NHIRD. Patients diagnosed with depressive disorder (ICD-9-CM codes 296.2X-296.3X, 300.4, and 311.X), bipolar disorder (ICD-9-CM codes 296.0, 296.1, 296.4, 296.5, 296.6, 296.7, 296.8, 296.80, and 296.89), or TB disease (ICD-9-CM codes 011–018) before TB contact were excluded.
To validate the definition of TB contact in this study, 300 outpatients were randomly selected from 1737 outpatients who had claim data with TB exposure (ICD-9-CM code V01.1 plus CXR order) between January 1, 2011 and December 31, 2013, at Taipei Veterans General Hospital. The medical records were reviewed and analyzed by 2 physicians. Among the 300 patients, the diagnosis of TB exposure was confirmed in 295 patients. Thus, the definition of TB contact we used had a sensitivity of 98.3%, and the interobserver agreement was excellent (k = 0.91, 95% confidence interval [CI] 0.89–0.93).
The control group was matched for age, sex, income level, and year of enrollment. Four controls were randomly selected for TB contact cases. Control participants were excluded if they had received a diagnostic code for TB contact, TB disease, depressive disorder, or bipolar disease before inclusion in the study. The contact and control groups were both followed until the occurrence of death, withdrawal from the NHI system, or December 31, 2012.
Variables and Outcome Measures
The comorbidities of study participants included diabetes (ICD-9 code 250), hypertension (ICD-9 code 401–405), congestive heart failure (ICD-9 code 428.0), coronary artery disease (ICD-9 code 410–414), cerebrovascular disease (ICD-9 code 430–437, excluding 432), asthma (ICD-9 code 493), chronic obstructive pulmonary disease (COPD) (ICD-9 code 491, 492, and 496), chronic kidney disease (ICD-9 code 580–587), liver cirrhosis (ICD-9 code 571.2 and 571.5), cancer (ICD-9 code 140–208), rheumatoid arthritis (ICD-9 code 714), and systemic lupus erythematosus (ICD-9 code 710). A person was considered to have a comorbidity only if the condition occurred in an inpatient setting or at 3 or more outpatient visits. Income level, an indicator of the socioeconomic status of the study participants, was calculated from the average monthly income of the insured person and grouped into 3 levels: low (≤20,000 New Taiwan Dollars [NTD]), intermediate (>20,000 to <40,000 NTD), and high (≥40,000 NTD).
Depressive disorder during the follow-up period, the primary outcome, was defined as ICD-9-CM codes 296.2X-296.3X, 300.4, and 311.X (Chen MH 2014) and a psychiatrist-assigned diagnosis.18 The development of active TB disease in the follow-up period, which was defined by compatible ICD-9-CM codes (010–018 in ICD-9-CM), was also recorded and validated by the prescription of at least 2 anti-TB medications for more than 28 days.6
Statistical Analysis
First, the demographic data of the study participants were analyzed. Continuous data are presented as mean ± standard deviation (SD). The normality of continuous variables was checked by the Kolmogorov-Smirnov test. The independent t test and Mann-Whitney U test were used to compare continuous variables when the distribution was normal and non-normal, respectively. Categorical data were expressed as number (%) and were analyzed by the Pearson chi-square test when appropriate. Second, the follow-up person-years were calculated to measure the incidence of depressive disorder and TB disease in both groups. The Kaplan-Meier method and the log-rank test were used to depict the curve of event rates and examined the differences between groups. Subsequently, the crude hazard ratios (HRs) and the 95% CIs were calculated by univariate Cox proportional-hazards regression models. Finally, to identify independent risk factors for depressive disorder, a multivariable Cox regression model was used to calculate adjusted HRs (aHRs) and 95% CIs after adjusting for age, sex, income level, and comorbidities, including diabetes, hypertension, congestive heart failure, coronary artery disease, cerebrovascular disease, asthma, COPD, chronic kidney disease, liver cirrhosis, cancer, rheumatoid arthritis, and systemic lupus erythematous. A 2-tailed P value of <0.05 was considered to indicate statistical significance. All data management and analyses were performed using the SAS 9.4 software package (SAS Institute, Cary, NC).
RESULTS
Study Enrollment and Characteristics of Population
On the basis of the validated definition, we identified 12,334 TB contacts from January 1, 2008 to December 31, 2012. As shown in Figure 1, 9046 patients were included in the TB contact group after exclusion, and another 36,184 individuals without TB contact were randomly selected for the control group. The numbers of TB contacts who were enrolled in year 2008, 2009, 2010, 2011, and 2012 were 1463 (16.2%), 1625 (17.9%), 1779 (19.7%), 1984 (21.9%), and 2195 (24.3%), respectively. Of the total 45,230 study participants, 648 (1.4%) were diagnosed with depressive disorder during a mean follow-up period of 2.5 ± 1.4 years, yielding an incidence rate of 5.7 per 1000 person-years.
FIGURE 1.

Flow chart of this study.
The overall mean age of the participants was 44.7 ± 16.8 years, and 19% of them were aged ≥60 years. In addition, 56.0% of the enrolled patients were women, and more than one-third of these patients were classified as of low-income level (Table 1). For each cohort, 127 (1.40%) patients in the TB contact cohort and 521 (1.44%) in the control group developed depressive disorder (P = 0.840). The incidence rate of depressive disorder per 1000 person-years was 6.3 in the TB contacts and 5.5 in the control group (P = 0.221). In addition, 67 (0.74%) patients in the TB contact cohort and 88 (0.24%) in the control group developed active TB disease during the follow-up periods (P < 0.001). The incidence rate of active TB per 1000 person-years was 3.3 in the TB contact cohort and 0.9 in the control group (P < 0.001). However, none of the 155 patients with active TB developed depressive disorder during the follow-up periods; there was no significant association between the 2 variables (P = 0.179).
TABLE 1.
Characteristics of Tuberculosis (TB) Contacts and Matched Controls
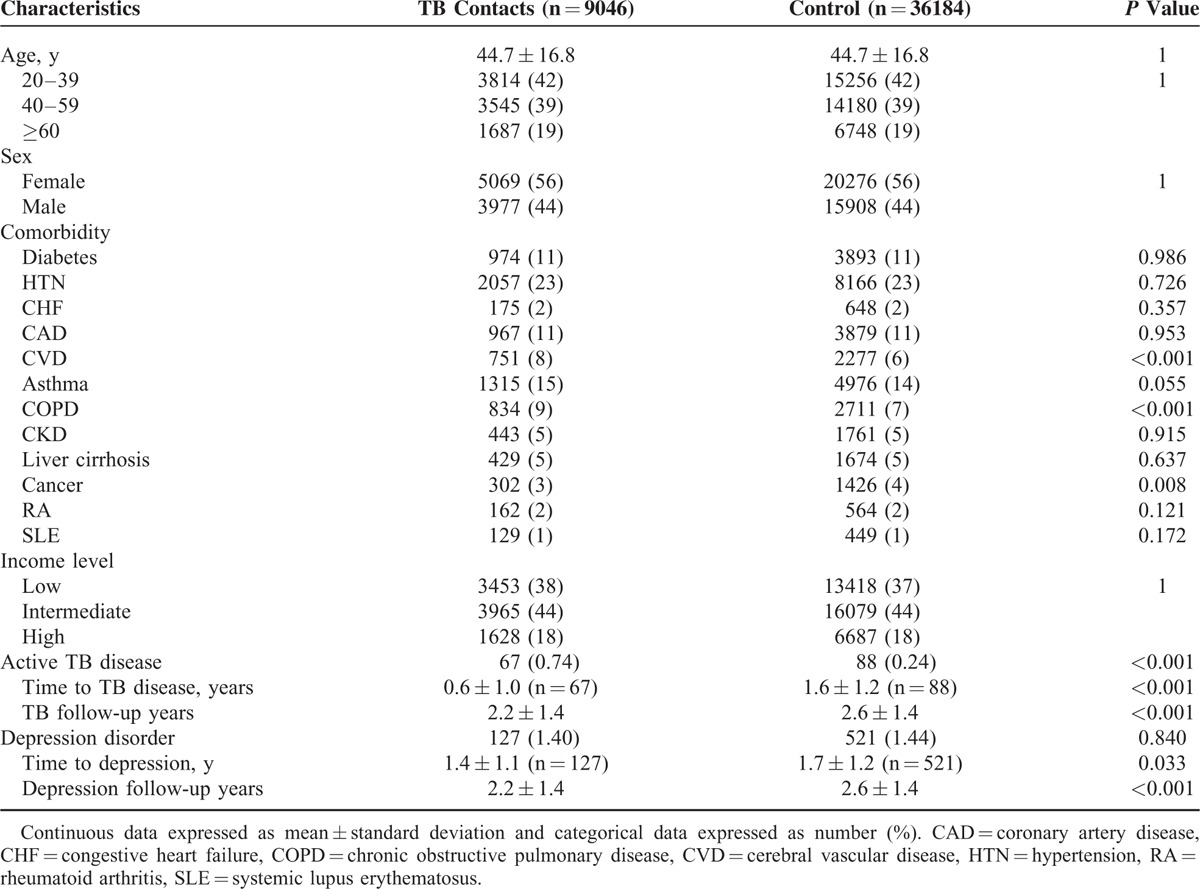
Subgroup Analysis of the Association Between TB Contact and Depression
Overall, the TB contact group did not have a significantly higher risk of developing depressive disorder than the control group, as shown by the results from the Cox regression models (aHR 1.12, 95% CI 0.92–1.36) (Table 2). Notably significant was that for the female population, the risk of depressive disorder was 1.3-fold higher in the TB contacts than in the controls before and after adjustment (aHR 1.34, 95% CI 1.07–1.68). In contrast, for men, there was no statistical difference in the risk of depressive disorder between TB contacts and controls (aHR 0.71, 95% CI 0.48–1.06). Also, for the low-income–level population, the risk of depressive disorder was 1.3-fold higher in the TB contacts than in the controls (aHR 1.37, 95% CI 1.02–1.83).
TABLE 2.
Subgroup Analysis of the Association Between TB Contact and Depression
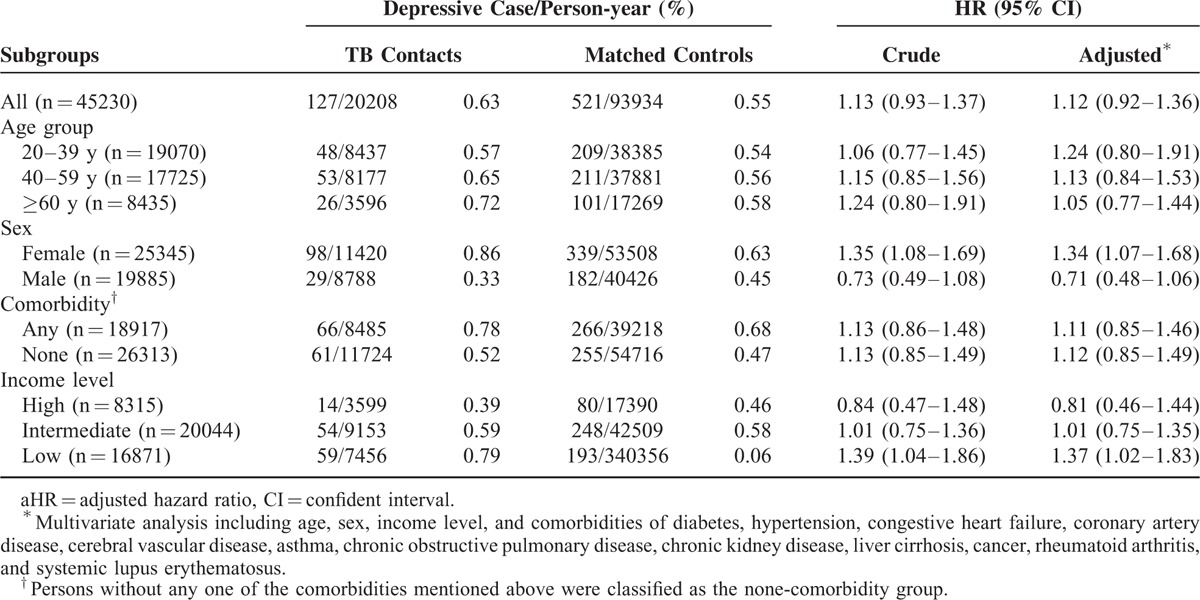
Impact of Sex and TB Contact on the Rate of Depression and Active TB
The Kaplan-Meier curves of depressive disorder in 4 subgroups stratified by sex and TB contact are shown in Figure 2A. Female TB contacts had the highest incidence rate of depressive disorder and male contact groups, the lowest (log-rank P < 0.001 for tread). There were significant differences in the curves between female contacts and controls (log-rank P < 0.001), but not between male contacts and controls (log-rank P = 0.112). The cumulative incidence of active TB in the 4 subgroups is also shown Figure 2B. Male TB contacts had the highest incidence rate for active TB, followed by female contacts, male controls, and female controls (log-rank P < 0.001 for trend). Overall, the TB contact cohort had a higher risk of acquiring active TB than the control cohort (aHR 3.28, 95% CI 2.38–4.52). The male contacts had a significantly higher incidence rate of active TB (log-rank P < 0.001), with a risk 2.9-fold (aHR 2.86, 95% CI 1.90–4.31) higher than that of male controls. Likewise, for the female population, female TB contacts had a 4.2-fold higher risk of active TB than the controls (aHR 4.20, 95% CI 2.49–7.08).
FIGURE 2.
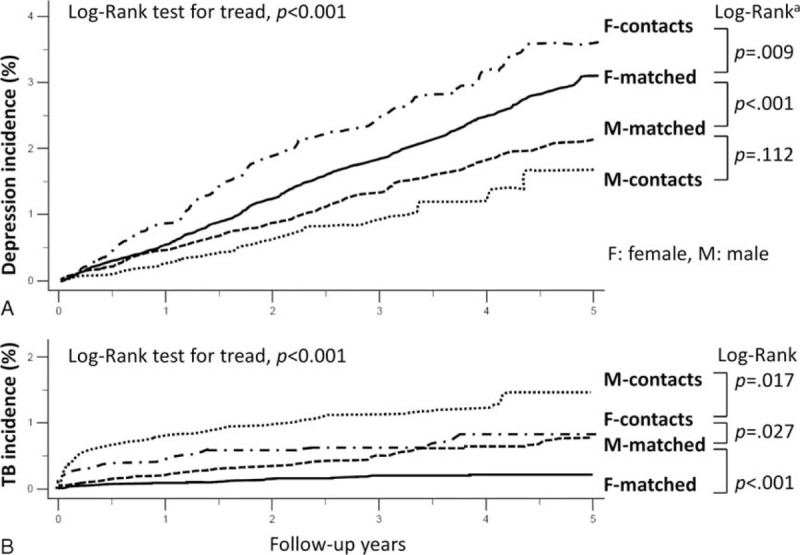
Cumulative incidences of outcomes in subgroups during follow-up periods. Cumulative incidence of depressive disorder (A) and that of active tuberculosis (TB) (B) in the 4 subgroups stratified by sex, and TB contact or matched control. aIn addition to the standard log-rank test, a weighted log-rank test was performed. According to the P values obtained by the Gehan-Breslow-Wilcoxon test, the Kaplan-Meier curve for female contacts was statistically significantly different from that for female matched controls (P = 0.002) and other groups (male matched controls, P < 0.001; male contacts, P < 0.001). In addition, the curve for female matched controls was also different from that for male matched controls (P < 0.001) and male contacts (P < 0.001). However, the curves for male matched controls and contacts did not differ significantly (P = 0.098).
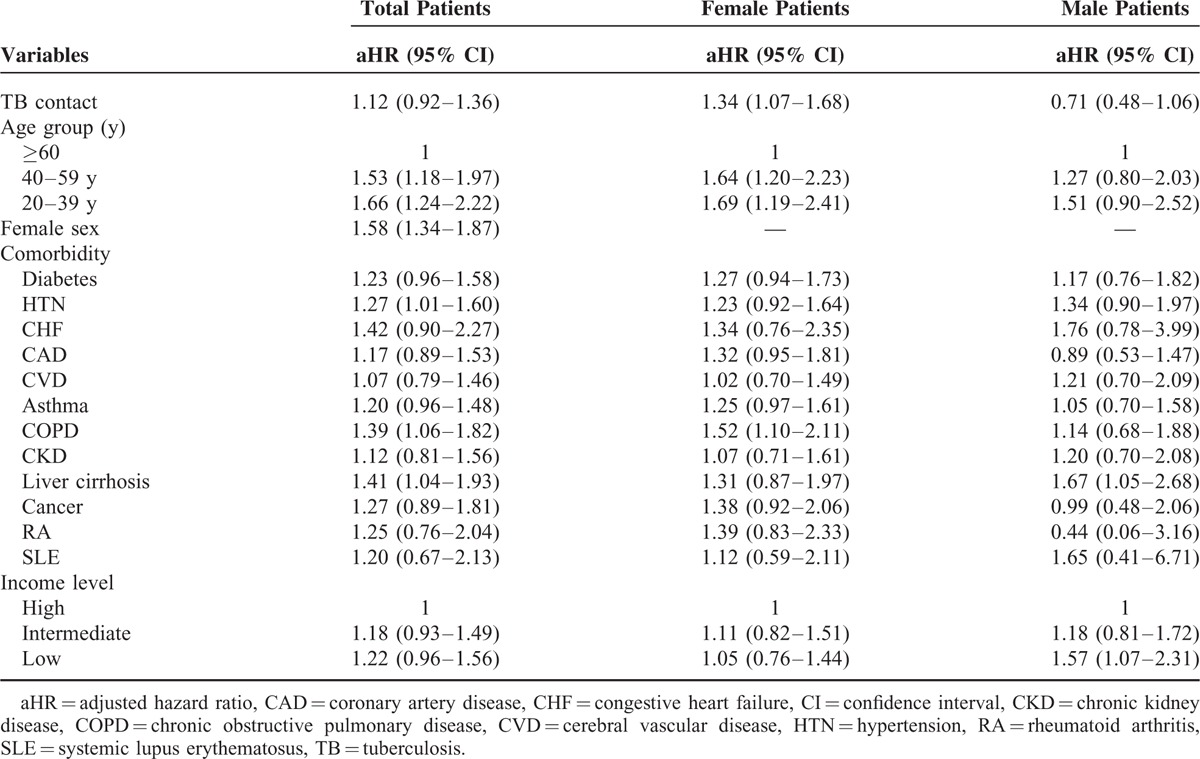
Independent Risk Factors for Depressive Disorder
For the whole population, TB contact was not an independent risk factor for depressive disorder (Table 3). For the female population, TB contact (aHR 1.34, 95% CI 1.07–1.68), age of 40–56 years (aHR 1.64, 95% CI 1.20–2.23), and of 20–39 years (aHR 1.69, 95% CI 1.19–2.41) (reference age of ≥60 y), and COPD (aHR 1.52, 95% CI 1.10–2.11) were risk factors for the development of depressive disorder. For the male population, liver cirrhosis (aHR 1.67, 95% CI 1.05–2.68) and lower-income level (aHR 1.57, 95% CI 1.07–2.31) (as compared with high-income level) were risk factors for the development of depressive disorder.
TABLE 3.
Multivariate Analyses of Risk Factors for Depressive Disorders in Male and Female Population
Annual Incidence Rate and Risk of Depression in Female TB Population
Since TB contact was significantly associated with the development of depressive disorder in the female population, the annual incidence rate of depressive disorder in female TB contacts and a matched cohort were compared. The corresponding aHRs are provided in Figure 3A. The case numbers at risk and incidence rate of depression are shown in Figure 3B. Within the first and second years of the follow-up periods, female TB contacts had a 1.5-fold higher risk of developing depressive disorder than the control group (aHR 1.49, 95% CI 1.03–2.14, P = .034; and aHR 1.53, 95% CI 1.05–2.23, P = .029, respectively). However, there was no difference in the annual risk of depression between female TB contacts and controls in the following 3 years. As shown in Figure 4A, the first 2-year depression rates of female contacts in stratified subgroups were higher than those of matched controls. Figure 4B shows a female subgroup analysis of the association between TB contact and depressive disorder within the first 2 years. Of note, low-income female TB contacts had a significantly increased risk of depression relative to controls, after adjustment for age, comorbidities, and income levels (aHR 1.70, 95% CI 1.13–2.54).
FIGURE 3.
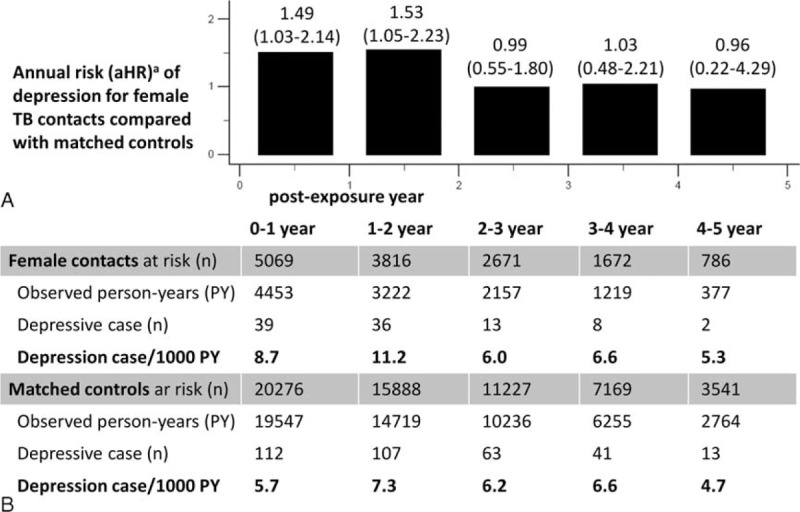
Annual risk and incidence rate of depression in female population. A, Annual risks of depression for female tuberculosis (TB) contacts compared with matched controls. B, The corresponding annual incidence rate of depression per 1000 person-years and absolute case numbers in both groups. aAdjusted factors in Cox regression model included age, sex, income level, and comorbidities of diabetes, hypertension, congestive heart failure, coronary artery disease, cerebral vascular disease, asthma, chronic obstructive pulmonary disease, chronic kidney disease, liver cirrhosis, cancer, rheumatoid arthritis, and systemic lupus erythematosus. Adjusted hazard ratio (aHR) with 95% confidence interval (95% CI) is provided.
FIGURE 4.
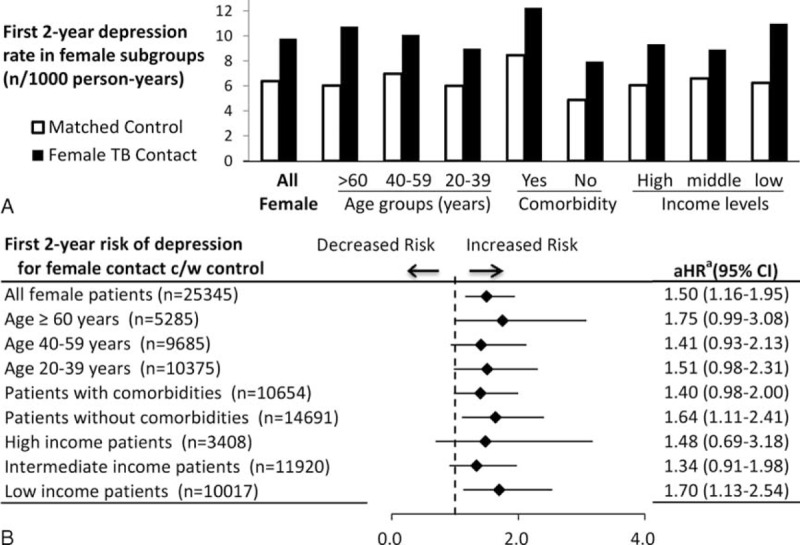
Subgroup analysis for first 2-year risk of depression in female population. A, The first 2-year depression rate per 1000 person-years of female TB contacts and matched controls in stratified subgroups. B, Subgroup analysis of the association between TB contacts and the first 2-year risk of depressive disorder in female population. aAdjusted factors in Cox regression model included age, sex, income level, and comorbidities of diabetes, hypertension, congestive heart failure, coronary artery disease, cerebral vascular disease, asthma, chronic obstructive pulmonary disease, chronic kidney disease, liver cirrhosis, cancer, rheumatoid arthritis, and systemic lupus erythematosus. Adjusted hazard ratio (aHR) with 95% confidence interval (95% CI) is provided. C/W = compared with.
DISCUSSION
This is the first population-based study to investigate the association between TB contact and the subsequent development of depressive disorder. Our novel finding is that female TB contacts had a significantly higher risk for developing depressive disorder than the general female population (aHR 1.34, 95% CI 1.07–1.68). In addition, the risks were significantly higher within the first and second years after exposure, but not different thereafter. Of note is that although TB contacts did have a higher risk of acquiring active TB than the matched controls, none of those with TB developed depressive disorder in the follow-up period.
Tuberculosis disease has significant impact on the patients’ health, quality of life, and psychosocial status.1–3 TB disease may also be transmitted from source cases to TB contacts, causing physical illness.19 However, little is known about the potential psychosocial impacts on TB contacts. In this study, we found that female TB contacts had a 1.5-fold higher risk of subsequent depressive disorder than the general female population of Taiwan in the first 2 years after exposure. Although women have been generally noted to be at a higher risk of depression than men in different clinical settings,20 this study targeted TB contacts without TB disease and discovered a sex difference in developing depression during follow-up periods. Interestingly, another population-based study in Taiwan that focused on active TB cases found a higher risk of subsequent depression in the male population than in the female population.5 We suspect that the difference in patient illnesses and management between the TB case and contacts may be associated with the discrepancy.
Several potential factors may explain the sex difference in the risk of depression in this study. First, since women are more vulnerable to stress and fear-based disorders, female TB contacts may be more prone than male contacts to psychosocial stress due to TB stigma and fear of infection.21,22 In Taiwan, adult TB contacts are asked to undergo CXR at clinics to rule out active TB disease in the 1st and 12th months after exposure, but are not routinely screened for LTBI to document an infection-free status.23 Worrying about TB infection and disease may be associated with psychological stress in women. The increased risk of depression within the first 2 years after TB exposure may be related to the fact that the risk of TB reactivation is highest within the first 2 years after infection.10
Second, female TB contacts may have depressive symptoms if they have to care for their household TB cases, and it is known that the household caregiving burden may cause depression.24 As previously reported, more than half of the TB contacts were household contacts in Taiwan and in other low-middle–income countries.25,26 Thus, a certain proportion of female TB contacts may have both postexposure risk of TB and family members with TB at home simultaneously. More importantly, if female TB contacts have to care for their TB relatives at home, they may experience psychological distress along with financial burdens due to reduced work opportunities.27 This may be supported by our finding of a 70% higher risk of depression in the low-income subgroup of female TB contacts than in controls (Figure 3B). In contrast, for socioeconomic reasons, male TB contacts may have to continue working outside the home rather than caring for family members with TB. Having social and financial stability, they may not be at risk of depression.
Finally, TB contacts may develop depressive disorder due to infection-related inflammation, either from subclinical infection immediately after exposure or TB reactivation from LTBI.28 However, in this study, none of the participants with active TB disease had either preceding depression or subsequent depression in the follow-up periods. Hence, depression due to postexposure TB disease was not observed in our follow-up periods. Further prospective study is warranted to clarify the association between the infection status of TB contacts and the risk of depression.
This cohort study confirmed the increased risk of TB disease after TB exposure and provides novel information on the risk of depressive disorder in female TB contacts. However, this study has some limitations. First, the study examined only the Taiwanese population in eastern Asia. Whether our findings can be generalized to people in different cultures and TB-burden areas is uncertain. Second, diagnoses of TB contact and depressive disorder that rely on administrative claims data recorded by physicians or hospitals may be less accurate than diagnoses made in a prospective clinical setting. However, the validation of the diagnosis of TB contacts (ICD-9-CM: V01.1 plus CXR) showed excellent accuracy in this study. Also, the diagnoses of depressive disorder were assigned by board-certified psychiatrists, so their diagnostic validity should be good. Third, we did not have detailed information on the exposure type of each TB contact in this retrospective study using data from the NHIRD. Hence, the different impacts of household or nonhousehold exposure on the risk of depression in the female population could not be investigated. Certain potential confounding factors, such as family history, occupation, personal habits, marital status, and caregiving burden, were not available in the NHIRD, despite their importance. Whether the increased risk of depression among female contacts is associated with burden of taking care of their TB spouses or family warrants further investigation. Fourth, reporting bias during contact investigation cannot be totally ruled out as a contributor for depression risk in female TB contacts. However, the current TB contact investigation protocol does not include psychological evaluations for both sexes in Taiwan. In addition, since the 2-year period of an increased risk of depression exceeds the 1-year period for contact investigation in Taiwan, reporting bias is less likely. Finally, the association between LTBI and the development of depression could not be evaluated in this retrospective study because LTBI screening was not routinely performed for adult contacts in Taiwan during the period studied.
In conclusion, this population-based study in a TB-endemic area determined that female TB contacts had a significantly higher risk of developing depressive disorder within the first 2 years postexposure. Hence, for TB contacts, clinicians should consider not only the risk of developing TB disease in both sexes but also the risk of depression in women, especially those with low incomes. Further prospective study is warranted to investigate the cause of depression in this group of individuals.
ACKNOWLEDGMENTS
The authors thank the Medical Science and Technology Building of Taipei Veterans General Hospital for providing research facilities. The authors are grateful to Dr Pei-Hung Chuang for his valuable contributions in data management and statistical analysis.
Authors’ contributions: SWP, YFY, VYS and WJS designed the study. SWP and YFY had full access to all of the data in the study, performed statistical analysis, and took responsibility for the accuracy of the data analysis. SWP, YFY, JYF, VYS and WJS provided the final interpretations of the results. SWP, YFY and JYF drafted the manuscript. YRK and WJS provided critical revision of the manuscript. JYF and WJS gave administrative, technical, and material support. WJS was the study supervisor and took responsibility for the integrity of the work. All authors read and approved the final manuscript.
Footnotes
Abbreviations: aHR = adjusted hazard ratio, CI = confidence interval, CXR = chest radiographic imaging, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, LHID = Longitudinal Health Insurance Database, LTBI = latent TB infection, NHIRD = National Health Insurance Research Database, NTD = New Taiwan Dollars, TB = tuberculosis.
S-WP and Y-FY contributed equally to this manuscript.
The authors do not have any conflicts of interest.
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report 2014. Available from http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf. [Google Scholar]
- 2.Bauer M, Leavens A, Schwartzman K. A systematic review and meta-analysis of the impact of tuberculosis on health-related quality of life. Qual Life Res 2013; 22:2213–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masumoto S, Yamamoto T, Ohkado A, et al. Prevalence and associated factors of depressive state among pulmonary tuberculosis patients in Manila, The Philippines. Int J Tuberc Lung Dis 2014; 18:174–179. [DOI] [PubMed] [Google Scholar]
- 4.Ugarte-Gil C, Ruiz P, Zamudio C, et al. Association of major depressive episode with negative outcomes of tuberculosis treatment. PLoS One 2013; 8:e69514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen TC, Wang CY, Lin CL, et al. People with tuberculosis are associated with a subsequent risk of depression. Eur J Intern Med 2014; 25:936–940. [DOI] [PubMed] [Google Scholar]
- 6.Yen YF, Chung MS, Hu HY, et al. Association of pulmonary tuberculosis and ethambutol with incident depressive disorder: a nationwide, population-based cohort study. J Clin Psychiatry 2015; 76:e505–511. [DOI] [PubMed] [Google Scholar]
- 7.Gazetta CE, Santos Mde L, Vendramini SH, et al. Tuberculosis contact control in Brazil: a literature review (1984–2004). Rev Lat Am Enfermagem 2008; 16:306–313. [DOI] [PubMed] [Google Scholar]
- 8.Feng JY, Chen SC, Lee MC, et al. Is 1-year follow-up adequate for adult tuberculosis contacts? Eur Respir J 2015; 45:1501–1504. [DOI] [PubMed] [Google Scholar]
- 9.Sloot R, Schim van der Loeff MF, Kouw PM, et al. Risk of tuberculosis after recent exposure. A 10-year follow-up study of contacts in Amsterdam. Am J Respir Crit Care Med 2014; 190:104410–104452. [DOI] [PubMed] [Google Scholar]
- 10.Taylor Z, Nolan CM, Blumberg HM, American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society of America. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep 2005; 54:1–81. [PubMed] [Google Scholar]
- 11.Courtwright A, Turner AN. Tuberculosis and stigmatization: pathways and interventions. Public Health Rep 2010; 125 Suppl 4:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan PC, Huang LM, Suo J. It is time to deal with latent tuberculosis infection in Taiwan. J Formos Med Assoc 2009; 108:901–903. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control, Department of Health, R.O.C. (Taiwan). Taiwan Tuberculosis Control Report 2012. Available from http://www.cdc.gov.tw/uploads/files/201303/9ea28ba2–69c7–4f27-af3b-55be5ec7e35c.pdf. [Google Scholar]
- 14.Chan PC, Tsai YF, Feng CF, et al. Treatment of latent tuberculosis infection in Taiwan: the present and future. Taiwan Epidemiol Bull 2013; 29:40–49. [Google Scholar]
- 15.Cheng TM. Taiwan's new national health insurance program: genesis and experience so far. Health Aff (Millwood) 2003; 22:61–76. [DOI] [PubMed] [Google Scholar]
- 16.Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011; 20:236–242. [DOI] [PubMed] [Google Scholar]
- 17.Lin CC, Lai MS, Syu CY, et al. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc 2005; 104:157–163. [PubMed] [Google Scholar]
- 18.Chen MH, Su TP, Chen YS, et al. Higher risk of developing major depression and bipolar disorder in later life among adolescents with asthma: a nationwide prospective study. J Psychiatr Res 2014; 49:25–30. [DOI] [PubMed] [Google Scholar]
- 19.Chee CB, Sester M, Zhang W, et al. Diagnosis and treatment of latent infection with Mycobacterium tuberculosis. Respirology 2013; 18:205–216. [DOI] [PubMed] [Google Scholar]
- 20.Simonds VM, Whiffen VE. Are gender differences in depression explained by gender differences in co-morbid anxiety? J Affect Disord 2003; 77:197–202. [DOI] [PubMed] [Google Scholar]
- 21.Maeng LY, Milad MR. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav 2015; [Epub ahead of print] DOI: 10.1016/j.yhbeh.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudelson P. Gender differentials in tuberculosis: the role of socio-economic and cultural factors. Tuber Lung Dis 1996; 77:391–400. [DOI] [PubMed] [Google Scholar]
- 23.Chan PC, Yang CH, Chang FY. Scaling up of latent tuberculosis infection treatment for close contacts of tuberculosis in Taiwan. J Formos Med Assoc 2011; 110:733–736. [DOI] [PubMed] [Google Scholar]
- 24.Khalaila R, Cohen M. Emotional suppression, caregiving burden, mastery, coping strategies and mental health in spousal caregivers. Aging Ment Health 2015; [Epub ahead of print] DOI:10.1080/13607863.2015.1055551. [DOI] [PubMed] [Google Scholar]
- 25.Chen Chang-Hsun. TB Contact Tracing Plus Project, 2012. Available from https://www.cdc.gov.tw/uploads/files/1fc2b9fc-83a2-4cac-a631-421a3c043d82.pdf (article in Chinese). [Google Scholar]
- 26.Fox GJ, Barry SE, Britton WJ, et al. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J 2013; 41:140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oshio T. How is an informal caregiver's psychological distress associated with prolonged caregiving? Evidence from a six-wave panel survey in Japan. Qual Life Res 2015; [Epub ahead of print] DOI: 10.1007/s11136-015-1041-4. [DOI] [PubMed] [Google Scholar]
- 28.Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]


