Abstract
Intravenous immunoglobulin (IVIg) has been proven most effective in treating Guillain–Barré syndrome (GBS). Corticosteroids as an add-on therapy have been prescribed in severe GBS cases. However, the efficacy of intravenous corticosteroids combined with IVIg in dealing with severe GBS remains unclear. We explored the therapeutic effects of different therapeutic regimens on the short-term prognosis of GBS patients, especially the severe cases.
We retrospectively analyzed the clinical data of 527 adult patients with GBS who were prescribed to different treatments from 2003 to 2014. The therapeutic effect of a treatment was evaluated by the improvement of Hughes Functional Grading Scale (HFGS) and Medical Research Council (MRC) sum score.
With comparable incidence of infectious complications (P > 0.05), more mechanically ventilated patients were found improvement after IVIg treatment than combination IVIg with intravenous corticosteroids (MRC: 97% vs. 72.4%, P < 0.05; HFGS: 97% vs. 72.4%, P < 0.05). As to bedridden patients without mechanical ventilation, incidence of infectious complications (P > 0.05) and ratio of patients who were improved after IVIg were insignificantly different from the combination therapy (MRC: 89.6% vs. 86.5%; HFGS: 69.6% vs. 61.5%; both P > 0.05), even if the intravenous corticosteroids were initiated within 7 days after onset (P > 0.05). In addition, supportive treatment was sufficient for patients who were able to walk with help (HFGS = 3) and mildly affected (HFGS < 3) when compared with IVIg and intravenous corticosteroids.
IVIg is sufficient to GBS patients who are unable to walk (HFGS > 3), while corticosteroids are detrimental for short-term prognosis in mechanically ventilated patients when used in combination with IVIg. Further prospective and randomized studies are warranted to validate this finding.
INTRODUCTION
Guillain–Barré syndrome (GBS) is an acute inflammatory disorder of the peripheral nervous system, and it is an important cause of acute neuromuscular paralysis.1,2 Patients with GBS usually presented as rapidly progressive, symmetric flaccid weakness of the extremities accompanied with sensory disturbance.1 Although the clinical course, severity, and prognosis of different subtypes of GBS are variable, intravenous immunoglobulin (IVIg) and plasma exchange (PE) are proven effective treatments.1,3 Despite various treatment options, GBS patients could have severe clinical signs or residual functional deficits, especially the severe cases. Corticosteroids as an immunosuppressant and anti-inflammatory agent were postulated to be effective in treating GBS, as were in the animal model of GBS.4 However, studies have shown that corticosteroids were overall ineffective in GBS when administered alone.5–7 Nevertheless, it remains unclear whether add-on use of corticosteroid may exert a possible surplus effect to the already proven beneficial effect of IVIg in GBS. In 1994, a study by Dutch GBS group assessed the possible synergistic effect of the combination IVIg with methylprednisolone and found that 76% patients in the combination therapy group improved at least 1 grade of GBS disability scale compared with 53% patients in the IVIg-treated group.8 Moreover, the median time to regain unaided walking was also shorter when methylprednisolone was used in combination with IVIg,8 indicating that corticosteroid add-on therapy was superior to IVIg alone. Similar results were found when adjustments were made in a randomized double-blind, placebo-controlled, and multicentre study by van Koningsveld et al9, which compared the therapeutic effect between IVIg alone and combination methylprednisolone with IVIg. They found that 68% patients in the IVIg combined with methylprednisolone treated group had improved by at least 1 disability grade scales by 4 weeks compared with 56% of the IVIg plus placebo treated group, which barely reached statistical significance after adjustments for age and disease severity. Based on these data, intravenous corticosteroids as an add-on therapy have been recommended in treatment of severe or protracted GBS cases.9,10 Empirically, however, add-on use of steroids on the basis of IVIg appears ineffective, in particular for the severe GBS patients. Hence we retrospectively compared the therapeutic effect of different treatments to GBS, so as to investigate whether a synergic benefit still exists when corticosteroids are used in combination with IVIg in severe GBS patients.
SUBJECTS AND METHODS
Subjects
This retrospective study was approved by the ethics committee of the First Hospital of Jilin University, Changchun, China. The records of the patients were anonymized and deidentified before analysis. From 2003 to 2014, adult patients who were admitted to Department of Neurology of the First Hospital of Jilin University and fulfilled the diagnostic criteria of GBS were enrolled.11 Patients were excluded if they were younger than 16 years old, or diagnosed as Miller Fisher syndrome, chronic inflammatory demyelinating polyneuropathy, Bickerstaff encephalitis, or acute transverse myelitis. Critical illness polyneuropathy as the most common cause of acute flaccid paralysis in hospital was also excluded.12 As we aimed to compare the effects of different therapeutic regimens to GBS, those who were died or discharged within 5 days after admission or those without available evaluations of the clinical severity and functional impairment at admission and at discharge were ruled out from the study. For all the recruited patients in the study, the data on age, sex, preceding infections, severity and distribution of weakness, sensory disturbances and reflexes in arms and legs, cranial nerve deficits, time from onset to admission, time from onset to nadir and infectious complications (mainly pneumonia) which was diagnosed during hospitalization were collected.
Evaluation of Clinical Severity and Functional Impairment
The motor function deficits of enrolled patients were assessed by the Hughes Functional Grading Scale (HFGS), a widely accepted scale of disability for GBS patients. The HFGS score was illustrated in Table 1.13 In addition, muscle weakness was evaluated by Medical Research Council (MRC) sum score, ranging from 0 (tetraparalytic) to 60 (normal strength).14 The disease nadir was defined as the highest HFGS score or the lowest MRC sum score.
TABLE 1.
The Hughes Functional Grading Scale (HFGS)

Grouping and Therapeutic Effect Assessment
Treatment was immediately started when the clinical diagnosis was established after admission, whereas before the nadir of disease. IVIg is significantly more likely to be completed with comparable efficiency to PE when started within 2 weeks from onset; while IVIg after PE does not confer significant extra benefit as well.15 Thus IVIg is the first-line treatment option for GBS patients who were unable to walk independently (HFGS ≥ 3) in our department, while intravenous corticosteroids as an add-on therapy were administrated to those who deteriorated despite the use of IVIg. Of note is that if patients refused IVIg or PE (unaffordable for most cases), they received either intravenous corticosteroids or supportive treatments. According to the therapeutic regimens, all the enrolled patients fell into one of the following groups: Group 1: standard intravenous immunoglobulin treatment (IVIg, 0.4 g/kg/day, for 5 consecutive days); Group 2: combination therapy: combined standard IVIg treatment with intravenous corticosteroids; Group 3: intravenous corticosteroids (methylprednisolone or dexamethasone); Group 4: supportive treatment by using neurotrophic drugs (eg, vitamin B1, B12, etc.) instead of IVIg or intravenous corticosteroids. In Groups 2 and 3, the category and dose of the intravenous corticosteroids were variable; for the patients with mechanical ventilation, pulse methylprednisolone (500 mg for 5 days) or dexamethasone (15 or 20 mg for 7 days) were usually prescribed and gradually tapered; while for those without mechanical ventilation, intravenous methylprednisolone (500 or 250 or 80 mg for 5 days,) or dexamethasone (15 and 10 mg for 7 days) were administrated and gradually tapered. As the hospital stay was variable, the therapeutic effect was evaluated by the difference of HFGS and MRC sum score between nadir and 4 weeks after different treatments for the mechanically ventilated patients, while for those who did not require mechanical ventilation, the therapeutic effect was evaluated by the difference of HFGS and MRC sum score between nadir and 2 weeks after treatment. It is considered to be effective if the HFGS score of a patient has decreased by at least 1 grade or the MRC sum score has increased by 5 or more points after different therapies.16
Statistical Analysis
Statistical analysis was performed with SPSS version 17.0 software (IBM, West Grove, PA). Categorical data were presented as proportions, while continuous data were presented as means and standard deviations if normally distributed while as medians and interquartile ranges if abnormally distributed. Differences in proportions were tested by the Chi-square tests and differences in continuous variables were tested by Student t tests. For all statistical tests, P < 0.05 was considered significant.
RESULTS
Demographic Features of Enrolled Patients With GBS
A total of 527 adult patients were enrolled in the retrospective study, and flow chart is demonstrated in Figure 1. The baseline demographics of the recruited patients are shown in Table 2. The mean age of the recruited patients was 41-year-old with a male predilection (61.9%). Three hundred forty-three (65.1%) patients had antecedent infections, who usually accompanied with hyporeflexia or areflexia and sensory disturbance. Cranial nerve involvement was found in 212 patients (40.2%), among which the facial nerve palsy was the most common, followed by glossopharyngeal and vagus nerve deficits. Around 14.0% of patients required mechanical ventilation. A treatment before the nadir of disease was immediately initiated when the clinical diagnosis was confirmed after admission. Approximately 93.7% of patients received a treatment within 2 weeks after onset. According to the treatment modalities, the enrolled patients could be divided into 4 groups, and the comparisons of the demographic features of each group are shown in Table 3. We found that 81.2% patients with an HFGS more than 3, that is, the severe cases, either received IVIg or a combined therapy (IVIg plus intravenous corticosteroids). In addition, we found that the clinical severity was different among groups. Thus we compared the therapeutic effect of different treatments in patients with different nadir HFGS.
FIGURE 1.
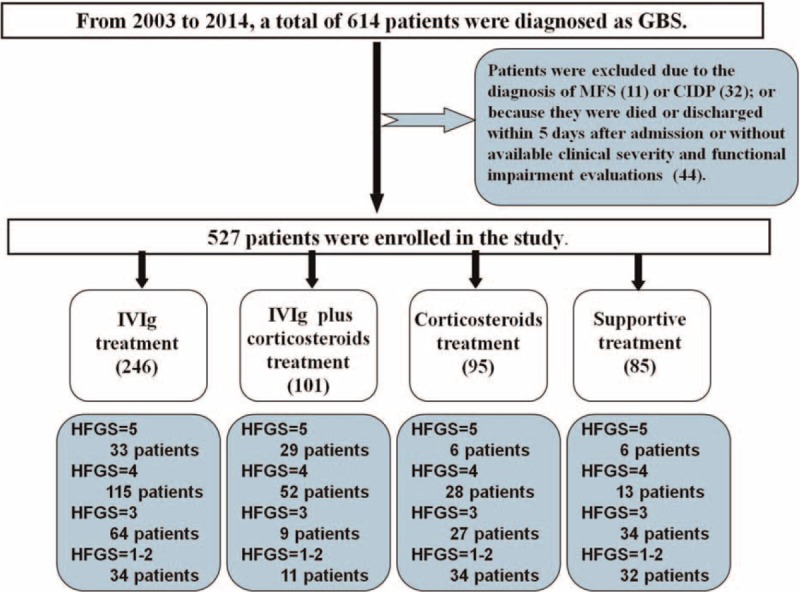
Flow chart of the study. From 2003 to 2014, a total of 614 patients who were admitted to the Department of Neurology of the First Hospital of Jilin University were diagnosed as Guillain–Barré syndrome (GBS). Thirty-two patients with later-confirmed chronic inflammatory demyelinating polyneuropathy (CIDP) and 11 patients with Miller Fisher syndrome (MFS) were excluded from the study. In addition, 44 patients who were died or discharged within 5 days after admission and those without available evaluations of the clinical severity and functional impairment during hospitalization were ruled out as well. Finally, 527 patients with GBS were enrolled and were divided into 4 groups according to the treatment modality, that is, intravenous immunoglobulin (IVIg) treatment group (246 patients), IVIg plus intravenous corticosteroids treatment group (101 patients), intravenous corticosteroids treatment group (95 patients), and supportive treatment group (85 patients). In each group, according to the Hughes Functional Grading Scale (HFGS) score, the patients were further divided into different subgroups.
TABLE 2.
Demographic Features of Patients With GBS
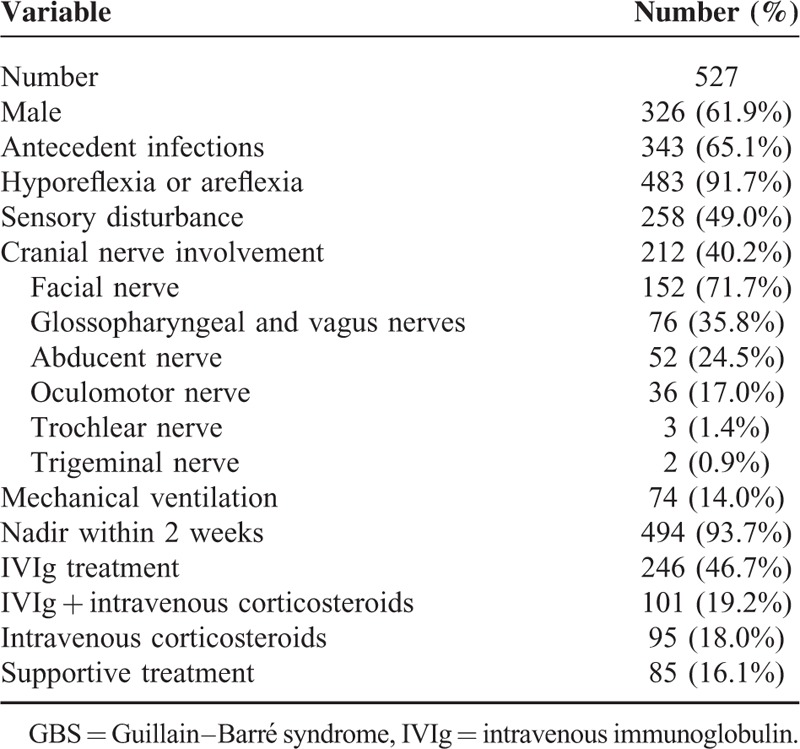
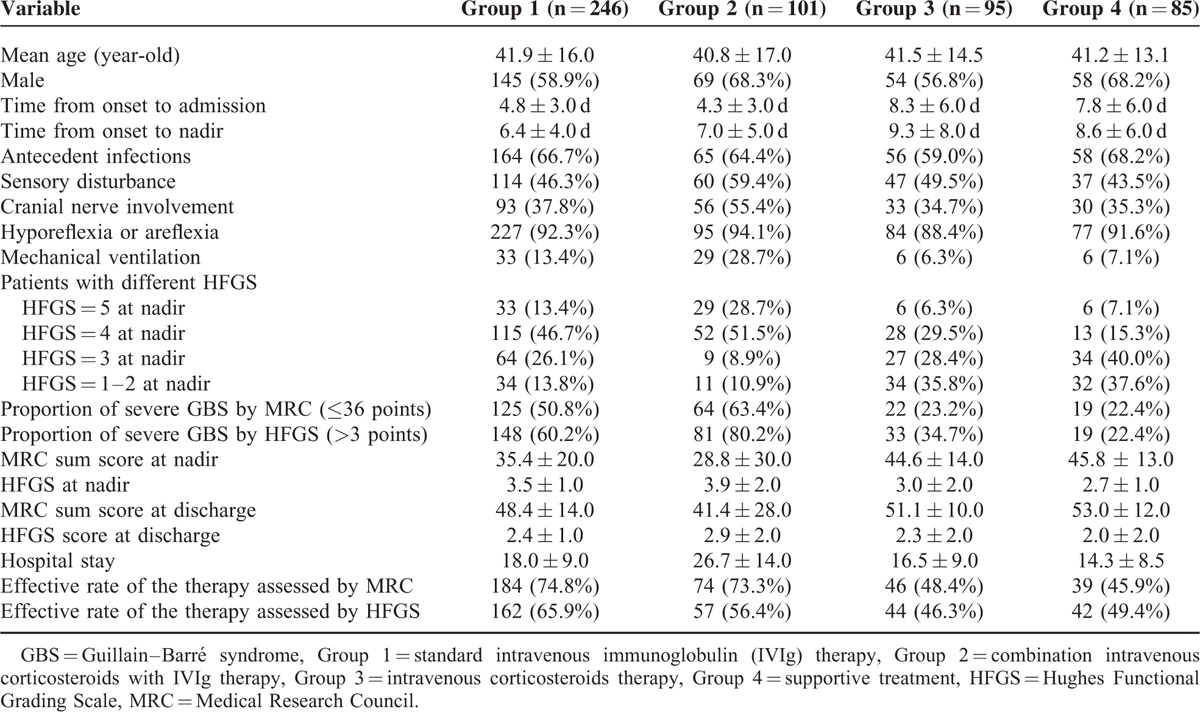
TABLE 3.
Comparisons of the Demographic Features of Patients With GBS in Different Groups
IVIg was More Effective in Treating Mechanically Ventilated Patients
As addition of intravenous corticosteroids was recommended in treating severe GBS, we further investigated whether there was a synergistic effect of combination intravenous corticosteroids with IVIg in treating severe GBS patients who required mechanical ventilation. Not only the clinical signs in the IVIg group and in the combination therapy group were comparable (P > 0.05), but the incidence of the infectious complications in the 2 groups was not significantly different (P > 0.05, Table 4). Except for the same HFGS score, the clinical severity revealed by MRC at nadir between the 2 groups was comparable (16.8 vs. 16.3, P > 0.05), as shown in Figure 2A. More patients were found improvement after IVIg treatment as assessed by the difference of MRC or HFGS (MRC: 97% vs. 72.4%; HFGS: 97% vs. 72.4%; both P < 0.05), as shown in Figure 2B. We did not found a synergistic effect of intravenous corticosteroid when used in combination with IVIg in the patients requiring mechanical ventilation.
TABLE 4.
Comparisons of Clinical Features of the Mechanically Ventilated GBS in Different Treatment Groups
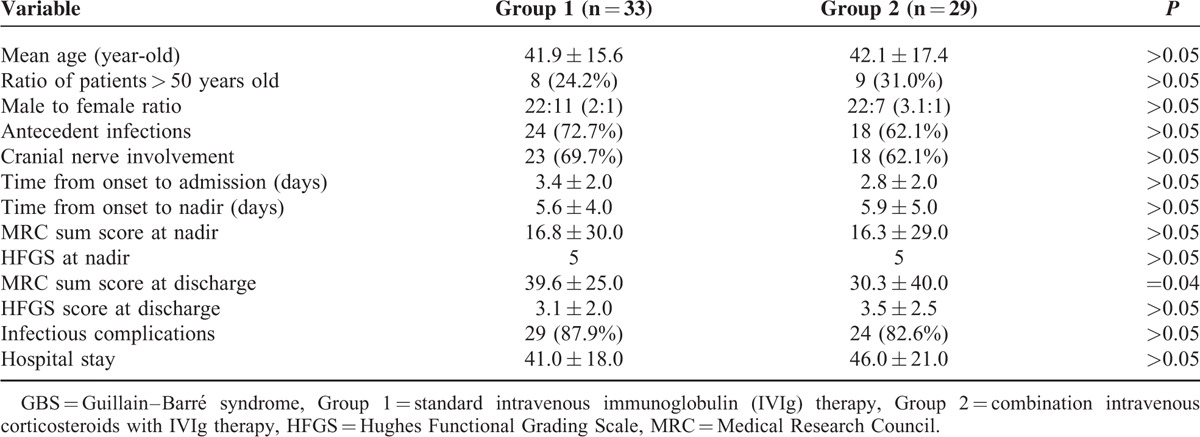
FIGURE 2.
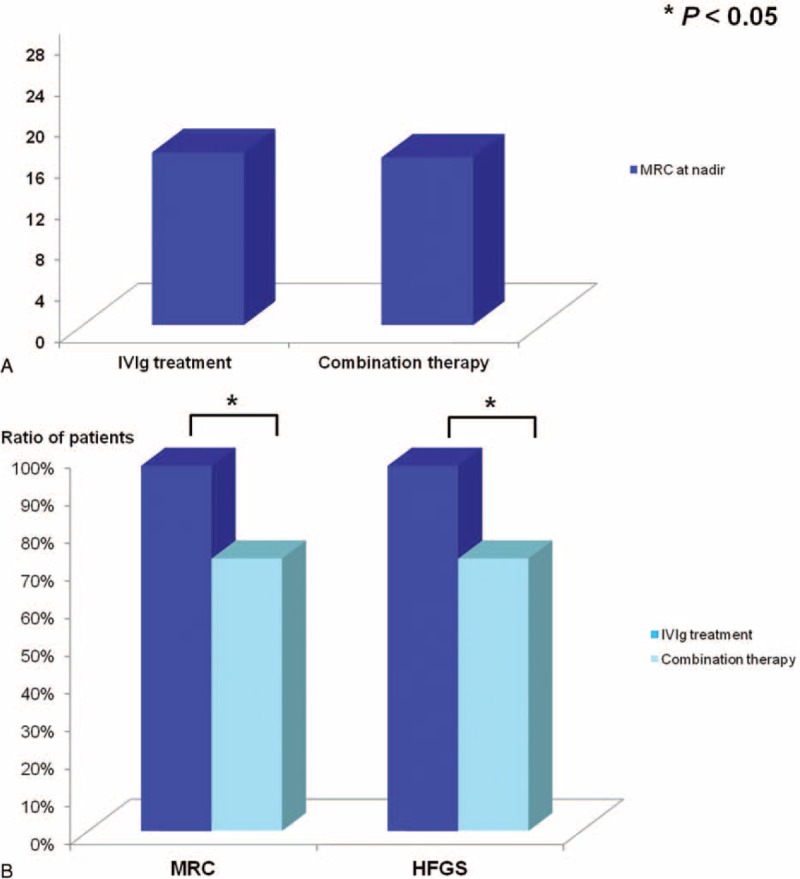
Intravenous immunoglobulin (IVIg) was more effective in treating mechanically ventilated Guillain–Barré syndrome (GBS) patients. The clinical severity revealed by Medical Research Council (MRC) at nadir of the mechanically ventilated GBS patients in the IVIg treatment groups was 16.8 while it was 16.3 in the combination therapy group (P > 0.05), indicating the comparable muscle weakness between the 2 groups (A). Moreover, more patients were found improvement after IVIg treatment as assessed by the improvement of MRC sum score (97%) and Hughes Functional Grading Scale (HFGS) score (97%) compared with that in the combination therapy group (MRC: 72.4%; HFGS: 72.4%) (B).
Combined Use of Intravenous Corticosteroids and IVIg Appeared Detrimental to Mechanically Ventilated Patients
As above mentioned, the clinical severity and the incidence of the infectious complications of the mechanically ventilated GBS patients in the IVIg-treated group were comparable with the combination therapy group (P > 0.05). Of note was that the combination therapy was less effective than IVIg to the mechanically ventilated patients as demonstrated by the different ratio of patients who were found improvement after different treatments (MRC: 72.4% vs. 97%; HFGS: 72.4% vs. 97%; both P < 0.05). Thus, intravenous corticosteroids when used in combination with IVIg appear detrimental to mechanically ventilated patients.
Intravenous Corticosteroids Did Not Synergize With IVIg in Treating Bedridden Patients Without Mechanical Ventilation
We further investigated whether intravenous corticosteroids when used in combination with standard IVIg were superior to the IVIg alone in treating bedridden GBS patients without mechanical ventilation (HFGS = 4). Totally, 115 bedridden patients without mechanical ventilation fell into the IVIg treatment group, while 52 were in the combination therapy group. The mean age in the IVIg group was 43-year-old, which was similar to that in the combination therapy group (41-year-old). Time from onset to admission (4.0 vs. 4.8, P > 0.05) and time from onset to nadir (5.7 vs. 6.7, P > 0.05) were also comparable between the 2 groups. As shown in Figure 3A, although the incidence of the cranial nerve involvement was higher in the combination group (46.2% vs. 29.6%, P < 0.05), the MRC scores at nadir and at discharge in the 2 groups were not significantly different (P > 0.05) (Fig. 3B), as well as the HFGS score (P > 0.05) (Fig. 3C). The hospital stay in the IVIg treatment group was 14 days compared with 18 days in the combination therapy group. The incidence of the infectious complications was not significantly different between the 2 groups (15.7% vs. 23.1%, P > 0.05). In addition, ratio of patients who showed improvement after treatment was not significant different between the 2 groups revealing by the difference of MRC sum score and HFGS score (MRC: 89.6% vs. 86.5%; HFGS: 69.6% vs. 61.5%; both P > 0.05), as demonstrated in Figure 3D. Collectively, intravenous corticosteroids did not synergize with IVIg in treating nonmechanically ventilated but bedridden GBS patients.
FIGURE 3.
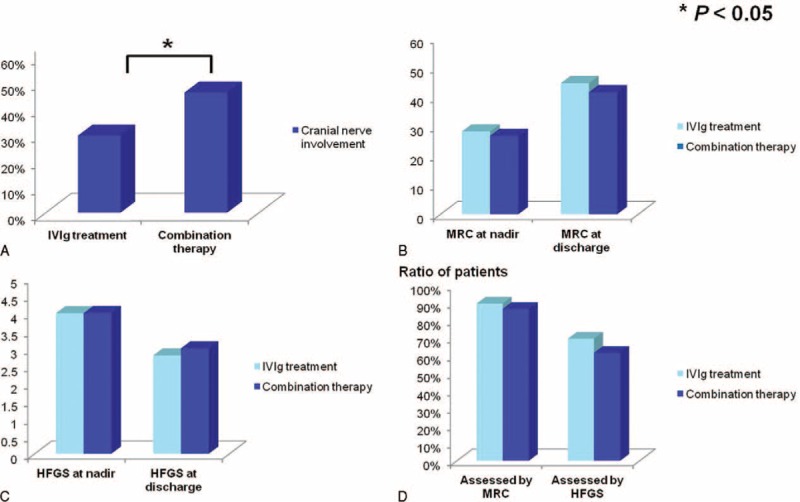
Comparisons of clinical signs of bedridden Guillain–Barré syndrome (GBS) patients without mechanical ventilation in different treatment groups. The incidence of the cranial nerve involvement of the nonmechanically ventilated but bedridden patients in the combination therapy group was higher than the intravenous immunoglobulin (IVIg) treatment group (46.2% vs. 29.6%, P < 0.05) (A). Similarly, the Medical Research Council (MRC) sum score at nadir (28.2 vs. 26.7, P > 0.05) and at discharge (44.7 vs. 41.6, P > 0.05) were comparable between the 2 groups (B), as well as the Hughes Functional Grading Scale (HFGS) score at nadir (4.0 vs. 4.0, P > 0.05) and at discharge (2.8 vs. 3.0, P > 0.05) (C). The efficiency reflected by the ratio of patients who were improved after treatment was not different between the 2 groups either assessed by improvement of MRC (89.6% vs. 86.5%, P > 0.05) or HFGS (69.6% vs. 61.5%, P > 0.05) (D).
Most of the enrolled patients received a treatment within 2 weeks before the nadir of disease. As corticosteroids might inhibit the macrophage repair processes whereby interfering with the recovery of GBS, we further compared the therapeutic effect of different treatments which started within 7 days after onset. One hundred fifty three bedridden patients without mechanical ventilation received a therapy within 7 days, among which 108 fell into the IVIg treatment group. Table 5 demonstrates the comparisons of the clinical signs and the therapeutic efficiency between the IVIg-treated group and the combination therapy group. We found that the clinical data (including the median age, the ratio of males and older patients, cranial nerve involvement, etc.), as well as the disease severity at nadir (MRC and HFGS) between the 2 groups were not different (P > 0.05). Similarly, we found that the efficacy as evaluated by the ratio of patients with improvement of MRC and HFGS was insignificantly different between the 2 groups (both P > 0.05), indicating that intravenous corticosteroids when used in combination with IVIg was not superior to IVIg alone in treating bedridden GBS patients without mechanical ventilation.
TABLE 5.
Comparisons of Clinical Signs of the Bedridden GBS Patients Without Mechanical Ventilation in Different Treatment Groups Initiated Within 7 Days After Onset
Supportive Treatment was Sufficient for Patients who were Able to Walk With or Without Assistance
Patients who were able to walk 5 m across an open space with help (HFGS = 3) were divided into the IVIg treatment group (64 patients), the intravenous corticosteroids group (27 patients), and the supportive treatment group (34 patients) in present study. The mean age was 40-year-old, 39-year-old, and 42-year-old in the IVIg treatment group, the intravenous corticosteroids group, and the supportive treatment group, respectively, which was comparable (P > 0.05). As shown in Figure 4A, time from onset to admission and to nadir were 6.0 d and 7.1 in the IVIg-treated group, 7.7 d and 8.5 d in the corticosteroids group, 7.1 d and 7.9 d in the supportive treatment group, respectively, which were not significantly different among the 3 groups (P > 0.05). The MRC sum score at nadir and at discharge (Fig. 4B), and the HFGS score at nadir and at discharge were also comparable (P > 0.05). Although the ratio of patients responsive to the treatment as assessed by the improvement of MRC or HFGS was higher in the IVIg-treated group; however, it did not reach a significant difference among the 3 groups (MRC: 70.3% vs. 55.6% vs. 58.8%, HFGS: 67.2% vs. 48.1% vs. 58.8%; both P > 0.05), as shown in Figure 4C. Therefore, supportive treatment appear sufficient for GBS patients who were able to walk with help (HFGS = 3).
FIGURE 4.
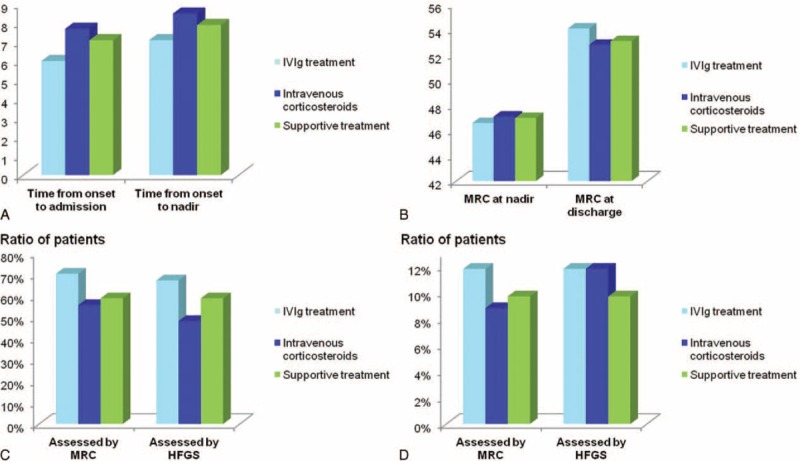
Supportive treatment was sufficient to patients who were able to walk with or without assistance. In patients who were able to walk with help, time from onset to admission and to nadir were 6.0 d and 7.1 d in intravenous immunoglobulin (IVIg) treatment group, 7.7 d and 8.5 d in intravenous corticosteroids group, 7.1 d and 7.9 d in supportive treatment group, respectively, which were not significantly different (P > 0.05) (A). MRC at nadir and at discharge were 46.6 and 54.1, 47.1 and 52.8, 47.0 and 53.1 in the 3 groups, which was as well comparable (B). Assessed by improvement of the Medical Research Council (MRC) sum score and Hughes Functional Grading Scale (HFGS) score, ratio of patients who were improved was respectively 70.3% and 67.2% in the IVIg group, 55.6% and 48.1% in intravenous corticosteroids group, 58.8% and 58.8% in supportive treatment group, which was comparable among the 3 groups (P > 0.05) (C). Thus, supportive treatment was sufficient to GBS patients who were able to walk with help (HFGS = 3). Furthermore, for patients with GBS who could walk unaided (HFGS = 1–2), the ratio of patients who were found improvement was 11.8% and 11.8% after IVIg treatment, which was similar to the intravenous corticosteroids group (8.8% and 11.8%) and supportive treatment group (9.7% and 9.7%) (D).
In addition, 111 patients were able to walk unaided (HFGS = 1–2), and 100 of them fell into either the IVIg treatment group (34 patients), the intravenous corticosteroids group (34 patients), or the supportive treatment group (32 patients). No significant difference was observed in these patients regardless of treatment options, as shown in Figure 4D, implying that supportive treatment was also sufficient to deal with mildly affected GBS patients.
DISCUSSION
In the study, we retrospectively explored the therapeutic efficacy of intravenous corticosteroids combined with IVIg in dealing with severe GBS. We first found that IVIg treatment was more effective while intravenous corticosteroids when used in combination with IVIg appeared detrimental for mechanically ventilated GBS patients. For the bedridden GBS patients without mechanical ventilation, the corticosteroid add-on therapy was not superior to IVIg alone, that is, intravenous corticosteroids did not synergize with IVIg in treating these patients. As to patients who were able to walk with or without help, supportive treatment was sufficient.
The pathogenesis of GBS is generally ascribed to the involvement of both the cellular and humoral immunity.17,18 Due to the autoimmune nature of the disease, the immunotherapy is usually prescribed. Both IVIg and PE have been proven effective in treating GBS patients, who were unable to walk independently (HFGS ≥ 3), while addition IVIg after PE treatment does not confer significant extra benefit.1,15 However, GBS remains a potentially life-threatening disease as even with the above proven effective options, some patients had a severe clinical signs or had residual deficits. In this regard, more effective therapeutic strategies remain in urgent need.
Theoretically, corticosteroids with the capability of anti-inflammatory and immunosuppressive activities are expected to reduce inflammation and lessen nerve damage in inflammatory neuropathy. Corticosteroids have been shown to hasten recovery when used in large doses in experimental autoimmune neuritis, an animal model of GBS.4 As to patients with GBS, oral corticosteroids might slow recovery, while intravenous methylprednisolone alone does not produce significant benefit or harm.6,7,19 Thus corticosteroids alone were not recommended for treatment of the patients with GBS. In 1994, a study by Dutch GBS group found that 76% GBS patients in the combination of corticosteroids with IVIg therapy group improved by at least 1 disability grade scales compared with 53% patients in the IVIg alone-treated group.8 Similarly, a double-blind, placebo-controlled, multicentre, and randomized study by van Koningsveld et al9 found that 68% patients in the IVIg combined with methylprednisolone treated group had improved by at least 1 disability grade scales by 4 weeks compared with 56% of the IVIg plus placebo treated group, which barely reached statistical significance after adjustments for age and disease severity, suggestive of a synergistic effect of corticosteroids when used in combination with IVIg. Thus, intravenous corticosteroids as an add-on therapy have been recommended in treatment of severe or protracted GBS patients by some authors.9,10 However, Hughes proposed that there was no synergy between IVIg and steroids when took into consideration all the available evidence, and the lack of benefits from corticosteroids might be due to the harmful effects of corticosteroids on denervated muscle or its inhibition on macrophage repair processes.20,21 Collectively, the benefit of intravenous corticosteroids as an add-on therapy remains controversial at present.
We found that IVIg treatment is more effective for mechanically ventilated GBS patients, while intravenous corticosteroids when used in combination with IVIg appeared detrimental with comparable incidence of infectious complications. This was inconsistent with the study conducted by van Koningsveld et al. It is noteworthy that the recruited patients in the study by van Koningsveld et al were GBS patients with an HFGS score of 3 or worse. However, the ratio of mechanically ventilated GBS patients was not reported. Thus the efficacy of combination therapy in treating mechanically ventilated GBS has thus far underdetermined.9 The mechanisms of the detrimental role of intravenous corticosteroids for mechanically ventilated patients could not be ascribed to the incidence of infectious complications according to the present study. Except for its detrimental effects on denervated muscle or its inhibition on macrophage repair processes,20,21 it might be also related to the hyperglycemia caused by corticosteroids, which might lead to the damage of the peripheral nervous,22,23 as increased blood glucose concentrations requiring insulin were significantly more common in corticosteroid-treated GBS patients.7 In another study, we found that levels of fasting glucose in serum were correlated with the clinical severity of patients with GBS (unpublished data). Thus, the detrimental role of intravenous corticosteroids when used in combination with IVIg in treating mechanically ventilated GBS may be ascribed to the increased blood glucose concentrations. Further prospective and randomized studies are warranted to validate this finding.
Moreover, we found that the combination therapy was not superior to IVIg alone for bedridden GBS patients without mechanical ventilation. As corticosteroids might interfere with the repair processes of GBS due to its inhibitory effects on macrophage,20 we further compared the therapeutic effect of different treatments which initiated within 7 days to the bedridden patients without mechanical ventilation. Similar results were found as well. Thus, IVIg was sufficient to the bedridden GBS patients without mechanical ventilation (HFGS = 4). As van Koningsveld et al found a significant difference between methylprednisolone plus IVIg and IVIg combined with placebo when adjustments were made including a low number of days between onset of weakness and randomization, comparison of efficiency between IVIg with the combination therapy, which initiated earlier for bedridden GBS patients without mechanical ventilation are needed to validate this finding.
Currently, the immunotherapy is usually started if the patients are not able to walk 5 m unaided (HFGS ≥ 3).19 As to the “mildly affected GBS,” which has been defined as being able to walk with or without assistance (HFGS ≤ 3), the efficiency of different therapeutic regimens has not been extensively studied.19,24 Hence we further compared the efficiency of different treatments to mildly affected patients, and found that supportive treatment was sufficient in treating the mildly affected GBS (HFGS < 3). Of note is that for those GBS patients who are able to walk with help (HFGS = 3), the immunotherapy was recommended from previous studies. Moreover, a French randomized study investigated the effect of PE in patients who could walk with or without aid whereas could not run (HFGS = 2–3), and revealed that motor recovery was faster in those who received 2 PE sessions than those received no PE.25 Based on this study, there might be an indication to treat mildly affected GBS patients with PE, but it should be kept in mind that no randomized placebo-controlled trials have assessed the effect of different treatments for mildly affected patients with GBS. From our retrospective study, although the efficiency was better in the IVIg group whereas not significant different from that in the intravenous corticosteroids group and the supportive treatment group for GBS patients who are able to walk with help (HFGS = 3). Similarly, supportive treatment was also sufficient for the patents with GBS who are able to walk independently.
There are some limitations of our study. Due to the retrospective nature of the study, the use of intravenous corticosteroids was not consistent. In addition, it has been proposed that combination of IVIg with corticosteroids might be effective in a distinct subgroup of patients, such as those with anti-GM1 antibodies.26 However, limited by the sample size, we did not investigate the subtype-specific responsiveness to corticosteroids. Due to the retrospective nature of our study, the therapeutic effect was evaluated by the difference of HFGS and MRC sum score between nadir and 4 weeks after different treatments for the mechanically ventilated patients, while for those who did not require mechanical ventilation, the therapeutic effect was evaluated by the difference of HFGS and MRC sum score between nadir and 2 weeks after treatment. We failed to make a follow-up on patients who had not received electrophysiological evaluation and those who still needed mechanical ventilator when they were discharged. Thus, the electrophysiological data and the duration of mechanical ventilation were unavailable in some patients. The correlation between different subtypes of GBS and the prognosis awaits further validation.
In summary, IVIg is sufficient to GBS patients who are unable to walk (HFGS > 3), while corticosteroids are detrimental for mechanically ventilated GBS patients when used in combination with IVIg.
Footnotes
Abbreviations: GBS = Guillain–Barré syndrome, HFGS = Hughes Functional Grading Scale, IVIg = intravenous immunoglobulin, MRC = Medical Research Council, PE = plasma exchange.
The work was supported by grants from the National Natural Science Foundation of China (no. 81271294, 81241147 and 81471216), the Young Scholars Program of Norman Bethune Health Science Center of Jilin University (no. 2013205035), the Young Scholars Program of the First Hospital of Jilin University (no. JDYY42013003), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (no. 3C113BK73428).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.van den Berg B, Walgaard C, Drenthen J, et al. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol 2014; 10:469–482. [DOI] [PubMed] [Google Scholar]
- 2.Wakerley BR, Yuki N. Infectious and noninfectious triggers in Guillain-Barré syndrome. Expert Rev Clin Immunol 2013; 9:627–639. [DOI] [PubMed] [Google Scholar]
- 3.Zhang HL, Zheng XY, Zhu J. Th1/Th2/Th17/Treg cytokines in Guillain-Barré syndrome and experimental autoimmune neuritis. Cytokine Growth Factor Rev 2013; 24:443–453. [DOI] [PubMed] [Google Scholar]
- 4.Watts PM, Taylor WA, Hughes RA. High-dose methylprednisolone suppresses experimental allergic neuritis in the Lewis rat. Exp Neurol 1989; 103:101–104. [DOI] [PubMed] [Google Scholar]
- 5.Guillain-Barré Syndrome Steroid Trial Group. Double-blind trial of intravenous methylprednisolone in Guillain-Barré syndrome. Lancet 1993; 341:586–590. [PubMed] [Google Scholar]
- 6.Hughes RA, Swan AV, van Doorn PA. Corticosteroids for Guillain-Barré syndrome. Cochrane Database Syst Rev 2010; 2:CD001446. [DOI] [PubMed] [Google Scholar]
- 7.Hughes RA, van Doorn PA. Corticosteroids for Guillain-Barré syndrome. Cochrane Database Syst Rev 2012; 8:CD001446. [DOI] [PubMed] [Google Scholar]
- 8.The Dutch Guillain-Barré Study Group. Treatment of Guillain-Barré syndrome with high-dose globulins combined with methylprednisolone: a pilot study. Ann Neurol 1994; 35:749–752. [DOI] [PubMed] [Google Scholar]
- 9.van Koningsveld R, Schmitz PI, Meché FG, et al. Effect of methyprednisolone when added to standard treatment with intravenous immunoglobulin for Guillain-Barré syndrome: randomized trial. Lancet 2004; 363:192–196. [DOI] [PubMed] [Google Scholar]
- 10.Shahar E. Current therapeutic options in severe Guillain-Barré syndrome. Clin Neuropharmacol 2006; 29:45–51. [DOI] [PubMed] [Google Scholar]
- 11.Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol 1990; 27:S21–S24. [DOI] [PubMed] [Google Scholar]
- 12.Zhou CK, Wu LM, Ni FM, et al. Critical illness polyneuropathy and myopathy: a systematic review. Neural Regen Res 2014; 9:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes RA, Newsom-Davis JM, Perkin GD, et al. Controlled trial prednisolone in acute polyneuropathy. Lancet 1978; 2:750–753. [DOI] [PubMed] [Google Scholar]
- 14.Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve 1991; 14:1103–1109. [DOI] [PubMed] [Google Scholar]
- 15.Hughes RA, Swan AV, Van Doorn PA. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst Rev 2014; 9:CD002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fokke C, van den Berg B, Drenthen J, et al. Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain 2014; 137:33–43. [DOI] [PubMed] [Google Scholar]
- 17.Sheikh KA, Zhang G, Gong Y, et al. An anti-ganglioside antibody-secreting hybridoma induces neuropathy in mice. Ann Neurol 2004; 56:228–239. [DOI] [PubMed] [Google Scholar]
- 18.Tatsumoto M, Koga M, Gilbert M, et al. Spectrum of neurological diseases associated with antibodies to minor gangliosides GM1b and GalNAc-GD1a. J Neuroimmunol 2006; 177:201–208. [DOI] [PubMed] [Google Scholar]
- 19.Hughes RA, Swan AV, Raphaël JC, et al. Immunotherapy for Guillain-Barré syndrome: a systematic review. Brain 2007; 130:2245–2257. [DOI] [PubMed] [Google Scholar]
- 20.Hughes RA. Treatment of Guillain-Barré syndrome with corticosteroids: lack of benefit? Lancet 2004; 363:181–182. [DOI] [PubMed] [Google Scholar]
- 21.Rich MM, Pinter MJ, Kraner SD, et al. Loss of electrical excitability in an animal model of acute quadriplegic myopathy. Ann Neurol 1998; 43:171–179. [DOI] [PubMed] [Google Scholar]
- 22.Li G, Sun C, Wang Y, et al. A clinical and neuropathological study of Chinese patients with diabetic peripheral neuropathy. PLoS One 2014; 9:e91771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zychowska M, Rojewska E, Przewlocka B, et al. Mechanisms and pharmacology of diabetes neuropathy-experimental and clinical studies. Pharmacol Rep 2013; 65:1601–1610. [DOI] [PubMed] [Google Scholar]
- 24.van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet 2008; 7:939–950. [DOI] [PubMed] [Google Scholar]
- 25.The French Cooperative Group on Plasma Exchange in Guillain-Barré Syndrome. Appropriate number of plasma exchanges in Guillain-Barré syndrome. Ann Neurol 1997; 41:298–306. [DOI] [PubMed] [Google Scholar]
- 26.Susuki K, Yuki N. Effect of methylprednisolone in patients with Guillain-Barré syndrome. Lancet 2004; 363:1236–1237. [DOI] [PubMed] [Google Scholar]


