Abstract
Objective:
The purpose of the study was to evaluate acute normal tissue reactions and treatment compliance in a randomized clinical trial on 7-days-a-week post-operative radiotherapy (p-CAIR) vs post-operative concurrent radiochemotherapy (p-RTCT) in locally advanced cancer of the oral cavity/oropharynx. The sample analyzed at present represents approximately 30% of the intended future trial size.
Methods:
The patients were randomly assigned to receive 63 Gy in 1.8-Gy fractions 7 days a week (n = 44) or 63 Gy in 1.8-Gy fractions 5 days a week with concurrent cisplatin 80–100 mg per square metre of body surface area on Days 1, 22 and 43 of the course of radiotherapy (n = 40). Acute mucosal reactions were scored using the modified Dische system.
Results:
15 (17.9%) patients, including 5 patients in p-CAIR and 10 patients in p-RTCT, did not comply with the assigned radiation treatment, mostly because of rapid tumour progression or deteriorating general performance. In p-RTCT, 22 (55%) patients received less than the intended three courses of chemotherapy mostly owing to haematological toxicity. The average maximum mucosal severity score was 14.2 in p-CAIR compared with 13.4 in p-RTCT; the difference was not statistically significant (p = 0.31).
Conclusion:
The schedules compared (p-CAIR and p-RTCT) did not differ considerably with respect to acute mucosal reactions. Haematological toxicity in p-RTCT was elevated compared with p-CAIR. Both schedules were considered tolerable with respect to acute toxicity, which justifies further recruitment to the trial.
Advances in knowledge:
The results show that early mucosal reactions are comparable in both trial arms but haematological toxicity is more pronounced during radiochemotherapy.
INTRODUCTION
Despite improvements in surgical and radiation techniques, locoregional recurrences remain among the major causes of failure in combined treatment of locally advanced cancer of the oropharynx and oral cavity. Randomized trials demonstrated that post-operative concurrent administration of high-dose cisplatin with radiotherapy is more efficacious than post-operative radiotherapy (p-CAIR) alone.1,2 This resulted in acceptance of post-operative radiochemotherapy in adjuvant treatment after surgery for high-risk head and neck cancer (H&NC). The combined treatment that incorporates concurrent radiotherapy and chemotherapy is, however, associated with a substantial increase in adverse effects.1,2
An alternative approach in attempts to enhance the effectiveness of combined treatment for locally advanced H&NC is represented by the trials in which the overall radiation treatment time of p-CAIR was shortened, compared with standard fractionation. This may improve locoregional tumour control by hampering tumour repopulation that can be triggered by cell depletion from surgery and successive fractions of radiotherapy.3 The outcome of these trials is largely conflicting, with some of them demonstrating a significant improvement in locoregional control that favours accelerated p-CAIR,4 some demonstrating a non-significant trend towards such improvement,5,6 with others showing no beneficial effect of accelerated p-CAIR.7,8 Such disparity can be explained by the relatively small sample size of these trials, heterogeneity in patient selection criteria, diversity in dose-fractionation schedules and in time interval surgery-radiotherapy.
The trial that compared accelerated vs conventional p-CAIR for high-risk H&NC was performed in our institution and recruited 279 patients with cancer of the larynx, oral cavity and oropharynx.6 The results of this trial have shown a non-significant trend towards improvement in locoregional control in a whole group of 279 patients. A significant improvement in locoregional tumour control attributable to acceleration of p-CAIR was, however, demonstrated in a subgroup of 121 patients with cancer of the oropharynx/oral cavity. Our supposed ability to select the patients who may benefit from accelerated p-CAIR created the basis for the present trial.
Acute mucosal reactions of accelerated radiotherapy or radiochemotherapy and haematological toxicity of radiochemotherapy can be dose limiting and affect the overall tolerance of combined treatment. A prohibitive rate of consequential mucosal reactions was observed among few patients treated 7 days a week with 2.0 Gy per fraction in the initial attempts on continuous accelerated radiotherapy alone in locally advanced H&NC.9,10 Severe normal tissue complications are also frequently reported after concurrent radiochemotherapy. This illustrates the need for careful evaluation of acute normal tissue reactions providing, thus, the rationale for the present interim report from the trial.
METHODS AND MATERIALS
Eligibility criteria and randomization
The detailed eligibility criteria for this trial are available online http://www.controlled-trials.com/ISRCTN65457367. In short, the trial recruited patients with squamous-cell cancer of the oral cavity or oropharynx that were at intermediate and high risk of recurrence after surgery. The groups were well balanced with respect to the probable prognostic factors (Table 1). Only patients who could tolerate chemotherapy were recruited. Randomization was performed at the time of appointment for radiotherapy by telephone call to the trial office. The patients were randomly assigned to receive continuous 7-days-a-week p-CAIR (p-CAIR) or conventionally fractionated post-operative concurrent radiochemotherapy (p-RTCT). The protocol of the study was approved by the local bioethical committee in accordance with the national regulations.
Table 1.
Characteristics of the patients by treatment group
| Variable | Subgroups | p-CAIR (n = 44) | RT-CH (n = 40) | p-value |
|---|---|---|---|---|
| Age (median: 57; range 40–76) (years) | <57 years | 22 (50.0%) | 19 (47.5%) | 0.85 |
| >57 years | 22 (50.0%) | 21 (52.5%) | ||
| Sex | M | 33 (75.0%) | 28 (70.01%) | 0.69 |
| F | 11 (25.0%) | 12 (30.0%) | ||
| Tumour site | Oral cavity | 21 (47.7%) | 21 (52.5%) | 0.71 |
| Oropharynx | 23 (52.3%) | 19 (47.5%) | ||
| cT stage | T1, T2 | 27 (61.4%) | 25 (62.5%) | 0.95 |
| T3, T4 | 17 (38.6%) | 15 (37.5%) | ||
| cN stage | cN0 | 13 (29.5%) | 8 (20.0%) | 0.93 |
| cN1–3 | 31 (70.5%) | 32 (80.0%) | ||
| Pathological margin | Negative | 29 (65.9%) | 31 (77.5%) | 0.25 |
| Positive | 15 (34.1%) | 9 (22.5%) | ||
| Grade | G1 | 5 (11.4%) | 8 (20.0%) | 0.82 |
| G2 | 30 (68.2%) | 23 (57.5%) | ||
| G3 | 3 (6.8%) | 5 (12.5%) | ||
| Uncertain | 6 (13.6%) | 4 (10.0%) | ||
| Number of invaded nodes | 0 | 11 (25.0%) | 11 (27.5%) | 0.76 |
| 1 | 10 (22.7%) | 10 (25.0%) | ||
| >1 | 21 (47.7%) | 18 (45.0%) | ||
| Uncertain | 2 (4.4%) | 1 (2.5%) | ||
| Interval surgery-XRT | ≤9 weeks | 10 (22.7%) | 8 (20.0%) | 0.59 |
| >9 weeks | 34 (77.3%) | 32 (80.0%) | ||
| ZUBROD | 0 | 18 (40.9%) | 19 (47.5%) | 0.60 |
| 1 | 26 (59.1%) | 21 (52.5%) |
cN, clinically assessed nodal stage; cT, clinically assessed tumour stage; F, female; M, male; p-CAIR, post-operative radiotherapy; XRT, radiotherapy; ZUBROD, general performance score.
Surgical interventions
Only the patients who had undergone macroscopically complete major surgery were recruited. The protocol required p-CAIR to begin as soon as possible, i.e. as adequate healing had occurred and radiation-treatment plan had been approved. This may occur 4–6 weeks after the surgery, but with increasing complexity of radiation-treatment techniques and waiting lists for radiotherapy, the interval surgery-radiotherapy was frequently longer than originally intended.
Post-operative radiotherapy
The prescribed total dose, dose per fraction and radiation-treatment technique were the same in both arms of the trial; the assigned treatments differed, however, with respect to the overall radiation-treatment time: it was 5 weeks in p-CAIR and 7 weeks in p-RTCT. The total dose at sites considered to be at intermediate/high risk of recurrence was 63 Gy in 1.8 Gy per fraction. The rationale for such dose was provided by earlier studies.11
The dose delivered to the electively treated areas was 45 Gy; supraclavicular nodes were electively treated whenever the pathological specimen revealed involvement of the neck nodes. In patients assigned to continuous 7-days-a-week p-CAIR, “large” portals covering clinical target volume were irradiated to the total dose of 45 Gy and were treated 5 days a week (from Monday to Friday). By contrast, “small” portals, limited to the areas considered to be at intermediate/high risk of recurrence, excluded the spinal cord and were treated 7 days a week.
In patients assigned to conventional post-operative radiochemotherapy, “large” portals were irradiated 5 days a week to the total dose of 45 Gy, i.e. over the first 5 weeks of treatment, while “small fields” were irradiated at Weeks 6–7.
A three-dimensional (3D) treatment-planning system was used: the fields and multileaf collimator arrangement were individually optimized to ensure optimal target coverage and protection of the critical organs. The protocol allowed the use of intensity-modulated radiotherapy or 3D conformal radiotherapy. The spinal cord, parotid glands, bones and larynx were considered among the critical organs and the commonly accepted dose constrains were observed. Radiation dose was prescribed and specified in the reference point according to the guidelines of the International Commission on Radiation Units Report 50 and 62. Thermoplastic masks were used for immobilization. The patients were treated using linear accelerators with 6-MV photons. The quality assurance procedures included repeated in vivo dosimetry, image-guided radiotherapy procedures (kilovoltage portals or cone beam CT), double check of treatment plans and portals, pre-treatment and weekly audits during therapy.
Chemotherapy
Chemotherapy consisted of cisplatin 80–100 mg per square metre of body surface area on Days 1, 22 and 43 of the course of radiotherapy. The protocol of the trial that allowed a cisplatin dose of 100 mg per square metre was amended after completion of the pilot study, allowing the use of cisplatin doses in the range of 80–100 mg, with lower doses prescribed to the patients with ZUBROD 1 performance status and/or comorbidities.
Scoring and analyzing of acute mucosal and haematological reactions
Acute mucosal reactions were scored using the modified Dische system.12 This system places emphasis on both morphological and functional radiation effects. The system used in the present study incorporates both compulsory and optional elements of Dische scale including the data on morphology and distribution of mucositis, intensity of mucosal erythema, oedema, bleeding, ulceration, dysphagia and pain. Because optional elements of the scoring system were used, the maximum possible score was 32, compared with 24 in the first continuous accelerated radiotherapy trial.9 The score was evaluated every week during radiotherapy, then 2, 4 and 8 weeks after its completion. The follow-up visits were performed thereafter every 6 months. Such scoring frequency reflects a compromise between research objectives of the trial and clinical responsibilities of a busy radiotherapy department.
Both the time prevalence and maximum total severity score were analyzed. The relationship between the maximum total score and fractionation scheme, primary tumour site, patient age and weight was analyzed using a polychotomous logistic regression.13 An individual maximum score of reaction, Smax, was related to patient and treatment characteristics through the equation:
where β’s are the estimates of regression coefficients.
The haematological reactions were evaluated using the Radiation Therapy Oncology Group (RTOG) scoring system.
Supportive treatment
Supportive anti-inflammatory treatment was given whenever the severity score of acute mucositis exceeded 10. Corticosteroids and antibiotics were prescribed when the severity score was ≥16. Tube feeding was used when symptoms related to acute mucositis necessitated parenteral feeding for more than 1week. Prophylactic hydration and antiemetic agents were given to patients who received p-RTCT. Granulocyte colony-stimulating factors were given to patients with Grade ≥3 neutropenia and/or symptomatic febrile neutropenia.
Study end points
The present study focuses on secondary outcome measures of the trial such as acute mucosal reactions, acute radiation and haematological morbidity. The primary end point of the trial (cumulative incidence of locoregional recurrences) as well as other secondary outcome measures will be addressed in future reports.
RESULTS
Treatment compliance
The report includes 84 consecutively recruited patients: 44 patients were randomly assigned to receive post-operative continuous 7-day-a-week-radiotherapy (p-CAIR) and 40 patients to post-operative radiochemotherapy (p-RTCT). This represents approximately 30% of the intended future trial size. Table 2 summarizes the reasons for non-compliance of radiation treatment (5 patients in p-CAIR and 10 patients in p-RTCT). Radiation doses that were higher than that in the trial protocol were given because of detectable tumour recurrence during the interval surgery-radiotherapy or tumour progression during radiotherapy; lower radiation doses were given to patients who refused to complete treatment or to those with rapid local progression and deteriorating performance status.
Table 2.
Radiotherapy (RT) non-compliance
| Variable | p-CAIR (n = 44) | RT-CH (n = 40) |
|---|---|---|
| Death before RT | 0 (0%) | 2 (5.0%) (1 febrile neutropenia, 1 dissemination of cancer) |
| RT doses higher than in the protocol (70–72 Gy) | 4 (13.6%) | 2 (5.0%) |
| RT doses lower than in the protocol (20.0–55.8 Gy) | 0 (0%) | 4 (10.0%) |
| Surgery for nodal recurrence before RT | 0 (0%) | 1 (2.5%) |
| Consent withdrawal | 1 (2.3%) | 0 (0%) |
| Distant metastases before RT | 0 (0%) | 1 (2.5%) |
| Total | 5 (11.4%) | 10 (25.0%) |
p-CAIR, post-operative radiotherapy; RT-CH, radiochemotherapy.
Out of 40 patients assigned to p-RTCT, 18 (45%) patients received 3 courses of chemotherapy, 15 (37.5) patients received 2 courses and 2 patients received 1 course. Five (12.5%) patients did not receive chemotherapy because of recurrences before the onset of p-RTCT, rapid local progression and deteriorating performance status of the patient, development of distant metastases and withdrawal of informed consent (Table 2). The reasons for the reduction in the number of chemotherapy courses were Grade 3–4 neutropenia (10 patients, including 1 toxic death), Grade 2–3 thrombocytopenia (4 patients), Grade 2 anaemia (2 patients) and 1 case of disease progression during p-RTCT.
Unplanned radiation-treatment gaps
Out of 69 patients (39 patients in p-CAIR and 30 patients in p-RTCT) who complied with the assigned radiation schedule, 34 (87.2%) patients in p-CAIR had no treatment gaps compared with 21 (70.0%) patients in p-RTCT. The range of gap duration was 4–14 days (median, 9 days) in p-CAIR compared with 2–16 days (median, 3 days) in p-RTCT.
The actual mean overall radiation-treatment time was 36.3 days in p-CAIR [standard error of the mean (Std) ± 3.7] compared with 50.5 days (Std ± 6.4) in p-RTCT.
Total severity score of acute mucosal reactions in the course of treatment
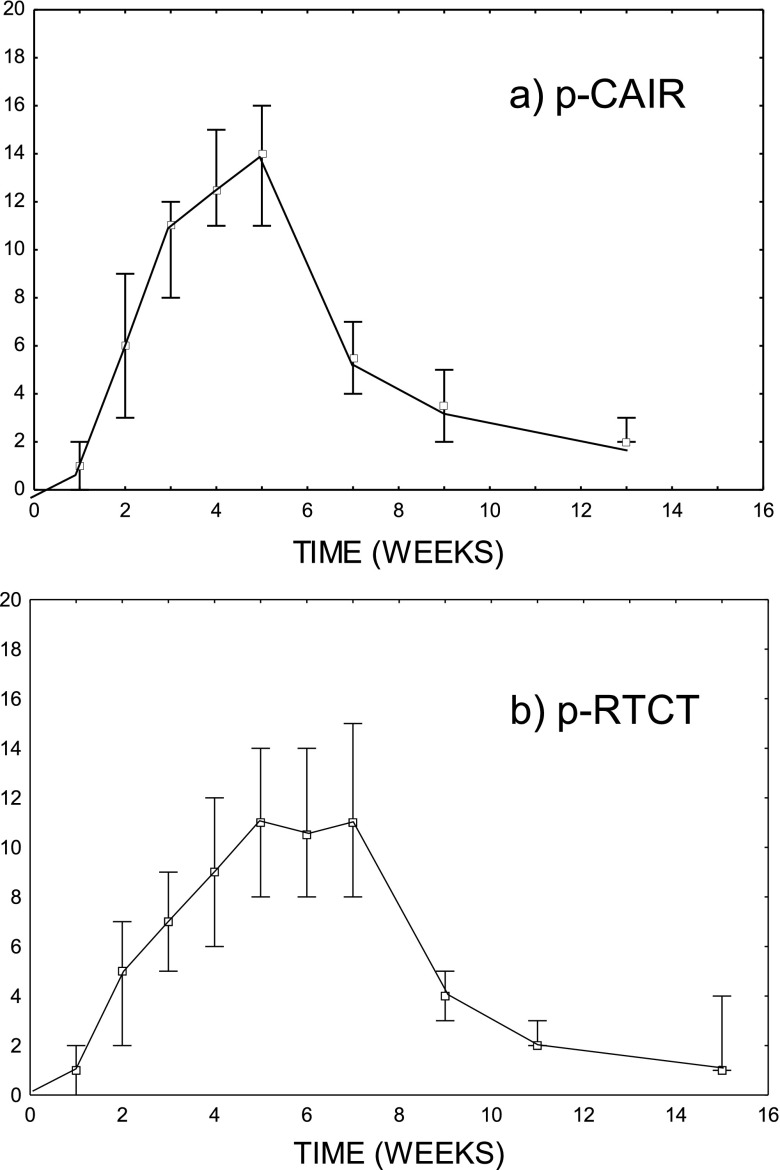
Figure 1a,b illustrates the average total severity score as a function of time in two arms of the trial. The highest average severity score in p-CAIR of about 14 was at Week 5, i.e. at the end of radiotherapy. For p-RTCT, a plateau in the average severity score of about 11 was observed at Weeks 5–7. The average maximum total severity score was higher in p-CAIR than in p-RTCT (14.2 vs 13.4); such a difference, however, was not statistically significant (p = 0.31).
Figure 1.
The average total severity score as a function of time in two arms of the trial: (a) post-operative radiotherapy (p-CAIR) and (b) post-operative concurrent radiochemotherapy (p-RTCT).
The average residual severity score 2 months after radiotherapy was similar in both trial arms and was, most frequently, associated with persistent mucosal erythema. Overall, the peak acute mucosal reactions were slightly higher in p-CAIR, but the duration of reaction was similar in both trial arms.
Incidence and duration of confluent mucositis
The prevalence of confluent mucositis was calculated as the proportion of patients having confluent mucositis at the course of treatment relative to the total number of patients. Most of the patients experienced confluent mucositis during the course of treatment, irrespective of the treatment arm; hence, the proportions were similar (86.6% for p-RTCT vs 83.9% for p-CAIR, p = 0.96). Also, the average duration of confluent mucositis was similar in both arms (2.6 weeks, Std ± 1.5, in p-CAIR vs 2.5 weeks, Std ± 1.8, in p-RTCT).
Factors influencing the maximum severity of mucosal reactions
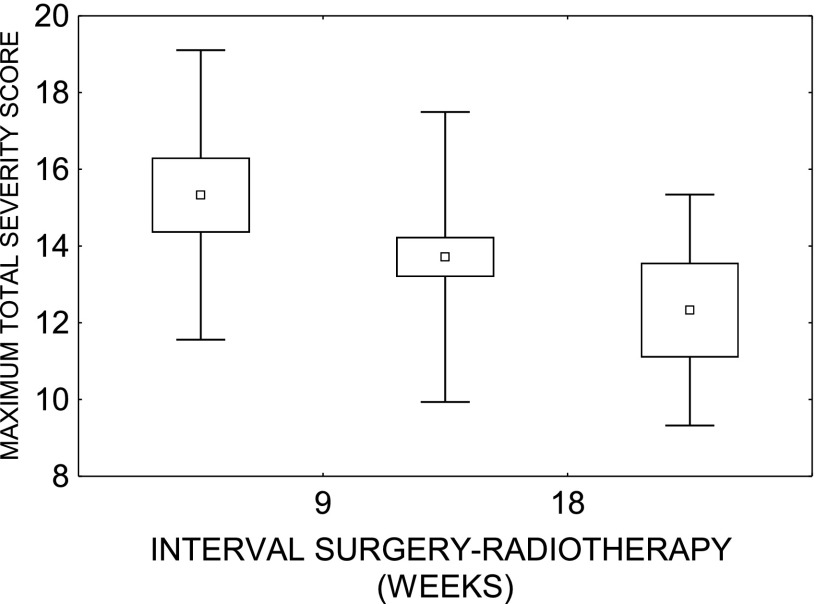
12 variables [age, gender, weight, T, N status, ZUBROD, trial arm, irradiated volume, treatment technique (3D vs intensity-modulated radiotherapy), haemoglobin concentration, interval surgery-radiotherapy and tumour site (oral cavity vs oropharynx)] were considered in the analysis. Multivariate polychotomous regression demonstrated that only time interval surgery-radiotherapy significantly influenced the maximum severity score: patients with short interval had more severe reactions (Figure 2).
Figure 2.
Peak total severity score as a function of time interval surgery-radiotherapy.
Haematological reactions
In spite of supportive treatment, haematological reactions were more severe in p-RTCT, including one patient who died owing to febrile neutropenia. The peak incidence of Grade 2–3 reactions in p-RTCT vs p-CAIR was as follows: leukopenia 63% vs 7%; neutropenia 48% vs 4%; lymphopenia 92% vs 61%; trombocytopenia 11% vs 0%; and anaemia 15% vs 7%.
Comparison of the white blood cell count at the beginning and at the end of radiotherapy indicated a higher decrease in this parameter for p-RTCT (−2.75 per cubic millimetre, Std ± 2.7, vs −0.23 per cubic millimetre, Std ± 2.3, for p-RTCT and p-CAIR, respectively; p<0.0001).
Likewise, comparison of haemoglobin concentration at the beginning and at the end of radiotherapy indicated a higher decrease in this parameter for p-RTCT (−1.98 g dl−1, Std ± 1.76, vs 0.10 g dl−1, Std ± 2.2, for p-RTCT and p-CAIR, respectively; p = 0.00015).
Comparison of the platelet count at the beginning and at the end of radiotherapy indicated a higher decrease in this parameter for p-RTCT (−1.8 per cubic millimetre, Std ± 65, vs −27 per cubic millimetre, Std ± 100, for p-RTCT and p-CAIR, respectively); the difference, however, was not significant (p = 0.31).
Out of the 12 variables considered, p-RTCT (p < 0.00001) and oral cavity primary site (p = 0.03) were related to significantly higher peak grade of neutropenia. A significant impact of primary tumour site for the peak grade of neutropenia (with more severe toxicity in oral cavity tumours) was also found in subsets of patients treated with p-RTCT (p = 0.01).
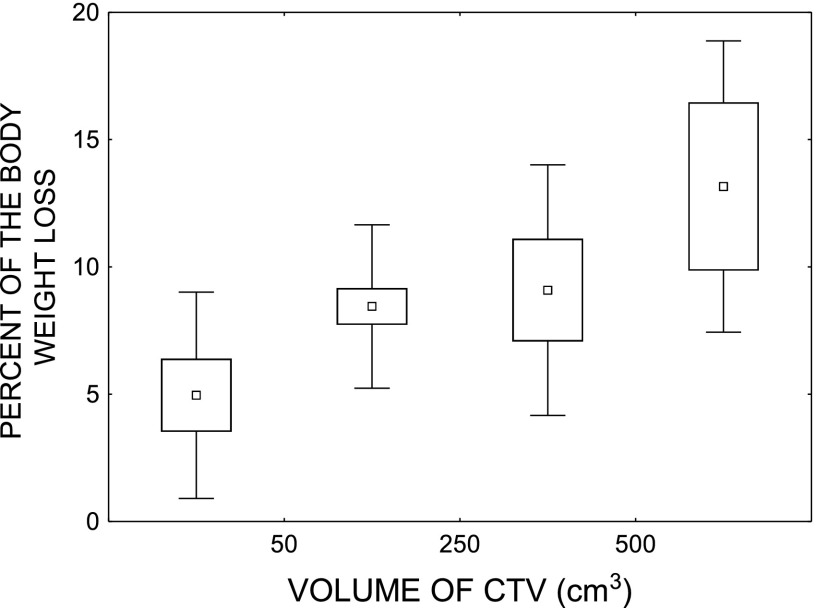
Weight loss
The average weight loss during the course of p-CAIR was 8.3% (Std ± 3.5) compared with 7.1% (Std ± 5.2) in p-RTCT (p = 0.31). Out of 12 variables (the same that were considered in the analysis of total severity score), only the irradiated volume significantly and independently influenced weight loss. Figure 3 illustrates the percentage of the body weight loss as a function of the volume of clinical target volume.
Figure 3.
Percent of the body weight loss as a function of the volume of clinical target volume (CTV).
DISCUSSION
General outcome of the present research
The data from clinical trials on accelerated radiotherapy or RTCT indicate that acute normal tissue reactions can affect the therapeutic index of combined treatment, leading (in extreme) to toxic deaths or consequential tissue necrosis. In spite of clinical importance, there are relatively few studies specifically devoted to quantification and prediction of acute normal tissue reactions in the combined treatment for H&NC. The pivotal reports from clinical trials are focused on primary end points, providing only a brief description of acute normal tissue reactions.1,2 They do not attempt to quantify the observed acute morbidity.
The present study provides a detailed assessment of acute reactions in p-CAIR and p-RTCT in locally advanced cancer of the oral cavity/oropharynx. The main finding is that p-CAIR and p-RTCT do not differ much with respect to acute mucosal reactions. On the other hand, haematological toxicity in p-RTCT is considerably elevated compared with p-CAIR.
Treatment compliance was relatively poor in this series, with disease progression before the onset of radiotherapy in some cases. This may be related to longer than originally intended interval surgery-radiotherapy, but also to biological aggressiveness of H&NC in Silesian population, with majority of human papilloma virus-negative tumours.14 Another factor contributing to non-compliance was adverse reactions related to p-RTCT that included one toxic death, deterioration of general performance status and unwillingness of patients to complete adjuvant treatment.
The present study does not address the long-term outcomes of the trial. Clearly, however, the data on acute tolerance of treatment must be considered while evaluating the overall therapeutic index of p-RTCT and p-CAIR.
Acute mucosal reactions
In general, acute mucosal reactions in both arms of the trial were severe, but tolerable. The peak reaction in p-CAIR was slightly higher, but its duration shorter than in p-RTCT. Consequential tissue necrosis was not observed in this series. Short interval surgery-radiotherapy predisposed to more severe mucosal reactions. This may reflect the impaired ability of normal tissues that heal after surgery to overcome radiation injury. It may also support an inference which suggests that unduly short interval surgery-radiotherapy may be detrimental, just as unduly prolonged is.3,6
Weight loss was related to irradiated volume; volume did not, however, affect the maximum severity score of mucosal reactions. Peak severity score may, thus, not precisely reflect the overall impact of mucositis since the score may be high, even though the affected area is small.
Haematological toxicity
The present data illustrate that acute haematological toxicity of p-RTCT is of concern, even though the investigated treatment schedule was less intense than that investigated before.1,2 The fraction doses of 1.8 Gy per fraction (instead of 2.0 Gy) and range of cisplatin doses (80–100 mg m−2, instead of 100 mg m−2) made treatment more tolerable.
In spite of reduction in treatment intensity, only 45% of the patients received three courses of chemotherapy. Such numbers are comparable with the published data, with 64% of the patients receiving three courses of chemotherapy in the European Organisation for Research and Treatment of Cancer trial1 (only 49% received the third course without delay) and 61% in RTOG trial.2 Both trials1,2 recruited, however, patients with laryngeal cancer who tended to better tolerate p-CAIR, compared to those with cancer of the oral cavity/oropharynx.15
Patients with tumours located in the oral cavity had significantly higher grade of neutropenia than patients with cancer of the oropharynx. A clinical significance of this finding is, however, unclear.
CONCLUSION
The schedules compared (p-CAIR and p-RTCT) did not differ considerably with respect to acute mucosal reactions. Short interval surgery-radiotherapy predisposed to more severe reactions. Haematological toxicity in p-RTCT was elevated compared with p-CAIR, with only 45% of the patients receiving the complete three courses of concurrent chemotherapy. Both schedules were considered tolerable with respect to acute toxicity, which justifies further recruitment to the trial.
FUNDING
This study was sponsored by the Polish Ministry of Science and Higher Education, grant number: N402 1801 34. The following number was assigned upon registration of the protocol of the trial: ISRCTN65457367.
Contributor Information
Rafal Suwinski, Email: rafals@io.gliwice.pl.
Grzegorz Wozniak, Email: gwozniak.md@gmail.com.
Maciej Misiolek, Email: msmisiolek@gmail.com.
Magdalena Jaworska, Email: magdajawor@interia.pl.
Maciej Kozaczka, Email: kozaczkam@interia.pl.
Wieslaw Bal, Email: wbal@op.pl.
Elzbieta Nowara, Email: enowara@io.gliwice.pl.
Leszek Miszczyk, Email: leszek@io.gliwice.pl.
REFERENCES
- 1.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004; 350: 1945–52. doi: 10.1056/NEJMoa032641 [DOI] [PubMed] [Google Scholar]
- 2.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004; 350: 1937–44. doi: 10.1056/NEJMoa032646 [DOI] [PubMed] [Google Scholar]
- 3.Suwinski R, Sowa A, Rutkowski T, Wydmanski J, Tarnawski R, Maciejewski B. Time factor in postoperative radiotherapy: a multivariate locoregional control analysis in 868 patients. Int J Radiat Oncol Biol Phys 2003; 56: 399–412. [DOI] [PubMed] [Google Scholar]
- 4.Awwad HK, Lotayef M, Shouman T, Begg AC, Wilson G, Bentzen SM, et al. Accelerated hyperfractionation (AHF) compared to conventional fractionation (CF) in the postoperative radiotherapy of locally advanced head and neck cancer: influence of proliferation. Br J Cancer 2002; 86: 517–23. doi: 10.1038/sj.bjc.6600119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang KK, Trotti A, Brown BW, Garden AS, Foote RL, Morrison WH, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001; 51: 571–8. doi: 10.1016/S0360-3016(01)01690-X [DOI] [PubMed] [Google Scholar]
- 6.Suwinski R, Bankowska-Wozniak M, Majewski W, Idasiak A, Maciejewski A, Ziółkowska E, et al. Randomized clinical trial on 7-days-a-week postoperative radiotherapy for high-risk squamous cell head and neck cancer. Radiother Oncol 2008; 87: 155–63. doi: 10.1016/j.radonc.2008.02.009 [DOI] [PubMed] [Google Scholar]
- 7.Sanguineti G, Richetti A, Bignardi M, Corvo' R, Gabriele P, Sormani MP, et al. Accelerated versus conventional fractionated postoperative radiotherapy for advanced head and neck cancer: results of a multicenter phase III study. Int J Radiat Oncol Biol Phys 2005; 61: 762–71. doi: 10.1016/j.ijrobp.2004.07.682 [DOI] [PubMed] [Google Scholar]
- 8.Langendijk JA, Kaanders JH, Doornaert P, Burlage FR, van den Ende PL, Oei SB, et al. Postoperative accelerated radiotherapy (POPART) versus conventional postoperative radiotherapy (CPORT) in squamous cell head and neck cancer: A multicenter prospective randomized study of the Dutch Head and Neck Cooperative Study Group. J Clin Oncol 2010; 28: 15. [Google Scholar]
- 9.Maciejewski B, Skladowski K, Pilecki B, Taylor JM, Withers RH, Miszczyk L, et al. Randomized clinical trial on accelerated 7 days per week fractionation in radiotherapy for head and neck cancer. Preliminary report on acute toxicity. Radiother Oncol 1996; 40: 137–45. doi: 10.1016/0167-8140(96)01776-8 [DOI] [PubMed] [Google Scholar]
- 10.Skladowski K, Maciejewski B, Golen M, Tarnawski R, Slosarek K, Suwinski R, et al. Continuous accelerated 7-days-a-week radiotherapy for head-and-neck cancer: long-term results of phase III clinical trial. Int J Radiat Oncol Biol Phys 2006; 66: 706–13. doi: 10.1016/j.ijrobp.2006.05.026 [DOI] [PubMed] [Google Scholar]
- 11.Peters LJ, Goepfert H, Ang KK, Byers RM, Maor MH, Guillamondegui O, et al. Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. Int J Radiat Oncol Biol Phys 1993; 26: 3–11. doi: 10.1016/0360-3016(93)90167-T [DOI] [PubMed] [Google Scholar]
- 12.Dische S, Warburton MF, Jones D, Lartigau E. The recording of morbidity related to radiotherapy. Radiother Oncol 1989; 16: 103–8. doi: 10.1016/0167-8140(89)90026-1 [DOI] [PubMed] [Google Scholar]
- 13.Bentzen SM, Saunders MI, Dische S, Bond SJ. Radiotherapy-related early morbidity in head and neck cancer: quantitative clinical radiobiology as deducted from the CHART trial. Radiother Oncol 2001; 60: 123–35. doi: 10.1016/S0167-8140(01)00358-9 [DOI] [PubMed] [Google Scholar]
- 14.Snietura M, Piglowski W, Jaworska M, Mucha-Malecka A, Wozniak G, Lange D, et al. Impact of HPV infection on the clinical outcome of p-CAIR trial in head and neck cancer. Eur Arch Otorhinolaryngol 2011; 268: 721–6. doi: 10.1007/s00405-010-1396-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suwinski R., Bankowska-Wozniak M., Majewski W., Sowa A, Idasiak A, Ziolkowska E, et al. : Randomized clinical trial on continuous 7-days-a-week postoperative radiotherapy for high-risk squamous cell head-and-neck cancer: a report on acute normal tissue reactions. Radiother Oncol 2005; 77: 58–64. doi: 10.1016/j.radonc.2005.07.007 [DOI] [PubMed] [Google Scholar]