Abstract
The aim of this study was to investigate the association between 2 polymorphisms (ie, rs10877887 and rs13293512) in the promoter regions of let-7 and the risk of papillary thyroid carcinoma (PTC).
A case-control study of 618 PTC patients and 562 controls was conducted. The rs10877887 polymorphism was genotyped by using polymerase chain reaction-restriction fragment length polymorphism and the rs13293512 polymorphism was genotyped by using a TaqMan Genotyping Assay. The results were confirmed by DNA sequencing.
The rs10877887 polymorphism had reduced risks of PTC in heterozygous comparison, dominant model, and overdominant model (TC vs TT: adjusted odds ratio [OR] = 0.73, 95% confidence interval [95% CI] = 0.58–0.94, P = 0.01; TC/CC vs TT: adjusted OR = 0.79, 95% CI = 0.63–1.00, P = 0.047; TC vs TT/CC: adjusted OR = 0.73, 95% CI = 0.57–0.92, P = 0.007, respectively). Stratified analyses showed that PTC patients carrying the rs10877887 CC genotype were more likely to have multiple tumors (adjusted OR = 1.71, 95% CI = 1.03–2.86, P = 0.04), and PTC patients carrying the rs13293512 TC + CC or CC were more likely to develop N0 status (TC/CC vs TT: adjusted OR = 0.64, 95% CI = 0.43–0.94, P = 0.02; CC vs TC/TT: adjusted OR = 0.50, 95% CI = 0.33–0.77, P = 0.001, respectively).
Our study suggests that the rs10877887 polymorphism may be associated with the risk of PTC and the rs13293512 polymorphism may correlate to lymph node metastasis in PTC.
INTRODUCTION
Papillary thyroid carcinoma (PTC), accounting for more than 80% of the thyroid cancer, is the most common endocrine carcinoma among women.1 The incidence of PTC is sharply increasing worldwide in recent years.2,3 Although PTC shows relatively good prognosis, cervical lymph node metastases and aggressive subset are highly associated with the risk of recurrence or death.4,5 In addition, the etiology and pathogenesis of PTC are not fully clarified.6 These issues, therefore, have driven the development of molecular biomarker for the diagnosis and prognosis of PTC.
MicroRNAs (miRNAs), known as a class of endogenous noncoding small RNAs, can suppress gene expression at the posttranscriptional level by binding to the 3′-untranslated region of target messenger RNAs.7,8 Increasing evidence has identified that miRNAs are involved in cell proliferation, apoptosis, and differentiation.9–11 Previously, the expression of let-7f-1 has been demonstrated to be downregulated in PTC.12 Overexpression of let-7 in TPC-1 cells can suppress tumor cell proliferation,13 indicating that let-7 plays key roles in PTC carcinogenesis.
Recently, 2 single-nucleotide polymorphisms (SNPs) (ie, rs10877887 and rs13293512), located in the promoter regions of let-7, have been predicted to affect the affinity with transcription factor and interferon regulatory factor binding site.14,15 Xie et al16 found that the rs10877887 was associated with survival of hepatocellular carcinoma. Liang et al17 demonstrated that the rs10877887 and rs13293512 had correlation with the susceptibility of major depressive disorder. To date, however, no analyses have been conducted to assess the association between the 2 polymorphisms and PTC risk. The aim of this case-control study was to investigate the possible correlation of the 2 polymorphisms with the susceptibility to PTC in a Chinese Han population.
MATERIALS AND METHODS
Study Population
Between January 2010 and October 2014, a total of 618 consecutive patients with PTC at West China Hospital, Sichuan University, were enrolled. Patients were diagnosed on the basis of pathological result of an ultrasonography-guided, fine needle aspiration biopsy or resected specimens. The exclusion criteria in the case group were patients combined with other cancers. Detailed clinical data, including age, gender, tumor node metastasis (TNM) status, and multiplicity of tumor, were retrieved from medical records. In addition, 562 healthy subjects who came to the hospital for routine physical examination were recruited at the same institution during the same period. The controls were frequency-matched to the cases by age and gender. Individuals with thyroid disease or a personal or family history of cancer were excluded from the control group. All subjects were unrelated ethnic Han Chinese. The study was approved by the ethics committee of the hospital, and written informed consent was obtained from all subjects enrolled in this study.
Genotyping
According to the manufacturer's protocol, whole-genomic DNA was obtained from 200 μL ethylene diamine tetraacetic acid-anticoagulated peripheral blood using a commercial isolation kit (Bioteke, Beijing, China). The rs10877887 polymorphism was analyzed by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method, using the following primer sequences: 5′-AACCAGTTGGTGTCTGACTGC-3′ (forward) and 5′-CCACCGCTCTGAAGAGAGAA-3′ (reverse). DNA template was amplified in a total volume of 10 μL reaction mixture including 5 μL 2 × Power Taq PCR MasterMix, 0.5 mM of each primer. The PCR reaction conditions were as follows: 94°C for 2 minutes, followed by 35 cycles of 30 seconds at 94 °C, 30 seconds at 59°C, and 30 seconds at 72 °C, with a final elongation at 72°C for 10 minutes. PCR product was digested at 55°C for 2 hours with Fau I restriction enzyme (New England BioLabs Inc, Beverly, MA). The Fau I for allele C is cuttable, manifesting 2 fragments of 106 bp and 31 bp, and the Fau I for allele T is uncuttable, manifesting 1 fragment of 137 bp. Gel pictures were visualized by 2 independent researchers with a blindness of cases and controls. Genotyping of the rs13293512 polymorphism was detected using a TaqMan SNP genotyping assay. The genotyping results were verified by DNA sequencing.
Statistical Analysis
All statistical analyses were carried out using SPSS software (SPSS version 17.0; SPSS Inc., Chicago, IL). Genotype and allele frequencies of the 2 SNPs were obtained by direct computing. Hardy-Weinberg equilibrium was tested by Chi-square test. SNPstats were applied to obtain genotypic association tests in a case-control pattern.18 Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to evaluate the association between the rs10877887 and rs13293512 polymorphisms and PTC risk.
RESULTS
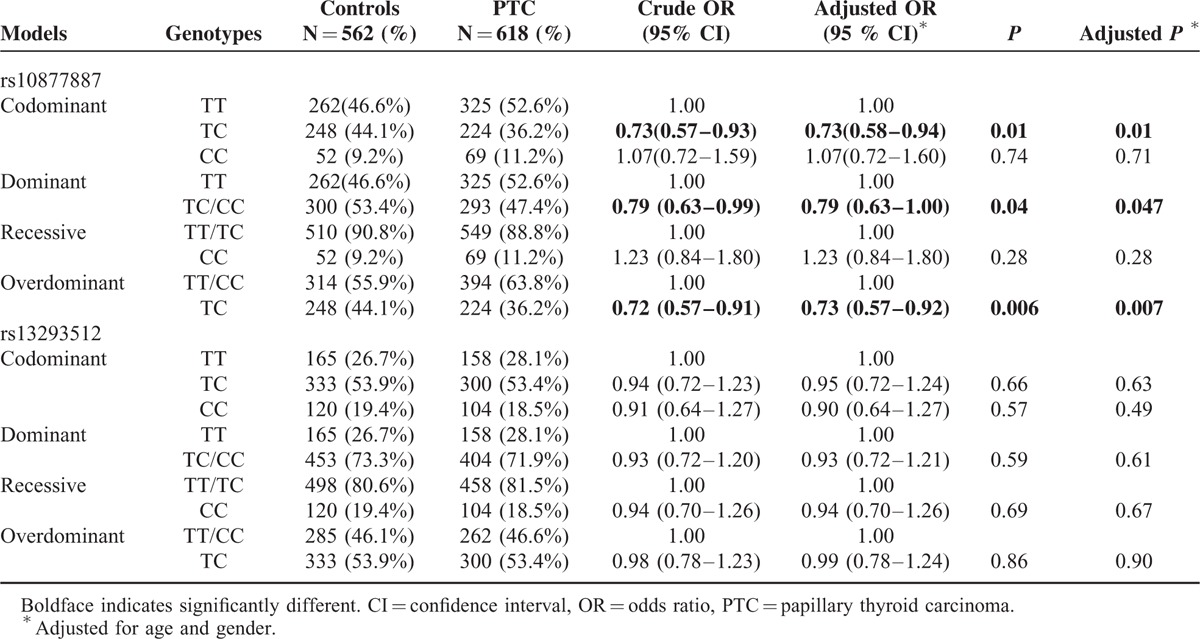
The clinical characteristics of the cases and controls are presented in Table 1. There were no significant differences in age and gender between the 2 groups (P > 0.05). The genotype distributions of the 2 polymorphisms in the control group conformed to Hardy-Weinberg equilibrium. The genotype and allele frequencies of the 2 polymorphisms in the promoters of let-7 are summarized in Table 2. For the rs10877887 polymorphism, reduced risks of PTC were observed in heterozygous comparison, dominant model, and overdominant model (TC vs TT: adjusted odds ratio [OR] = 0.73, 95% confidence interval [95% CI] = 0.58–0.94, P = 0.01; TC/CC vs TT: adjusted OR = 0.79, 95% CI = 0.63–1.00, P = 0.047; TC vs TT/CC: adjusted OR = 0.73, 95% CI = 0.57–0.92, P = 0.007, respectively). No significant differences were observed in the genotype distributions of the rs13293512 between the 2 groups.
TABLE 1.
Characteristics of the Study Population in the 2 Groups
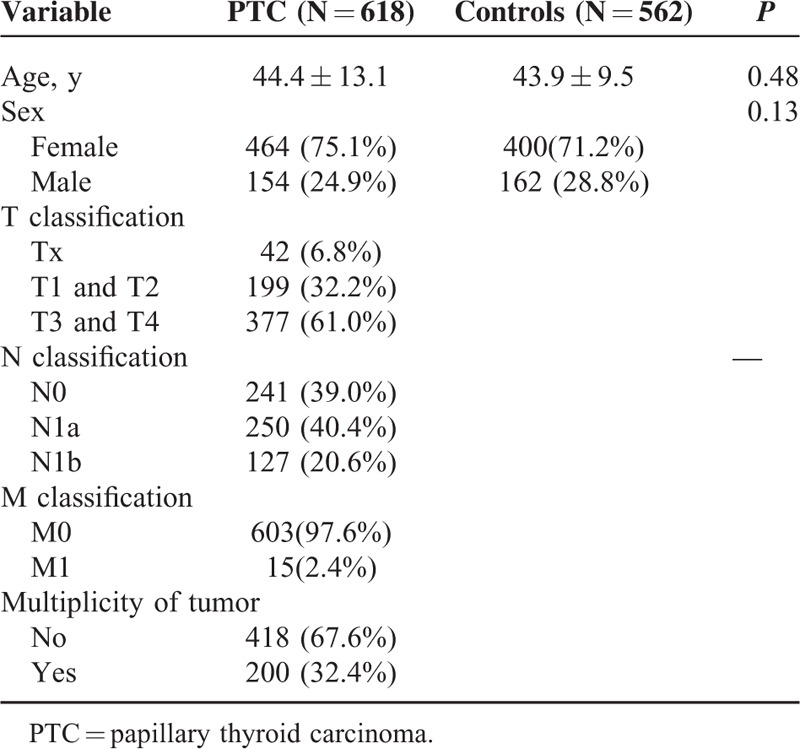
TABLE 2.
Genotype Distributions of the rs10877887 and rs13293512 Polymorphisms in PTC Patients and Controls
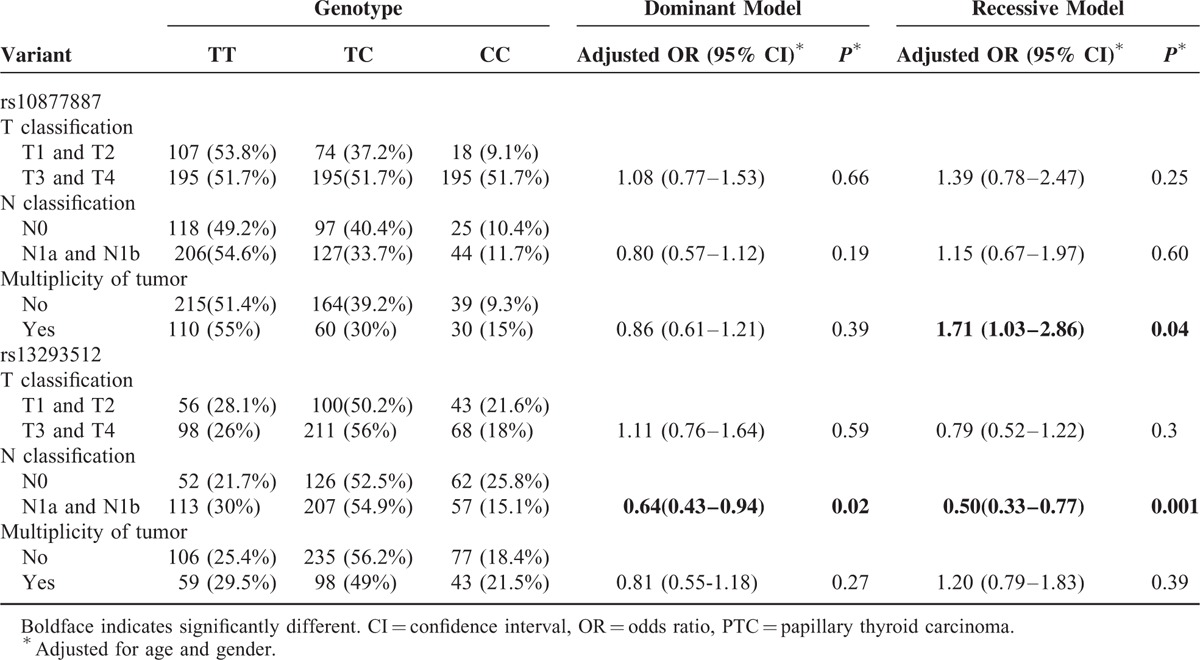
Stratification analyses were performed according to lymph node metastasis status and multiplicity of tumor. As summarized in Table 3, the frequency of the rs10877887 CC genotype in PTC patients with multiple tumors was higher than that in patients with a single tumor (adjusted OR = 1.71, 95% CI = 1.03–2.86, P = 0.04). Moreover, the frequency of the rs13293512 TC/CC or CC genotype was significantly lower in N1a + N1b group than that in N0 group in a dominant model and recessive model (TC/CC vs TT: adjusted OR = 0.64, 95% CI = 0.43–0.94, P = 0.02; CC vs TC/TT: adjusted OR = 0.50, 95% CI = 0.33–0.77, P = 0.001, respectively).
TABLE 3.
Stratified Analyses of Association Between the 2 Polymorphisms in the Promoters of Let-7 and Clinical Features of PTC Patients
DISCUSSION
To the best of our knowledge, this is the first study to investigate the association between the rs10877887 and rs13293512 polymorphisms in the promoter regions of let-7 and PTC risk in a Chinese population. We found that the rs10877887 TC heterozygote was significantly associated with a decreased risk of PTC. Stratified analyses demonstrated that PTC patients carrying the rs10877887 CC genotype were more likely to have multiple tumors, and PTC patients carrying the rs13293512 TC + CC or CC were more likely to develop N0 status. These results implied that the rs10877887 and rs13293512 polymorphisms may play a critical role in the etiology of PTC.
It is well known that let-7 is considered as a tumor suppressor gene, which is involved in the tumorigenesis of several cancers, including PTC.19–21 Regarding let-7 related polymorphisms, previous studies have identified that the let-7 binding site rs712 polymorphism was associated with the risk of gastric cancer, colorectal cancer, non-small cell lung cancer, and oral squamous cell carcinoma,22–25 but not with the risk of PTC and nasopharyngeal carcinoma.26,27 These results indicated that the rs712 polymorphism may play different roles in diverse human cancer types. Recently, Xie et al16 found that the rs10877887 polymorphism in the promoter of let-7 was associated with overall survival of hepatocellular carcinoma. Shen et al28 reported that the rs10877887 polymorphism was associated with an increased risk of lung adenocarcinoma risk. The increased risk was also observed in patients with major depressive disorder.17 In agreement with these positive results, we found that rs10877887 polymorphism was associated with a decreased risk of PTC, indicating that the rs10877887 polymorphism in the promoter of let-7 may have a critical role in the susceptibility to PTC.
With regard to the mechanism of the 2 polymorphisms in PTC risk, Pallante et al12 reported that the expression of let-7f-1 was significantly downregulated in PTC as compared with normal thyroid tissues. Wang et al29 demonstrated that miR-let-7i was downregulated in PTC group with lymph node (LN) metastasis compared with the group without LN metastasis group. Over-regulation of let-7 can reduce proliferation and dedifferentiation of TPC-1 cells in vitro by suppressing mitogen-activated protein kinase activation.13 It is evident that SNP in the promoter, primary, or precursor of miRNA may affect the expression of mature miRNA, and finally play key roles in human cancer susceptibility.30–33 Taken together, we speculated that the rs10877887 TC genotype may result in higher expression of let-7, which contribute to the risk of PTC. The positive results in stratification analyses indicated that the rs10877887 and rs13293512 polymorphisms may be used as biomarkers for tumor multiplicity and lymph nodule metastasis. However, the molecular mechanism needs to be done.
This study has several limitations. Firstly, relatively small number of sample size was included in this study, which may limit the statistical power. Secondly, we were unable to investigate the correlation between the genotype and serum level of let-7, due to lack of available data of the expression of let-7 in PTC. Finally, subjects enrolled in the study were all ethnic Han Chinese, which cannot be applicable to other populations.
In conclusion, the results of this study demonstrated that the rs10877887 polymorphism in the promoter of let-7 may contribute to the risk of PTC in the Chinese population. Stratified analyses revealed associations between the rs10877887 and rs13293512 polymorphisms and lymph nodule metastasis and multiplicity of tumor. However, further investigations with larger volume are still needed to confirm our findings in different populations.
Footnotes
Abbreviations: CIs = confidence intervals, OR = odds ratio, PCR-RFLP = polymerase chain reaction-restriction fragment length polymorphism, LN = lymph nodePTC papillary thyroid carcinoma, SNP = single-nucleotide polymorphism, TNM = tumor node metastasis.
Jingqiang Zhu, Linbo Gao, and Lin Zhang designed the research. Yichao Wang, Tao Wei, Junjie Xiong, Peng Chen, and Xunli Wang performed the experiments and carried out statistical analysis. Yichao Wang, Jingqiang Zhu, and Linbo Gao drafted the article. All authors read and approved the final manuscript.
This work was supported by grants from the Special Research Foundation of Doctoral Priority to the Development of Field Project (No. 20110181130013), National Natural Science Foundation of China (No. 81302149), Distinguished Young Scientist of Sichuan University (No. 2013SCU04A38), the Science & Technology Pillar Program of Sichuan Province (No. 2014SZ0001), and the Ph.D. Programs Foundation of Ministry of Education of China (No. 20130181120011).
No competing financial interests exist.
REFERENCES
- 1.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009; 19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 2.Sipos JA, Mazzaferri EL. Thyroid cancer epidemiology and prognostic variables. Clin Oncol (R Coll Radiol) 2010; 22:395–404. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Semenciw R, Ugnat AM, et al. Increasing thyroid cancer incidence in Canada, 1970-1996: time trends and age-period-cohort effects. Br J Cancer 2001; 85:1335–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings AL, Goldfarb M. Thyroid carcinoma metastases to axillary lymph nodes: report of two rare cases of papillary and medullary thyroid carcinoma and literature review. Endocr Pract 2014; 20:e34–e37. [DOI] [PubMed] [Google Scholar]
- 5.Sakorafas GH, Sampanis D, Safioleas M. Cervical lymph node dissection in papillary thyroid cancer: current trends, persisting controversies, and unclarified uncertainties. Surg Oncol 2010; 19:e57–e70. [DOI] [PubMed] [Google Scholar]
- 6.Wang P, Lv L, Qi F, et al. Increased risk of papillary thyroid cancer related to hormonal factors in women. Tumour Biol 2015; 36:5127–5132. [DOI] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116:281–297. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchiya S, Okuno Y, Tsujimoto G. MicroRNA: biogenetic and functional mechanisms and involvements in cell differentiation and cancer. J Pharmacol Sci 2006; 101:267–270. [DOI] [PubMed] [Google Scholar]
- 9.Li LM, Hu ZB, Zhou ZX, et al. Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Res 2010; 70:9798–9807. [DOI] [PubMed] [Google Scholar]
- 10.Jackson BL, Grabowska A, Ratan HL. MicroRNA in prostate cancer: functional importance and potential as circulating biomarkers. BMC Cancer 2014; 14:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JC, Gundara JS, Glover A, et al. MicroRNA expression profiles in the management of papillary thyroid cancer. Oncologist 2014; 19:1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pallante P, Visone R, Ferracin M, et al. MicroRNA deregulation in human thyroid papillary carcinomas. Endocr Relat Cancer 2006; 13:497–508. [DOI] [PubMed] [Google Scholar]
- 13.Ricarte-Filho JC, Fuziwara CS, Yamashita AS, et al. Effects of let-7 microRNA on cell growth and differentiation of papillary thyroid cancer. Transl Oncol 2009; 2:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mudduluru G, Vajkoczy P, Allgayer H. Myeloid zinc finger 1 induces migration, invasion, and in vivo metastasis through Axl gene expression in solid cancer. Mol Cancer Res 2010; 8:159–169. [DOI] [PubMed] [Google Scholar]
- 15.Kröger A, Köster M, Schroeder K, et al. Activities of IRF-1. J Interferon Cytokine Res 2002; 22:5–14. [DOI] [PubMed] [Google Scholar]
- 16.Xie K, Liu J, Zhu L, et al. A potentially functional polymorphism in the promoter region of let-7 family is associated with survival of hepatocellular carcinoma. Cancer Epidemiol 2013; 37:998–1002. [DOI] [PubMed] [Google Scholar]
- 17.Liang Y, Zhao G, Sun R, et al. Genetic variants in the promoters of let-7 family are associated with an increased risk of major depressive disorder. J Affect Disord 2015; 183:295–299. [DOI] [PubMed] [Google Scholar]
- 18.Solé X, Guinó E, Valls J, et al. SNPStats: a web tool for the analysis of association studies. Bioinformatics 2006; 22:1928–1929. [DOI] [PubMed] [Google Scholar]
- 19.Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 microRNA family. Cell 2005; 120:635–647. [DOI] [PubMed] [Google Scholar]
- 20.Akao Y, Nakagawa Y, Naoe T. let-7 microRNA functions as a potential growth suppressor in human colon cancer cells. Biol Pharm Bull 2006; 29:903–906. [DOI] [PubMed] [Google Scholar]
- 21.Kumar MS, Erkeland SJ, Pester RE, et al. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc Natl Acad Sci U S A 2008; 105:3903–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li ZH, Pan XM, Han BW, et al. A let-7 binding site polymorphism rs712 in the KRAS 3’ UTR is associated with an increased risk of gastric cancer. Tumour Biol 2013; 34:3159–3163. [DOI] [PubMed] [Google Scholar]
- 23.Pan XM, Sun RF, Li ZH, et al. A let-7 KRAS rs712 polymorphism increases colorectal cancer risk. Tumour Biol 2014; 35:831–835. [DOI] [PubMed] [Google Scholar]
- 24.Chin LJ, Ratner E, Leng S, et al. A SNP in a let-7 microRNA complementary site in the KRAS 3’ untranslated region increases non-small cell lung cancer risk. Cancer Res 2008; 68:8535–8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang WY, Chien YC, Wong YK, et al. Effects of KRAS mutation and polymorphism on the risk and prognosis of oral squamous cell carcinoma. Head Neck 2012; 34:663–666. [DOI] [PubMed] [Google Scholar]
- 26.Pan XM, Jia J, Guo XM, et al. Lack of association between let-7 binding site polymorphism rs712 and risk of nasopharyngeal carcinoma. Fam Cancer 2014; 13:93–97. [DOI] [PubMed] [Google Scholar]
- 27.Jin H, Liang Y, Wang X, et al. Association between a functional polymorphism rs712 within let-7-binding site and risk of papillary thyroid cancer. Med Oncol 2014; 31:221. [DOI] [PubMed] [Google Scholar]
- 28.Shen LQ, Xie YZ, Qian XF, et al. A single nucleotide polymorphism in the promoter region of let-7 family is associated with lung cancer risk in Chinese. Genet Mol Res 2015; 14:4505–4512. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Zhang H, Zhang P, et al. Upregulation of miR-2861 and miR-451 expression in papillary thyroid carcinoma with lymph node metastasis. Med Oncol 2013; 30:577. [DOI] [PubMed] [Google Scholar]
- 30.Duan R, Pak C, Jin P. Single nucleotide polymorphism associated with mature miR-125a alters the processing of pri-miRNA. Hum Mol Genet 2007; 16:1124–1131. [DOI] [PubMed] [Google Scholar]
- 31.Mishra PJ, Bertino JR. MicroRNA polymorphisms: the future of pharmacogenomics, molecular epidemiology and individualized medicine. Pharmacogenomics 2009; 10:399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landi D, Moreno V, Guino E, et al. Polymorphisms affecting micro-RNA regulation and associated with the risk of dietary-related cancers: a review from the literature and new evidence for a functional role of rs17281995 (CD86) and rs1051690 (INSR), previously associated with colorectal cancer. Mutat Res 2011; 717:109–115. [DOI] [PubMed] [Google Scholar]
- 33.Gao LB, Li LJ, Pan XM, et al. A genetic variant in the promoter region of miR-34b/c is associated with a reduced risk of colorectal cancer. Biol Chem 2013; 394:415–420. [DOI] [PubMed] [Google Scholar]


