Abstract
To identify the anatomy and pathology of chest wall malformations presenting for consideration for corrective surgery or as a possible chest wall “mass”, and to review the common corrective surgical procedures. Congenital chest wall deformities are caused by anomalies of chest wall growth, leading to sternal depression or protrusion, or are related to failure of normal spine or rib development. Cross-sectional imaging allows appreciation not only of the involved structures but also assessment of the degree of displacement or deformity of adjacent but otherwise normal structures and differentiation between anatomical deformity and neoplasia. In some cases, CT is also useful for surgical planning. The use of three-dimensional reconstructions, utilizing a low-dose technique, provides important information for the surgeon to discuss the nature of anatomical abnormalities and planned corrections with the patient and often with their parents. In this pictorial essay, we discuss the radiological features of the commonest congenital chest wall deformities and illustrate pre- and post-surgical appearances for those undergoing surgical correction.
TECHNIQUE
At the Royal Brompton Hospital, we perform unenhanced volumetric low-dose chest CT during full inspiration. Patients are scanned from lung apices to costophrenic recesses, with topogram parameters of 120 kV and 35 mAs. Standard scan parameters are 80–120 kV with a quality reference mAs of 20 mAs, rotation time of 0.5 s, collimation of 128 × 0.6 mm, slice thickness of 1 mm and a pitch of 1.2. The studies are performed with dose modulation. The usual dose–length product for a child is 100 mGy cm and for an adult is 200 mGy cm. The raw data are post-processed to create volume-rendered images. Appropriate windowing is chosen to emphasize the skeleton, costal cartilages and muscles, and skin in turn.
One of the difficulties with CT in this regard is displaying both bony and cartilaginous information at the same time. Therefore, our standard technique is to provide a variety of volume-rendered images, in suitable anatomical projections, varying from bone to cartilage to soft-tissue rendering, building up anatomical layers, and displaying the net effect of underlying skeletal abnormality on overlying skin contour.
PECTUS EXCAVATUM
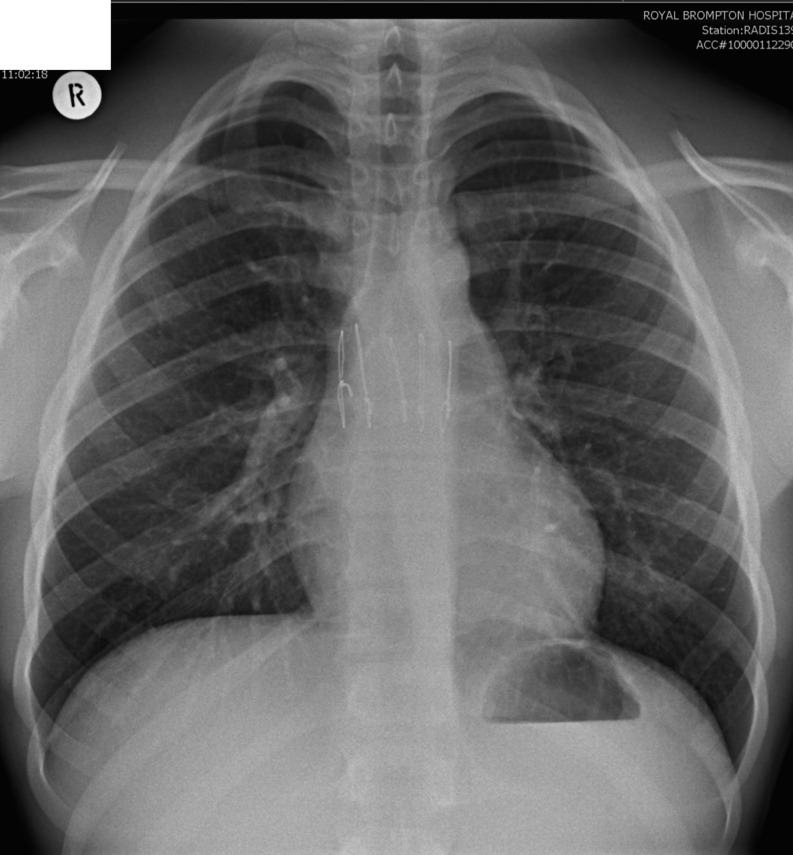
Pectus excavatum, also known as funnel chest or trichterbrust, is the most common congenital deformity of the sternum. Its incidence is approximately 1 in 400 births, afflicting males more than females. It is uncommon among African Americans and Latinos.1 Although the majority of pectus excavatum cases are congenital, around 15% of cases appear later during adolescent development. These are frequently associated with abnormalities of the connective tissues such as Marfan's disease, Ehlers–Danlos syndrome and Noonan syndrome. Pectus excavatum is characterized by the presence of deep sternal depression causing the ribs on each side to protrude more anteriorly than the sternum. The sternal and cartilaginous depression causes a reduction in the pre-vertebral space, which gives rise to leftward displacement and axial rotation of the heart. Other features that can be seen on chest radiographs include an indistinct right heart border, decreased density of the heart, horizontal posterior ribs and vertical anterior ribs (Figure 1). Although pectus excavatum is usually detected clinically, CT may be used to quantify the severity of the deformity, especially when surgical intervention is being considered (Figures 2 and 3). Extreme cases of pectus excavatum with an eccentric chest depression to the right are known as the “Grand Canyon” deformity, and this is associated with a higher post-operative complication rate (Figure 4). Severity may be graded using the Haller index, which is a ratio of the maximum internal transverse diameter of the chest divided by the minimum anteroposterior diameter (Figure 5). Haller et al2 have suggested that an index of >3.25 would require surgical correction. In normal children, the Haller index ranges from 1.9 to 2.7, with the Haller index for children under 2 years of age being markedly lower than that in older children. Females between the ages of 0–6 and 12–18 years may have a higher index than their male counterparts. The phase of the respiratory cycle may affect the Haller index, with inspiration yielding a value significantly lower than that of the expiratory phase.
Figure 1.
A 24-year-old female patient being considered for surgical correction of pectus excavatum. Three consecutive radiographs demonstrate subsequent corrective surgeries. (a) Pre-operative chest radiograph. (b) The patient initially underwent a Nuss procedure. Note the post-operative right-sided pneumothorax and subcutaneous surgical emphysema. (c) She subsequently needed the Nuss bar removed, which was then exchanged for a Ravitch bar.
Figure 2.
(a) Pre-operative axial CT of the same 24-year-old patient demonstrating pectus excavatum. Note the sternal depression and tilting, with leftward displacement of the heart. (b) Sagittal reconstructions.
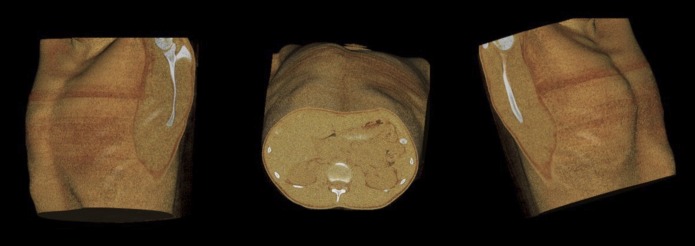
Figure 3.
Pre-operative three-dimensional surface reconstructions of the same 24-year-old patient showing sternal depression and tilting.
Figure 4.

Grand Canyon pectus excavatum three-dimensional cutaway.
Figure 5.
The Haller index of the same 24-year-old patient (ratio of A to B).
Pectus excavatum is easily diagnosed in childhood but is commonly ignored. Recent literature suggests that many patients experience detrimental cardiovascular and respiratory physiological changes as they mature. The exact reason remains elusive but may be due to decreasing chest wall compliance with increasing age. Pectus excavatum correction may improve the quality of life in patients, both physically and psychosocially. Surgeons may delay a repair until well after the pubertal growth spurt, to increase the chances of successful repair. This could be assessed by measuring patients' height until it plateaus. If the patient is severely affected psychosocially, the operation may be brought forward after discussion.
The two most common surgical procedures used to correct pectus excavatum are the Nuss procedure and the modified Ravitch procedure. The Nuss procedure is also known as minimally invasive repair of pectus excavatum.
Minimally invasive repair of pectus excavatum involves implanting a curved metallic bar retrosternally through small lateral incisions (Figures 6 and 7). This allows immediate correction of the deformity in childhood, since chest wall structures are less rigid. The procedure is less popular in adults, who require more force due to a more rigid chest wall, leading to more complications. The Nuss bar, also known as a strut, is shaped intraoperatively to fit the deformity of each patient. The strut is inserted in a concave-up position. The Nuss bar is usually removed several years after placement. The main advantage of the Nuss procedure is cosmetic requiring smaller skin incisions.
Figure 6.

CT of a 37-year-old male patient with a Nuss bar deep in to the sternum.
Figure 7.

Three-dimensional reconstructions of the same 37-year-old patient with a Nuss bar.
The original Ravitch procedure dates back to 1949 and involves resection of all sternal attachments and the whole length of the deformed costal cartilages. The sternum is then bent sharply anteriorly, fracturing the posterior cortical lamella in the process. The original technique had a high rate of recurrence due to a lack of sternal stabilization. This technique has since been modified in various ways, to provide sternal stabilization with less extensive cartilage resections. The modern Ravitch procedure usually supports the chest wall with metal bar implantations.
There is conflicting evidence regarding the outcomes of both techniques. Nasr et al3 performed a systematic analysis, which did not identify any differences in the overall complication rates and length of hospital stay. However, the study did conclude that the rates of reoperation, post-operative haemothorax and pneumothorax were higher in the Nuss group (odds ratio of 5.68, 1.57 and 5.60, respectively, with p ≤ 0.05).
At our institution, along with lung function tests, including dynamic lung volumes and flow volume loop, we routinely perform pre-operative chest MRI to rule out any signs of Marfan's, with evaluation of the aortic arch. Transthoracic echocardiograms are used to assess valve anatomy; if this investigation is not satisfactory, a transoesophageal echocardiogram is performed.
Chest radiographs are useful to visualize the position of the Nuss and Ravitch bars and to detect complications related to bar slippage. Some institutions perform lateral chest radiographs, although we find these to be of limited value and hence not routinely performed. Immediate post-operative complications include transient pleural effusions, wound infections, haematoma, bar migration and pneumothorax. Delayed complications such as bar migrations can be identified on chest radiographs and CT (Figures 1b and Figures 8–10).
Figure 8.
(a) Pre-operative CT of a 21-year-old female patient. (b) Post-modified Ravitch procedure CT. The right anterior end of the Ravitch bar had migrated inferiorly and invaginated the lung parenchyma. The bar was subsequently removed.
Figure 10.

Three-dimensional surface reconstruction of the same 51-year-old patient with migration of the Ravitch bar.
Figure 9.
51-year-old male with a previous modified Ravitch procedure presented with chest tightness. An initial chest radiograph demonstrated migration of the Ravitch bar inferiorly and eccentric to the right.
In the immediate post-operative period, a baseline radiograph is taken on the same day if a chest drain is not present; otherwise, it is taken on either the same day or the following day. A radiograph is taken after the drain is removed. A pneumothorax is not a common complication, but if it is present, it is usually treated conservatively. Further radiographs may be necessary depending on the patient's clinical status. All patients are seen at 6 weeks to assess their pain control, and a follow-up radiograph is taken then. Patients who have undergone the Nuss procedure are followed up with yearly radiographs for 2 years, before bar removal. Those who have undergone the modified Ravitch procedure will not have further chest radiographs, even up till bar removal in 8–12 months' time (Figure 11). Post-operatively, lung function tests and echocardiograms are performed to look for improvement in the cardiorespiratory functional status.
Figure 11.
Chest radiograph of a patient after removal of the Ravitch bar. His sternum was transected horizontally as demonstrated by his sternotomy wires.
PECTUS CARINATUM
Pectus carinatum is colloquially referred to as “pigeon chest”. It is the second most common chest wall congenital deformity, although much less common than pectus excavatum. Its incidence varies in literature, approximately 1 in 1000 with a predilection for males.4 It is commonly undertreated and seen as a cosmetic deformity only, despite the potential to invoke psychosocial issues and low self-esteem. It is characterized by convex anterior protrusion of the sternum and costochondral joints and is believed to be the result of a disproportionate overgrowth of the costochondral cartilages. The chest radiograph is often unremarkable (Figure 12), and CT can further demonstrate the chest wall features (Figure 13). There is an association with family history, connective tissue disease and scoliosis. Pectus carinatum can be classified into the chondrogladiolar or chondromanubrial types. The former involves protrusion of the gladiolus or sternal body and can be asymmetric. The latter type is rare and involves protrusion of the manubrium. Initial lateral chest radiographs will demonstrate sternal protrusion, but subsequent imaging with CT allows quantification of the severity using the Haller index, as in pectus excavatum. In pectus carinatum, a lower index indicates more severe deformity. A study quoted the average Haller index of patients requiring surgical correction as 1.8.5 Surgical correction is reserved for moderate or severe deformities and involves the Ravitch and Nuss procedures modified appropriately to correct the protruding defect. Recent literature has shown the increasing popularity of compressive bracing as a non-invasive alternative, especially in children whose chest wall is still pliable and growing.
Figure 12.
Radiograph of a patient with pectus carinatum, which is unremarkable.
Figure 13.
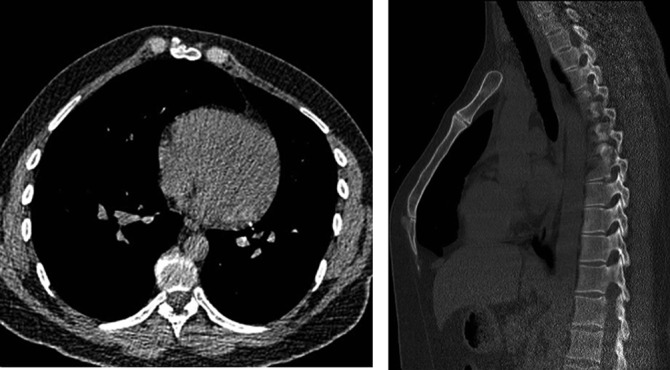
Axial and sagittal CT reconstructions of the same patient with pectus carinatum.
PECTUS ARCUATUM
Pectus arcuatum, or “wave-like chest”, is a rare condition with an unknown aetiology. The term is used to describe mixed deformities which contain both excavatum and carinatum either along the longitudinal or axial axis and is also known as a pouter pigeon chest (Figures 14 and 15). It involves a protrusion at the upper part of the sternum involving the manubriosternal junction and the second to fifth rib cartilages, with premature sternal ossification. There may be an associated excavatum deformity at the lower sternum in up to one-third of cases. The pouter pigeon chest can be appreciated on sagittal CT images although traditionally lateral chest radiography has been used. Imaging with CT allows calculation of the angle of Louis in order to determine the severity of the deformity. The normal angle of Louis is between 145° and 175°. Surgical correction is recommended in patients with an angle of 130°.
Figure 14.

A 24-year-old female patient with mixed deformity consisting of both pectus excavatum and carinatum, creating a “rolling” appearance.
Figure 15.
Three-dimensional reconstructions of the same 24-year-old female with asymmetrical pectus carinatum.
Surgical correction involves a wide wedge transverse sternotomy at the angle of Louis and subperichondrial resection of the adjacent costal cartilages. Like pectus carinatum, there is increasing popularity to use orthoptic bracing as a non-invasive alternative.
POLAND SYNDROME
Poland syndrome is a non-genetic congenital abnormality and the aetiology is unknown. The most popular theory is that it is due to hypoplasia of the subclavian artery. It occurs in 1 : 7000 to 1 : 100,000 live births.6 It is characterized by partial or total absence of the pectoral muscles and is most commonly unilateral. There is a range of breast involvement, varying from mild hypoplasia to complete absence (amastia). The nipple–areolar complex is usually affected. Poland syndrome is associated with rib cage anomalies, with up to 60% of cases including aplasia or hypoplasia of the ribs.7 Other associations include hand involvement (varying from mild shortening of the phalanges to syndactyly), lung herniation and dextrocardia.
On chest radiography, the affected side appears to have increased transradiancy due to reduction in volume of the overlying soft tissues. The differentials of this appearance are wide, including rotation, endobronchial foreign bodies, bullous diseases, Swyer–James syndrome and diaphragmatic hernia. Sometimes, this may not always be apparent on chest radiographs (Figure 16), and CT is useful to demonstrate the absence of the greater pectoral muscle and any associated musculoskeletal anomalies of the chest wall (Figure 17). Surgical correction is only required if there is a rib defect large enough to cause a lung herniation or if there are concerns of injury to the heart or lungs. Adolescent females with amastia may also require cosmetic reconstruction. Some surgeons now employ a laparoscopic omental flap reconstruction to improve breast symmetry, reducing recovery time and complication rates.
Figure 16.
Chest radiograph of a patient with Poland's syndrome with an unremarkable chest radiograph.
Figure 17.

Maximum intensity projection image of the same patient now demonstrating Poland's syndrome. In addition to the absence of right sided pectoral muscles, there is also abnormal sternal protrusion.
CHEST WALL HYPOPLASIA
There is a wide range of chest wall hypoplasia due to thoracospinal growth asymmetry and developmental arrest, which causes spinal scoliosis when focal. Many conditions are eponymously named and are associated with a range of cardiac defects and distal limb deformities. With advancements in genetics, there is now a better understanding of the pattern of inheritance with these conditions. An example of such an eponymous condition is Jeune syndrome, otherwise known as asphyxiating thoracic dystrophy. It is a very rare symmetrical deformity, recessively inherited with an incidence of between 1 in 100,000 and 130,000 live births.8 Patients have either a uniformly narrow thorax or a bell-shaped thorax (Figure 18). Chest wall rigidity causes respiratory restriction and subsequent recurrent infections, with the short ribs and dysplastic costochondral junctions of a bell-shaped thorax causing more severe symptoms. Most patients do not usually survive beyond 2 years of age, with morbidity mostly attributed to associated renal deformities. Management mainly consists of supportive ventilation, and surgical options include lateral thoracic expansions, distraction sternoplasty, thoracoplasty and a modified Nuss procedure. Those who survive beyond infancy are then closely monitored for respiratory complications and other comorbidities.
Figure 18.
Three-dimensional reconstruction of the bell-shaped thorax of a neonate with Jeune syndrome.
RIB OSTEOCHONDROMAS
Osteochondromas or exostoses are benign bone lesions which result from a segment of epiphyseal growth plate which has been separated from the main epiphysis. These bony spurs can be solitary or associated with multiple exostoses. Patients with multiple hereditary exostoses may have coexisting rib osteochondromas. Exostoses projecting outwards may be palpable, whereas internal exostoses may be an incidental finding on imaging only (Figures 19 and 20). They may be completely asymptomatic and cause only cosmetic deformities but may cause complications such as spontaneous haemothorax, pneumothorax or lacerations to the diaphragm. Rarely, osteochondromas may degenerate into malignant chondrosarcoma.
Figure 19.

Right fifth rib exostosis directed into the thoracic cavity.
Figure 20.
Three-dimensional reconstruction of the same patient with a right fifth rib bony exostosis.
NORMAL VARIATION
There are normal asymptomatic variations of the anterior chest wall that may be incidental on imaging. Donnelly et al9 estimated this to be as common as 30% of children on retrospective review of thoracic CT studies obtained for other reasons. These deformities may include a tilted sternum, convex anterior ribs or prominent costal cartilages. Clinicians should be cautioned against overzealous imaging. In a further study by Donnelly et al10 of 27 children who underwent cross-section imaging for palpable, asymptomatic anterior chest wall lesions, none needed further intervention.
CONCLUSION
There are a variety of congenital conditions affecting the chest wall which commonly present at birth or in childhood, the commonest being pectus excavatum and carinatum. We have demonstrated the characteristic radiographic and CT appearances of these conditions. In our institution cross-sectional imaging with CT using low-dose techniques is reserved for patients being considered for corrective surgery.
Contributor Information
Sze M Mak, Email: makszemun@doctors.org.uk.
Basrull N Bhaludin, Email: drbhaludin@gmail.com.
Sahar Naaseri, Email: naaseri@doctors.org.uk.
Francesco Di Chiara, Email: franzdik2501@gmail.com.
Simon Jordan, Email: s.jordan@rbht.nhs.uk.
Simon Padley, Email: s.padley@imperial.ac.uk.
REFERENCES
- 1.Fokin AA, Steuerwald NM, Ahrens WA, Allen KE. Anatomical, histologic, and genetic characteristics of congenital chest wall deformities. Semin Thorac Cardiovasc Surg 2009; 21: 44–57. doi: 10.1053/j.semtcvs.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 2.Haller JA, Jr, Kramer SS, Lietman SA. Use of CT scans in selection of patients for pectus excavatum surgery: a preliminary report. J Pediatr Surg 1987; 22: 904–6. doi: 10.1016/S0022-3468(87)80585-7 [DOI] [PubMed] [Google Scholar]
- 3.Nasr A, Fecteau A, Wales PW. Comparison of the Nuss and the Ravitch procedure for pectus excavatum repair: a meta-analysis. J Pediatr Surg 2010; 45: 880–6. doi: 10.1016/j.jpedsurg.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 4.Coelho MS, Guimaraes PS. Pectus carinatum. [In Portuguese.] J Bras Pneumol 2007; 33: 463–74. doi: 10.1590/S1806-37132007000400017 [DOI] [PubMed] [Google Scholar]
- 5.Fonkalsrud EW. Open repair of pectus excavatum with minimal cartilage resection. Ann Surg 2004; 240: 231–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freitas Rda S, o Tolazzi AR, Martins VD, Knop BA, Graf RM, Cruz GA. Poland's syndrome: different clinical presentations and surgical reconstructions in 18 cases. Aesthetic Plast Surg 2007; 31: 140–6. [DOI] [PubMed] [Google Scholar]
- 7.Goretsky MJ, Kelly RE, Jr, Croitoru D, Nuss D. Chest wall anomalies: pectus exacavatum and pectus carinatum. Adolesc Med Clin 2004; 15: 455–71. doi: 10.1016/j.admecli.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 8.Oberklaid F, Danks DM, Mayne V, Campbell P. Asphyxiating thoracic dysplasia. Clinical, radiological, and pathological information on 10 patients. Arch Dis Child 1977; 52: 758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnelly LF, Frush DP, Foss JN, O'Hara SM, Bisset GS, 3rd. Anterior chest wall: Frequency of anatomic variations in children. Radiology 1999; 212: 837–40. doi: 10.1148/radiology.212.3.r99se16837 [DOI] [PubMed] [Google Scholar]
- 10.Donnelly LF, Taylor CN, Emery KH, Brody AS. Asymptomatic, palpable, anterior chest wall lesions in children: is cross-sectional imaging necessary? Radiology 1997; 202: 829–31. doi: 10.1148/radiology.202.3.9051041 [DOI] [PubMed] [Google Scholar]