Abstract
To investigate the effects of dipeptidyl peptidase-4 (DPP-4) inhibitors on the risk of acute pancreatitis in patients with type 2 diabetes.
This nationwide population-based cohort study used the diabetes patients dataset of Taiwan's National Health Research Insurance Research Database. Patients with newly diagnosed type 2 diabetes between January 1, 2008 and December 31, 2009 and no history of acute pancreatitis were selected. This cohort was followed from the index date to the onset of acute pancreatitis or December 31, 2011. The main outcome measure was the hazard ratio (HR) for acute pancreatitis associated with DPP-4 inhibitor use. Cox proportional-hazards regression analyses were adjusted for alcohol use, hypertriglyceridemia, cholelithiasis, neoplasm, and Diabetes Complications Severity Index (DCSI) score. Subgroup analyses stratified by age and sex were conducted.
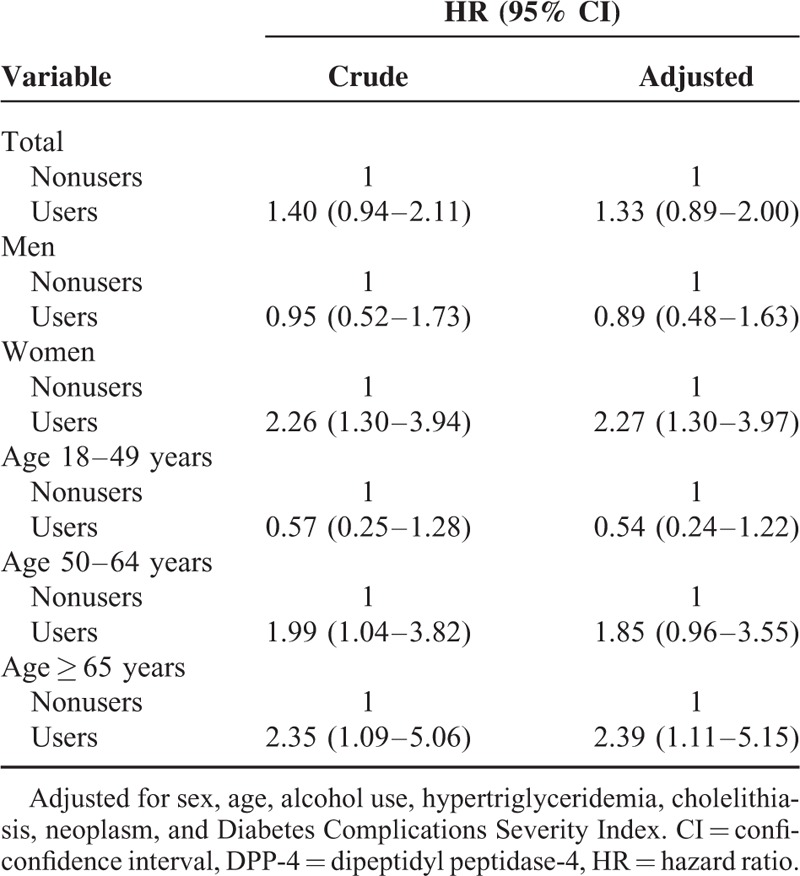
The study cohort comprised 114,141 patients. Significant interaction effects were observed between sex and age (HR 0.80, 95% confidence interval [CI] 0.64–0.99) and age and DCSI score (HR 0.83, 95% CI: 0.71–0.97). In subgroup analyses, significant risks of acute pancreatitis were noted in female and elderly DPP-4 inhibitor users. Among women, the risk of acute pancreatitis was significantly higher among DPP-4 inhibitor users than among nonusers (HR 2.27, 95% CI: 1.30–3.97). This risk was also significantly higher in users than in nonusers among patients aged >65 years (HR 2.39, 95% CI: 1.11–5.15).
Female and elderly DPP-4 inhibitor users had significantly elevated risks of acute pancreatitis development. Further well-conducted studies are needed to confirm our findings.
INTRODUCTION
Dipeptidyl peptidase-4 (DPP-4) inhibitors inhibit glucagon secretion by increasing the endogenous blood level of active incretin, thereby prolonging incretin action and stimulating insulin secretion in a glucose-dependent manner.1 Overall, DPP-4 inhibitors have modest effects in lowering the plasma glucose level and reducing the risk of hypoglycemia compared with other antidiabetic agents, and they have no substantial effect on body weight.2,3
However, the possible association of DPP-4 inhibitor use with pancreatitis has raised concern; events of acute pancreatitis, including fatal hemorrhagic or necrotizing pancreatitis, have been reported in the postmarketing period for these drugs.4–6 Previous observational studies of this association, based on clinical databases from various countries, have yielded inconsistent results due to differences in medications, study populations and designs, as well as inadequate duration of follow-up and adjustment for confounders such as comorbidity and disease severity.7–9 Some studies have linked these drugs to an increased risk of acute pancreatitis, whereas others have shown that this risk is not increased in DPP-4 inhibitor users compared with nonusers. The association between DPP-4 inhibitor use and acute pancreatitis thus remains inconclusive.
Given the increasing number of patients receiving prescriptions for DPP-4 inhibitors and concerns regarding the significant risks of morbidity and mortality caused by acute pancreatitis in these patients, we conducted a large population-based cohort study to determine whether the use of DPP-4 inhibitors is associated with an increased risk of acute pancreatitis in patients with newly diagnosed type 2 diabetes in Taiwan.
METHODS
The ethics committee for clinical research of Taichung Veterans General Hospital approved this study. Data were scrambled using a double-scrambling protocol to protect individuals’ privacy. As data were anonymized before analysis, informed consent was not required.
Study Population
Taiwan's National Health Insurance (NHI) program, initiated in March 1995, covers approximately 99.9% of the 23.74 million residents of Taiwan.10 The country's National Health Research Institute cooperated with the Bureau of NHI to establish and maintain a medical claims database (the National Health Insurance Research Database [NHIRD]) for research use. This database contains demographic information and claims data, including diagnoses and hospital and outpatient prescriptions. Data in the NHIRD that could be used to identify patients or care providers are scrambled before being released to researchers. Diagnoses recorded in the database are coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).
Based on the registration files and original claims data from the NHIRD, diabetes datasets have been constructed. For the present study, we used the 1999–2011 Longitudinal Cohort of Diabetes Patients Database (LHDB), which consists of deidentified secondary data from a random sample of 120,000 patients with incident diabetes diagnoses per year, representing the majority (about 70%) of this population in Taiwan. Data matching any diabetes-related ICD-9-CM codes (250.xx, 648.8x, 775.1x, 790.2x, and 648.0x) were selected to construct this dataset.11 The dataset contained medical records of all selected individuals for the period 2008 to 2011. Several studies have confirmed that the LHDB is representative of the Taiwanese diabetic population.12,13
We selected a cohort of patients with newly diagnosed type 2 diabetes between January 1, 2008 and December 31, 2009 from the LHDB, excluding patients with evidence of acute pancreatitis before diabetes onset and those aged <18 years. The study cohort was followed from the index date to the onset of acute pancreatitis (ICD-9-CM code 577.0) or December 31, 2011.
Variable Definitions
Patients with at least 3 ambulatory claims with an ICD-9-CM code of 250.x2 during the inclusion period were classified as having type 2 diabetes. The severity of diabetes was defined using the Diabetes Complications Severity Index (DCSI; 0, 1, or ≥2).14–16 Acute pancreatitis was defined by the registry of a primary or secondary ICD-9-CM code of 577.0 during hospitalization. Baseline comorbidities were defined according to corresponding ICD-9-CM codes registered in 3 outpatient claims before the acute pancreatitis index date: hypertriglyceridemia (ICD-9-CM code 272.1), cholelithiasis (ICD-9-CM code 574), alcohol use (ICD-9-CM code 292, 303), biliary cancer (ICD-9-CM code 156), pancreatic cancer (ICD-9-CM code 157), and hepatocellular carcinoma (ICD-9-CM code 155).
Medication Use
As the marketing of DPP-4 inhibitors was authorized recently in Taiwan (2009, 2011, 2011, and 2012 for sitagliptin, saxagliptin, vildagliptin, and linagliptin, respectively), linagliptin was not included in our study because it was not available during the study period. We identified all patients who received new prescriptions for DPP-4 inhibitors, including sitagliptin, saxagliptin, and vildagliptin, as monotherapy or in combination with other drugs, during the study period.
Statistical Analysis
We used descriptive statistics to summarize the characteristics of DPP-4 inhibitor users and nonusers. The 2 groups were compared using independent Student t tests for parametric continuous data and Chi-squared tests for categorical data. Subgroup analyses stratified by age and sex were conducted. A Cox proportional-hazards regression model with time-varying covariates was used to evaluate the association between DPP-4 inhibitor use and acute pancreatitis, with calculation of adjusted hazard ratios (HRs) and 95% confidence intervals (CIs). The models were adjusted for alcohol use, hypertriglyceridemia, cholelithiasis, neoplasm, and DCSI score. Survival time was calculated from the date of diabetes onset to the date of acute pancreatitis onset or the end of the study period (December 31, 2011). Two-tailed P values <0.05 were considered to be significant. Data analyses were conducted using SAS software (version 9.3 for Windows; SAS Institute, Inc., Cary, NC).
RESULTS
A total of 115,756 patients with new diagnoses of type 2 diabetes registered between January 1, 2008 and December 31, 2009 were identified. We excluded 1160 patients with past histories of acute pancreatitis and 455 patients aged <18 years. The analysis thus included 114,141 patients (10,877 DPP-4 inhibitor users, 103,264 nonusers) with new-onset type 2 diabetes. The characteristics of the study population are presented in Table 1. Compared with nonusers, DPP-4 inhibitor users were younger (56.88 ± 13.36 vs. 53.42 ± 12.81 years, P < 0.0001) and had higher DCSI scores (0.37 ± 0.62 vs. 0.51 ± 0.73, P < 0.0001). The prevalence of comorbidities (alcohol use, hypertriglyceridemia, cholelithiasis, and neoplasms [biliary cancer, pancreatic cancer, and hepatocellular carcinoma]) did not differ significantly between users and nonusers. During follow-up period, 25 DPP-4 inhibitor users and 486 nonusers had developed acute pancreatitis.
TABLE 1.
Baseline Characteristics of DPP-4 Inhibitor Users and Nonusers
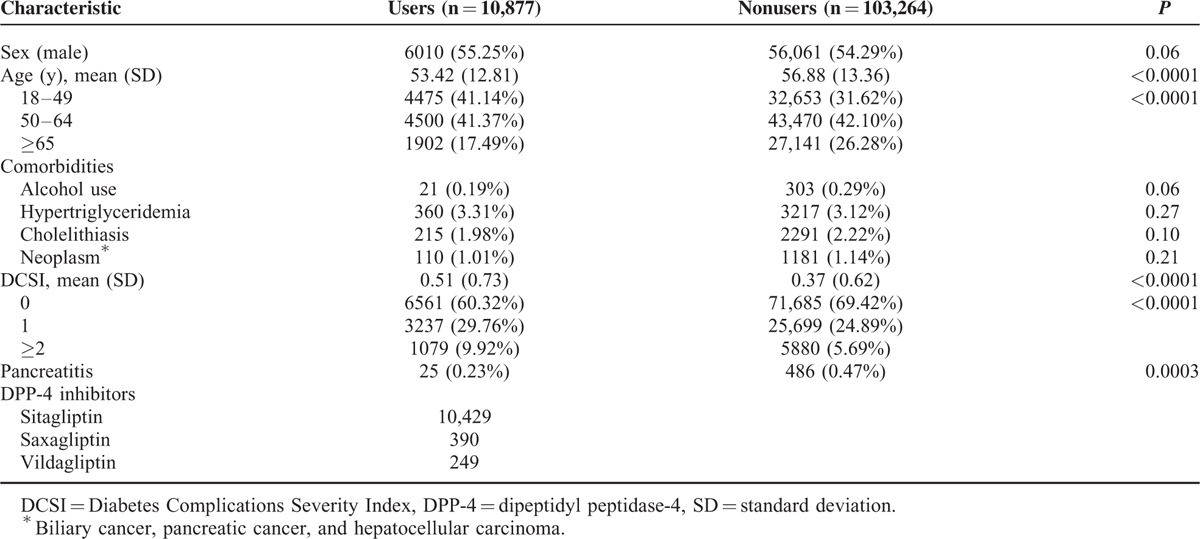
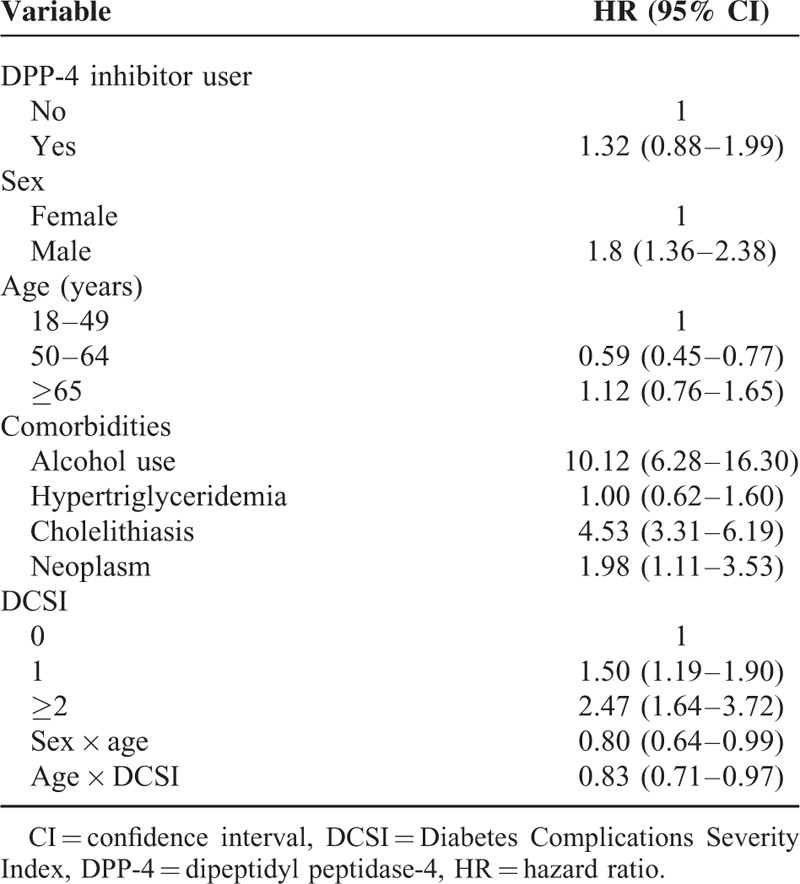
After controlling for age, sex, comorbidities, and DCSI score, the risk of acute pancreatitis was not significantly higher among DPP-4 inhibitor users than among nonusers (HR 1.32, 95% CI: 0.88–1.99). The risk of acute pancreatitis development during the follow-up period was significantly higher among men (vs. women; HR 1.80, 95% CI: 1.36–2.38), those who consumed alcohol (vs. no alcohol consumption; HR 10.12, 95% CI: 6.28–16.30), patients with cholelithiasis (vs. no cholelithiasis; HR 4.53, 95% CI: 3.31–6.19), those with neoplasms (vs. no neoplasm; HR 1.98, 95% CI: 1.11–3.53), and patients with DCSI scores ≥2 (vs. DCSI = 0; HR 2.47, 95% CI: 1.64–3.72; Table 2). Significant interaction effects were observed between sex and age (HR 0.80, 95% CI: 0.64–0.99) and between age and DCSI score (HR 0.83, 95% CI: 0.71–0.97).
TABLE 2.
DPP-4 Inhibitor Use and the Likelihood of Acute Pancreatitis (Cox Proportional-Hazard Model With Time Varying Covariates)
Stratified subgroup analyses revealed significant differences in the risk of acute pancreatitis according to DPP-4 inhibitor use (Table 3). Female, but not male, DPP-4 inhibitor users had a significant risk of acute pancreatitis development. Among women, analyses controlling for age, comorbidities, and DCSI score showed that the risk of acute pancreatitis was significantly higher in DPP-4 inhibitor users than in nonusers (HR 2.27, 95% CI: 1.30–3.97). In age-stratified analyses, the risk of acute pancreatitis was significant in DPP-4 inhibitor users aged ≥65 years, but not in those aged <65 years. Among patients aged >65 years, users had a significantly higher risk of acute pancreatitis than did nonusers (HR 2.39, 95% CI: 1.11–5.15).
TABLE 3.
Subgroup Analyses of Associations Between DPP-4 Inhibitor Use With Acute Pancreatitis
DISCUSSION
The results of our cohort study revealed significant interactions of age with sex and DCSI score in the estimation of acute pancreatitis risk among DPP-4 inhibitor users. We found increased risks in subgroups after stratification by sex and age. Among women and patients aged >65 years, the risk of acute pancreatitis development was significantly higher in DPP-4 inhibitor users than in nonusers.
Gallstone disease, alcohol consumption, and cigarette smoking have been found to be important risk factors for acute pancreatitis.17–19 In Taiwan, the overall prevalence of gallstone disease is 5.0% (4.6% in men, 5.4% in women), with no significant sex difference20; alcohol consumption and cigarette smoking are more prevalent in men than in women.21,22 Taiwan has particularly high male smoking prevalence and much lower female prevalence.22 Smoking and alcohol consumption patterns also differ between younger and older populations.22 Differences in the risk of acute pancreatitis may be related to differences between male and female characteristics, including body composition, fat distribution, sex hormones, physical activity, and lifestyle.23 Obesity has been associated with the severity of this disease,24 and the age-adjusted prevalence of obesity was higher in men than in women of Taiwan.25 In this study, the identification of significant interaction effects prompted stratified analyses, which revealed age- and sex-related differences in the risk of acute pancreatitis that were not apparent in the analysis of the total population. In Table 1, the crude ratio of acute pancreatitis between the 2 groups were compared using Chi-squared tests, which is quite significant (P = 0.0003). In Table 2, a Cox proportional-hazards regression model with time-varying covariates was used to evaluate the association between DPP-4 inhibitor use and acute pancreatitis after controlling for age, sex, comorbidities, and DCSI score, the risk of acute pancreatitis was not significantly higher among DPP-4 inhibitor users than among nonusers (HR 1.32, 95% CI: 0.88–1.99). Besides, in Table 2, significant interaction effects were observed between sex and age (HR 0.80, 95% CI: 0.64–0.99) and between age and DCSI score (HR 0.83, 95% CI: 0.71–0.97). It means that the effect of age on acute pancreatitis development is different among men and women, and among different DCSI score. In such condition, it is necessary to perform stratified analysis to separate the data and run multiple models (Table 3). Further studies are needed to confirm our results and explore underlying mechanisms.
The results of this study are consistent with those of a meta-analysis of randomized controlled trials26 and some observational studies examining the effects of DPP-4 inhibitors on acute pancreatitis.8,9,27,28 In a retrospective cohort study utilizing the Medco National Integrated Database in United States, Garg et al27 demonstrated an increased incidence of acute pancreatitis in diabetic compared with nondiabetic patients, but they found no association between the use of exenatide or sitagliptin and acute pancreatitis. Using data from the UK's Clinical Practice Research Datalink, Faillie et al8 found that the use of incretin-based drugs was not associated with an increased risk of acute pancreatitis compared with the use of sulfonylureas. Chou et al9 conducted a population-based nested case–control study using Taiwan's NHIRD. The results showed that the risks of acute pancreatitis among current and past DPP-4 inhibitor users were not significantly higher than that among nonusers. In a nationwide population-based case–control study utilizing medical databases in Denmark, Thomsen et al28 revealed no significantly increased risk of acute pancreatitis in users of incretin-based drugs, including glucagon-like peptide-1 receptor agonists and DPP-4 inhibitors. However, our results contrast with those of 2 case–control studies.7,29 In a population-based case–control study using a large US administrative database, Singh et al7 found that treatment with sitagliptin and exenatide was associated with increased odds of hospitalization for acute pancreatitis in patients aged 18 to 65. Soranna et al29 showed that incretin-based drug use significantly increased the risk of acute pancreatitis development in a nested-case–control study based on a healthcare database with a cohort of 166,591 patients aged ≥40 years from the Lombardy region of Italy. Inconsistent results of these observational studies may due to limited statistical power, inadequate following time and confounding factors, such as comorbidities and diabetes disease severity.
Our study has several strengths. We used the LHDB, which enabled examination of a large sample representing the entire diabetic population of Taiwan. To avoid bias in our assessment of the risk of acute pancreatitis due to the duration of diabetes, which may increase this risk,30,31 we selected patients with newly diagnosed diabetes. We adjusted for a large number of confounders for which data were available, including alcohol use, hypertriglyceridemia, cholelithiasis, neoplasm, and DCSI score. We also investigated the elderly population, which has rarely been included in previous studies. Our survival analyses included time-dependent variables, which improves the accuracy of results.32 We also accounted for interactions of age with sex and DCSI score in examining associations between DPP-4 inhibitor use and acute pancreatitis development by performing stratified subgroup analyses.
However, our findings must be interpreted with caution, given the inherent limitations of the retrospective observational design and reliance on insurance and pharmacy claims data, which may be incomplete and incorporate unknown confounders. The claims data lacked some patient information, such as body mass index, family history, smoking habit, blood pressure, lipid profile, and hemoglobin A1c level. Misclassification of pancreatitis may have occurred because we did not have access to clinical records, radiological images, or diagnostic markers, such as serum amylase and lipase levels. Drug exposure may also have been misclassified because this information was derived from pharmacy claims, and we cannot guarantee that patients ingested the medication. Furthermore, immortal time bias may affect the results of cohort studies in favor of the treatment group.33 As the marketing of DPP-4 inhibitors was authorized recently in Taiwan (2009, 2011, 2011, and 2012 for sitagliptin, saxagliptin, vildagliptin, and linagliptin, respectively), the LHDB contained data for a limited follow-up period (about 2 years). Linagliptin was not included in our study because it was not available during the study period. The longer-term safety of DPP-4 inhibitors should thus be investigated further. Finally, we did not impute for missing data in our analyses.
CONCLUSIONS
This population-based cohort study revealed significant interaction effects between sex and age, and age and DCSI score, on the association between DPP-4 inhibitor use and acute pancreatitis among patients with new-onset type 2 diabetes. Subgroup analyses showed that female DPP-4 inhibitor users and those aged >65 years had significantly increased risks of acute pancreatitis development. Our findings provide both reassurance and reason for caution to physicians and high-risk patients using these drugs. Further large well-conducted observational studies are needed to confirm our results.
Acknowledgments
We thank the staff members of the Bureau of National Health Insurance, Department of Health, and the National Health Research Institutes for providing and managing, respectively, the National Health Insurance Research Database.
Footnotes
Abbreviations: CI = confidence interval, DCSI = Diabetes Complications Severity Index, DPP-4 = dipeptidyl peptidase-4, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, LHDB = Longitudinal Cohort of Diabetes Patients Database, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, SD = standard deviation.
The authors have no conflicts of interest to disclose.
This study was supported by grant TCVGH-1013803B from Taichung Veterans General Hospital, Taichung, Taiwan.
REFERENCES
- 1.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 2008; 60:470–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fakhoury WK, Lereun C, Wright D. A meta-analysis of placebo-controlled clinical trials assessing the efficacy and safety of incretin-based medications in patients with type 2 diabetes. Pharmacology 2010; 86:44–57. [DOI] [PubMed] [Google Scholar]
- 3.Aroda VR, Henry RR, Han J, et al. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin Ther 2012; 34:1247–1258. [DOI] [PubMed] [Google Scholar]
- 4.Girgis CM, Champion BL. Vildagliptin-induced acute pancreatitis. Endocr Pract 2011; 17:e48–e50. [DOI] [PubMed] [Google Scholar]
- 5.Nakata H, Sugitani S, Yamaji S, et al. Pancreatitis with pancreatic tail swelling associated with incretin-based therapies detected radiologically in two cases of diabetic patients with end-stage renal disease. Intern Med (Tokyo, Japan) 2012; 51:3045–3049. [DOI] [PubMed] [Google Scholar]
- 6.Kunjathaya P, Ramaswami PK, Krishnamurthy AN, et al. Acute necrotizing pancreatitis associated with vildagliptin. JOP 2013; 14:81–84. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Chang HY, Richards TM, et al. Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med 2013; 173:534–539. [DOI] [PubMed] [Google Scholar]
- 8.Faillie JL, Azoulay L, Patenaude V, et al. Incretin based drugs and risk of acute pancreatitis in patients with type 2 diabetes: cohort study. BMJ (Clinical Research Ed) 2014; 348:g2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou HC, Chen WW, Hsiao FY. Acute pancreatitis in patients with type 2 diabetes mellitus treated with dipeptidyl peptidase-4 inhibitors: a population-based nested case-control study. Drug Saf 2014; 37:521–528. [DOI] [PubMed] [Google Scholar]
- 10.Cheng SH, Chiang TL. The effect of universal health insurance on health care utilization in Taiwan. Results from a natural experiment. JAMA 1997; 278:89–93. [DOI] [PubMed] [Google Scholar]
- 11.Lin CC, Lai MS, Syu CY, et al. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc 2005; 104:157–163. [PubMed] [Google Scholar]
- 12.Chang SH, Wu LS, Chiou MJ, et al. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol 2014; 12:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shih CJ, Wu YL, Lo YH, et al. Association of hypoglycemia with incident chronic kidney disease in patients with type 2 diabetes: a nationwide population-based study. Medicine 2015; 94:e771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang HY, Weiner JP, Richards TM, et al. Predicting costs with Diabetes Complications Severity Index in claims data. Am J Manag Care 2012; 18:213–219. [PubMed] [Google Scholar]
- 15.Chen HL, Hsiao FY. Risk of hospitalization and healthcare cost associated with Diabetes Complication Severity Index in Taiwan's National Health Insurance Research Database. J Diabetes Complications 2014; 28:612–616. [DOI] [PubMed] [Google Scholar]
- 16.Young BA, Lin E, Von Korff M, et al. Diabetes Complications Severity Index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care 2008; 14:15–23. [PMC free article] [PubMed] [Google Scholar]
- 17.Sadr-Azodi O, Andren-Sandberg A, Orsini N, et al. Cigarette smoking, smoking cessation and acute pancreatitis: a prospective population-based study. Gut 2012; 61:262–267. [DOI] [PubMed] [Google Scholar]
- 18.Unal E, Atalay S, Tolan HK, et al. Biliopancreatic duct injection of ethanol as an experimental model of acute and chronic pancreatitis in rats. Int J Clin Exp Med 2015; 8:304–310. [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura Y, Arata S, Takada T, et al. Gallstone-induced acute pancreatitis. J Hepatobiliary Pancreat Sci 2010; 17:60–69. [DOI] [PubMed] [Google Scholar]
- 20.Chen CH, Huang MH, Yang JC, et al. Prevalence and risk factors of gallstone disease in an adult population of Taiwan: an epidemiological survey. J Gastroenterol Hepatol 2006; 21:1737–1743. [DOI] [PubMed] [Google Scholar]
- 21.Chuang YC, Chuang KY. Gender differences in relationships between social capital and individual smoking and drinking behavior in Taiwan. Soc Sci Med (1982) 2008; 67:1321–1330. [DOI] [PubMed] [Google Scholar]
- 22.Wen CP, Levy DT, Cheng TY, et al. Smoking behaviour in Taiwan, 2001. Tob Control 2005; 14 suppl 1:i51–i55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng TS, Lin HY. Gender and age disparity in health-related behaviors and behavioral patterns based on a National Survey of Taiwan. Int J Behav Med 2008; 15:14–20. [DOI] [PubMed] [Google Scholar]
- 24.Martinez J, Sanchez-Paya J, Palazon JM, et al. Is obesity a risk factor in acute pancreatitis? A meta-analysis. Pancreatology 2004; 4:42–48. [DOI] [PubMed] [Google Scholar]
- 25.Chu NF. Prevalence of obesity in Taiwan. Obes Rev 2005; 6:271–274. [DOI] [PubMed] [Google Scholar]
- 26.Monami M, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and pancreatitis risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16:48–56. [DOI] [PubMed] [Google Scholar]
- 27.Garg R, Chen W, Pendergrass M. Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. Diabetes Care 2010; 33:2349–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomsen RW, Pedersen L, Moller N, et al. Incretin-based therapy and risk of acute pancreatitis: a nationwide population-based case-control study. Diabetes Care 2015; 38:1089–1098. [DOI] [PubMed] [Google Scholar]
- 29.Soranna D, Bosetti C, Casula M, et al. Incretin-based drugs and risk of acute pancreatitis: a nested-case control study within a healthcare database. Diabetes Res Clin Pract 2015; 108:243–249. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Perez A, Schlienger RG, Rodriguez LA. Acute pancreatitis in association with type 2 diabetes and antidiabetic drugs: a population-based cohort study. Diabetes Care 2010; 33:2580–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girman CJ, Kou TD, Cai B, et al. Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes Obes Metab 2010; 12:766–771. [DOI] [PubMed] [Google Scholar]
- 32.Allison PD. Survival Analysis Using SAS: A Practical Guide. Cary, North Carolina State: SAS Institute; 2010. [Google Scholar]
- 33.Levesque LE, Hanley JA, Kezouh A, et al. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ (Clinical Research Ed) 2010; 340:b5087. [DOI] [PubMed] [Google Scholar]


