Abstract
The human immunodeficiency virus-1 transgenic (HIV-1Tg) rat is a non-infectious rodent model for HIV-1 infection which develops altered immune-responses similar to those in persons infected with HIV-1. HIV-1Tg and F344 rats respond significantly different to morphine, ethanol, nicotine and other psychostimulants, although the molecular mechanisms underlying these differences remain largely undetermined. Here, we compared expression of 52 immune-related genes in the prefrontal cortex (PFC), nucleus accumbens (NAc), and ventral tegmental area (VTA) of HIV-1Tg and F344 rats treated with either nicotine (0.4 mg/kg nicotine, base, s.c.) or saline for 27 days, to identify differentially expressed genes in the presence of HIV-1 with and without nicotine treatment. Using quantitative RT-PCR array, we measured RNA expression levels. Results showed that RNA expression of CASP3, CCL5, CX3CL1, CX3CR1, IL1α, LRF4, LFR7, TGFβ1 and TLR4 in NAc, CCL2, CCL5, TGFβ1 and TLR4 in PFC, and CASP3, CX3CR1, IFNα1, IL1β and IL6 in VTA was significantly modulated in HIV-1Tg rats compared with F344 rats. IL1α showed a 58% (P = 0.000072) decrease and IRF6 showed a 93.7% increase (P = 0.000227) in the NAc of HIV-1Tg compared with F344 rats; results remained significant after correction for multiple testing. We also found that several genes were significantly modulated by nicotine in HIV-1Tg rats while only a small number of immune-related genes were altered by nicotine in F344 rats. These findings imply that HIV-1 viral proteins greatly impact immune function and alter responsiveness to nicotine in certain immune-related genes.
Keywords: nicotine, HIV-1 transgenic rat, CNS immunity, prefrontal cortex, nucleus accumbens, ventral tegmental area
Introduction
HIV-1 infection involves the actions of HIV-1 viral proteins on targeted cells of the immune system, such as macrophages and T-lymphocytes (Cicala et al., 2002, Verani et al., 2005, Jiang and Aiken, 2006). Like the peripheral immune system, the central nervous system (CNS) is also highly vulnerable to HIV-1 infection. The brain regions targeted by HIV-1 virus include the prefrontal cortex region (PFC), nucleus accumbens (NAc), and ventral tegmental area (VTA) (Midde et al., 2011, Nesil et al., 2015). Although combination anti-retroviral therapy (cART) leads to a temporary reversal of HIV dementia (Portegies, 1995, Gisslen et al., 1998, Gendelman et al., 1998), both HIV-associated neurocognitive disorders (HAND) and HIV-associated dementia (HAD) are resistant to cART and become more severe as the lifespan of HIV-1 infected patients is prolonged (McArthur et al., 2003).
To better understand the effects of HIV-1 on neurocognitive function, an HIV-1 transgenic (HIV-1Tg) rat model, which carries a gag-pol deleted HIV-1 genome under the control of the HIV-1 viral promoter and expresses 7 of the 9 HIV-1 genes, was developed (Reid et al., 2001). Although there is no viral replication in this animal model, viral proteins are expressed in various organs including blood (Reid et al., 2001, Mazzucchelli et al., 2004). The HIV-1Tg rat develops characteristics similar to those of humans infected with HIV-1, which include immune-response alterations, pathologies with advancing age, T-cell abnormalities and kidney failure (Ray et al., 2003, Ray et al., 2010, Ray et al., 2009). Furthermore, the HIV-1Tg rat also shows alterations in sensitivity to many psychostimulants, including morphine, alcohol and nicotine (Sarkar and Chang, 2013, Homji et al., 2012).
Since its development in 2001, the HIV-1Tg rat model has been used to study the impact of HIV-1 viral proteins on molecular, cellular, systemic, and behavioral levels. This model has gained favor in many laboratories, including ours, as a model to mimic the state of HIV-1 patients given cART. Li and colleagues discovered that several immune-related genes and pathways are altered in the brain of the HIV-1Tg rat (Li et al., 2013). Among these altered genes and pathways, many are related to cytokines such as interferon, interleukins and chemokines (Li et al., 2013). In addition, this group also found that, in HIV-1Tg rats, signaling for TLR in the PFC, interferon in the HIP, and IL-4 in the STR are significantly altered (Li et al., 2013).
It is generally accepted that, while nicotine is a substance of abuse, it also exerts positive effects in the CNS. Various studies have demonstrated nicotine’s ability to modulate immune regulations (Sopori, 2002, Chang et al., 2014, Cui and Li, 2010). At a dose comparable to that achieved by “average to heavy” smokers, nicotine upregulates expression of inflammatory mediators, cytokines (IL-1β, TNFα, and IL-18), chemokines (CCL2 and CX(3)CL1), and adhesion molecules (ICAM-1, VCAM-1, and P-selectins) in mice (Bradford et al., 2011). Pretreatment with nicotine results in upregulation of IL-8 in human gingival epithelial cells (HGECs) and this upregulation is attenuated when cells are pretreated with non-selective nicotinic acetylcholine receptor (nAChR) antagonists (Kashiwagi et al., 2012). In human lamina propria (LPT) cells, T-bet, an integral player in coordinating immune responses, is upregulated following nicotine treatment (Kikuchi et al., 2008).
While it is clear that nicotine affects immune responses, the research is not unanimous regarding its effect on inflammation which has been implicated in the neurodegenerative effects of HIV-1 (Chang et al., 2014). For example, some studies suggest that nicotine suppresses inflammation (Nizri et al., 2009, Sugano et al., 1998, Thomsen and Mikkelsen, 2012) while others suggest that nicotine has opposite effects in cells such as aortic vascular smooth muscle cells (Wang et al., 2012). After treating SH-SY5Y cells with nicotine, Cui et al. (Cui et al., 2012) reported alterations in 14 biological pathways (Cui et al., 2012). Of the pathways significantly modulated by nicotine in SH-SY5Y cells, the Toll Like Receptor (TLR) and death receptor (DR) pathways are integral components of the innate immune system which is responsible for inflammatory responses. Further, downstream pathways of TLR and DR signaling and the expression of genes associated with innate immunity such as nuclear factor κB (NF-κB) were also affected by nicotine (Cui et al., 2012).
Specifically for this HIV-1Tg rat model, we found that the expression a significant number of genes related to neuroprotective function and Wnt/β-catenin is restored by treatment with nicotine (Cao et al., 2013). More specifically, in the PFC, genes related to Wnt/β-catenin signaling are restored to a level comparable to F344 control rats. it has been demonstrated that Wnt/β-catenin signaling has been reported to play a major role in the early development of the nervous system and activation of this pathway has a neuroprotective effect in adults (Toledo et al., 2008). Thus, nicotine’s restoration of Wnt β-catenin pathways and related genes has implications for enhanced neuronal survival in the brain of HIV-1Tg rat and treatment of various other disorders.
Further, following nicotine treatment, N-methyl-d-aspartate (NMDA) receptor function is restored, and fibroblast growth factor 9 (Fgf9) expression is increased as is Wnt5a expression which has been linked to synaptic plasticity and NMDA regulation. In the hippocampus, nicotine regulates the overexpression of a phospholipase C (Plch1) and decreases oxidative stress in HIV-1Tg rats. Nicotine has a similar effect on oxidative stress in the striatum and also restores the function of the tricarboxylic acid cycle in this brain region. This study demonstrated that nicotine has the ability to regulate a number of neurodegenerative effects of HIV-1 viral proteins in various brain regions (Cao et al., 2013).
Following from the aforementioned studies and given the fact that, despite the fact that individuals with HIV-1 are more likely to smoke cigarettes (Vigorito et al., 2015, Browning et al., 2013; Tesoriero et al., 2010), there is a lack of research investigating the potential therapeutic potential of nicotine, the current study sought, for the first time, to determine (1) the effects of HIV-1 viral proteins on the expression of immune-related genes in three brain regions which are associated with cognitive function and are known to be affected by HIV-1 and (2) the effect of nicotine on the expression of immune-related genes using the HIV-1Tg rat model.
Materials and Methods
Animals
Male HIV-1Tg rats and F344 control rats between 7 and 8 weeks were purchased from Harlan Laboratories (Indianapolis, IN). All rats were group-housed in standard plastic rat cages, maintained in a temperature-controlled environment with a 12 h light/dark cycle, and fed a standard rat diet and water ad libitum. All experimental procedures were conducted during the light cycle and approved by the University of Virginia Animal Care and Use Committee. Rats were divided into 4 groups with 5–6 animals per group: HIV-1Tg control; F344 control; HIV-1Tg nicotine; and F344 nicotine.
Nicotine treatment and tissue collection
Nicotine hydrogen tartrate (Sigma, St Louis, MO) was dissolved in 0.9% physiological saline solution. F344 and HIV-1Tg rats were injected subcutaneously (s.c.) with either saline or nicotine once per day (0.4 mg/kg/day) for 27 d before tissue collection, a procedure commonly used to study pharmacological effects of nicotine in our and other groups (Gutala et al., 2006, Matta et al., 2007, Nesil et al., 2015). The concentration of nicotine was calculated as nicotine free base, pH 7.0, according to Matta et al. (Matta et al., 2007) and based on our previous reported (Song et al., 2015, Nesil et al., 2015).
On Day 28, the animals were sacrificed and brain regions were collected. Using a rat brain matrix (Kent Scientific, Torrington, CT), 1 mm slices were taken from each brain. The slices that contained the prefrontal cortex (PFC), ventral tegmental area (VTA) and nucleus accumbens (NAc) were identified according to the rat brain atlas (Paxinos and Watson, 2005). Tissue from the regions of interest was collected from each brain using a 1.5 mm brain punch (Stoelting, Wood Dale, IL), and stored at −80°C until use.
RNA extraction and quantitative RT-PCR analysis
Total RNA was isolated using the Trizol agent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The purity and quantity of total RNA were measured at optical densities of 260 nm and 280 nm with NanoDrop 2000c (Thermo Scientific, Waltham, MA, USA). The primers for all 52 immune-related genes were designed using Primer Express (v. 3.0) software. To avoid amplifying genomic DNA, two primers of each gene were designed to span at least one intron. A custom-designed RT-PCR array was used to measure gene expression as described previously (Cui et al., 2013, Cao et al., 2011, Wei et al., 2011). Each pair of primers and their amplicon sequences were tested using the Basic Local Alignment Search Tool (BLAST; http://blast.ncbi.nlm.nih.gov/Blast.cgi) to ensure the specificity of the designed primers for the targeted genes. Dissociation curves were generated to check the specificity of the primers before including them in the qRT-PCR array. All primer sequences are listed in Table 1.
Table 1.
Primer sequences of immune-related genes used for quantitative RT-PCR analysis in this study
| Gene Symbol | Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| CASP1 | Caspase 1 | CCTCAAGTTTTGCCCTTTAGAAAT | TGAAAGTCTGTGCTGCAGATAATG |
| CASP3 | Caspase 3 | AGGCCGACTTCCTGTATGCTT | TGACCCGTCCCTTGAATTTC |
| CCL2 | Chemokine (C-C motif) ligand 2 | CCCAAAGAAGCTGTAGTATTTG | TCTGGACCCATTCCTTATTG |
| CCL3 | Chemokine (C-C motif) ligand 3 | TGAGACCAGCAGCCTTTGCT | CAGATCTGCCGGTTTCTCTTG |
| CCL5 | Chemokine (C-C motif) ligand 5 | GTCGTCTTTGTCACTCGAAGGA | GATGTATTCTTGAACCCACTTCTTCTC |
| CCL11 | Chemokine (C-C motif) ligand 11 | TCACGGCCACTTCCTTCAC | GGGATCTTCTTACTGGTCATGGTAA |
| CCR1 | Chemokine (C-C motif) receptor 1 | GGGACCTTGAACCTTGAATTCATA | TGAGTCTGTGTTTCCAGAGTACTTTGT |
| CCR2 | Chemokine (C-C motif) receptor 2 | GGTCTGCTACGGGCTCACA | CCCGGCGTTTCTGTCTCAT |
| CCR3 | Chemokine (C-C motif) receptor 3 | CAGTCCTCGCTATCCA | TTTAGCTCTTCCTCTCCTCAT |
| CCR5 | Chemokine (C-C motif) receptor 5 | TCATAAAACCAGTGTGAAACAAATTG | TCATGTTACCCACAAAACCAAAGA |
| CX3CL1 | Chemokine (C-X3-C motif) ligand 1 | CTGGCCGCGTTCTTTCAT | TGGCACGTGATGTTGCATTT |
| CX3CR1 | Chemokine (C-X3-C motif) receptor 1 | GGGTGAGTGGCTGGCACTT | GATCCAATTCCGGGAAGGA |
| CXCL10 | Chemokine (C-X-C motif) ligand 10 | GGCCATAGGAAAACTTGAAATCA | CTCTTCTCATTGTTCTTTTTCATTGTG |
| IFNα1 | Interferon-α1 | CCTGGTGGTGGTGAGCTACTG | GCTCTCTTGTTCCTGAGGTTATGAG |
| IFNαR1 | Interferon (α, β and ω) receptor 1 | CAGTGCACACATCGCTTCAGA | ATTTTATTTGCCACTGTGGTGTTTAT |
| IFNβ1 | Interferon β1, fibroblast | GCGTTCCTGCTGTGCTTCTC | TGCTAGTGCTTTGTCGGAACTG |
| IFNγ | Interferon γ | CATTCATGAGCATCGCCAAGT | TCTGATGAGTTCATTGACAGCTTTG |
| IFNγR1 | Interferon γ receptor 1 | CCCCTTTCTCCATGATGACAGA | GCCGGGACAACTATTGTAAGGA |
| IL1α | Interleukin 1α | GCCATAGCCCATGATTTAGAAGA | TGCTTGACGATCCTTATCAATTTG |
| IL1β | Interleukin 1β | AACAGGCTCATCTGGGATCCT | TCAGGGACAGTTGCCATAGCT |
| IL1R1 | Interleukin 1 receptor, type I | AAGTGGAATGGGTCGGAAATT | TGAAGGGTGTTCCAAAAACTGA |
| IL1R2 | Interleukin 1 receptor, type II | TGCACACAGGAAGGGTTTCTG | AACCAGCAATCGTCTCCAAGA |
| IL2 | Interleukin 2 | TTCATTTTCCAGGCACTGAAGA | CCATGATGCTCACGTTTAAATTT |
| IL4 | Interleukin 4 | CAGACGTCCTTACGGCAACA | TGCGAAGCACCCTGGAA |
| IL5 | Interleukin 5 | CCATGAGCACAGTGGTGAAAGA | CGTCTCATTGCTCGTCAACAG |
| IL6 | Interleukin 6 | GCCCTTCAGGAACAGCTATGA | TGTCAACAACATCAGTCCCAAGA |
| IL6R | Interleukin 6 receptor | GCCCTTGCTGGTGGATGTT | GAGAGGGAGTGCTGCTTGGA |
| IL8Rα | Interleukin 8 receptor, α | GGTCTGCTACGGGCTCACA | CCCGGCGTTTCTGTCTCAT |
| IL10 | Interleukin 10 | CCCTGGGAGAGAAGCTGAAGA | CACTGCCTTGCTTTTATTCTCACA |
| IL10Rα | Interleukin 10 receptor, α | GCGCACACGGGACAGAAC | GCTGGAAAAATCTGGCTTCAA |
| IL11 | Interleukin 11 | CTGGATAGCGCTGTCCTCTTG | TGTCTCTCATCTGTGCAGCTAGTTG |
| IL12α | Interleukin 12α | TCTGGACCTGCCAAGTGTCTT | TGGCCGTCCTCACCATGT |
| IL12β | Interleukin 12β | AGAGGAGGAGTGTAACCAGAAAGGT | CGCCCCTTTGCATTGG |
| IL13 | Interleukin 13 | GCAACATCACACAAGACCAGAAG | TGTCAGGTCCACGCTCCAT |
| IL15 | Interleukin 15 | CCTTCTCAACAGTCACTTCTT | ACAGAGGCCAACTGGATA |
| IL16 | Interleukin 16 | CCAGTTCCCATCATCGTTAGC | CCAAACTTGACGCCTTCCA |
| IL17β | Interleukin 17β | AGCTGCAGGCTGACTTAATGG | AGATGGAGATGGCAAGAAGGAA |
| IL18 | Interleukin 18 | AAACCCGCCTGTGTTCGA | TCAGTCTGGTCTGGGATTCGT |
| IL33 | Interleukin 33 | CCAAGGAACTTCACTGCTAACAGA | GTAACATCCATTCTCCAAAACAAAAC |
| IRF1 | Interferon regulatory factor 1 | CATTCCCGGGTACCCTCTGT | TCTCTAGCCAGGGTCTCATTCG |
| IRF2 | Interferon regulatory factor 2 | AAGGAAACAACGCCTTCAGAGT | TTCTCTGTCTTTGGTTTCTTTCCTTT |
| IRF3 | Interferon regulatory factor 3 | CTGGCTCAGGAACTGCACTGT | CCCAGGTCCAGCTGAGACA |
| IRF4 | Interferon regulatory factor 4 | CAGACTGCCGGCTGCATAT | CCAGGTTGCTAACATCATAGGTATGT |
| IRF6 | Interferon regulatory factor 6 | CATAGAGATTCCAAACGCTTCCA | CAAGCCTTAAAAATGGTGTTTTCTT |
| IRF7 | Interferon regulatory factor 7 | GCTCTGGAGAACAGGGAAGAAG | GTTTGCAACCCAGCATTTCCT |
| IRF8 | Interferon regulatory factor 8 | TGGTGACTGGGTATACTGCCTATG | TGCCCCCGTAGTAAAAGTTGA |
| IRF9 | Interferon regulatory factor 9 | GGAAGGAGCTGGCACAACTG | AGGAAGCGGATTCTCCATGA |
| TGFβ1 | Transforming growth factor, β1 | AGAAGTCACCCGCGTGCTA | TGTGTGATGTCTTTGGTTTTGTCA |
| TLR4 | Toll-like receptor 4 | CCCTCATGACATCCCTTATTC | CAATTTCTCACAACTTCAGTGG |
| TNF | Tumor necrosis factor (TNF superfamily, member 2) | CCTCAGCCTCTTCTCATT | GGAACTTCTCCTCCTTGT |
| TNFRSF1α | Tumor necrosis factor receptor superfamily, member 1α | TGCCCATCCAAAGAATAATTCC | TGGGCTTGGACAGTCACTCA |
| TNFRSF1β | Tumor necrosis factor receptor superfamily, member 1β | GCCTAGCCAAACTCCACACATC | TCAGTCCAACGATCAGACCAAT |
Two μg of total RNA was reverse-transcribed into the first-strand cDNA using Superscript II Reverse Transcriptase. The cDNA mixture was incubated at 25°C for 10 min, 42°C for 1.5 h, and 70°C for 15 min. The PCR amplification was conducted as described previously (Cui et al., 2013, Cao et al., 2011, Wei et al., 2011). Briefly, the product was amplified in a volume of 10 μl containing 5.0 μl of 2× Power SYBR Green PCR Master Mix (Applied Biosystems) and combined sense and antisense primers (2.5 μl; final concentration 20 nM) in a 384-well plate using the 7900HT Sequence Detection System (Applied Biosystems). The PCR conditions were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. A cycle threshold was assigned at the beginning of the logarithmic phase of the amplification, and differences in the Ct values of the control and nicotine-treated groups were used to determine the relative expression of genes of interest. Melting curve analysis was applied to characterize the specificity of the amplifications.
Statistical analysis
Data are expressed as mean ± S.E.M. Expression of each gene of interest was first normalized to the expression of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and then analyzed using a comparative Ct method (Winer et al., 1999). The relative expression of each gene was compared between the groups of HIV-1Tg control versus F344 control (to determine viral effects), HIV-1Tg nicotine versus HIV-1Tg saline (to determine the pharmacological effects of nicotine in the presence of viral proteins) and F344 nicotine versus F344 saline (to determine the pharmacological effects of nicotine in the absence of viral proteins) rats using the Student t-test. Significant alteration in mRNA expression was defined as a fold change > 20% with a P value < 0.05. All P values given in this report are original. Bonferroni correction was used to correct for multiple testing.
Based on the literature, any difference less than 20% between two groups is less likely to be biologically significant (Wang et al., 2007, Li et al., 2004). A power analysis conducted using Power Analysis and Sample Size (PASS) software (Hintze, 2000, Cohen, 1988) revealed that a sample size of 6 is sufficient to attain at least 85% power and detect 20% expression difference between two groups using a two-tailed two-sample t-test at the 5% significance level.
Results
Effect of HIV-1 viral proteins and nicotine on expression of immune-related genes in NAc
As shown in Table 2, our qRT-PCR array analysis revealed 18 of 52 immune-related genes are significantly changed (P < 0.05) by HIV-1 viral proteins or nicotine in the NAc of HIV-1Tg or F344 control rats. Genes upregulated by HIV-1 include CASP3 (43%, P=0.003), CX3C11 (31%, P=0.029), LRF4 (58%, P=0.024), and IRF7 (95%, P=0.00027). Further, CCL5 (40%, P=0.007), CX3CR1 (27%, P=0.012), IL1α (58%, P=0.000071), TGFβ1 (14%, P=0.023) and TLR4 (24%, P=0.03) are significantly downregulated in HIV-1Tg rats.
Table 2.
Effects of Nicotine and Viral Proteins on Immune-Related Genes in the Nucleus Accumbens of the F344 and HIV-1Tg Rats
| Gene Symbol | F344 rat (n=5–6 animals) | HIV-1Tg rat (n=5–6 animals) | Viral effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sal (Mean ± SD) | Nic (Mean ± SD) | Ratio Nic/Sal | Nic effect in F344 (P-value) | Sal (Mean ± SD) | Nic (Mean ± SD) | Ratio Nic/Sal | Nic effect in HIV-1Tg (P-value) | Ratio HIV-1Tg_Sal/ F344_Sal | P-value | |
| CASP3 | 8.1±0.8 | 11.4±2.9 | 1.41 | 0.039 | 11.6±1.8 | 11.2±2.8 | 0.97 | 0.756 | 1.43 | 0.003 |
| CCL3 | 0.085±0.018 | 0.15±0.041 | 1.76 | 0.005 | 0.15±0.074 | 0.20±0.11 | 1.33 | 0.443 | 1.76 | 0.054 |
| CCL5 | 0.25±0.0.044 | 0.21±0.16 | 0.84 | 0.531 | 0.15±0.046 | 0.18±0.10 | 1.17 | 0.97 | 0.60 | 0.007 |
| CCL11 | 0.034±0.023 | 0.061±0.027 | 1.81 | 0.127 | 0.0164±0.0062 | 0.051±0.027 | 3.08 | 0.023 | 0.48 | 0.149 |
| CCR2 | 0.077±0.027 | 0.054±0.016 | 0.71 | 0.137 | 0.065±0.020 | 0.191±0.082 | 2.92 | 0.012 | 0.84 | 0.463 |
| CX3CL1 | 275.1±64.8 | 297.3±90.7 | 1.08 | 0.635 | 359.9±36.3 | 259.2±44.1 | 0.72 | 0.003 | 1.31 | 0.029 |
| CX3CR1 | 38.7±6.6 | 32.8±5.5 | 0.85 | 0.126 | 28.2±5.3 | 28.1±4.3 | 0.99 | 0.958 | 0.73 | 0.012 |
| IL1α | 0.288±0.055 | 0.185±0.070 | 0.64 | 0.023 | 0.12±0.031 | 0.204±0.049 | 1.69 | 0.006 | 0.42 | 7.17E-05 |
| IL2 | 0.29±0.014 | 0.36±0.14 | 1.21 | 0.468 | 0.305±0.084 | 0.193±0.0792 | 0.63 | 0.040 | 1.05 | 0.937 |
| IL5 | 0.035±0.006 | 0.076±0.04 | 2.13 | 0.037 | 0.064±0.054 | 0.075±0.061 | 1.16 | 0.759 | 1.83 | 0.273 |
| IL8Rα | 0.14±0.06 | 0.232±0.13 | 1.59 | 0.184 | 0.20±0.049 | 0.10±0.019 | 0.52 | 0.002 | 1.43 | 0.114 |
| IL17β | 0.062±0.046 | 0.069±0.04 | 1.11 | 0.781 | 0.056±0.015 | 0.031±0.014 | 0.56 | 0.017 | 0.90 | 0.77 |
| IRF4 | 1.2±0.1 | 1.6±0.3 | 1.33 | 0.026 | 1.9±0.2 | 1.3±0.5 | 0.68 | 0.041 | 1.58 | 0.024 |
| IRF7 | 0.82±0.13 | 1.4±0.9 | 1.70 | 0.109 | 1.6±0.03 | 2.1±0.5 | 1.31 | 0.142 | 1.95 | 2.27E-04 |
| IRF9 | 8.1±0.8 | 11.4±2.2 | 1.41 | 0.002 | 9.2±1.4 | 8.8±2.0 | 0.96 | 0.741 | 1.14 | 0.139 |
| TGFβ1 | 3.5±0.3 | 3.3±0.3 | 0.94 | 0.517 | 3.0±0.2 | 3.2±0.3 | 1.01 | 0.88 | 0.86 | 0.023 |
| TLR4 | 1.73±0.32 | 1.70±0.31 | 0.97 | 0.825 | 1.32±0.2 | 1.3±0.1 | 0.98 | 0.843 | 0.76 | 0.030 |
| TNF | 0.32±0.096 | 0.29±0.062 | 0.89 | 0.454 | 0.25±0.1 | 0.13±0.065 | 0.52 | 0.044 | 0.78 | 0.217 |
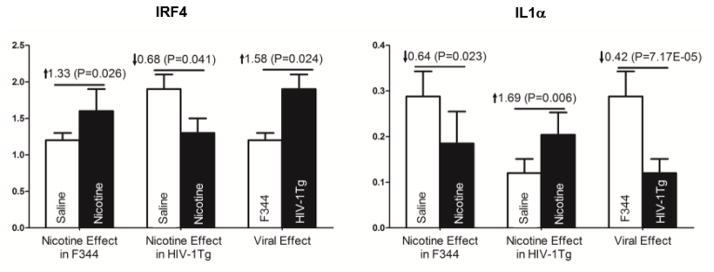
In the NAc of HIV-1Tg rats, nicotine significantly upregulated the expression of CCL11 (208%, P=0.023), CCR2 (192%, P=0.012) and IL1α (69%, P=0.006) and downregulated expression of CX3C11 (28%, P= 0.003), IL2 (37%, P= 0.04), IL8Rα (48%, P=0.002), IRF4 (32%, P = 0.041), and TNF (48%, P =0.044). In F344 control rats, CASP3 (41%, P=0.039), IL5 (213%, P=0.037), IRF4 (33%, P =0.026) and IRF9 (41%, P=0.002) were upregulated following nicotine treatment and only IL1α (36%, P=0.023) was downregulated. Of the genes affected, nicotine had opposite effects on IRF4 and IL1α in the NAc of HIV-1 and F344 rats. A comparison of the effects of HIV-1 and nicotine on IRF4 and IL1α expression in the NAc region is given in Figure 1.
Figure 1.
Regulatory effects of nicotine and HIV-1 viral proteins on IRF4 and IL1α in the nucleus accumbens of the HIV-1Tg and F344 rats following 27 days of either saline or nicotine (0.4 mg/kg) treatment. Nicotine treatment had a significantly different effect on IRF4 and IL1α expression in the nucleus accumbens of HIV-1Tg and F344 rats. Expression levels are reported as fold increase in mRNA levels. See Table 2 for detailed statistical analysis results (N = 5–6 animals per group).
Effect of HIV-1 viral proteins and nicotine on expression of immune-related genes in PFC
Of the 52 genes examined in the PFC, we found that the expression of CASP1 (38%, P=0.042), CCL5 (51%, P=0.026), TGFβ1 (23%, P=0.015), and TLR4 (465, P=0.010) were all downregulated and no gene was upregulated by HIV-1 viral proteins in HIV1-1Tg rats (see Table 3).
Table 3.
Effects of Nicotine and Viral Proteins on Immune-Related Genes in the PFC of the F344 and HIV-1Tg Rat
| Gene Symbol | F344 rat (n=5–6 animals) | HIV-1Tg rat (n=5–6 animals) | Viral effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sal (Mean ± SD) | Nic (Mean ± SD) | Ratio Nic/Sal | Nic effect in F344 (P-value) | Sal (Mean ± SD) | Nic (Mean ± SD) | Ratio Nic/Sal | Nic effect in HIV-1Tg (P-value) | Ratio HIV-1Tg_Sal/ F344_Sal | P-value | |
| CASP1 | 0.25±0.061 | 0.20±0.062 | 0.80 | 0.206 | 0.30±0.059 | 0.20±0.026 | 0.66 | 0.006 | 1.20 | 0.191 |
| CCL2 | 0.15±0.040 | 0.080±0.013 | 0.51 | 0.004 | 0.093±0.043 | 0.078±0.023 | 0.84 | 0.476 | 0.62 | 0.042 |
| CCL5 | 0.16±0.064 | 0.11±0.015 | 0.689 | 0.131 | 0.078±0.022 | 0.092±0.011 | 1.19 | 0.192 | 0.49 | 0.026 |
| CCR5 | 0.014±0.005 | 0.012±0.0073 | 0.92 | 0.752 | 0.015±0.004 | 0.010±0.0017 | 0.66 | 0.031 | 1.07 | 0.72 |
| CX3CR1 | 21.7±5.69 | 17.1±2.44 | 0.79 | 0.129 | 19.9±4.66 | 14.4±2.80 | 0.72 | 0.035 | 0.92 | 0.548 |
| IL1β | 1.48±0.51 | 1.30±0.42 | 0.88 | 0.512 | 1.59±0.27 | 1.00±0.31 | 0.63 | 0.014 | 1.07 | 0.943 |
| IL1R2 | 0.13±0.021 | 0.09±0.018 | 0.68 | 0.011 | 0.14±0.034 | 0.074±0.028 | 0.53 | 0.004 | 1.08 | 0.615 |
| IL8Rα | 0.36±0.060 | 0.045±0.024 | 0.12 | 0.267 | 0.91±0.81 | 0.027±0.017 | 0.30 | 0.025 | 2.53 | 0.254 |
| IL18 | 2.94±0.95 | 1.82±0.36 | 0.63 | 0.023 | 2.08±0.85 | 1.51±0.73 | 0.73 | 0.245 | 0.71 | 0.133 |
| TGFβ1 | 2.81±0.38 | 2.12±0.29 | 0.76 | 0.006 | 2.17±0.37 | 1.69±0.31 | 0.74 | 0.040 | 0.77 | 0.015 |
| TLR4 | 1.60±0.41 | 1.05±0.25 | 0.66 | 0.031 | 0.87±0.28 | 0.93±0.16 | 1.08 | 0.626 | 0.54 | 0.010 |
| TNF | 0.18±0.091 | 0.14±0.058 | 0.83 | 0.492 | 0.14±0.053 | 0.077±0.043 | 0.53 | 0.036 | 0.78 | 0.428 |
| TNFRSF1α | 3.83±0.47 | 3.15±0.35 | 0.82 | 0.019 | 3.37±0.72 | 2.47±0.29 | 0.73 | 0.017 | 0.88 | 0.225 |
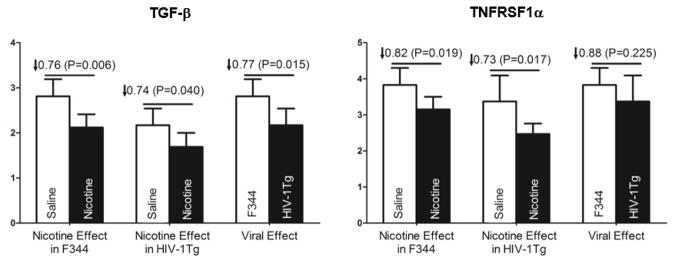
Following nicotine treatment, nine genes CASP1 (34%, P=0.006), CCR5 (34%, P=0.031), CX3CR1 (28%; P=0.035), ILL1β (37%, P=0.014), II1R2 (47%, P=0.004), II8Rα (70%, P=0.025), TGFβ1 (26%, P=0.04), TNF (47%, P=0.036), and TNFRSF1α (27%, P=0.017) were downregulated in HIV-1Tg rats whereas only genes CCL2 (49%, P=0.004), II1R2 (32%, P=0.011), II18 (37%, P=0.023), TGFβ1 (34% P=0.006), TLR4 (34%, P=0.031) and TNFRSF1α (28%, P=0.019) were downregulated in F344 rats. No genes were upregulated by nicotine in the PFC of either HIV-1Tg or F344 control rats. Of the genes modulated by nicotine, only TNFRSF1α and TGFβ1 were affected in both groups. A comparison of the effects of HIV-1 viral and nicotine on TGFβ1 and TNFRSF1α expression in the PFC region is given in Figure 2.
Figure 2.
Regulatory effects of nicotine and HIV-1 viral proteins on TGFβ1 and TNFRSFF1α in the prefrontal cortex of the HIV-1Tg and F344 rats following 27 days of either saline or nicotine (0.4 mg/kg) treatment. An independent-samples t-test revealed that expression of TGFβ1 and TNFRSFF1α was significantly regulated in HIV-1Tg rats compared to F33 controls. Expression levels are reported as fold increase in mRNA levels. See Table 3 for detailed statistical analysis results (N = 5–6 animals per group).
Effect of HIV-1 viral proteins and nicotine on expression of immune-related genes in VTA
With the same RT-PCR technology, we also examined expression of the 52 genes in the VTA region of HIV-1Tg and F344 rats treated with nicotine or without nicotine (Table 4). Comparisons of HIV-1Tg and F344 controls revealed that HIV-1 significantly downregulates expression of II6 (74%, P=0.000) and upregulates expression of CASP3 (37%, P=0.0001), CX3CR1 (44%, P=0.046) and IL1β (188%, P=0.0005) in the VTA region.
Table 4.
Effects of Nicotine and Viral Proteins on Immune-Related Genes in the VTA of the F344 and HIV-1Tg Rat
| Gene Symbol | F344 rat (n=5–6 animals) | HIV-1Tg rat (n=5–6 animals) | Viral effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sal (Mean ± SD) | Nic (Mean ± SD) | Ratio Nic/Sal | Nic effect in F344 (P-value) | Sal (Mean ± SD) | Nic (Mean ± SD) | Ratio Nic/Sal | Nic effect in HIV-1Tg (P-value) | Ratio HIV-1Tg_Sal/ F344_Sal | P-value | |
| CASP3 | 6.3±0.83 | 4.61±0.85 | 0.73 | 0.008 | 4.60±0.43 | 4.30±1.12 | 0.94 | 0.556 | 1.37 | 0.001 |
| CCL2 | 0.206±0.053 | 0.137±0.014 | 0.67 | 0.022 | 0.160±0.054 | 0.114±0.047 | 0.71 | 0.150 | 1.29 | 0.169 |
| CCR5 | 0.054±0.027 | 0.045±0.033 | 0.83 | 0.632 | 0.064±0.028 | 0.0196±0.0123 | 0.30 | 0.005 | 0.84 | 0.527 |
| CX3CR1 | 28.2±7.8 | 22.2±0.7 | 0.79 | 0.124 | 19.6±4.8 | 16.5±3.8 | 0.84 | 0.255 | 1.44 | 0.046 |
| IFNα1 | 1.05±0.12 | 1.15±0.18 | 1.10 | 0.283 | 0.79±0.17 | 0.7±0.16 | 0.88 | 0.359 | 1.33 | 0.015 |
| IL1β | 0.470±0.12 | 0.42±0.34 | 0.91 | 0.772 | 0.163±0.050 | 0.15±0.11 | 0.95 | 0.876 | 2.88 | 0.0005 |
| IL5 | 0.097±0.036 | 0.108±0.053 | 1.11 | 0.718 | 0.21±0.11 | 0.088±0.029 | 0.42 | 0.036 | 0.46 | 0.110 |
| IL6 | 0.072±0.055 | 0.0228±0.0368 | 0.31 | 0.192 | 0.28±0.17 | 0.257±0.289 | 0.91 | 0.874 | 0.26 | 0.033 |
| IL8Rα | 0.069±0.017 | 0.30±0.11 | 4.34 | 0.0009 | 0.13±0.10 | 0.062±0.033 | 0.45 | 0.118 | 0.53 | 0.137 |
| IL33 | 8.07±0.73 | 9.49±0.99 | 1.18 | 0.018 | 8.4±2.4 | 8.1±0.7 | 0.96 | 0.79 | 0.96 | 0.757 |
| IRF1 | 3.8±1.6 | 3.7±0.8 | 0.97 | 0.973 | 3.6±1.0 | 2.4±0.3 | 0.67 | 0.032 | 1.06 | 0.836 |
| IRF6 | 0.91±0.26 | 0.53±0.18 | 0.58 | 0.020 | 0.97±0.21 | 1.24±0.63 | 1.28 | 0.353 | 0.94 | 0.710 |
| TNF | 0.2±0.077 | 0.3±0.1 | 1.50 | 0.274 | 0.2±0.069 | 0.1±0.068 | 0.50 | 0.008 | 1.00 | 0.652 |
Nicotine treatment was found to downregulate CCR5 (70%, P=0.005), IL5 (58%, P=0.036), IRF1 (33%, P=0.032) and TNF (50%, P=0.008) expression in HIV-1Tg rats, and CASP3 (27%, P=0.008), CCL2 (33%, P=0.022) and IRF6 (42%, P=0.02) expression in F344 control rats. Further, IL8Rα is upregulated by nicotine only in F344 rats.
Discussion
In this study, we investigated 52 immune-related genes in the three brain regions implicated in addiction and HAND with three objectives. The first objective was to determine how HIV-1 viral proteins affect expression of these immune-related genes and the second and third objectives were to determine how nicotine affects expression of those genes in the presence (i.e., in HIV-1Tg rat) and absence (i.e., F344 genetic background control rat) of viral proteins. Our results showed that HIV-1 viral proteins have a significant impact on the expression of many immune-related genes in a brain region-dependent manner (Table 5). Further, our results showed that the pharmacological effect of nicotine on expression of these immune-related genes differed in the HIV-1Tg and F344 rats, suggesting that nicotine may have different effects on expression of those immune-related genes in the presence (i.e., HIV-1Tg rats) or absence (i.e., F344 control rats) of HIV-1 viral proteins. Importantly, our data showed that treatment with nicotine appears to have the opposite effects of viral proteins on a number of immune-related genes suggesting that nicotine could potentially reverse some of the negative effects of HIV-1 viral proteins.
Table 5.
Comparison of differentially expressed number genes by HIV-1 viral proteins and nicotine in the NAs, PFC and VTA regions
| Effect of HIV-1 viral proteins and/or nicotine | NAc | PFC | VTA | |||
|---|---|---|---|---|---|---|
| Up- regulated | Down- regulated | Up- regulated | Down- regulated | Up- regulated | Down- regulated | |
| Nicotine | 5 | 1 | 0 | 6 | 1 | 3 |
| HIV-1 viral proteins | 4 | 5 | 0 | 4 | 3 | 1 |
| Nicotine + HIV-1 viral proteins | 3 | 6 | 0 | 9 | 0 | 4 |
Of the differentially expressed genes reported in this paper, interleukin 1α (IL-1α) and Interferon regulatory factor 7 (IRF7) are two representative genes that are worthy of discussion. IL1α is a cytokine of the interleukin-1 family, which is responsible for the production of inflammation, as well as the promotion of fever and sepsis. In the NAC, we found that both HIV-1 viral proteins and nicotine reduced the expression of this gene. In the HIV-1Tg rats treated with saline, IL-1α expression was decreased by 58% and in F344 rats treated with nicotine, IL-1α expression was reduced by 36% compared with F344 saline controls. However, when the HIV-1Tg rats were treated with nicotine, we found the expression level of this gene was increased by 69%. These results suggest that, in the presence of HIV-1 viral proteins, nicotine works to not only reverse the suppression effect of HIV-1 viral proteins on IL-1α expression, but also further enhanced the expression of this gene. IL-1 production is induced by endotoxins as well as cytokines such as TNF which is also associated with chronic inflammation. It has been posited that neural inflammation plays a large role in the development and progression of HIV-associated neurocognitive disorder (HAND), thus, due it it’s involvement in regulation of both the innate and adaptive immunity and induction of fever and host in inflammation, IL-1α regulation may be a potential target for treatment of HAND and other inflammation-related disorders
In the case of Irf7, our results showed a 96% increase in expression in the NAc of HIV-1Tg rats. However, when treated with nicotine, HIV-1Tg rats exhibited no significant difference in NAc Irf7 expression compared to nicotine-treated F344 rats. These results suggest that nicotine suppressed the increased expression of IRF7 in the NAc resulting from HIV-1 viral proteins. Given that Irf7 is the master regulator of type I interferon production and is involved in oncogenesis and apoptosis (Ning et al., 2011), alteration of that pathway and upregulation of Irf7 suggest disturbances of the innate immune response and neuronal survival in the HIV-1Tg rat. Nicotine was able to decrease the effect of HIV-1 viral proteins on Irf7 expression, thus acting as a possible neuronal protectant.
As reported in previous studies which focused on the PFC, hippocampus, and striatum (Li et al., 2013, Cao et al., 2013), we found that HIV-1 viral proteins and nicotine affect expression of immune-related genes in a brain region-dependent manner. More specifically, we found that more genes were altered by nicotine, HIV-1 viral proteins, or the combination of both in the NAc than in the PFC or the VTA. Further, we found that no genes were up-regulated by either nicotine or HIV-1 viral proteins in the PFC, however this brain region had the highest number of significantly down-regulated genes. The VTA has the least number of genes altered by either nicotine, HIV-1 viral proteins, or the combination of the two variables. These results clearly indicate that the pathological effect of HIV-1 viral proteins and pharmacological effect of nicotine on immune-related genes differ between brain regions.
In our previous study, which was the first to perform deep-sequencing analysis of RNA transcripts associated with the HIV-1Tg rat, we discovered that three major pathways were effected by HIV-1 viral proteins; immune response-, neuronal survival-, and neurotransmission-related pathways. In the current study, we were able to expand on our previous research which focused on identifying genes and pathways altered in the prefrontal cortex, hippocampus, and striatum of the HIV-1Tg rat (Li et al., 2013).
Although it is well documented that nicotine is an addictive substance, it also exerts neuroprotective effects in the brain. For example, nicotine treatment restored signaling in genes related to Wnt/β-catenin to normal, restored NMDA receptor function, increased Fgf9 and Wnt5a expression in the PFC of HIV-1Tg rats (Cao et al., 2013). In the hippocampus, nicotine regulates the overexpression of phospholipase C (Plch1) and decreases oxidative stress. Nicotine has a similar effect on oxidative stress in the striatum and also restores the function of the TCA in this brain region. The effect of nicotine on oxidative stress in HIV-1Tg rats has been supported in another study recently reported by our group (Song et al., 2015). Together, these findings demonstrated that nicotine has the ability to regulate a number of neurodegenerative effects of HIV-1 viral proteins in the brain.
In addition to those in vivo studies, modulation of the innate immune system is also seen in a neuronal SH-SY5Y neuroblastoma cell model. After treating these cells with nicotine, Cui et al reported alterations in 14 pathways. Among these pathways are the TLR pathway and the death receptor pathway, which are integral components of the innate immune system (Cui et al., 2012). Further, downstream pathways of TLR and DR signaling and the expression of genes associated with innate immunity such as nuclear factor κB (NF-κB) were also affected by nicotine. These findings demonstrate that nicotine can regulate multiple innate immune-related pathways, and our data thus provide new clues to the molecular mechanisms underlying nicotine’s regulatory effects on neurons in the NAc, PFC, and VTA.
In sum, this study demonstrates the HIV-1 viral proteins have significantly impacted expression of a number of immune-related genes in different brain regions. Furthermore, we found that the pharmacological effects of nicotine on expression of immune-related genes differed in the brains of HIV-1Tg rats compared with F344 control rats, suggesting that HIV-1 viral proteins can modulate the response of immune system to nicotine. Finally, we showed the biological effect of HIV-1 viral proteins and nicotine on immune-related gene expression appears to be in a brain region-dependent manner.
Acknowledgments
The authors thank Drs. Shaolin Wang, Junran Cao, and Guohua Song for their assistance with sample collection during the experiments. This work was supported, in part, by US National Institutes of Health grants DA-016149 to SLC and DA-026356 to SLC and MDL.
Footnotes
Financial Disclosures: The authors claim no conflict interest regarding this report.
References
- BRADFORD ST, STAMATOVIC SM, DONDETI RS, KEEP RF, ANDJELKOVIC AV. Nicotine aggravates the brain postischemic inflammatory response. Am J Physiol Heart Circ Physiol. 2011;300:H1518–29. doi: 10.1152/ajpheart.00928.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAO J, DWYER JB, MANGOLD JE, WANG J, WEI J, LESLIE FM, LI MD. Modulation of cell adhesion systems by prenatal nicotine exposure in limbic brain regions of adolescent female rats. Int J Neuropsychopharmacol. 2011;14:157–74. doi: 10.1017/S1461145710000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAO J, WANG S, WANG J, CUI W, NESIL T, VIGORITO M, CHANG SL, LI MD. RNA Deep Sequencing Analysis Reveals That Nicotine Restores Impaired Gene Expression by Viral Proteins in the Brains of HIV-1 Transgenic Rats. PLoS One. 2013;8:e68517. doi: 10.1371/journal.pone.0068517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG SL, CONNAGHAN KP, WEI Y, LI MD. NeuroHIV and use of addictive substances. Int Rev Neurobiol. 2014;118:403–40. doi: 10.1016/B978-0-12-801284-0.00013-0. [DOI] [PubMed] [Google Scholar]
- CICALA C, ARTHOS J, SELIG SM, DENNIS G, JR, HOSACK DA, VAN RYK D, SPANGLER ML, STEENBEKE TD, KHAZANIE P, GUPTA N, YANG J, DAUCHER M, LEMPICKI RA, FAUCI AS. HIV envelope induces a cascade of cell signals in non-proliferating target cells that favor virus replication. Proc Natl Acad Sci U S A. 2002;99:9380–5. doi: 10.1073/pnas.142287999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN J. Statistical Power Analysis for the Behavioral Sciecnes. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- CUI WY, LI MD. Nicotinic Modulation of Innate Immune Pathways Via alpha7 Nicotinic Acetylcholine Receptor. J Neuroimmune Pharmacol. 2010:479–488. doi: 10.1007/s11481-010-9210-2. [DOI] [PubMed] [Google Scholar]
- CUI WY, WANG J, WEI J, CAO J, CHANG SL, GU J, LI MD. Modulation of innate immune-related pathways in nicotine-treated SH-SY5Y cells. Amino Acids. 2012;43:1157–69. doi: 10.1007/s00726-011-1171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUI WY, ZHAO S, POLANOWSKA-GRABOWSKA R, WANG J, WEI J, DASH B, CHANG SL, SAUCERMAN JJ, GU J, LI MD. Identification and characterization of poly(I:C)-induced molecular responses attenuated by nicotine in mouse macrophages. Mol Pharmacol. 2013;83:61–72. doi: 10.1124/mol.112.081497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENDELMAN HE, ZHENG J, COULTER CL, GHORPADE A, CHE M, THYLIN M, RUBOCKI R, PERSIDSKY Y, HAHN F, REINHARD J, JR, SWINDELLS S. Suppression of inflammatory neurotoxins by highly active antiretroviral therapy in human immunodeficiency virus-associated dementia. J Infect Dis. 1998;178:1000–7. doi: 10.1086/515693. [DOI] [PubMed] [Google Scholar]
- GISSLEN M, SVENNERHOLM B, FUCHS D, HAGBERG L. Neurological efficacy of stavudine, zidovudine, and lamivudine. Lancet. 1998;352:402–3. doi: 10.1016/s0140-6736(05)60499-0. [DOI] [PubMed] [Google Scholar]
- GUTALA R, WANG J, HWANG YY, HAQ R, LI MD. Nicotine modulates expression of amyloid precursor protein and amyloid precursor-like protein 2 in mouse brain and in SH-SY5Y neuroblastoma cells. Brain Res. 2006;1093:12–9. doi: 10.1016/j.brainres.2006.03.100. [DOI] [PubMed] [Google Scholar]
- HINTZE JL. 2000 Users’ Guide. Kaysville, UT: Number Cruncher Statistical Software; 2000. [Google Scholar]
- HOMJI NF, MAO X, LANGSDORF EF, CHANG SL. Endotoxin-induced cytokine and chemokine expression in the HIV-1 transgenic rat. J Neuroinflammation. 2012;9:3. doi: 10.1186/1742-2094-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG J, AIKEN C. Maturation of the viral core enhances the fusion of HIV-1 particles with primary human T cells and monocyte-derived macrophages. Virology. 2006;346:460–8. doi: 10.1016/j.virol.2005.11.008. [DOI] [PubMed] [Google Scholar]
- KASHIWAGI Y, YANAGITA M, KOJIMA Y, SHIMABUKURO Y, MURAKAMI S. Nicotine up-regulates IL-8 expression in human gingival epithelial cells following stimulation with IL-1beta or P. gingivalis lipopolysaccharide via nicotinic acetylcholine receptor signalling. Arch Oral Biol. 2012;57:483–90. doi: 10.1016/j.archoralbio.2011.10.007. [DOI] [PubMed] [Google Scholar]
- KIKUCHI H, ITOH J, FUKUDA S. Chronic nicotine stimulation modulates the immune response of mucosal T cells to Th1-dominant pattern via nAChR by upregulation of Th1-specific transcriptional factor. Neurosci Lett. 2008;432:217–21. doi: 10.1016/j.neulet.2007.12.027. [DOI] [PubMed] [Google Scholar]
- LI MD, CAO J, WANG S, WANG J, SARKAR S, VIGORITO M, MA JZ, CHANG SL. Transcriptome sequencing of gene expression in the brain of the HIV-1 transgenic rat. PLoS One. 2013;8:e59582. doi: 10.1371/journal.pone.0059582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI MD, KANE JK, WANG J, MA JZ. Time-dependent changes in transcriptional profiles within five rat brain regions in response to nicotine treatment. Brain Res Mol Brain Res. 2004;132:168–80. doi: 10.1016/j.molbrainres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- MATTA SG, BALFOUR DJ, BENOWITZ NL, BOYD RT, BUCCAFUSCO JJ, CAGGIULA AR, CRAIG CR, COLLINS AC, DAMAJ MI, DONNY EC, GARDINER PS, GRADY SR, HEBERLEIN U, LEONARD SS, LEVIN ED, LUKAS RJ, MARKOU A, MARKS MJ, MCCALLUM SE, PARAMESWARAN N, PERKINS KA, PICCIOTTO MR, QUIK M, ROSE JE, ROTHENFLUH A, SCHAFER WR, STOLERMAN IP, TYNDALE RF, WEHNER JM, ZIRGER JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- MAZZUCCHELLI R, AMADIO M, CURRELI S, DENARO F, BEMIS K, REID W, BRYANT J, RIVA A, GALLI M, ZELLA D. Establishment of an ex vivo model of monocytes-derived macrophages differentiated from peripheral blood mononuclear cells (PBMCs) from HIV-1 transgenic rats. Mol Immunol. 2004;41:979–84. doi: 10.1016/j.molimm.2004.06.023. [DOI] [PubMed] [Google Scholar]
- MCARTHUR JC, HAUGHEY N, GARTNER S, CONANT K, PARDO C, NATH A, SACKTOR N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–21. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- MIDDE NM, GOMEZ AM, HARROD SB, ZHU J. Genetically expressed HIV-1 viral proteins attenuate nicotine-induced behavioral sensitization and alter mesocorticolimbic ERK and CREB signaling in rats. Pharmacol Biochem Behav. 2011;98:587–97. doi: 10.1016/j.pbb.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESIL T, CAO J, YANG Z, CHANG SL, LI MD. Nicotine attenuates the effect of HIV-1 proteins on the neural circuits of working and contextual memories. Mol Brain. 2015;8:43. doi: 10.1186/s13041-015-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NING S, PAGANO JS, BARBER GN. IRF7: activation, regulation, modification and function. Genes Immun. 2011;12:399–414. doi: 10.1038/gene.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIZRI E, IRONY-TUR-SINAI M, LORY O, ORR-URTREGER A, LAVI E, BRENNER T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J Immunol. 2009;183:6681–8. doi: 10.4049/jimmunol.0902212. [DOI] [PubMed] [Google Scholar]
- PAXINOS G, WATSON C. The rat brain in stereotxic coordinates. Academic Press Inc; San Diego: 2005. [Google Scholar]
- PORTEGIES P. Review of antiretroviral therapy in the prevention of HIV-related AIDS dementia complex (ADC) Drugs. 1995;49(Suppl 1):25–31. doi: 10.2165/00003495-199500491-00007. discussion 38–40. [DOI] [PubMed] [Google Scholar]
- RAY PE, LIU XH, ROBINSON LR, REID W, XU L, OWENS JW, JONES OD, DENARO F, DAVIS HG, BRYANT JL. A novel HIV-1 transgenic rat model of childhood HIV-1-associated nephropathy. Kidney Int. 2003;63:2242–53. doi: 10.1046/j.1523-1755.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- RAY R, MITRA N, BALDWIN D, GUO M, PATTERSON F, HEITJAN DF, JEPSON C, WILEYTO EP, WEI J, PAYNE T, MA JZ, LI MD, LERMAN C. Convergent evidence that choline acetyltransferase gene variation is associated with prospective smoking cessation and nicotine dependence. Neuropsychopharmacology. 2010;35:1374–82. doi: 10.1038/npp.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAY R, TYNDALE RF, LERMAN C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J Neurogenet. 2009;23:252–61. doi: 10.1080/01677060802572887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REID W, SADOWSKA M, DENARO F, RAO S, FOULKE J, JR, HAYES N, JONES O, DOODNAUTH D, DAVIS H, SILL A, O’DRISCOLL P, HUSO D, FOUTS T, LEWIS G, HILL M, KAMIN-LEWIS R, WEI C, RAY P, GALLO RC, REITZ M, BRYANT J. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98:9271–6. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARKAR S, CHANG SL. Ethanol concentration-dependent alterations in gene expression during acute binge drinking in the HIV-1 transgenic rat. Alcohol Clin Exp Res. 2013;37:1082–90. doi: 10.1111/acer.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONG G, NESIL T, CAO J, YANG Z, CHANG SL, LI MD. Nicotine mediates expression of genes related to antioxidant capacity and oxidative stress response in HIV-1 transgenic rat brain. J Neurovirol. 2015 doi: 10.1007/s13365-015-0375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOPORI M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372–7. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- SUGANO N, SHIMADA K, ITO K, MURAI S. Nicotine inhibits the production of inflammatory mediators in U937 cells through modulation of nuclear factor-kappaB activation. Biochem Biophys Res Commun. 1998;252:25–8. doi: 10.1006/bbrc.1998.9599. [DOI] [PubMed] [Google Scholar]
- THOMSEN MS, MIKKELSEN JD. The alpha7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF-alpha release from microglia. J Neuroimmunol. 2012;251:65–72. doi: 10.1016/j.jneuroim.2012.07.006. [DOI] [PubMed] [Google Scholar]
- TOLEDO EM, COLOMBRES M, INESTROSA NC. Wnt signaling in neuroprotection and stem cell differentiation. Prog Neurobiol. 2008;86:281–96. doi: 10.1016/j.pneurobio.2008.08.001. [DOI] [PubMed] [Google Scholar]
- VERANI A, GRAS G, PANCINO G. Macrophages and HIV-1: dangerous liaisons. Mol Immunol. 2005;42:195–212. doi: 10.1016/j.molimm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- WANG J, GUTALA R, SUN D, MA JZ, SHEELA RC, TICKU MK, LI MD. Regulation of platelet-derived growth factor signaling pathway by ethanol, nicotine, or both in mouse cortical neurons. Alcohol Clin Exp Res. 2007;31:357–75. doi: 10.1111/j.1530-0277.2006.00331.x. [DOI] [PubMed] [Google Scholar]
- WANG Y, ZHANG F, YANG W, XUE S. Nicotine induces pro-inflammatory response in aortic vascular smooth muscle cells through a NFkappaB/osteopontin amplification loop-dependent pathway. Inflammation. 2012;35:342–9. doi: 10.1007/s10753-011-9324-6. [DOI] [PubMed] [Google Scholar]
- WEI J, WANG J, DWYER JB, MANGOLD J, CAO J, LESLIE FM, LI MD. Gestational nicotine treatment modulates cell death/survival-related pathways in the brains of adolescent female rats. Int J Neuropsychopharmacol. 2011;14:91–106. doi: 10.1017/S1461145710000416. [DOI] [PubMed] [Google Scholar]
- WINER J, JUNG CK, SHACKEL I, WILLIAMS PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–9. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]