Abstract
Objective:
To study the potential nephroprotective role of agomelatine in rat renal tissue in cases of contrast-induced nephrotoxicity (CIN). The drug's action on the antioxidant system and proinflammatory cytokines, superoxide dismutase (SOD) activity, levels of glutathione (GSH) and malondialdehyde (MDA) and the gene expression of interleukin-6 (IL-6), tumour necrosis factor (TNF)-α and nuclear factor kappa B (NF-κB) was measured. Tubular necrosis and hyaline and haemorrhagic casts were also histopathologically evaluated.
Methods:
The institutional ethics and local animal care committees approved the study. Eight groups of six rats were put on the following drug regimens: Group 1: healthy controls, Group 2: GLY (glycerol), Group 3: CM (contrast media—iohexol 10 ml kg−1), Group 4: GLY+CM, Group 5: CM+AGO20 (agomelatine 20 mg kg−1), Group 6: GLY+CM+AGO20, Group 7: CM+AGO40 (agomelatine 40 mg kg−1) and Group 8: GLY+CM+AGO40. The groups were evaluated by one-way analysis of variance and Duncan's multiple comparison test.
Results:
Agomelatine administration significantly improved the serum levels of blood urea nitrogen (BUN) and creatinine, SOD activity, GSH and MDA. The use of agomelatine had substantial downregulatory consequences on TNF-α, NF-κB and IL-6 messenger RNA levels. Mild-to-severe hyaline and haemorrhagic casts and tubular necrosis were observed in all groups, except in the healthy group. The histopathological scores were better in the agomelatine treatment groups.
Conclusion:
Agomelatine has nephroprotective effects against CIN in rats. This effect can be attributed to its properties of reducing oxidative stress and inhibiting the secretion of proinflammatory cytokines (NF-κB, TNF-α and IL-6).
Advances in knowledge:
CIN is one of the most important adverse effects of radiological procedures. Renal failure, diabetes, malignancy, old age and non-steroidal anti-inflammatory drug use pose the risk of CIN in patients. Several clinical studies have investigated ways to avoid CIN. Theophylline/aminophylline, statins, ascorbic acid and iloprost have been suggested for this purpose. Agomelatine is one of the melatonin ligands and is used for affective disorders and has antioxidant features. In this study, we hypothesized that agomelatine could have nephroprotective, antioxidant and anti-inflammatory effects against CIN in rats.
INTRODUCTION
Contrast-induced nephrotoxicity (CIN) is the worsening of renal function (an increase in levels of creatinine by more than one-fourth or 44 μmol l−1) that occurs within 72 h after the administration of a contrast material in the absence of any other aetiology.1 Although its incidence has been reported to be low,2 it is one of the most important adverse effects of radiological procedures3 and is related to an increase in morbidity and mortality.4 This iatrogenic complication following the administration of contrast materials is a common cause of hospital-acquired renal insufficiency and accounts for about 11% of cases,5 occurring during diagnostic and interventional procedures.6 Renal failure, diabetes, malignancy, old age and non-steroidal anti-inflammatory drug use have all been found to be associated with a higher risk of CIN.2 CIN occurs more commonly in patients with underlying non-symptomatic kidney pathologies. It has become the third leading cause of hospital-acquired acute renal failure with the increasing use of contrast media (CM) for diagnostic and interventional procedures. In this regard, it is important to thoroughly investigate this issue in animal models.
Pre-treatment with glycerol can be used to induce pre-existing renal damage in rats.7 By administering glycerol, we induced CIN in the rats to mimic any underlying pathology. Iohexol is a low-osmolar non-ionic radiocontrast agent that has improved safety and tolerability compared with classic radiocontrast agents. For these reasons, iohexol became the most common radiocontrast agent used in clinical and experimental “contrast-induced nephropathy” research.8,9 We therefore chose to use this contrast agent in our experimental model.
Several clinical studies have investigated ways to avoid CIN. Drugs such as theophylline/aminophylline, statins, ascorbic acid and iloprost have been suggested to be helpful in reducing the occurrence of this condition; nevertheless, a detailed evaluation of their impact is needed.10–13
Agomelatine is a melatonergic receptor agonist and a selective serotonin 5HT2C receptor antagonist that has shown antidepressant efficacy. Melatonin receptor type (MT)1 and MT2 receptors are predominating in the human foetal kidney cortex area and have been disclosed as renoprotective agents, as they are generally attributed to have the debated antioxidant capability.14,15 Melatonin has several applications related to the renal system and its production is impaired in chronic renal failure and it cannot protect the kidneys from inflammation which can cause renal damage.16,17 In this context, to our knowledge, there has not been a study to date on the nephroprotective role of agomelatine for CIN aggravated by glycerol in rats.
In this study, we aimed to investigate the possible nephroprotective role of agomelatine. To appreciate the relationship between the nephroprotective action of agomelatine, the antioxidant system and proinflammatory cytokines, we measured the superoxide dismutase (SOD) activity, levels of glutathione (GSH) and malondialdehyde (MDA) and the gene expression of interleukin-6 (IL-6), tumour necrosis factor (TNF)-α and nuclear factor kappa B (NF-κB) in the renal tissue of rats with CIN.
METHODS AND MATERIALS
The study protocol was approved by the institutional ethics and local animal care committees. Animal experimentations and trials were implemented in a manner that was consistent with the national guiding principles.
Animals
In total, 48 female albino Wistar rats (250–300 g) were used in the study. They were obtained from our university experimental animal laboratory. The rats were stored in typical plastic cages on sawdust bedding in a well-ventilated room at 22°C under precise light conditions (14/10 h light/dark cycle). Standard rat chow and tap water were provided ad libitum.
Chemicals
Clinical substances were obtained from Sigma Chemical Company (Germany), agomelatine (Valdoxan® 25 mg, 28 tablets) was obtained from Les Laboratories Servier (Istanbul, Turkey), iohexol (Omnipaque™ 300 mgI ml−1, 100 ml) from Opakim Medical Products (Istanbul, Turkey) and 100% glycerol from Bikar (Istanbul, Turkey).
Experimental design
Eight sets of six rats in separate cages were administered the following drugs:
Group 1: healthy control group
Group 2 (GLY): glycerol only
Group 3 (CM): contrast media (iohexol 10 ml kg−1)
Group 4 (GLY+CM): glycerol + contrast media (iohexol 10 ml kg−1)
Group 5 (CM+AGO20): contrast media (iohexol 10 ml kg−1) + agomelatine 20 mg kg−1 (po)
Group 6 (GLY+CM+AGO20): glycerol + contrast media (iohexol 10 ml kg−1) + agomelatine 20 mg kg−1 (po)
Group 7 (CM+AGO40): contrast media (iohexol 10 ml kg−1) + agomelatine 40 mg kg−1 (po)
Group 8 (GLY+CM+AGO40): glycerol + contrast media (iohexol 10 ml kg−1) + agomelatine 40 mg kg−1 (po).
The contrast media-induced nephrotoxicity model
Kidney injury was induced in Groups 2, 4, 6 and 8 by intramuscular administration of 25% glycerol as a single dose of 10 ml kg−1 body weight after 24-h water deprivation. The dose was divided among the legs. After glycerol injection, drinking water was resumed ad libitum.
24 h after glycerol injection, nephrotoxicity was induced with intravenous administration of iohexol (contrast media, CM) as a single dose of 10 ml kg−1 body weight over a period of 2 min to the animals in Groups 3–8 via the tail vein.8
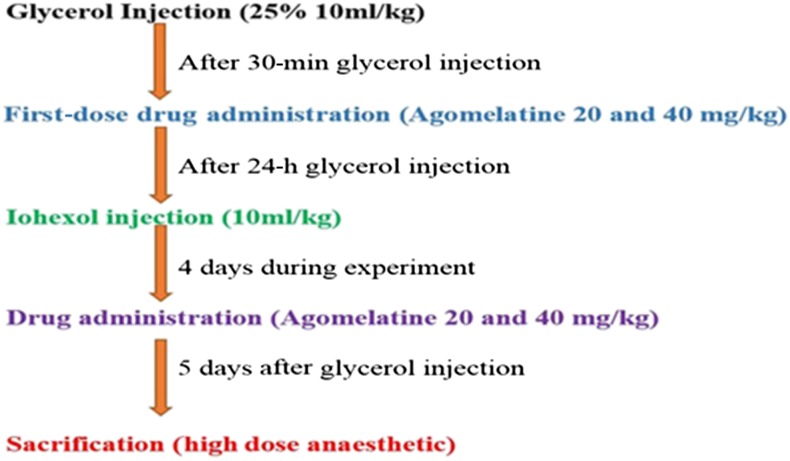
30 min after glycerol injection, agomelatine (dissolved in isotonic sodium chloride solution) was administered orally to the animals in Groups 5–8. Agomelatine administration was repeated on the second, third and fourth days (Figure 1).
Figure 1.
Summary of experimental procedures.
Following the drug administration period, the study was finished on the fifth day after glycerol injection by an overdose of a general anaesthetic (thiopental sodium, 50 mg kg−1). Under anaesthetic conditions, blood samples were obtained by intracardiac method. Kidney tissues were dissected out immediately. Half of one kidney was kept at −80°C for biochemical evaluation; the rest was kept in an RNA stabilization reagent for molecular analysis, and the other kidney was fixed in formalin for pathological examination. Serum was instantly separated by centrifugation at 4000 rpm for 10 min at 4°C and stored at −80°C until it was analysed.
Determination of serum BUN and creatinine concentrations
Serum blood urea nitrogen (BUN) (Lot No: B0382A, S.R.I, Italy) and creatinine (Lot No: B0914A, S.R.I, Italy) levels were detected using commercially available kits. All analyses were performed in a ChemWell® 2910 Automated EIA and Chemistry analyzer (Awareness Technology®, Inc., Palm City, FL).
Biochemical examination of kidneys
After surgery, the tissues were stored at −80°C. All samples were first perfused with PBS/heparin and then pulverized in liquid nitrogen using a TissueLyser II (Qiagen, Ankara, Turkey) grinding jar set. Nearly 100 mg of the pulverized tissue was homogenized in 1 ml of PBS homogenate buffer in an Eppendorf tube® using the TissueLyser II. Samples were then centrifuged. SOD,18 GSH19 and MDA20 levels from the supernatant of each sample and standard were measured at room temperature in duplicate via modified methods with an enzyme-linked immunosorbent assay reader. The average absorbance of each sample and standard was calculated. A standard curve was plotted, and the equation was obtained from the absorbance of the standards. The linear SOD, GSH and MDA concentrations were calculated according to this equation. The SOD, GSH and MDA levels in the tissues were expressed as U mg−1 protein, nmol mg−1 protein and nmol mg−1 protein, respectively. Obtained data were presented as mean ± standard deviation from 1 mg of protein.
Molecular investigations of kidney tissues
Total RNA extraction and cDNA synthesis
Tissues (20 mg) were briefly stabilized in an RNA stabilization reagent (RNAlater®; Qiagen, Hilden, Germany) and then disrupted using the TissueLyser II (2 × 2 min for kidney tissue; 2 × 5 min, Qiagen). Total RNA was purified in Qiacube using the RNeasy Mini Kit (Qiagen) according to manufacturer instructions. The RNA samples were reverse transcribed into complementary DNA (cDNA) using a high-capacity cDNA reverse transcription kit (Applied Biosystem®; Hernakim, Istanbul, Turkey). From 10 μl, total RNA was treated with 2 μl 10× RT buffer, 0.8 μl 25× dNTPs mix, 2 μl 10× RT Random Primers, 1 μl MultiScribe® reverse transcriptase and 4.2 μl DEPC-H2O. Reverse transcription was carried out at 25°C for 10 min, followed by 120 min at 37°C and finally 5 min at 85°C using a Veriti® 96-well thermal cycler (Applied Biosystem). The cDNA concentration and quality were assessed and quantified using the Epoch™ Spectrophotometer System and Take3 plate (Bio Tek®;Pera Medikal, Istanbul, Turkey).
Relative quantification of gene expression
Relative TNF-α, IL-6 and NF-κB expression analyses were performed with StepOne Plus™ Real Time PCR System technology (Applied Biosystems) using synthesized cDNA from rat kidney RNAs. A quantitative polymerase chain reaction (qPCR) was run using a TaqMan® probe mix based on the TaqMan probe-based technology (Applied Biosystems). A real-time PCR was performed using primers generated for rat TNF-α (Rn00562055_m1), rat IL-6 (Rn01410330_m1), rat NF-κB (Rn01399583_m1) and rat β-actin (Rn00667869_m1). The results are expressed as relative-fold, compared with control groups. Expression data for β-actin in each tissue were used as endogenous controls. For each tissue, triplicate determinations were performed in a 96-well optical plate for both targets using 9 μl of cDNA (100 ng), 1 μl of Primer Perfect Probe mix and 10 μl of QuantiTect® Probe polymerase chain reaction (PCR) Master mix (Qiagen) in each 20-μl reaction. The plates were heated for 2 min at 50°C and for 10 min at 95°C. Subsequently, 40 cycles of 15 s at 94°C and cycles of 60 s at 60°C were applied. All data are expressed as fold change in expression, compared with the expression in other animal groups, using the 2−ΔΔCt method.21
Histological procedure
Kidneys from the rats in all groups were obtained, sectioned frontally and fixed in 10% neutral formalin for 48–72 h. Tissues were then processed routinely and embedded in paraffin wax, from which 4–5-μm-thick serial sections were cut. All tissue sections were stained with haematoxylin and eosin for histopathology and periodic acid–Schiff (PAS) stain for basement membrane assessment and examined under a light microscope (BX51, Olympus, Japan). For histopathological assessment, tubular necrosis, hyaline casts and haemorrhagic casts were evaluated and scored independently by a pathologist blinded to the experiment. A minimum of five fields for each kidney slide at 100× magnification was evaluated and assigned scores for severity of changes as follows: Grade 0: − (negative), Grade 1: +1 (mild), Grade 2: +2 (moderate), Grade 3: +3 (severe) and Grade 4: +4 (most severe). The other sections were stored for assessing the immunohistochemistry.
Statistical analysis
For statistical analysis, we used SPSS® v. 20.0 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). Results are presented as means ± standard deviation. Comparisons between groups were performed using one-way analysis of variance and Duncan's multiple comparison test. Significance was accepted at p < 0.05.
RESULTS
Serum concentrations of BUN and creatinine
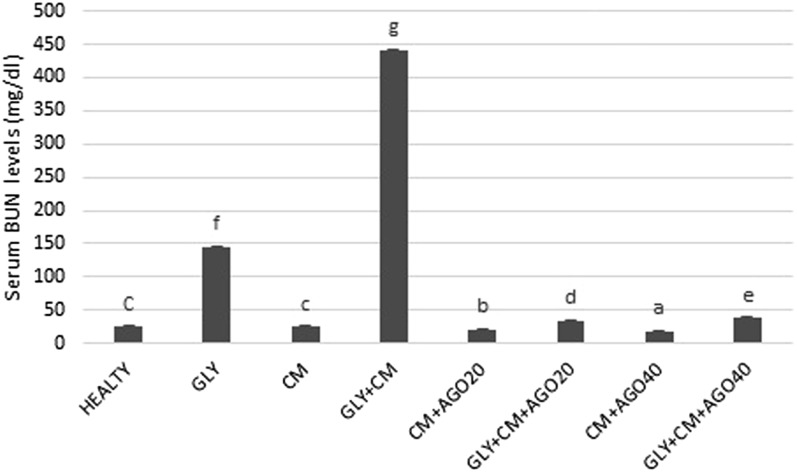
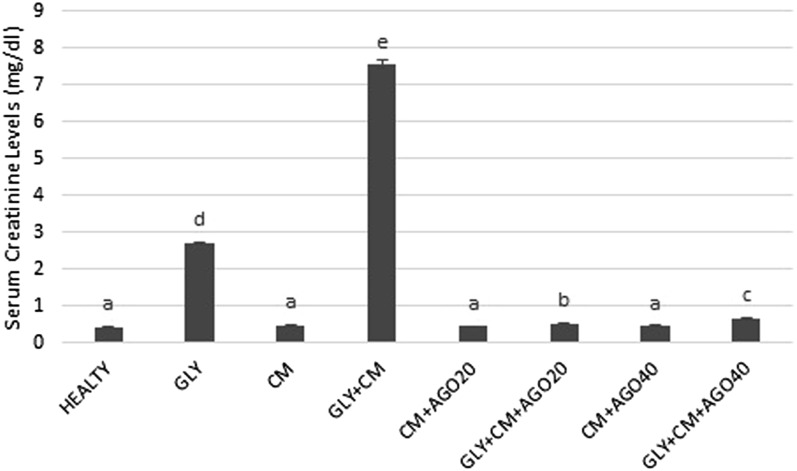
It was observed that BUN and creatinine serum levels significantly increased in the rats with GLY+CM-induced nephrotoxicity compared with the other groups (p < 0.05). Agomelatine administration in groups of 5–8 significantly decreased the activity of the serum enzymes compared with the GLY+CM group (p < 0.05). The changes in the serum BUN and creatinine levels are shown in Figures 2 and 3, respectively.
Figure 2.
The serum blood urea nitrogen (BUN) levels (mg dl−1) of all the experimental groups. Means in the same column with the same letter are not significantly different; means in the same column with different letters indicate significant differences between the groups. AGO20 (agomelatine 20 mg kg−1); AGO40 (agomelatine 40 mg kg−1); CM, contrast media; GLY (glycerol).
Figure 3.
The serum creatinine levels of all the experimental groups. Means in the same column with the same letter are not significantly different; means in the same column with different letters indicate significant differences between the groups. AGO20 (agomelatine 20 mg kg−1); AGO40 (agomelatine 40 mg kg−1); CM, contrast media; GLY (glycerol).
Biochemical results for kidney tissue
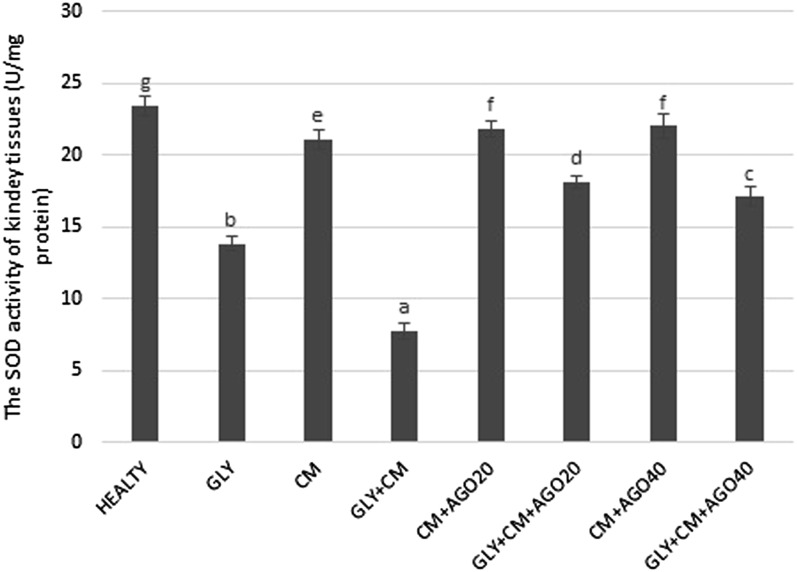
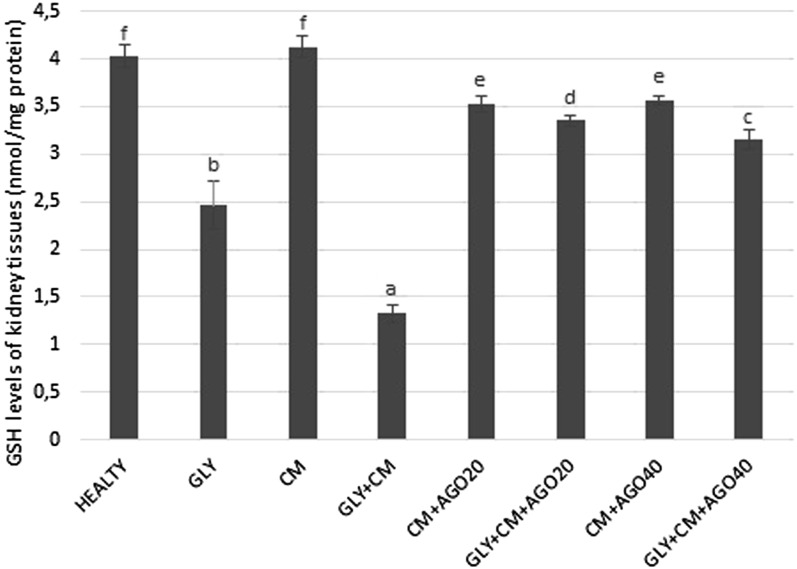
In the present study, SOD activity and levels of GSH and lipid peroxidation (MDA) were evaluated in all rat kidneys. SOD activity was significantly decreased in the GLY+CM group compared with that in the healthy group (p < 0.05). In addition, there were significant differences between the GLY and GLY+CM groups (p < 0.05). In the treatment groups, SOD activity was significantly improved compared with the GLY+CM group, as shown in Figure 4 (p < 0.05). GSH levels significantly decreased in the GLY+CM group compared with that in the healthy group (p < 0.05). There were also significant differences between the GLY and GLY+CM groups (p < 0.05). As seen in Figure 5, GSH levels were significantly ameliorated in the drug treatment groups when compared with that in the GLY+CM group (p < 0.05).
Figure 4.
The tissue superoxide dismutase (SOD) activities of all the experimental groups. Means in the same column with the same letter are not significantly different; means in the same column with different letters indicate significant differences between the groups. AGO20 (agomelatine 20 mg kg−1); AGO40 (agomelatine 40 mg kg−1); CM, contrast media; GLY (glycerol).
Figure 5.
The tissue glutathione (GSH) levels of all the experimental groups. Means in the same column with the same letter are not significantly different; means in the same column with different letters indicate significant differences between the groups. AGO20 (agomelatine 20 mg kg−1); AGO40 (agomelatine 40 mg kg−1); CM, contrast media; GLY (glycerol).
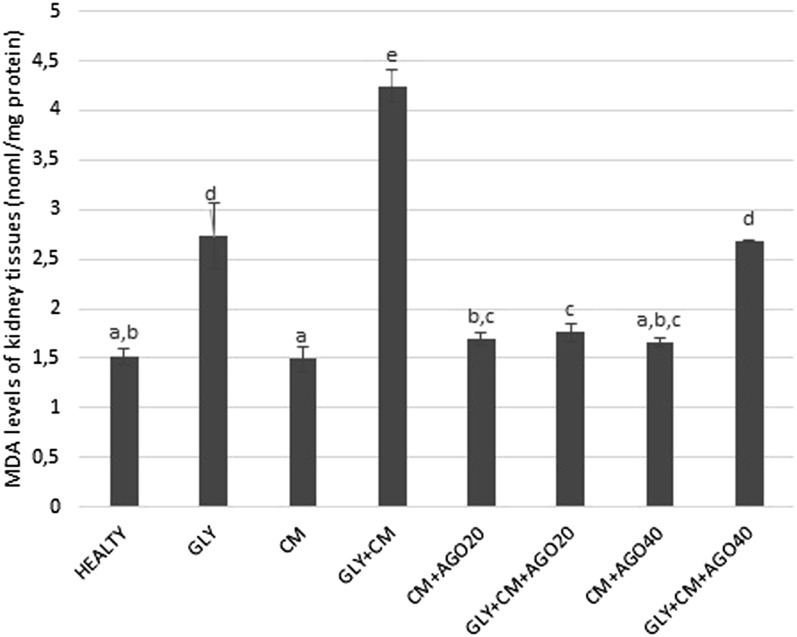
As shown in Figure 6, the MDA levels were significantly increased in the GLY+CM group compared with the healthy group (p < 0.05). In addition, there were significant differences between the GLY and GLY+CM groups (p < 0.05). The MDA levels in groups of 5–8 were significantly improved with the administration of agomelatine compared to the GLY+CM group (p < 0.05).
Figure 6.
The tissue malondialdehyde (MDA) levels of all the experimental groups. Means in the same column with the same letter are not significantly different; means in the same column with different letters indicate significant differences between the groups. AGO20 (agomelatine 20 mg kg−1); AGO40 (agomelatine 40 mg kg−1); CM, contrast media; GLY (glycerol).
Molecular results
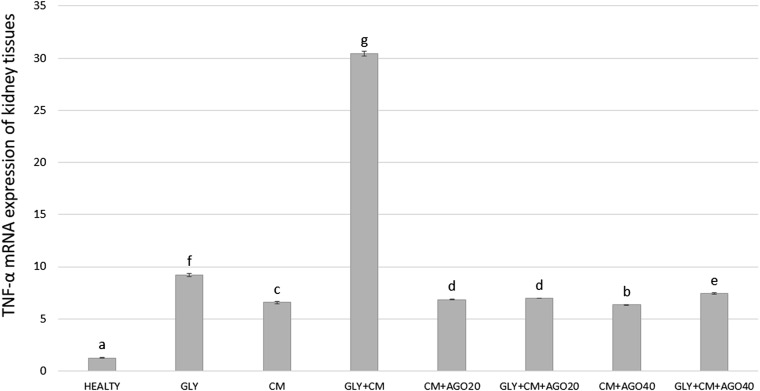
We investigated TNF-α, IL-6 and NF-κB messenger RNA (mRNA) expressions in the kidney tissue of rats using real-time PCR. As shown in Figure 7, TNF-α gene expression increased in the GLY+CM groups (30.46-fold) compared with that in the healthy group (p < 0.05). Agomelatine had a significant downregulatory effect on TNF-α mRNA expression, by 6.95-fold after 20 mg kg−1 doses and 7.40-fold for 40 mg kg−1 doses (p < 0.05).
Figure 7.
The tissue TNF-α messenger RNA (mRNA) expression of all the experimental groups. Means in the same column with the same letter are not significantly different; means in the same column with different letters indicate significant differences between the groups. AGO20 (agomelatine 20 mg kg−1); AGO40 (agomelatine 40 mg kg−1); CM, contrast media; GLY (glycerol).
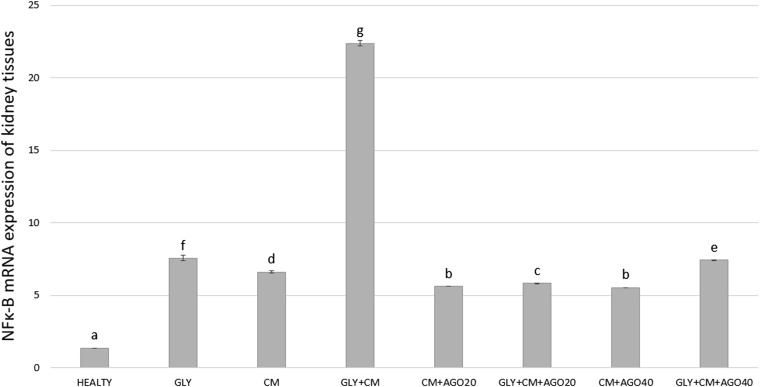
As shown in Figure 8, compared with the control group, the NF-κB mRNA level was significantly higher in the GLY+CM group, by 22.37-fold (p < 0.05). NF-κB expression in the rat kidney tissues decreased by 5.82-fold and 7.45-fold in the treatment groups compared with that in the control group to nearly healthy levels (p < 0.05).
Figure 8.
The tissue NFκ-β messenger RNA (mRNA) expression of all the experimental groups. Means in the same column with the same letter are not significantly different; means in the same column with different letters indicate significant differences between the groups. AGO20 (agomelatine 20 mg kg−1); AGO40 (agomelatine 40 mg kg−1); CM, contrast media; GLY (glycerol).
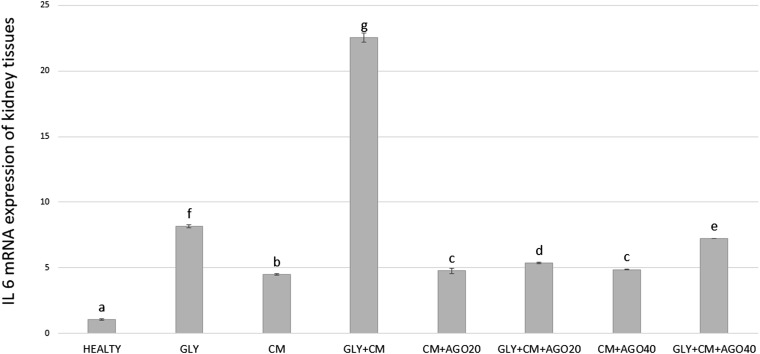
Compared with the control group, the IL-6 mRNA level was significantly higher (22.55-fold; p < 0.05) in the GLY+CM groups (Figure 9). Agomelatine had a significant downregulatory effect on IL-6 mRNA expression in the treatment groups when compared with the GLY+CM group (5.36- and 7.21-fold, respectively; p < 0.05).
Figure 9.
The tissue IL-6 messenger RNA (mRNA) expression of all the experimental groups. Means in the same column with the same letter are not significantly different; means in the same column with different letters indicate significant differences between the groups. AGO20 (agomelatine 20 mg kg−1); AGO40 (agomelatine 40 mg kg−1); CM, contrast media; GLY (glycerol).
Histopathological results
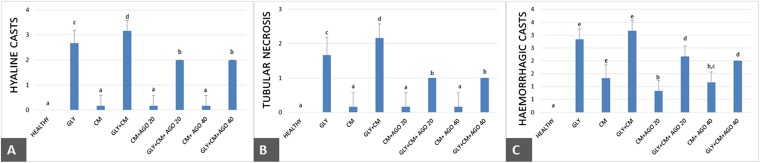
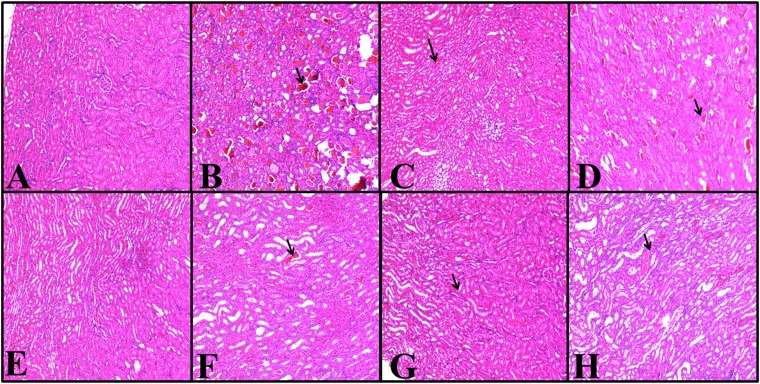
The histopathology of the kidneys of the animals in all groups was examined and scored; the results are given in Figure 10. Rats in the healthy group did not have any histopathological lesions (Figure 11), but severe lesions were seen in both the cortex and medulla of the glycerol-treated rats. Mild-to-severe hyaline and haemorrhagic casts and tubular necrosis were observed in all groups, except in the healthy group (Figure 11). The histopathological scores were better in the agomelatine treatment groups when compared with that in the GLY+CM groups.
Figure 10.
(a–c) Effects of agomelatine on histopathological hyaline cast (a) tubular necrosis (b) and haemorrhagic cast (c) scores in rats' kidneys. Means in the same column by the same letter are not significantly different from Duncan's multiple comparison test (p = 0.05). Results are expressed as means ± SD (n = 6). AGO20 (agomelatine 20 mg kg−1); AGO40 (agomelatine 40 mg kg−1); CM, contrast media; GLY (glycerol); SD, standard deviation.
Figure 11.
(a–h) Haematoxylin and eosin results in rats' kidney tissues; magnification 100×. Healthy group (a), GLY (glycerol) group (b), CM (contrast media) group (c), GLY+CM group (d), CM+AGO20 (agomelatine 20 mg kg−1) group (e), GLY+CM+AGO20 group (f), CM+AGO40 (agomelatine 40 mg kg−1) group (g) and GLY+CM+AGO 40 group (h). (a) Normal structure of the kidney in the control group. (b) Hyaline and haemorrhagic casts are seen severely and moderately; tubular necrosis is also present. (c) Only haemorrhagic casts are seen moderately. Hyaline and tubular necrosis are observed mildly. (d) Haemorrhagic and hyaline casts are seen severely. Moderate tubular necrosis is present. (e) Only haemorrhagic casts are seen mildly. (f) Both hyaline and haemorrhagic casts are seen moderately. Tubular necrosis is also present mildly. (g) Only haemorrhagic casts are seen mildly. (h) Hyaline and haemorrhagic casts are seen moderately; tubular necrosis is observed mildly.
DISCUSSION
In our study, we focused on the restorative effects of agomelatine for rats with contrast-induced nephropathy; we found that agomelatine administration significantly decreased the elevated serum concentrations of both BUN and creatinine to nearly healthy levels. Biochemical results for SOD activity, GSH and MDA levels in the kidney tissue, as well molecular results for TNF-α, NF-κB and IL-6, showed the ameliorative effects of agomelatine on kidney function. Histological scores in terms of hyaline and haemorrhagic casts and tubular necrosis were also improved by the administration of agomelatine.
Three different pathophysiological mechanisms lead to CIN: the first being haemodynamic effects (renal medullary hypoxia); the second, the direct effects of the contrast medium molecule causing tubular cell toxicity; and the third being endogenous biochemical changes such as induction in oxygen-free radicals and/or a reduction in antioxidant enzyme activity. In addition, it is thought that activation of cytokine-induced inflammatory mediators by reactive free radicals may be a possible mechanism,22–27 and apoptosis may also play a part in the development of CIN.22,28 However, the main mechanism by which CIN develops is still unclear; so, in clinical examinations, a variety of ways of avoiding CIN has been investigated.
Agomelatine is a selective melatonergic (MT1/MT2) agonist and a 5-HT2C antagonist.29 It has been reported to have antioxidant,30 anti-inflammatory31 and memory-facilitating effects.32 Agonistic regulation of melatonin receptors has been said to improve locomotor activity and protect the cholinergic system,32 as well as attenuate mitochondrial oxidative damage.32 Melatonin has both lipophilic and hydrophilic properties. It can diffuse without difficulty into subcellular partitions and shield against free radical-mediated damage. The latest literature states that melatonin averts nephrotoxicity triggered by several drugs.33–38 In particular, Gazi et al39 demonstrated the defensive effect of melatonin administration in rat radiocontrast nephrotoxicity by reducing BUN and creatinine levels.40 However, these studies have not measured oxidative and molecular parameters to indicate whether regeneration of the kidneys occurred after injury. In the present literature, there is no study about CIN related to agomelatine.
Serum creatinine is the most frequently used parameter for evaluating kidney function, along with BUN.41 In previous studies, in which nephrotoxicity was created by glycerine and CM, increased levels of serum BUN and creatinine were shown in parallel with elevated oxidative stress and inflammation.7 In our study, we detected large increases in serum BUN and creatinine levels in the GLY and GLY+CM groups compared with the healthy group. We also showed that agomelatine, in both groups, ameliorated the serum BUN and creatinine levels of nephrotoxic rats. These decreases in the levels of serum BUN and creatinine, which are important clinical findings of CIN, suggested that agomelatine may have prevented CM-induced nephrotoxicity.
Another possible marker of CIN is oxidative stress. Changes in SOD activity, GSH and MDA levels develop owing to generation of free radicals.42 Glycerol, when injected intramuscularly, causes renal GSH depletion.43 Baykara et al42 in their study on propolis, a potential nephroprotective agent against a specific contrast agent (diatrizoate), claimed that propolis protects the renal tissue against diatriozate toxicity, free radicals and other adverse effects by restoring MDA, GSH and SOD levels. In another CIN study, decreases in SOD levels in nephrotoxicity groups increased with ebselen treatment.44 In cisplatin-induced nephrotoxicity, significantly increased MDA levels were observed in groups administered cisplatin.45 In this study, MDA levels dramatically increased in the GLY and GLY+CM groups compared with that in the healthy group. In parallel with this, SOD activity and GSH levels for the groups of 5–8 in the GLY and GLY+CM groups dramatically decreased. Agomelatine administration improved SOD activity and GSH levels compared with the GLY and GLY+CM groups. Similar antioxidant effects of agomelatine have been demonstrated in previous studies.46
The inflammatory process is a factor acting in the pathogenesis of nephrotoxicity. In this process, macrophages released due to inflammation increase the production of proinflammatory cytokines in addition to oxidant release.47–49 Previous studies demonstrated that contrast media administration led to increased levels of proinflammatory cytokines in the kidneys.43 IL-6 and TNF-α are associated with CIN severity 35,39 and it has been shown that radiocontrast media administration increases NF-κB.50 TNF-α is a proinflammatory cytokine that further recruits numerous mediators associated with tissue damage. In the animal model of nephrotoxicity, TNF-α is of great importance in the activation of inflammatory response.35 Another important inflammatory cytokine, IL-6, exhibits many physiological effects.36 Contrast media administration was shown to lead to an increase in the levels of IL-6 in the kidney,38 while TNF-α led to an increase in NF-κB.51,52
In our study, NF-κB upregulation, in parallel with contrast media and glycerol administration, correlated with the increasing levels of TNF-α and IL-6. However, doses of agomelatine caused significant downregulation of NF-κB, TNF-α and IL-6 expression, in comparison with GLY and GLY+CM groups. In a previous study, we demonstrated that agomelatine has an anti-inflammatory effect in cases of paracetamol hepatotoxicity; agomelatine administration also significantly improved TNF-α and IL-6 levels.46 The results of the present study indicate that agomelatine has a potential nephroprotective effect against CIN.
In various studies, tubular necrosis and epithelial injury have been shown to be significant indicators in nephrotoxicity injury;53 tubular epithelial damage, tubular necrosis, hyaline and haemorrhagic casts have been found in these investigations.54 In the development of nephropathy, significant damage to haemorrhagic renal tubules with hyaline occurs and is related to tubular injury. We determined positive effects of agomelatine administration on tubular structure.
In conclusion, our study showed that agomelatine has nephroprotective, antioxidant and anti-inflammatory effects against CIN in rats. This effect is brought about by a reduction in oxidative stress and inhibition of the secretion of proinflammatory cytokines (NF-κB, TNF-α and IL-6) that trigger the initiation of inflammation in the kidney. This animal model can be a reflection of CM-induced nephropathy in patients with underlying renal insufficiency. We can suggest that agomelatine can be a drug of choice for both radiologists and nephrologists before CM administration in patients with renal insufficiency. However, more detailed future clinical studies are required to better clarify the protective effects of agomelatine in CIN.
Contributor Information
Adem Karaman, Email: drkaraman77@yahoo.com.
Busra Diyarbakir, Email: busradiyarbakir@gmail.com.
Irmak Durur-Subasi, Email: irmakdurur@yahoo.com.
Duygu Kose, Email: duygum.46@hotmail.com.
Asli Özbek-Bilgin, Email: asli.ozbek.bilgin@hotmail.com.
Atilla Topcu, Email: atopcu@gmail.com.
Cemal Gundogdu, Email: cemalg@atauni.edu.tr.
Afak Durur-Karakaya, Email: afakdurur@yahoo.com.
Zafer Bayraktutan, Email: gbayraktutan@atauni.edu.tr.
Fatih Alper, Email: fatihrad@yahoo.com.
REFERENCES
- 1.Morcos SK, Thomsen HS, Webb JA. Contrast-media-induced nephrotoxicity: a consensus report. Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR). Eur Radiol 1999; 9: 1602–13. doi: 10.1007/s003300050894 [DOI] [PubMed] [Google Scholar]
- 2.Moos SI, van Vemde DN, Stoker J, Bipat S. Contrast induced nephropathy in patients undergoing intravenous (IV) contrast enhanced computed tomography (CECT) and the relationship with risk factors: a meta-analysis. Eur J Radiol 2013; 82: e387–99. doi: 10.1016/j.ejrad.2013.04.029 [DOI] [PubMed] [Google Scholar]
- 3.Abujudeh HH, Gee MS, Kaewlai R. In emergency situations, should serum creatinine be checked in all patients before performing second contrast CT examinations within 24 hours? J Am Coll Radiol 2009; 6: 268–73. doi: 10.1016/j.jacr.2008.09.014 [DOI] [PubMed] [Google Scholar]
- 4.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 2002; 105: 2259–64. doi: 10.1161/01.CIR.0000016043.87291.33 [DOI] [PubMed] [Google Scholar]
- 5.McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol 2008; 51: 1419–28. doi: 10.1016/j.jacc.2007.12.035 [DOI] [PubMed] [Google Scholar]
- 6.Zhang T, Shen LH, Hu LH, He B. Statins for the prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Am J Nephrol 2011; 33: 344–51. doi: 10.1159/000326269 [DOI] [PubMed] [Google Scholar]
- 7.Al-Otaibi KE, Al Elaiwi AM, Tariq M, Al-Asmari AK. Simvastatin attenuates contrast-induced nephropathy through modulation of oxidative stress, proinflammatory myeloperoxidase, and nitric oxide. Oxid Med Cell Longev 2012; 2012: 831748. doi: 10.1155/2012/831748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan SB, Liu FY, Luo JA, Wu HW, Liu RH, Peng YM, et al. Nephrotoxicity of high- and low-osmolar contrast media. The protective role of amlodipine in a rat model. Acta Radiol 2000; 41: 503–7. [DOI] [PubMed] [Google Scholar]
- 9.Seeliger E, Sendeski M, Rihal CS, Persson PB. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J 2012; 33: 2007–15. doi: 10.1093/eurheartj/ehr494 [DOI] [PubMed] [Google Scholar]
- 10.Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol 2011; 21: 2527–41. doi: 10.1007/s00330-011-2225-0 [DOI] [PubMed] [Google Scholar]
- 11.Dussol B, Morange S, Loundoun A, Auquier P, Berland Y. A randomized trial of saline hydration to prevent contrast nephropathy in chronic renal failure patients. Nephrol Dial Transplant 2006; 21: 2120–6. doi: 10.1093/ndt/gfl133 [DOI] [PubMed] [Google Scholar]
- 12.Attallah N, Yassine L, Musial J, Yee J, Fisher K. The potential role of statins in contrast nephropathy. Clin Nephrol 2004; 62: 273–8. doi: 10.5414/CNP62273 [DOI] [PubMed] [Google Scholar]
- 13.Spargias K, Alexopoulos E, Kyrzopoulos S, Iokovis P, Greenwood DC, Manginas A, et al. Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation 2004; 110: 2837–42. doi: 10.1161/01.CIR.0000146396.19081.73 [DOI] [PubMed] [Google Scholar]
- 14.Song Y, Chan CW, Brown GM, Pang SF, Silverman M. Studies of the renal action of melatonin: evidence that the effects are mediated by 37 kDa receptors of the Mel1a subtype localized primarily to the basolateral membrane of the proximal tubule. FASEB J 1997; 11: 93–100. [DOI] [PubMed] [Google Scholar]
- 15.Sehajpal J, Kaur T, Bhatti R, Singh AP. Role of progesterone in melatonin-mediated protection against acute kidney injury. J Surg Res 2014; 191: 441–7. doi: 10.1016/j.jss.2014.04.025 [DOI] [PubMed] [Google Scholar]
- 16.Koch BC, van der Putten K, Van Someren EJ, Wielders JP, Ter Wee PM, Nagtegaal JE, et al. Impairment of endogenous melatonin rhythm is related to the degree of chronic kidney disease (CREAM study). Nephrol Dial Transplant 2010; 25: 513–19. doi: 10.1093/ndt/gfp493 [DOI] [PubMed] [Google Scholar]
- 17.Quiroz Y, Ferrebuz A, Romero F, Vaziri ND, Rodriguez-Iturbe B. Melatonin ameliorates oxidative stress, inflammation, proteinuria, and progression of renal damage in rats with renal mass reduction. Am J Physiol Renal Physiol 2008; 294: F336–44.doi: 10.1152/ajprenal.00500.2007 [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem 1988; 34: 497–500. [PubMed] [Google Scholar]
- 19.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 1968; 25: 192–205. doi: 10.1016/0003-2697(68)90092-4 [DOI] [PubMed] [Google Scholar]
- 20.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351–8. doi: 10.1016/0003-2697(79)90738-3 [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–8. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 22.Romano G, Briguori C, Quintavalle C, Zanca C, Rivera NV, Colombo A, et al. Contrast agents and renal cell apoptosis. Eur Heart J 2008; 29: 2569–76. doi: 10.1093/eurheartj/ehn197 [DOI] [PubMed] [Google Scholar]
- 23.Pucelikova T, Dangas G, Mehran R. Contrast-induced nephropathy. Catheter Cardiovasc Interv 2008; 71: 62–72. doi: 10.1002/ccd.21207 [DOI] [PubMed] [Google Scholar]
- 24.Heyman SN, Reichman J, Brezis M. Pathophysiology of radiocontrast nephropathy: a role for medullary hypoxia. Invest Radiol 1999; 34: 685–91. doi: 10.1097/00004424-199911000-00004 [DOI] [PubMed] [Google Scholar]
- 25.Katholi RE, Woods WT, Jr, Taylor GJ, Deitrick CL, Womack KA, Katholi CR, et al. Oxygen free radicals and contrast nephropathy. Am J Kidney Dis 1998; 32: 64–71. doi: 10.1053/ajkd.1998.v32.pm9669426 [DOI] [PubMed] [Google Scholar]
- 26.Deray G, Baumelou B, Martinez F, Brillet G, Jacobs C. Renal vasoconstriction after low and high osmolar contrast agents in ischemic and non ischemic canine kidney. Clin Nephrol 1991; 36: 93–6. [PubMed] [Google Scholar]
- 27.Bakris GL, Gaber AO, Jones JD. Oxygen free radical involvement in urinary Tamm-Horsfall protein excretion after intrarenal injection of contrast medium. Radiology 1990; 175: 57–60. doi: 10.1148/radiology.175.1.2315505 [DOI] [PubMed] [Google Scholar]
- 28.Hizoh I, Haller C. Radiocontrast-induced renal tubular cell apoptosis: hypertonic versus oxidative stress. Invest Radiol 2002; 37: 428–34. doi: 10.1097/00004424-200208000-00003 [DOI] [PubMed] [Google Scholar]
- 29.Ladurelle N, Gabriel C, Viggiano A, Mocaer E, Baulieu EE, Bianchi M. Agomelatine (S20098) modulates the expression of cytoskeletal microtubular proteins, synaptic markers and BDNF in the rat hippocampus, amygdala and PFC. Psychopharmacology (Berl) 2012; 221: 493–509. doi: 10.1007/s00213-011-2597-5 [DOI] [PubMed] [Google Scholar]
- 30.Aguiar CC, Almeida AB, Araujo PV, Vasconcelos GS, Chaves EM, do Vale OC, et al. Effects of agomelatine on oxidative stress in the brain of mice after chemically induced seizures. Cell Mol Neurobiol 2013; 33: 825–35. doi: 10.1007/s10571-013-9949-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molteni R, Macchi F, Zecchillo C, Dell'agli M, Colombo E, Calabrese F, et al. Modulation of the inflammatory response in rats chronically treated with the antidepressant agomelatine. Eur Neuropsychopharmacol 2013; 23: 1645–55. doi: 10.1016/j.euroneuro.2013.03.008 [DOI] [PubMed] [Google Scholar]
- 32.Gupta S, Sharma B. Pharmacological benefits of agomelatine and vanillin in experimental model of Huntington's disease. Pharmacol Biochem Behav 2014; 122: 122–35. doi: 10.1016/j.pbb.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 33.Parlakpinar H, Sahna E, Ozer MK, Ozugurlu F, Vardi N, Acet A. Physiological and pharmacological concentrations of melatonin protect against cisplatin-induced acute renal injury. J Pineal Res 2002; 33: 161–6. doi: 10.1034/j.1600-079X.2002.02910.x [DOI] [PubMed] [Google Scholar]
- 34.Anwar MM, Meki AR. Oxidative stress in streptozotocin-induced diabetic rats: effects of garlic oil and melatonin. Comp Biochem Physiol A Mol Integr Physiol 2003; 135: 539–47. [DOI] [PubMed] [Google Scholar]
- 35.Hannemann J, Baumann K. Cisplatin-induced lipid peroxidation and decrease of gluconeogenesis in rat kidney cortex: different effects of antioxidants and radical scavengers. Toxicology 1988; 51: 119–32. doi: 10.1016/0300-483X(88)90143-6 [DOI] [PubMed] [Google Scholar]
- 36.Montilla P, Túnez I, Muñoz MC, López A, Soria JV. Hyperlipidemic nephropathy induced by adriamycin: effect of melatonin administration. Nephron 1997; 76: 345–50. doi: 10.1159/000190202 [DOI] [PubMed] [Google Scholar]
- 37.Ozbek E, Turkoz Y, Sahna E, Ozugurlu F, Mizrak B, Ozbek M. Melatonin administration prevents the nephrotoxicity induced by gentamicin. BJU Int 2000; 85: 742–6. [DOI] [PubMed] [Google Scholar]
- 38.Nava M, Romero F, Quiroz Y, Parra G, Bonet L, Rodriguez-Iturbe B. Melatonin attenuates acute renal failure and oxidative stress induced by mercuric chloride in rats. Am J Physiol Renal Physiol 2000; 279: F910–8. [DOI] [PubMed] [Google Scholar]
- 39.Gazi S, Altun A, Erdogan O. Contrast-induced nephropathy: preventive and protective effects of melatonin. J Pineal Res 2006; 41: 53–7. doi: 10.1111/j.1600-079X.2006.00336.x [DOI] [PubMed] [Google Scholar]
- 40.Nasri H, Tavakoli M, Ahmadi A, Baradaran A, Nematbakhsh M, Rafieian-Kopaei M. Ameliorative effect of melatonin against contrast media induced renal tubular cell injury. Pak J Med Sci 2014; 30: 261–5. [PMC free article] [PubMed] [Google Scholar]
- 41.Yilmaz M, Aydinalp A, Okyay K, Tekin A, Bal UA, Bayraktar N, et al. Comparison of carvedilol and metoprolol for preventing contrast-induced nephropathy after coronary angiography. Cardiorenal Med 2015; 5: 199–207. doi: 10.1159/000381964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baykara M, Silici S, Ozcelik M, Guler O, Erdogan N, Bilgen M. In vivo nephroprotective efficacy of propolis against contrast-induced nephropathy. Diagn Interv Radiol 2015; 21: 317–21. doi: 10.5152/dir.2015.14075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slusser SO, Grotyohann LW, Martin LF, Scaduto RC, Jr. Glutathione catabolism by the ischemic rat kidney. Am J Physiol 1990; 258: F1546–53. [DOI] [PubMed] [Google Scholar]
- 44.Ozgur T, Tutanc M, Zararsiz I, Motor S, Ozturk OH, Yaldiz M, et al. The protective effect of ebselen on radiocontrast-induced nephrotoxicity. Ren Fail 2012; 34: 991–7. doi: 10.3109/0886022x.2012.706880 [DOI] [PubMed] [Google Scholar]
- 45.Sahu BD, Kalvala AK, Koneru M, Mahesh Kumar J, Kuncha M, Rachamalla SS, et al. Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-kappaB activation and antioxidant defence. PLoS One 2014; 9: e105070. doi: 10.1371/journal.pone.0105070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karakus E, Halici Z, Albayrak A, Polat B, Bayir Y, Kiki I, et al. Agomelatine: an antidepressant with new potent hepatoprotective effects on paracetamol-induced liver damage in rats. Hum Exp Toxicol 2013; 32: 846–57. doi: 10.1177/0960327112472994 [DOI] [PubMed] [Google Scholar]
- 47.Cadirci E, Altunkaynak BZ, Halici Z, Odabasoglu F, Uyanik MH, Gundogdu C, et al. Alpha-lipoic acid as a potential target for the treatment of lung injury caused by cecal ligation and puncture-induced sepsis model in rats. Shock 2010; 33: 479–84. doi: 10.1097/SHK.0b013e3181c3cf0e [DOI] [PubMed] [Google Scholar]
- 48.Salvemini D, Wang ZQ, Bourdon DM, Stern MK, Currie MG, Manning PT. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur J Pharmacol 1996; 303: 217–20. doi: 10.1016/0014-2999(96)00140-9 [DOI] [PubMed] [Google Scholar]
- 49.Yayla M, Halici Z, Unal B, Bayir Y, Akpinar E, Gocer F. Protective effect of Et-1 receptor antagonist bosentan on paracetamol induced acute liver toxicity in rats. Eur J Pharmacol 2014; 726: 87–95. doi: 10.1016/j.ejphar.2014.01.022 [DOI] [PubMed] [Google Scholar]
- 50.Xu X, Wu T, Ding X, Zhu J, Zou J, He J. The role of nuclear factor-kappaB in rats of radiocontrast-media-induced nephropathy. J Biochem Mol Toxicol 2008; 22: 416–21. doi: 10.1002/jbt.20256 [DOI] [PubMed] [Google Scholar]
- 51.Sanchez-Capelo A. Dual role for TGF-beta1 in apoptosis. Cytokine Growth Factor Rev 2005; 16: 15–34. [DOI] [PubMed] [Google Scholar]
- 52.Yan X, Liu Z, Chen Y. Regulation of TGF-beta signaling by Smad7. Acta Biochim Biophys Sin (Shanghai) 2009; 41: 263–72. doi: 10.1093/abbs/gmp018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naziroglu M, Yoldas N, Uzgur EN, Kayan M. Role of contrast media on oxidative stress, Ca(2+) signaling and apoptosis in kidney. J Membr Biol 2013; 246: 91–100. doi: 10.1007/s00232-012-9512-9 [DOI] [PubMed] [Google Scholar]
- 54.Droguett A, Krall P, Burgos ME, Valderrama G, Carpio D, Ardiles L, et al. Tubular overexpression of gremlin induces renal damage susceptibility in mice. PLoS One 2014; 9: e101879. doi: 10.1371/journal.pone.0101879 [DOI] [PMC free article] [PubMed] [Google Scholar]