Abstract
We conducted a retrospective, cross-sectional study to analyze predisposing factors, clinical features, and microbiological characteristics of patients with microbial keratitis hospitalized over 10 years.
The medical records of 558 patients who were diagnosed with microbial keratitis and admitted to Chang Gung Memorial Hospital (CGMH), a referral center in Taiwan, from January 1, 2003 to December 31, 2012 were reviewed. Demographics, predisposing factors, isolated organisms, treatment, and hospital stay were recorded. Yearly trends were tested using a linear-by-linear association.
Contact lens wear was the most common predisposing factor (31.4%), followed by ocular and systemic diseases (26.3%) and trauma (23.5%). Contact lens-related infectious keratitis increased year by year (P = 0.011). Pseudomonas aeruginosa was the most commonly isolated organism (28%), followed by fungi (17.6%) and coagulase-negative Staphylococcus (5.4%). Except for Serratia marcescens, the identified organisms did not change over 10 years. Most bacterial infections were controlled using antimicrobial treatment, but more than half of patients with fungal keratitis required surgical interventions. The mean hospital stay was 13.7 ± 11.5 days. Previous ocular surgery, large ulcer size, nontuberculous myycobacteris infection, and surgery during admission were related to prolonged hospital stay.
In Taiwan, contact lens-related pseudomonal keratitis remained the most common cause of microbial keratitis in patients hospitalized from 2003 to 2012.
INTRODUCTION
Microbial keratitis is a major cause of ocular morbidity and visual disability worldwide. Several risk factors such as contact lens wear, trauma, ocular surface disease, ocular surgery, and systemic disease have been reported to predispose patients to corneal infections. Management of microbial keratitis commonly involves obtaining corneal scrapings for microbiological studies, and then, empiric broad-spectrum treatment is typically initiated before culture results are available. Appropriate empiric therapy is selected by practitioners on the basis of epidemiological information such as predisposing factors and the spectrum of causative organisms. However, such information may vary by geographic region. For example, trauma is a common risk factor for fungal keratitis in developing agricultural countries,1 whereas contact lens wear is the main risk factor for bacterial keratitis in developed countries.2–6 Thus, it is essential to establish related information on microbial keratitis, including patient-specific risk factors and the most likely causative organisms, which would facilitate the development of effective strategies for the prevention, diagnosis, and treatment of microbial keratitis.
In Taiwan, a study from a university hospital reported that contact lens-related pseudomonas keratitis was the most common form of microbial keratitis from 1992 to 2001.7 Conducting periodic surveys of infectious keratitis is crucial for updating the local information for reference by clinicians because epidemiologic patterns may change over time. Therefore, the present study collected data on the predisposing factors, clinical manifestations, spectrum of microorganisms, and treatments for patients with microbial keratitis who were admitted to and treated at Chang Gung Memorial Hospital (CGMH), a major teaching hospital in Northern Taiwan, from 2003 to 2012.
METHODS
This study was conducted as a retrospective, cross sectional design, and in accordance with the Declaration of Helsinki and was approved by the Institutional Research Ethics Board of CGMH, Taiwan (IRB102-4073B). Consent was waived because of the retrospective design of the project and the anonymous analysis of the data.
We retrospectively reviewed the medical records of 558 patients with infectious keratitis who were admitted to CGMH from January 1, 2003 to December 31, 2012. We identified patients by using a computerized diagnostic code search. We included patients with negative culture results if they presented with an epithelial defect and stromal infiltrate and responded favorably to antimicrobial treatment. Admission criteria were primarily severe (ie, potentially sight-threatening) keratitis and the need for intensive topical antimicrobials.
Information on age, sex, predisposing factors, clinical features, microbiological results, treatments, and visual acuity (if recorded) was collected. We defined an ulcer as being central if it encroached within 2 mm of fixation, peripheral if it involved a zone within 2 mm from the limbus, and paracentral if it was between the central and peripheral zone. Corneal ulcers were defined as being small (<2 mm), medium (2–6 mm), or large (>6 mm). Corneal scrapings were obtained using a surgical blade and directly inoculated into blood agar, chocolate agar, modified Sabouraud agar, Lowenstein–Jensen agar slant, and thioglycolate broth. Corneal scrapings of patients with clinical characteristics suggestive of Acanthamoeba keratitis were inoculated into nonnutrient agar seeded with Escherichia coli. The various media were routinely incubated for a week or longer depending on the media before the final culture result was obtained. A positive culture was defined as growth of at least 3 colonies along the line of inoculation on one solid medium on the basis of the criteria in a previous study.8
Before the result of corneal ulcer culture could be obtained, levofloxacin (0.5%) alone or a combination of 2 fortified antibiotics (25 mg/mL of cefazolin and 25 mg/mL of amikacin) was administered topically once per hour. Antibiotics were modified on the basis of the culture results and the clinical response. Topical natamycin and amphotericin B (0.1%) were applied hourly for mold and yeast infection, respectively. Topical polyhexamethylene biguanide (0.02%) was applied for Acanthamoeba keratitis.
For data presentation, we arbitrarily divided the study years into 2 periods; the first half was from 2003 to 2007, and the second half was from 2008 to 2012. Mantel-Haenszel linear-by-linear association χ2 test was used to detect the trends over the 10-year period. Categorical variables were analyzed using a χ test; continuous variables were analyzed using analysis of variance (ANOVA). Simple linear regression for univariate analysis was used to identify the factors associated with hospital stay. Multiple linear stepwise regression was performed after univariate analysis. A P value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS software, Version 22 (IBM, Armonk, NY).
RESULTS
Demographics and Clinical Features
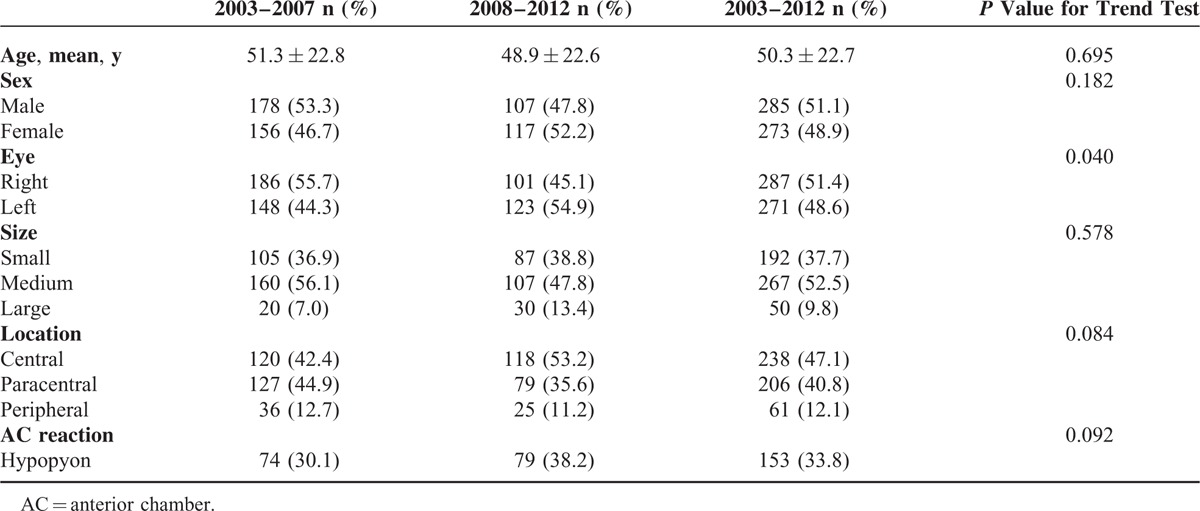
During the 10-year study period, 558 patients were included in this study, of which 285 (51.1%) and 273 (48.9%) were male and female, respectively (Table 1). The mean age of patients was 50.3 ± 22.7 years (range, 2–100 yr). The right eye was involved in 287 patients and the left eye was involved in 271 patients. The corneal ulcer was small in 192 eyes (37.7%), medium in 267 eyes (52.5%), and large in 50 eyes (9.8%). The location of the corneal ulcer was central in 238 eyes (47.1%), paracentral in 206 eyes (40.8%), and peripheral in 61 eyes (12.1%). The presence of hypopyon was noted in 153 eyes (33.8%). Except for laterality, trends for demographics and clinical features were not statistically significant.
TABLE 1.
Demographics and Clinical Features of Microbial Keratitis
Predisposing Factors
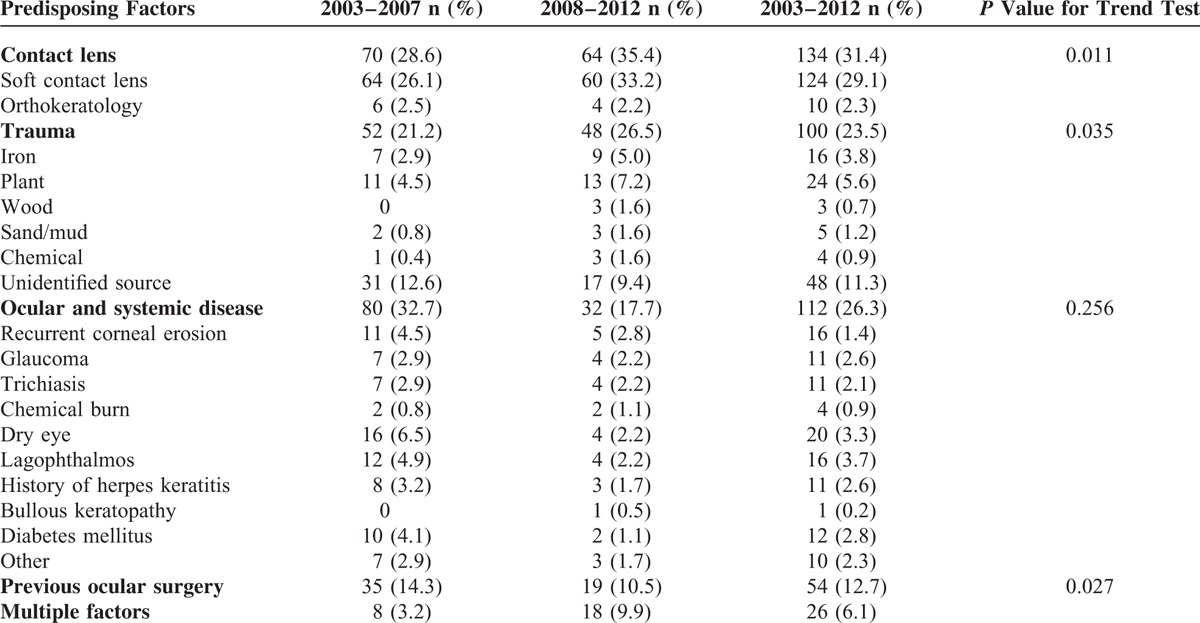
Risk factors for microbial keratitis were identified in 426 patients (76.3%). The most common risk factor was contact lens wear (31.4%), followed by systemic and ocular diseases (26.3%), trauma (23.5%), and previous ocular surgery (12.7%) (Table 2). A total of 54 patients (12.7%) had at least 2 predisposing factors for corneal ulcers. Materials causing ocular trauma included plant (n = 24), iron (n = 16), mud (n = 5), chemical (n = 4), wood (n = 3), and unidentified sources (n = 48). Lagophthalmos (n = 16) and dry eye (n = 14) were the most common ocular surface diseases. The trend test showed that during the 10-year study period, the proportion of patients wearing contact lenses and with trauma increased (P = 0.011 and P = 0.035, respectively), and the rate of previous ocular surgery decreased (P = 0.027).
TABLE 2.
Predisposing Factors for Microbial Keratitis
Microbiological Analysis
Positive culture results were obtained in 353 patients (63.3%). Two hundred thirty-eight (42.7%) patients were administered topical antibiotics before referral, and 88 patients had negative culture results. Regarding isolates, 210 bacterial isolates (59.9%), 62 fungal isolates (17.6%), 8 nontuberculous mycobacteria (NTM) isolates (2.3%), and 2 Acanthamoeba isolates (0.6%) were identified (Table 3). Seventy-one patients had polymicrobial infections (20.1%). Among bacterial isolates, gram-negative bacteria (37.4%) were more common than gram-positive bacteria (22.1%). The most commonly isolated bacterium was Pseudomonas aeruginosa (28%), followed by coagulase-negative Staphylococcus (CNS, 5.4%) and Staphylococcus aureus (4.5%). The percentage of Serratia marcescens decreased significantly (P = 0.033); the identified organisms did not change over 10 years.
TABLE 3.
Isolated Organisms in Microbial Keratitis
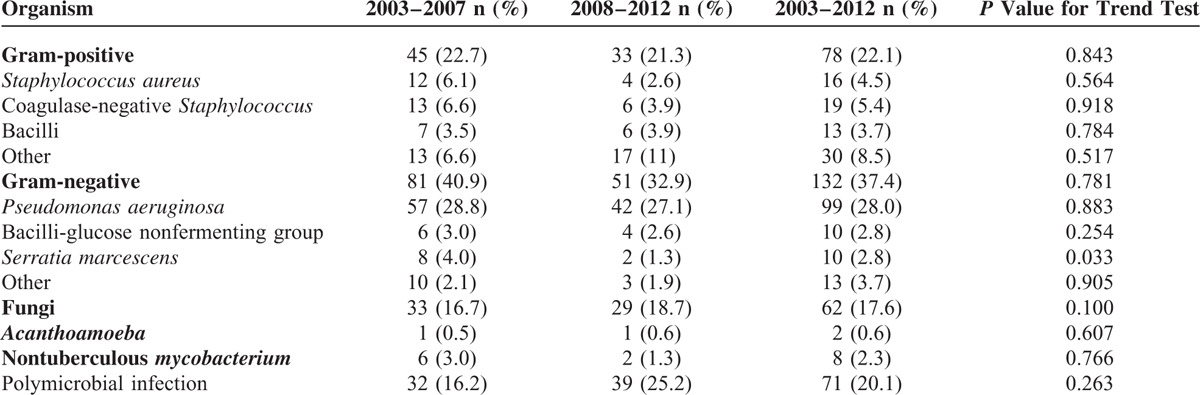
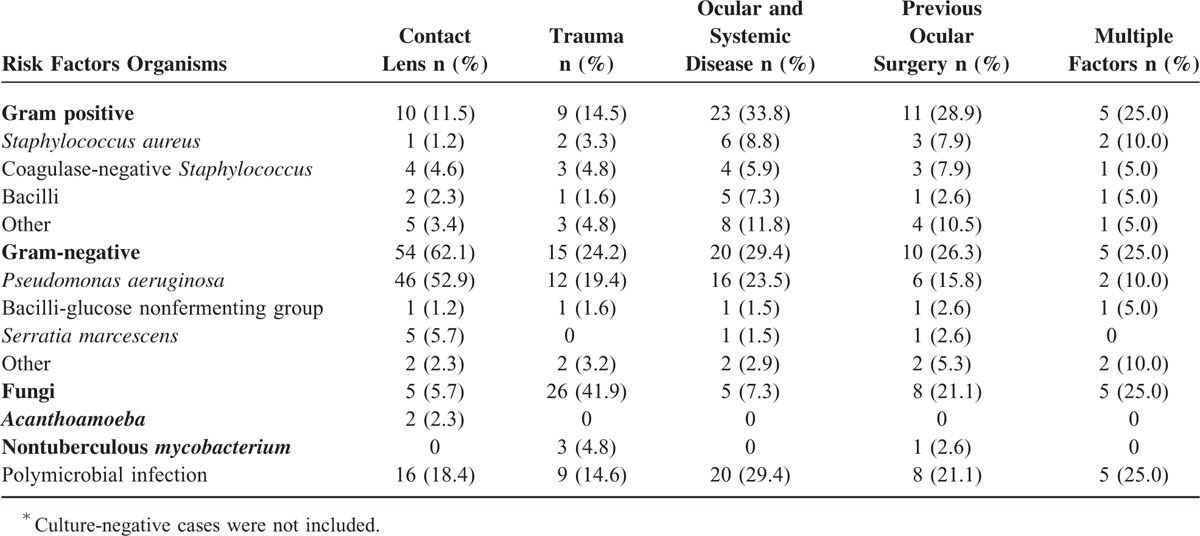
Table 4 lists isolated organisms from patients with different risk factors for microbial keratitis. Gram-negative bacteria, particularly P. aeruginosa, mainly accounted for contact lens-related keratitis (52.9%). Fungi were dominant in trauma-related keratitis (41.9%). Keratitis associated with ocular and systemic diseases was mainly caused by bacteria (33.8% and 29.4% for gram-positive and gram-negative bacteria, respectively).
TABLE 4.
Risk Factors Versus Isolated Organisms in Microbial Keratitis∗
TREATMENT
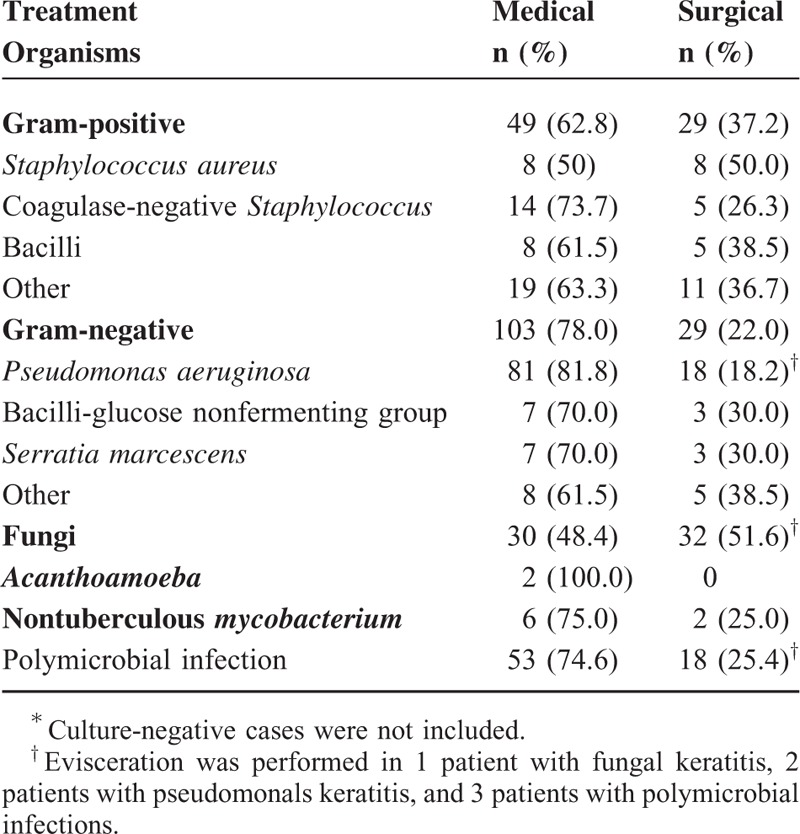
All patients were initially treated with empiric antimicrobials, which were adjusted on the basis of the clinical response and the results of the drug susceptibility test. Overall, 69.5% of the patients were cured by antimicrobials solely. Medical treatment was successful for patients with gram-positive bacterial infections (62.8%), gram-negative bacterial infections (78%), NTM infections (75%), Acanthamoeba infections (100%), and polymicrobial infections (74.6%) (Table 5). However, 51.6% of patients with fungal keratitis required additional surgical interventions. Surgical procedures included amniotic membrane transplantation, patch graft, lamellar keratectomy, penetrating keratoplasty, and evisceration. One patient with fungal keratitis, 2 patients with pseudomonal keratitis, and 3 patients with polymicrobial infections underwent evisceration to eradicate the infections.
TABLE 5.
Treatment for Microbial Keratitis∗
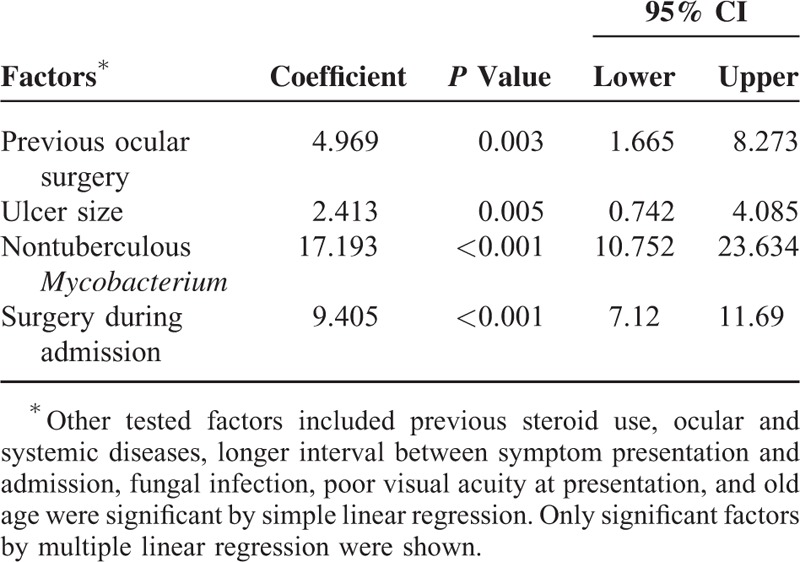
The mean hospital stay was 13.7 ± 11.5 days. Longer hospital stay was correlated with previous steroid use, ocular and systemic diseases, longer interval between symptom presentation and admission, previous ocular surgery, large ulcer size, fungal infection, NTM infection, poor visual acuity at presentation, old age, and surgery during admission (all P < 0.05 by simple linear regression). In multiple linear stepwise regression analysis, 4 factors including previous ocular surgery, large ulcer size, NTM infection, and surgery during admission were associated with longer hospital stays (Table 6).
Table 6.
Factors Affecting Hospital Bed Days: Multiple Linear Regression
DISCUSSION
Severe infectious keratitis is a leading cause of corneal blindness. Optimal clinical practice for the prevention and treatment of microbial keratitis should account for patient-specific risk factors and possible causative organisms in different regions. In this study, we focused on patients admitted with microbial keratitis; first, we ensured that only patients with severe infection leading to admission were included in this study, and second, we used the same (or as similar as possible) criteria as used in a previous report conducted at another university hospital in Northern Taiwan between 1992 and 20017 to provide updated regional epidemiological information. Our findings showed that contact lens wear remained the leading risk factor for inpatient microbial keratitis, and the trend increased significantly over the 10-year study period; P. aeruginosa was the most common causative organism.
In the current study, risk factors for microbial keratitis from 2003 to 2012 were contact lens wear, ocular and systemic diseases, trauma, and previous ocular surgery, in descending order, which was similar to the results of a previous Taiwanese report.7 In Taiwan, the leading risk factor for microbial keratitis was still contact lens wear, which was also reported in the United States, Western Europe Australia, and Hong Kong.2,8–11 Rattanatam et al12 found that the number of patients with contact lens-related microbial keratitis decreased in their hospital, suggesting that a higher number of such patients were treated in the community after the introduction of fluoroquinolones. By contrast, our study demonstrated an increasing trend in the rate of contact lens-related microbial keratitis in our hospital over the 10-year study period (P = 0.011). In a prospective, population-based study of contact lens-related microbial keratitis in Australia,13 risk factors for infections included overnight use, poor storage case hygiene, smoking, Internet purchase of contact lenses, less than 6 months wear experience, and higher socioeconomic class; however, new lens types did not reduce the incidence of infection. Because of the retrospective design of this study, it was difficult to correlate contact lens wearing modalities, hygiene, and other factors with infections; however, approximately 35.8% of patients had overnight use in our study. Wearing contact lenses is popular in Taiwan, which is a country with high prevalence of refractive errors, and contact lens-related microbial keratitis has become a public health concern. In 2012, the Ophthalmological Society of Taiwan launched the “corneal health for care network” to advocate the 3 C's for contact lens wearers: consulting a physician on how to fit contact lenses correctly (correct), cleaning and maintaining contact lens (care), and receiving regular health check-ups (check). This campaign is anticipated to facilitate decreases in the rate of contact lens-related microbial keratitis in Taiwan.
In this study, the most common causative organisms were bacteria, followed by fungi; gram-negative bacteria were more common than gram-positive bacteria, and P. aeruginosa was the most commonly identified isolate. Our findings are consistent with those of a previous Taiwanese report.7 The spectrum of microorganisms accounting for microbial keratitis differ depending on geographic location, climate, and etiology.5 For example, gram-positive bacteria are predominant in temperate climate regions,2,14,15 whereas gram-negative bacteria and fungi are prevalent in tropical regions;1pseudomonas species are associated with contact lens-related infections,4,16 whereas fungi are related to trauma caused by plants.1,17 Taiwan is located in a subtropical area. The predominance of P. aeruginosa infection in Taiwanese studies may reflect both the geographic prevalence of the microorganism and contact lens-related keratitis. Fungi are also crucial causative organisms of microbial keratitis in Taiwan; similarly, this might be due to the geography and climate, and injury caused by plant materials.
Notably, 4 of 5 previous studies in Europe and Taiwan investigating microbiological findings of hospitalized patients with microbial keratitis have reported that gram-negative bacteria are the most common causative organism.2,4,7,8,16 This finding might suggest that microbial keratitis caused by gram-negative bacteria, particularly pseudomonas species, tends to be severe and progresses rapidly, thus requiring admission.
In the current study, medical treatment was successful in 69.5% of patients; more than half of the patients with fungal keratitis required additional surgical interventions to control infections. Medical treatment of fungal keratitis, particularly deep-seated infections, is often unsatisfactory because of delayed diagnosis, inadequate drug penetration, and slow response to therapy.18 Surgical intervention to remove infectious elements and necrotic tissue may increase drug penetration and shorten the clinical course.18 However, therapeutic corneal transplantation and even destructive surgery may be indicated for severe keratitis with a poor response to medical therapy or when severe complications supervene.
Long hospital stay has a potential impact on patients of working age. Long hospital stay also has effects on financial resources, staffing, and turnover rate of beds in the public health service. In this study, the mean hospital stay was 13.7 days, and longer hospital stays were associated with previous ocular surgery, large ulcer size, NTM infection, and surgery during admission. A study of hospitalized patients with infectious keratitis in New Zealand reported that longer hospital stay was associated with the presence of hypopyon, larger ulcers, previous ocular surgery, and poor visual acuity.19 As expected, large ulcers or surgery during admission prolonged hospital stay. Older age and longer duration from symptom onset to diagnosis were noted in patients with previous ocular surgery (data not shown), which might explain the relationship between longer hospital stay and previous ocular surgery. NTM has a relatively slow growth rate,20 and the infection can mimic that caused by other pathogens.21 Thus, the delay in diagnosis and treatment of NTM infections might prolong hospital stay. Morbidities caused by microbial keratitis can be assessed on the basis of surgical intervention, hospital stay, and visual loss. In this study, we did not analyze the predictors for poor visual outcome in microbial keratitis because of visual assessments with variable follow-up intervals. However, the study in New Zealand19 reported that longer hospital stays were associated with poor visual acuity both at presentation and final assessment.
Our study had the following limitations. Because of its retrospective design, data such as risk factors were incomplete. We included cases with both positive and negative culture results, and we could not exclude the possibility that cultured isolates were contaminated. Moreover, physicians have used diverse protocols for treating patients. Finally, because our patients were admitted to a tertiary referral hospital in Taiwan, the results cannot be generalized.
In conclusion, P. aeruginosa was the most common causative organism, and contact lens wear was the most common risk factor for microbial keratitis; there was a significant increase in the percentage of contact lens-related keratitis during the 10-year study period in Taiwan. The majority of patients with microbial keratitis were cured through medical treatment, but a high proportion of patients with fungal keratitis required surgical interventions. Microbial keratitis has the potential to cause devastating visual impairment and major costs to the public health system; our findings provide updated information and facilitate future prevention and treatment of microbial keratitis in Taiwan.
ACKNOWLEDGMENT
The authors thank Tao-Chun Peng, Department of Family and Community Medicine, Tri-Service General Hospital for assistance in statistical analyses.
Footnotes
Abbreviations: ANOVA = analysis of variance, CGMH = Chang Gung Memorial Hospital, CNS = coagulase-negative stalylococcus, NTM = nontuberculous myycobacteris.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Gopinathan U, Garg P, Fernandes M, et al. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center in South India. Cornea 2002; 21:555–559. [DOI] [PubMed] [Google Scholar]
- 2.Bourcier T, Thomas F, Borderie V, et al. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol 2003; 87:834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keay L, Edwards K, Naduvilath T, et al. Microbial keratitis predisposing factors and morbidity. Ophthalmology 2006; 113:109–116. [DOI] [PubMed] [Google Scholar]
- 4.Saeed A, D’Arcy F, Stack J, et al. Risk factors, microbiological findings, and clinical outcomes in cases of microbial keratitis admitted to a tertiary referral center in Ireland. Cornea 2009; 28:285–292. [DOI] [PubMed] [Google Scholar]
- 5.Shah A, Sachdev A, Coggon D, et al. Geographic variations in microbial keratitis: an analysis of the peer-reviewed literature. Br J Ophthalmol 2011; 95:762–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ni N, Nam EM, Hammersmith KM, et al. Seasonal, geographic, and antimicrobial resistance patterns in microbial keratitis: 4-year experience in eastern Pennsylvania. Cornea 2015; 34:296–302. [DOI] [PubMed] [Google Scholar]
- 7.Fong CF, Tseng CH, Hu FR, et al. Clinical characteristics of microbial keratitis in a university hospital in Taiwan. Am J Ophthalmol 2004; 137:329–336. [DOI] [PubMed] [Google Scholar]
- 8.Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea 2008; 27:22–27. [DOI] [PubMed] [Google Scholar]
- 9.Schaefer F, Bruttin O, Zografos L, et al. Bacterial keratitis: a prospective clinical and microbiological study. Br J Ophthalmol 2001; 85:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yildiz EH, Airiani S, Hammersmith KM, et al. Trends in contact lens-related corneal ulcers at a tertiary referral center. Cornea 2012; 31:1097–1102. [DOI] [PubMed] [Google Scholar]
- 11.Ng AL-K, To KK-W, Yuen LH, et al. Predisposing factors, microbial characteristics, and clinical outcome of Microbial Keratitis in a tertiary centre in Hong Kong: a 10-year experience. J Ophthalmol 2015; 2015: Article ID 769436, 9 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rattanatam T, Heng WJ, Rapuano CJ, et al. Trends in contact lens-related corneal ulcers. Cornea 2001; 20:290–294. [DOI] [PubMed] [Google Scholar]
- 13.Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology 2008; 115:1655–1662. [DOI] [PubMed] [Google Scholar]
- 14.Orlans HO, Hornby SJ, Bowler IC. In vitro antibiotic susceptibility patterns of bacterial keratitis isolates in Oxford, UK: a 10-year review. Eye (Lond) 2011; 25:489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lichtinger A, Yeung SN, Kim P, et al. Shifting trends in bacterial keratitis in Toronto: an 11-year review. Ophthalmology 2012; 119:1785–1790. [DOI] [PubMed] [Google Scholar]
- 16.Dart JK. Predisposing factors in microbial keratitis: the significance of contact lens wear. Br J Ophthalmol 1988; 72:926–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panda A, Satpathy G, Nayak N, et al. Demographic pattern, predisposing factors and management of ulcerative keratitis: evaluation of one thousand unilateral cases at a tertiary care centre. Clin Experiment Ophthalmol 2007; 35:44–50. [DOI] [PubMed] [Google Scholar]
- 18.Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect 2013; 19:210–220. [DOI] [PubMed] [Google Scholar]
- 19.Wong T, Ormonde S, Gamble G, et al. Severe infective keratitis leading to hospital admission in New Zealand. Br J Ophthalmol 2003; 87:1103–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bullington RH, Jr, Lanier JD, Font RL. Nontuberculous mycobacterial keratitis. Report of two cases and review of the literature. Arch Ophthalmol 1992; 110:519–524. [DOI] [PubMed] [Google Scholar]
- 21.Moorthy RS, Valluri S, Rao NA. Nontuberculous mycobacterial ocular and adnexal infections. Surv Ophthalmol 2012; 57:202–235. [DOI] [PubMed] [Google Scholar]


