Abstract
Immunologic checkpoint marker B7 homolog 1 (B7-H1) plays a fundamental role in the initiation and progression of gastric cancer (GC); however, the clinicopathologic significance and prognostic value of B7-H1 in GC remains controversial. In this study, we aimed to assess their relationship through a meta-analysis.
Medline/PubMed, EMBASE, the Cochrane Library databases, and Grey literature were searched up to August 10, 2015, for eligible studies of the association between B7-H1 expression and overall survival in GC. The hazard ratio and its 95% confidence interval (CI) were calculated from the included studies. Moreover, the odds ratio (OR) was also extracted to evaluate the association between the clinicopathologic parameters of participants and B7-H1 expression.
Five studies involving 481 patients were included in the meta-analysis. The pooled results showed that positive B7-H1 expression was a negative predictor for overall survival with hazard ratio of 1.74 (95% CI: 1.40–2.17; Pheterogeneity = 0.146) in GC. Additionally, increased B7-H1 was found to be significantly associated with positive lymph node metastasis (OR = 2.61, 95% CI: 1.78–3.84; Pheterogeneity = 0.004) and poorer tumor stage (OR = 2.28, 95% CI: 1.39–3.74; Pheterogeneity = 0.006); however, higher B7-H1 expression was not significantly correlated with poorer tumor differentiation (OR = 1.29, 95% CI: 0.90–1.86; Pheterogeneity = 0.013) and bigger tumor size (OR = 1.18, 95% CI: 0.81–1.73; Pheterogeneity = 0.104).
The meta-analysis suggested that B7-H1 could act as a significant biomarker in the poor prognosis of gastric carcinoma.
INTRODUCTION
In 2015, an estimated 24,590 new cases in the United States will be diagnosed with gastric cancer (GC), with an estimated 10,720 deaths1; however, as one of the most fatal cancers, effective methods for early diagnosis and monitoring prognosis are currently unavailable. Recently, more and more studies focused on the new immunotherapeutic strategies, which could lead to a major breakthrough in the field of GC treatment.2 Immune checkpoint blockade with antibodies that target cytotoxic T lymphocyte-associated antigen 4 and the programmed cell death protein 1 pathway has shown to mediate tumor shrinkage, extend overall survival (OS) and demonstrate promise in a variety of malignancies.3 On the contrary, certain immunologic checkpoint markers have been reported in GC.4 Among them, B7 homolog 1 (B7-H1) has been the focus of research.
B7-H1, also known as programmed death-L1 (PD-L1) or CD274, is an important member of the B7/CD28 costimulatory factor superfamily. It is a surface glycoprotein known to be expressed on a majority of tumor cells and other immune cells including conventional CD4+ and CD8+ T cells, dendritic cells, macrophages, and Tregs.5 Under normal circumstance, B7-H1 is expressed to maintain the homeostasis of immune response; however, tumor cells exploit it and upregulate its expression to protect themselves from cytolysis by activated T cells. The co-inhibitory characteristic of B7-H1 molecule is attributed to binding to its receptor, programmed death 1 on tumor-specific T cells, which leads to their apoptosis and then provides an immune escape for tumor cells.6 Accumulating evidence has shown that B7-H1 expression is associated with clinicopathologic and immunologic factors in various human malignancies including esophageal,7 liver,8 colorectal,9 pancreatic,10 breast,11 cervical,12 lung,13 bladder,14 brain,15 and blood cancers.16 So there is an urgent need to obtain a further understanding of the potential relationship between B7-H1 and prognosis in cancer sufferers.
Moreover, some researchers have published their data with respect to B7-H1 expression and have raised concerns about the efficacy of B7-H1 as a specific prognostic factor in cancers; however, its prognostic role in GC is still under debate. In this study, we aimed to perform an up-to-date meta-analysis to reveal the clinicopathologic significance and prognostic value of B7-H1 in gastric carcinoma.
MATERIALS AND METHODS
Search Strategy
We searched several international databases including Medline/PubMed, EMBASE, the Cochrane Library databases, and Grey literature up to August 10, 2015. The key terms employed for literature retrieval included “B7-H1” or “PD-L1” or “CD274” or “B7 homolog 1” or “programmed death ligand-1” and “gastric cancer” or “gastric carcinoma” or “stomach tumor” and “survival” or “outcome” or “prognosis”. To obtain additional relevant manuscripts, conference summaries and reference lists missed in the retrieval were identified. We even contacted the corresponding authors to get additional information if necessary. All procedures were approved by the ethics committee for human experiments of Capital Medical University.
Selection Criteria
Studies were selected if they met the following criteria: they focused on GC; all selected cancer patients were pathologically confirmed; and correlation between B7-H1, clinicopathologic features, and OS was described.
Articles were excluded from the analyses based on the following criteria: non-English studies; non-human experiments; review articles, case studies, or letters; duplicate publication; and insufficient data to report the hazard ratios (HR) and 95% confidence interval (95% CI), or the Kaplan-Meier curve could not be extracted.
Data Extraction
All relevant data were screened and extracted by 2 independent investigators (F.X and G.S.F.). The quality of the selected articles was assessed according to the Newcastle-Ottawa Scale.17 The information including author, year of publication, country of origin, patient number, tumor stage, the cut off value, hazard ratio, follow-up, overexpression rates of B7-H1 and the quality scores of the enrolled studies were recorded for each study. To get the HRs and their 95% CI that were not reported in the articles, we digitized and extracted the data from the Kaplan-Meier curves using the software designed by Jayne F Tierney and Matthew R Sydes.18 To reach a consensus, any disagreement on a conflicting study was resolved by full discussion.
Statistical Analysis
The statistical analysis was performed according to the guidelines proposed by the Meta-Analysis of Observational Studies in Epidemiology group.19 HR with 95% CI was calculated using Stata SE12.0 (Stata Corporation, College Station, TX). The heterogeneity among studies was measured using the Q and I2 tests. A probability value of P < 0.1 and I2 ≥50% indicated the existence of significant heterogeneity.20 A fixed or random model was used depending on the heterogeneity analysis. The potential for publication bias was assessed using the Begg funnel plot and the Egger linear regression test. P value < 0.05 was considered statistically significant. All P values are two-tailed.
RESULTS
Search Results
The initial search returned a total of 149 articles. After screening the titles and abstracts, 144 irrelevant articles were excluded according to the search strategy above. As a result, a total of 5 studies were included in our meta-analysis. The detailed screening process was shown in Figure 1. All of these enrolled studies comprehensively evaluated the expression of B7-H1 and the OS rate.
FIGURE 1.
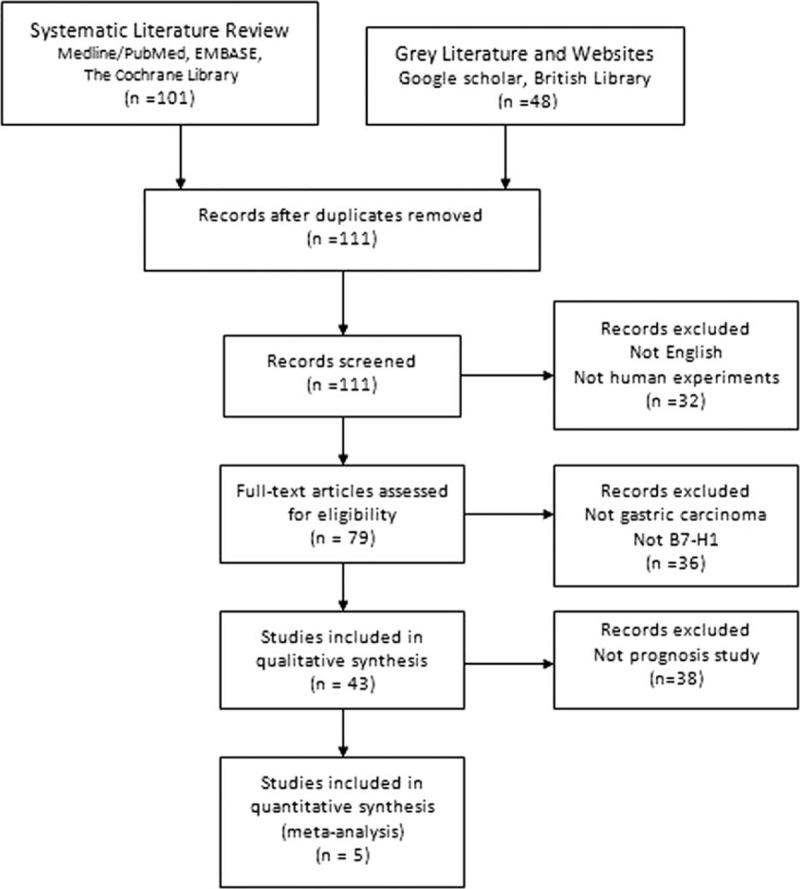
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart of the literature search.
Study Selection and Characteristics
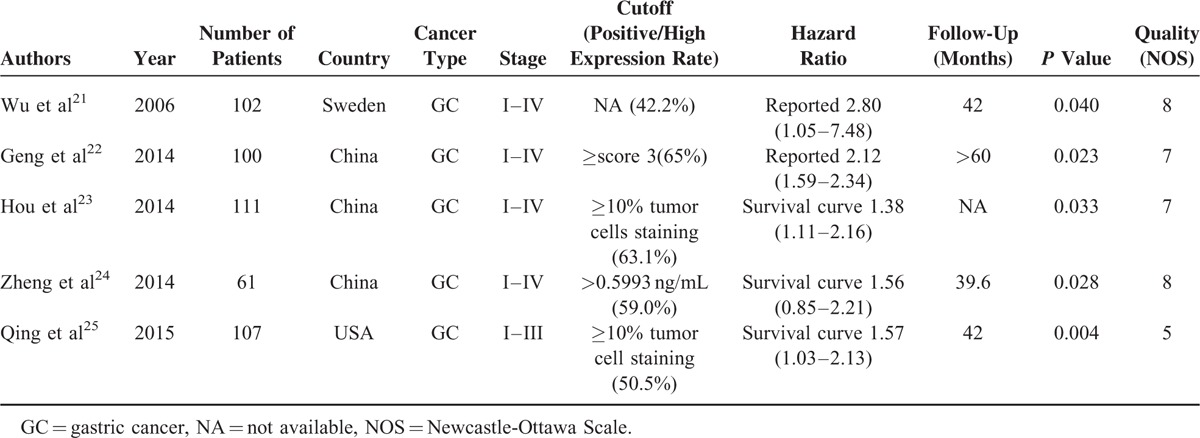
All features of the 5 eligible studies are listed in Table 1.21–25 The publication years of the eligible studies ranged from 2006 to 2015. The number of patients in each study ranged from 61 to 111 (mean sample size, 96 patients). The quality of the enrolled studies varied from 5 to 8, with a mean of 7. At least 3 studies reported the clinicopathologic features including tumor size, tumor differentiation, TNM stage, and lymph nodes metastasis. B7-H1 expression levels were measured in tumor tissue. The mean length of follow-up ranged from 39.6 to 60 months (Table 1). In all studies, none of the patients received neo-adjuvant radio- or chemotherapy prior to surgery.
TABLE 1.
Characteristics of Included Studies for Meta-Analyses
Correlation of B7-H1 Expression With Prognosis and Clinicopathologic Features
As shown in Figure 2, we found that elevated B7-H1 predicted a poor outcome with a pooled HR of 1.74 (95% CI: 1.40–2.17; Pheterogeneity = 0.146) in the random-effects model for 5 studies evaluating OS. To explore the potential source of heterogeneity among studies, “metareg” STATA command was conducted utilizing variables as year of publication, country, and analysis method (univariable vs mutivariable). The results showed that no variable included in the meta-regression contributed to the heterogeneity.
FIGURE 2.
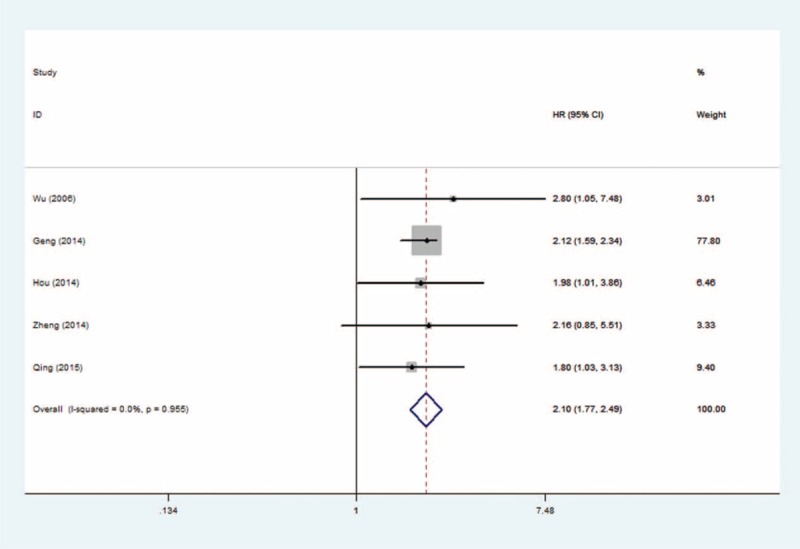
Forest plots of studies evaluating hazard ratios of B7 homolog 1 for overall survival.
The relationship between elevated B7-H1 and clinicopathologic parameters (reported in at least 3 studies) was presented in Figure 3. In GC, increased B7-H1 was found to be significantly associated with positive lymph node metastasis (OR = 2.61, 95% CI: 1.78–3.84; Pheterogeneity = 0.004) and poorer tumor stage (OR = 2.28, 95% CI: 1.39–3.74; Pheterogeneity = 0.006) but not with larger tumor size (OR = 1.18, 95% CI: 0.81–1.73; Pheterogeneity = 0.104) and poorer tumor differentiation (OR = 1.29, 95%CI: 0.90–1.86; Pheterogeneity = 0.013); however, no significant relationship was detected between B7-H1 overexpression and other clinical characteristics in gastric carcinoma due to limited studies (n = 2).
FIGURE 3.
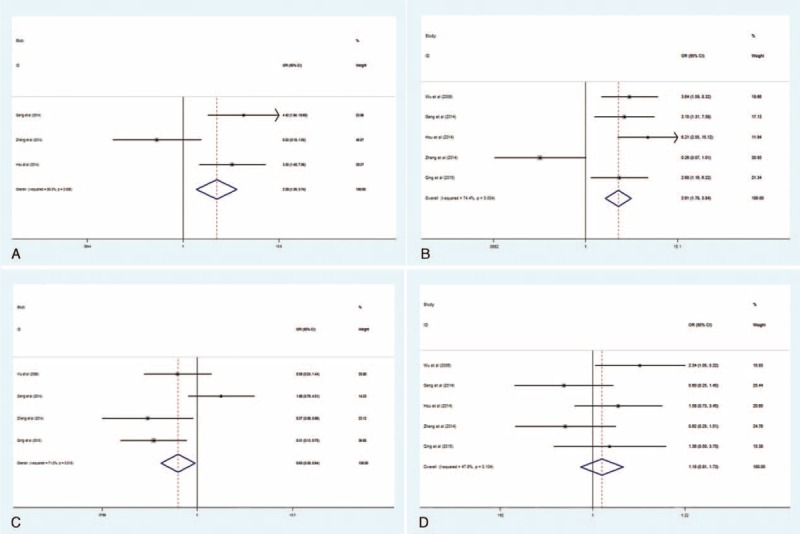
Forest plots of studies evaluating the association between B7 homolog 1 and clinical parameters in gastric cancer. A, TNM stage (advanced vs early); B, lymph node metastasis (present vs absent); C, differentiation (poor vs well/moderate); D, tumor size (large vs small).
Sensitivity Analysis
The selected studies were sequentially removed to investigate whether any single study could have an influence on the pooled results. As shown in Figure 4, the stable pooled HRs was found to be not significantly affected by each individual study.
FIGURE 4.
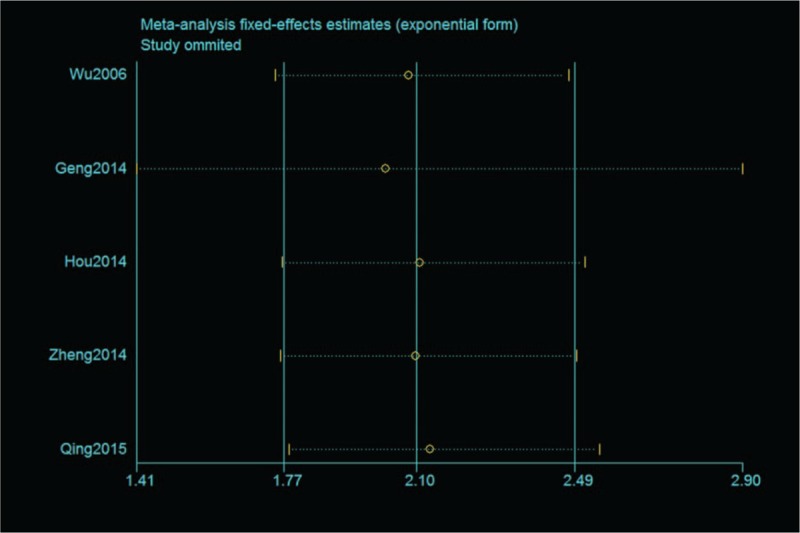
Effect of individual studies on the pooled hazard ratio for B7 homolog 1 and overall survival of patients.
Publication Bias
The figure of the Egger funnel plot (Fig. 5) did not show any evidence of obvious asymmetry (P = 0.806). The Egger linear regression test was performed to statistical test and no evidence for publication bias was detected either (P = 0.614).
FIGURE 5.
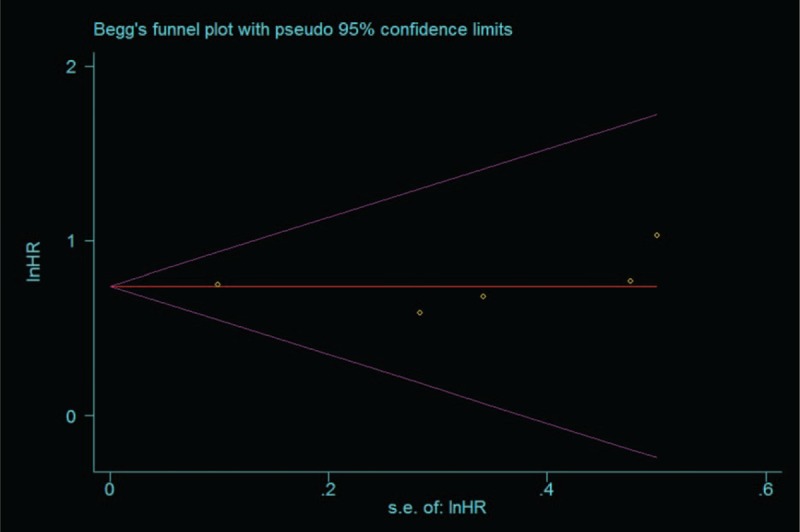
Begg funnel plots for all of the included studies reported with overall survival.
DISCUSSION
Up to now, the relationship between B7-H1 and the outcome of tumor sufferers remains inconclusive. Our current study chiefly concerned with the prognostic role of B7-H1 in GC. To the best of our knowledge, it is the first meta-analysis to investigate the prognostic role of B7-H1 in gastric carcinoma. The pooled results of our analysis indicated that positive B7-H1 expression significantly predicted poorer OS compared with negative/lower B7-H1 expression. Meta-regression was performed to investigate the source of heterogeneity; however, none of the variables including year of publication, detection method, and analysis method contributed to the heterogeneity in our meta-analysis. Additionally, when the clinicopathologic features were considered, the combined odds ratio(OR) was found to be significantly associated with high TNM stage and positive lymph node metastasis of stomach tumor. Based on these results, B7-H1 might act as a reliable biomarker in predicting clinical outcomes of GC.
The molecular mechanism of higher B7-H1 expression in gastric carcinoma remains controversial. Jiang et al26 demonstrated that high tumor B7-H1 was significantly increased after IFN-γ stimulation in GC, which was consistent with research of Loos et al27 on pancreatic cancer cell lines. In other digestive malignancies, Kuang et al28 reported that a fraction of B7-H1+ monocytes in peritumoral stroma were stimulated by autocrine tumor necrosis factor and interleukin 10, effectively suppressed tumor-specific T-cell immunity and contributed to the growth of human hepatocellular carcinoma. Song et al29 found that cancer progression and B7-H1 protein expression were independently related to the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) loss in colorectal cancer cells. All the same, the specific molecular mechanism of B7-H1 overexpression is still unclear. B7-H1 expression might be modulated through different pathways among different tumor cell types. Cancer cells also release immunosuppressive cytokines that upregulate B7-H1 expression and then aid the tumor cells to elude immune responses. Further, the early clinical experience of large phase I studies targeting PD-L1 pathway with monoclonal antibodies has received considerable attention.30 Although few official data are available in digestive cancers, the anti-B7-H1 antibody MPDL3280A reportedly has shown confirmed responses in metastatic gastric carcinoma.31 Our results suggest that anti-B7-H1 antibodies might be preferentially carried out on patients with GC in future clinical trials.
Although our results are promising, there are several limitations of this study need to be carefully taken into account. Firstly, as a novel prognostic marker in malignancies, B7-H1 just loomed in recent years and the sample size was relatively small. Secondly, a majority of the selected studies measured B7-H1 expression by IHC, the distinct antibodies used, the protocol of staining, the exact cell type and the method of scoring might account for the high variability in the frequencies reported by different authors. On the contrary, a different number of cut off values might have caused some of the heterogeneity observed here. Although triple subsets of B7-H1 threshold values revealed the similar results, a baseline referring to B7-H1 overexpression should be set up. Thirdly, few studies explored the association of patient prognosis and circulating B7-H1 expression, which might be more acceptable than tissue markers because they can be assayed before surgery and be monitored throughout the life. Moreover, due to lack of appropriate data, the association of B7-H1 and other clinical characteristics in gastric carcinoma was not explored. In the future, more multicentre studies with larger sample size are needed to present more reliable results of the clinical relevance and precise molecular explanation for the abnormal expression of B7-H1.
In conclusion, the current evidence firstly shows that an elevated B7-H1 is a negative predictor for survival in gastric carcinoma.
Footnotes
Abbreviations: B7-H1 = B7 homolog 1, CI = confidence interval, GC = gastric carcinoma, HR = hazard ratio, NOS = Newcastle-Ottawa Scale, OR = odds ratio, OS = overall survival, PD-L1 = programmed death-L1.
This study was supported by the National Science and Technology Support Program of China (2013BAI04B01).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res 2013; 19:1021–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu J, Lee-Gabel L, Nadeau MC, et al. Clinical evaluation of compounds targeting PD-1/PD-L1 pathway for cancer immunotherapy. J Oncol Pharm Prac 2014; 6:1–17. [DOI] [PubMed] [Google Scholar]
- 4.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015; 1:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afreen S, Dermime S. The immunoinhibitory B7-H1 molecule as a potential target in cancer: killing many birds with one stone. Hematol Oncol Stem Cell Ther 2014; 7:1–17. [DOI] [PubMed] [Google Scholar]
- 6.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother 2005; 54:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Deng H, Lu M, et al. B7-H1 expression associates with tumor invasion and predicts patient's survival in human esophageal cancer. Int J Clin Exp Pathol 2014; 7:6015–6023. [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Li G, Meng H, et al. Upregulation of B7-H1 expression is associated with macrophage infiltration in hepatocellular carcinomas. Cancer Immunol Immunother 2012; 61:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao LW, Li C, Zhang RL, et al. B7-H1 and B7-H4 expression in colorectal carcinoma: correlation with tumor FOXP3(+) regulatory T-cell infiltration. Acta Histochem 2014; 116:1163–1168. [DOI] [PubMed] [Google Scholar]
- 10.Bigelow E, Bever KM, Xu H, et al. Immunohistochemical staining of B7-H1 (PD-L1) on paraffin-embedded slides of pancreatic adenocarcinoma tissue. J Vis Exp 2013; 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2014; 146:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karim R, Jordanova ES, Piersma SJ, et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res 2009; 15:6341–6347. [DOI] [PubMed] [Google Scholar]
- 13.Boland JM, Kwon ED, Harrington SM, et al. Tumor B7-H1 and B7-H3 expression in squamous cell carcinoma of the lung. Clin Lung Cancer 2013; 14:157–163. [DOI] [PubMed] [Google Scholar]
- 14.Wang YH, Cao YW, Yang XC, et al. Effect of TLR4 and B7-H1 on immune escape of urothelial bladder cancer and its clinical significance. Asian Pac J Cancer Prev 2014; 15:1321–1326. [DOI] [PubMed] [Google Scholar]
- 15.Berghoff AS, Kiesel B, Widhalm G, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neurooncology 2014; 17:1064–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura H, Ishibashi M, Yamashita T, et al. Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia 2013; 27:464–472. [DOI] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25:603–605. [DOI] [PubMed] [Google Scholar]
- 18.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 21.Wu C, Zhu Y, Jiang J, et al. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 2006; 108:19–24. [DOI] [PubMed] [Google Scholar]
- 22.Geng Y, Wang H, Lu C, et al. Expression of costimulatory molecules B7-H1, B7-H4 and Foxp3 Tregs in gastric cancer and its clinical significance. Int J Clin Oncol 2014; 20:273–281. [DOI] [PubMed] [Google Scholar]
- 23.Hou J, Yu Z, Xiang R, et al. Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancer. Exp Mol Pathol 2014; 96:284–291. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Z, Bu Z, Liu X, et al. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin J Cancer Res 2014; 26:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qing Y, Li Q, Ren T, et al. Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer. Drug Des Devel Ther 2015; 9:901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang D, Xu YY, Li F, et al. The role of B7-H1 in gastric carcinoma: clinical significance and related mechanism. Med Oncol 2014; 31:268. [DOI] [PubMed] [Google Scholar]
- 27.Loos M, Giese NA, Kleeff J, et al. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett 2008; 268:98–109. [DOI] [PubMed] [Google Scholar]
- 28.Kuang DM, Zhao Q, Peng C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 2009; 206:1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song M, Chen D, Lu B, et al. PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer. PLoS One 2013; 8:e65821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012; 24:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabernero J, Hamid O, Powderly JD, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic CRC, gastric cancer (GC), SCCHN, or other tumors. J Clin Oncol 2013; 31:abstr3622. [Google Scholar]


