Abstract
25 years ago, on a Friday evening at 9 pm, the emergency department (ED) was full of patients with a wide range of clinical problems. Their investigations included plain radiographs, but no other imaging was included until the next working day. At present, many patients are receiving advanced imaging such as ultrasound, CT and MRI, often delivered out of hours—an obvious advance for patients or sometimes an unnecessary development? In this article, we will consider how to assess patient benefits and whether increased use of advanced imaging is an overall advance for patients. We will address the general implications for healthcare services which come with greater use of advanced imaging. We will then address the effect of advanced imaging on individual fictional ED patients with a variety of complaints.
GENERAL CONSIDERATIONS
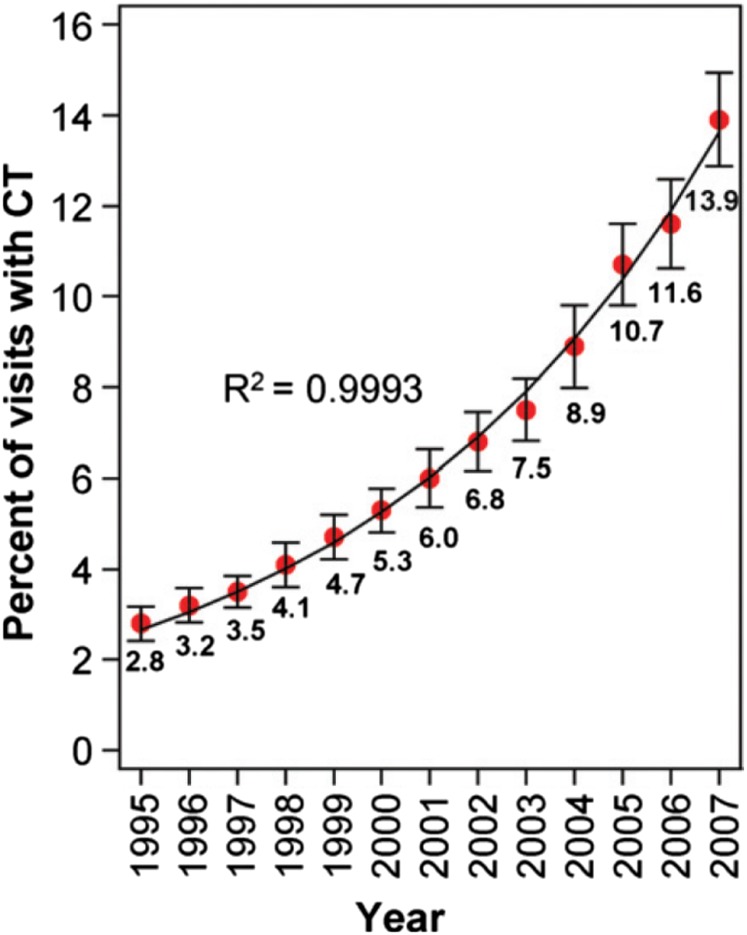
The use of advanced imaging for emergency department (ED) patients has taken off in the past 15 years, from 1998 to 2007, the use of CT or MRI for injured patients increased three fold in the USA,1 without an increase in the number of diagnosis of life-threatening conditions (such as skull fracture or cervical spine injury) or admission rates.1 For other ED presentations (such as headache and chest pain), a similar increase in CT use has been demonstrated, rising by a factor of almost five times between 1995 and 2007, with no evidence that this increased use is levelling off2 (Figure 1). This represents both increased use of scanning for the same indications (i.e. a lower “threshold”) and an increasing number of reasons to use advanced imaging.
Figure 1.
Exponential growth in advanced imaging use. Graph illustrates percentages of emergency department (ED) visits involving CT from 1995 to 2007. Data points indicate national estimates of percentages of ED visits involving CT based on the sample data. Solid line = exponential model based on the data. Error bars = 95% confidence intervals. Reproduced from Larson et al2 with permission from The Radiological Society of North America.
There are multiple factors which have both pulled and pushed the increased use of advanced imaging in the ED. Technology has been a “pull” factor for ultrasound, CT and MRI. Ultrasound machines have become smaller, more portable and with greater image quality, leading ED physicians to routinely use ultrasound at the bedside. Indeed, competence in basic ultrasound is a core training requirement for ED registrars in the UK with clearly defined measures of competency. similarly, in Australia, the use of ultrasound is regulated by Medicare reimbursement, and there are stringent training requirements. However, despite this, the use of ultrasound by non-radiologists without high standards of prescriptive training is increasing. CT in particular has improved in both image quality and scanner efficiency, leading to CT replacing plain films and conventional catheter angiography. Plain films are also being supplemented with MRI: the development of low-field peripheral MRI scanners has made it possible to perform dedicated musculoskeletal imaging for acute injuries.3 Ultrasound, CT and to a lesser extent MRI scanners have become increasingly available, and CT in particular has a greater proximity to EDs, allowing clinicians to easily access rapid, minimally invasive, high-resolution imaging which is reproducible.2,4 Other technological developments such as teleradiology and voice recognition enable a timely and accurate diagnosis to be communicated promptly to guide clinicians to appropriate treatment.2 Factors which “push” the clinician towards more advanced imaging include the introduction of the “four hour” ED rule (in the UK and Australia) as well as the type of hospital setting. Academic or urban centres demonstrate higher use of advanced imaging perhaps because patients are more likely to see a training grade doctor.1,2 Patient expectation and fear of malpractice litigation are other likely precipitators.1 In 1984, missed appendicitis was the most frequently successful malpractice claim against Emergency Physicians study; since then, the use of advanced imaging for suspected appendicitis has become almost routine (see fuller discussion later).5
Increased use of advanced imaging does have disadvantages. Cost is a consideration. At our institutions (three Academic Hospital Centres in the UK and Australia), the costs are as follows: abdominal X-ray (AXR), mean £31 ($47); ultrasound, mean £53 ($81); abdominopelvic CT, mean £196 ($ 298); and one-part MRI, mean £209 ($317). The mean cost of one night's patient stay on a standard medical ward is £673 (US$1021) across our institutions, whereas the cost of an outpatient's visit is £136 ($206). The cost to a patient is also important and requires a complex cost benefit calculation taking into account many variables including disruption of work and other responsibilities. An important, but easy to overlook, consequence is that increased use of advanced imaging has been shown to increase length of visit to the ED, leading to ED crowding and potentially indirectly increasing the risk of medical error.1 At the very least, this requires re-organization of ED services to accommodate patients awaiting imaging results with implications for healthcare planning and resource allocation, whether in private care, an insured system or in a public hospital.
Other disadvantages of advanced imaging include the effects of the risk of ionizing radiation (IR) from CT, which is particularly important in young patients. 1–2% of all cancers in the UK and USA may be associated with exposure to IR from CT which is one of the largest sources of radiation exposure overall.1,6 The effective doses of radiation from CT of the body range from 10 to 23 mSv (highest in trauma)7 compared with an annual background radiation dose of 3 mSv. On an individual level, 10-mSV CT in a 25-year-old patient is associated with a risk of induced cancer of 1 in 900 individuals and a risk of fatal cancer in 1 in 1800 individuals.4 Increasingly, it is recognized that despite the clinical benefit of increasing numbers of CTs being performed, too many are being performed overall—maybe up to one-third.8
Non-IR imaging modalities are also not perfect. While MRI offers exquisite soft tissue contrast resolution, its disadvantages are the relatively long scan times (a minimum of 15 min) and lack of 24/7 availability.4 Ultrasound is often available but carries the disadvantages of lack of reproducibility and limitations owing to operator experience, body habitus, guarding and presence of intraluminal or peritoneal air.9
How do we assess whether an imaging investigation is beneficial? Increased use of investigations may be instinctively seen to be an advance, but it is important to define whether an imaging technique is actually useful. Statistical measures of sensitivity, specificity and positive- and negative-predictive values may not translate from a study on a specific patient subgroup to a broad spectrum of unselected patients.4 Rather, if an advanced imaging test produces a higher level of diagnostic confidence and helps in patient management, it can be considered to have yielded positive findings.4,10
VIRTUAL EMERGENCY DEPARTMENT PATIENTS
Patient 1: 70-year-old male with generalized abdominal pain, has had previous surgery 20 years ago. Obstruction? Cause?
The acute abdomen is one of the most common ED presentations and accounts for 5–10% of visits to an ED11,12 (Table 1). Imaging in these patients increases clinical accuracy, changes decisions regarding management and increases diagnostic certainty.11,13,14 A prospective study of over 1000 patients at 6 hospitals across Netherlands, presenting with acute abdominal symptoms took clinical diagnosis 6 months after the episode as a gold standard (the OPTIMA study).11 Patients were allocated to six different management strategies such as (i) clinical diagnosis alone, (ii) clinical + AXR, (iii) ultrasound—all patients, (iv) CT—all patients, (v) ultrasound then CT if ultrasound is inconclusive or negative, (vi) ultrasound then CT if ultrasound is inconclusive. Table 1, reproduced from Lameris et al,11 illustrates the list of final diagnosis in patients included in the study.
Table 1.
Final diagnoses assigned by expert panel
| Final diagnoses in 1021 patients | Number (%) |
|---|---|
| Urgent | |
| Acute appendicitis | 284 (28) |
| Acute diverticulitis | 118 (12) |
| Bowel obstruction | 68 (7) |
| Acute cholecystitis | 52 (5) |
| Acute pancreatitis | 28 (3) |
| Gynaecological diseasesa | 27 (3) |
| Urological diseasesb | 22 (2) |
| Abscessc | 14 (1) |
| Perforated viscus | 13 (1) |
| Bowel ischaemia | 12 (1) |
| Pneumonia | 11 (1) |
| Retroperitoneal or abdominal wall bleeding | 9 (1) |
| Acute peritonitis | 3 (0.3) |
| Total urgent diagnoses | 661 (65) |
| Non-urgent | |
| Non-specific abdominal pain | 183 (18) |
| Gastrointestinal diseasesd | 56 (5) |
| Hepatic, pancreatic and biliary diseasese | 43 (4) |
| Inflammatory bowel disease | 30 (3) |
| Urological diseasesf | 20 (2) |
| Gynaecological diseasesg | 9 (1) |
| Malignancyh | 5 (0.5) |
| Hernia | 2 (0.2) |
| Other | 12 (1) |
| Total non-urgent diagnoses | 360 (35) |
Reproduced from Lameris et al11 with permission BMJ Publishing Group Ltd.
Ovarian torsion, pelvic inflammatory disease, bleeding/ruptured ovarian cyst.
Renal and ureteral stones with obstruction, hydronephrosis, pyelonephritis.
Intra-abdominal abscess, retroperitoneal abscess, hepatic abscess, tubo-ovarian abscess.
Gastritis, gastroenteritis, peptic ulcer, acute epiploic appendagitis, constipation.
Hepatic metastases, cholecystolithiasis, chronic pancreatitis.
Renal and ureteral stones without obstruction, urinary tract infection.
Ovulation pain/bleeding, endometriosis, menstrual pain, uterine myoma, benign adnexal cyst.
Pancreatic, gastrointestinal and kidney malignancies.
Relying on clinical diagnosis alone resulted in unacceptable number of false-positives (27%).11 What of the imaging strategies? AXR alone only alters the management of 4% of patients with acute abdomen but still carries a radiation dose (0.1–1.0 msV).4 Lameris et al11 found that relying on clinical findings and ultrasound alone resulted in an unacceptable number of false-negatives (30%). The best sensitivity (94%) and specificity (68%) was achieved with a strategy of CT after a negative or inconclusive ultrasound. This also had the advantage of halving the number of patients requiring CT.
Sala et al14 follow up patients with acute abdominal ED presentations for 6 months to establish a final diagnosis and assess the value of early CT vs traditional management of clinical assessment and AXR alone. In this study, early CT improved diagnostic certainty. It was also associated with a shorter hospital stay (median 4.2 days, compared with 5.3 days for patients who had AXR alone initially), although this finding was not statistically significant.14 Interestingly, the patients who had early CT required fewer subsequent investigations, such as magnetic resonance cholangiopancreatography (MRCP), Barium studies, and ultrasound. Furthermore, half of the standard protocol patient (clinical assessment and AXR initially) group eventually required CT.14
CT is well known to be an excellent test in suspected small and large bowel obstruction with high accuracy for the common causes of large bowel obstruction including neoplasm, volvulus and diverticulitis. When small bowel obstruction is suspected, CT has a high sensitivity and specificity which is related to the grade of obstruction (64% for low-grade obstruction and 80% for high-grade obstruction).4 CT can help differentiate between the 25% patients who will need surgery and the 75% who do not.4 The use of intravenous contrast improves diagnostic confidence of CT further, for example, increased enhancement of the bowel wall is seen in early ischaemia, whereas decreased or even absent contrast enhancement of the bowel wall is seen in advanced ischaemia.12
Patient 2: 20-year-old female with right lower quadrant pain and high clinical suspicion of appendicitis
Right lower quadrant pain is challenging because it has a long list of differential diagnosis including appendicitis (with an ED prevalence of 14%); however, history, examination and bloods are rarely enough to produce a confident and accurate diagnosis.4 Clinical evaluation of appendicitis leads to a false-negative diagnosis in 12–40% of patients—usually higher in females as gynaecological disease confounds the diagnosis.15 An accurate and timely diagnosis is important because a missed diagnosis results in prolonged time to treatment, increased risk of perforation and associated increases in morbidity and mortality compared with uncomplicated appendicitis. The average mortality rates for negative appendectomy, appendectomy for acute appendicitis and for perforated appendices are 0.14%, 0.24% and 1.66%, respectively.15
Because of this, a high rate of negative appendectomy has been traditionally been accepted (up to 40%).5 However, the medical and economic consequences of a negative appendectomy are increasingly being considered to be unacceptable leading to multiple studies evaluating imaging, including AXR, ultrasound, CT and MRI.16
A conventional X-ray is still performed for 50–75% of patients with suspected acute appendicitis, but there is no evidence of diagnostic value.4 In experienced hands, graded compression sonography has a proven diagnostic value and carries the advantage of no preparation and that ultrasound can be performed at the patient's bedside.17 A confident diagnosis of appendicitis (non-compressible appendix of >7 mm), periappendicial inflammation (local echogenicty) or abscess can also be made with a sensitivity of 74% and specificity of 97%.16,18 However, the normal appendix will only be seen in a minority of cases, and diagnostic specificity also suffers in the presence of local perforation.16
CT is more accurate than ultrasound, but the greatest sensitivity is obtained with ultrasound followed by CT in negative or inconclusive ultrasound.4 This approach reduces radiation exposure because CT is only needed in half of patients.11
CT has the advantage of not only demonstrating specific findings of appendicitis and associated perforation but also allows other diagnosis to be assessed such as colitis or epiploic appendagitis.12,19 Sensitivity of up to 100% and specificity of up to 97% have been described in studies as far back as 1998.16 The presence of an enlarged (>6 mm) appendix and adjacent fat infiltration have a high positive-predictive value for appendicitis.4 The normal appendix is identified in approximately half-routine abdominal CTs in asymptomatic adults, and a normal or non-visualised appendix virtually excludes appendicitis in 98% of patients.19
The use of intravenous (but not oral or rectal contrast) is commonly advocated particularly in patients lacking abdominal and pelvic fat, not only to increase diagnostic accuracy of appendicitis but also to improve visibility of alternate diagnoses such as inflammatory bowel disease and infectious ileocolitis.12 IV contrast has been found to be essential for diagnosis in 14% of patients and changed the diagnosis or treatment plan for 46% patients.9
The use of CT for possible appendicitis has skyrocketed—CT preceded emergent appendectomy in 18.5% of patients in 1998 vs 93.2% of patients in 2007 at Duke University Medical Centre.12,15 The increased use of CT is generally presumed to have favourably influenced the rate of negative appendectomy. Depending on the study, the rate of negative finding appendectomy has decreased to 14–19% (selective CT in equivocal patients) or 2–5% (routine CT on all patients).20 However, not all authors have shown an association between increased used of imaging and decreased negative appendectomy rate.21 In the study by Coursey et al (n = 925) of appendectomy patients over a 10-year period, the increased use of pre-operative CT coincided with a drop in the negative appendectomy rate for females under 45 years old from 43% to 7% (1998–2007). However, there was no change in the rate for females over 45 years or males of any age, possibly because the negative appendectomy rate in these groups was not very high to begin with.15
Both the false-positive and false-negative rate of CT varied each year—these values are of course affected by technical improvements and by the pre-test probability.15 This is relevant because as CT is increasingly being routinely used, the proportion of patients being investigated who have a diagnosis of appendicitis has decreased, for example, in one 2004 article, only 4% of patients with the clinical indication of suspected appendicitis actually had a diagnosis of appendicitis.22
Acute appendicitis is one area where cost-effective studies have been performed. Routine use of CT has been shown to be cost-effective, both by avoiding unnecessary operations (13 patients out of 100 patients) and by avoiding unnecessary hospital admissions (50 patient days), saving hospital resources overall $447 per patient in 1998.5
In summary, ultrasound followed by CT or routine CT alone (depending on local expertise) will provide a timely and accurate diagnosis of appendicitis and its complications including perforation and abscess formation. The advantages of avoiding an unnecessary operation as well as of exploring the differential diagnosis are of benefit to the patient. Equally the cost to the individual and to the healthcare provider may be well balanced by shorter hospital stay related to an uncomplicated post-operative recovery.
Patient 3: 32-year-old pregnant female, 20 weeks gestation, right lower quadrant pain
In pregnant females, although CT has a high accuracy, the risks of IR to the foetus have led to alternative diagnostic pathways.
Rapid accurate diagnosis in pregnancy is important because perforated appendix is associated with high foetal mortality (up to 37%),23 yet perforation is also more common in pregnancy with rates of up to 55%.24 Ultrasound is the obvious imaging modality of choice but has its limitations, with a higher rate of indeterminate examination in part because graded compression is difficult to achieve and the negative-predictive value of a non-visualised appendix is ≤90%.24
Current American Colleges of Radiology and Obstetrics and Gynaecology guidelines recommend pregnant females with suspected appendicitis should be evaluated with MRI when ultrasound findings are indeterminate and that CT should be avoided.12
MRI has a high negative-predictive value for diagnosing a normal appendix even in patients already screened by ultrasound (94–100%)24 and shares the advantage of lack of IR. Unenhanced T1 and T2 weighted images demonstrate peritoneal fat and the appendix with a high sensitivity (100%) and specificity (94%) for acute appendicitis.23 On MRI, as for other modalities, the presence of an appendix ≤6 mm in diameter, containing air or contrast material is suggestive of a normal appendix.24 Theoretical safety concerns regarding amniotic fluid heating and foetal hearing loss have not been a practical concern, and limited non-contrast-enhanced MRI is widely considered safe in pregnancy.23 In this scenario, ultrasound followed by MRI where ultrasound is indeterminate is the best imaging protocol which will avoid the high risks of missing an acute appendicitis.
Patient 4: 45-year-old female with right upper quadrant pain and known history of gallstones
In suspected acute calculous cholecystitis, ultrasound is the most appropriate initial investigation with a sensitivity of 88% and specificity of 80%, demonstrating gallbladder wall thickening, enlarged tender non-compressible gallbladder and adjacent infiltration/fluid collection.4 Ultrasound can also help in the decision regarding insertion of a percutaneous drain or performance of a delayed or prompt cholecystectomy (within 96 h of the onset of symptoms).4 If the patient does not have ultrasound first, then CT also shows good accuracy (sensitivity 92% and specificity 99%).4
MRCP is widely advocated as the next investigation in ultrasound-negative suspected common bile duct (CBD) stones (choledocholithiasis) and has a sensitivity of approximately 90% in detecting CBD stones when compared with ERCP.25,26 MRCP has the obvious advantage over ERCP of being non-invasive and less operator dependant. While endoscopic ultrasound equals MRCP in diagnostic capability, it requires endoscopy and is less widely available than MRCP.27 The availability of emergency/urgent MRCP across different hospital settings is generally less than for CT. A further limitation of MRCP is its spatial resolution which restricts the diagnosis of small calculi in the CBD. Although the lower limit of spatial resolution is difficult to quantify, in the study by Aubé et al25 comparing MRCP with endoscopic ultrasound, MRCP missed multiple 1 mm diameter stones floating in the CBD.
Patient 5: 76-year-old male with hip pain after fall at home, now unable to weight bear. Initial radiographs are negative for a fracture
In 2–10% of patients with a fractured neck of femur (NOF), the fracture is occult on the initial radiographs.28 Prompt diagnosis of a fracture is important, as the mortality rate in the first-year post-fracture is between 14% and 36%.28 Clinical signs do not help to identify the presence of an occult fracture: Hossain et al reviewed the value of MRI in 76 patients with pelvic pain after a fall and a negative radiograph. 35 patients had occult proximal pelvic fractures including subcapital and intertrochanteric NOF numbers, isolated greater trochanteric fractures and pubic rami numbers.29 In these patients, clinical signs such as pain on axial loading did not differentiate between patients with or without hip fractures. They recommended that MRI is offered to all patients with severe hip pain but normal X-rays after a fall.29
Bearing in mind that there are barriers to MRI use, the value of CT has to be explored, particularly as in practice CT is often the “second step” after negative or dubious plain radiographs. There are no good studies comparing CT and MRI in patients with hip pain after a fall. However, in patients without a clear history of trauma, MRI is certainly substantially better than CT at detecting insufficiency fractures.30 MRI detected 99% of fractures including number of the pubic ramus, sacrum and NOF, whereas CT only diagnosed 69% of patients. CT was particularly poor at the diagnosis of sacral and acetabular fractures (possibly because these are best seen in the coronal plane, requiring reformatting). MRI has the advantage over CT of being able to depict bone marrow oedema, fracture lines and surrounding soft tissue oedema, whereas CT can only depict trabecular or cortical fracture lines, focal sclerosis and adjacent resorption.
Cabarrus et al30 argued that in patients with a suspected insufficiency fracture, i.e. without a clear history of a fall, MRI is worthwhile, despite being more expensive and less available than CT, because the implications of a missed stress fracture both in terms of delayed treatment, additional days in complications and secondary complications of immobility.
Patient 6: 31-year-old female, right hand dominant, information technology worker who fell on her outstretched hand
She is the sole carer for two young children. Initial radiographs are normal but she has anatomical snuff box tenderness.
The scaphoid is the most commonly fractured carpal bone with a 16% prevalence of a true fracture in patients with clinically suspected fracture.31 Clinical examination has a high sensitivity and low specificity (average negative-predictive value of 74%),32 but initial radiographs only diagnose 75% of fractures.31 Plain radiograph interpretation has poor interobserver agreement, not improved by clinical experience.31 The non-union rate in conservatively treated fractures is 7.7%, increasing if treatment is delayed beyond 4 weeks.31 Therefore, patients with anatomical snuff box tenderness and a negative X-ray commonly undergo immobilization and further imaging and clinical review—the precise pathway varying from hospital to hospital. The rationale for immobilization is that an untreated scaphoid fracture may progress to non-union, avascular necrosis and osteoarthritis.
However, the evidence for immobilization and repeated radiographs is not at all clear: delayed radiographs may not improve diagnostic accuracy, and untreated radiologically occult fractures may not necessarily be more likely to progress to non-union.31 Meantime, three out of four patients with negative findings on the initial radiograph will likely undergo needless immobilization,32 and this overtreatment has an obvious impact on the patient's quality of life, ability to work as well as indirect economic costs to the patient and the healthcare system.33
A further area of controversy is whether occult fractures need to be treated. Like all fractures, scaphoid fractures may involve the cortex or the trabeculae alone. There is no definite evidence that trabecular fractures need treatment, or what the duration of treatment should be, if needed. It is recognized that fracture pattern is important in determining whether the fracture is likely to heal. A stable or incomplete fracture without cortical involvement is less likely to result in non-union.33 Therefore, trabeculae-only numbers may require shorter or no immobilization.
Both CT and MRI have a role in the identification of scaphoid fractures. MRI is considered the gold standard and limited sequences (<30-min study) have been advocated.32 MRI undoubtedly displays more trabeculae-only fractures than CT but multidetector CT (MDCT) is better at demonstrating cortical interruption as judged by the gold standard cortical fracture line at the 6-week follow-up.33 Cortical discontinuity is important as it implies increasing risk of displacement and therefore of non-union.33 However, MRI has the advantage of no IR and being able to demonstrate additional ligament injury.31,33 Both MRI and CT may demonstrate unsuspected fractures of other carpal bones.33 MDCT is, however, readily available and delivers negligible radiation if the arm is held about the head so that no other organs are in the primary beam of radiation.33
Jenkins et al calculated the theoretical cost of investigating a scaphoid fracture in the Scottish model. The cost of treating a patient with a fracture confirmed at 2-week review was £204, or £132 if no fracture is detected. The cost of using MRI and CT was £302 and £202, respectively.31 Hence, in a scenario with very limited healthcare resources, clinical examination, casting and delayed X-ray remain the most economic and safe option, with the disadvantage of routinely over treating patients.
When MRI is added to the usual management of suspected scaphoid fractures, healthcare costs increase when compared with traditional treatment. However, individual economic and productive costs may render this more cost-effective. At our institutions, the mean costs of wrist imaging are plain X-ray £31 ($47), CT £93 ($144), MRI £209 ($317), whereas the cost of an outpatient attendance is £136 ($206).
In the USA model, Dorsay et al32 calculated the cost of screening MRI to be $770, whereas the cost of traditional follow-up was $677, suggesting that the two models are fairly equivalent from a financial standpoint. However, when the cost to the patient of repeated hospital visits, and immobilization are all taken into account, the individual value of prompt MDCT or limited MRI becomes more convincing. In the case of our patient, the economical and practical disadvantages of being unnecessarily immobilized make a strong argument for CT or MRI. The choice between CT and MRI will be guided by local availability, but CT would be a reasonable choice to exclude a cortical fracture.33
14-year-old male, witnessed punch to head, no loss of consciousness, Glasgow Coma Scale 15, amnesia post-event for 10 min
Head injuries make up 10–20% of presentations to the ED,34 most of which are minor or mild. Of these, up to half of them are children aged below 15 years.35 The challenge is to identify the small proportion of patients at risk of a serious or fatal outcome. In younger children, the risk of non-accidental injury also needs to be considered.35 In the past, skull X-rays (SXRs) have often been performed, but these reveal a fracture in only 2% of cases. Admission to hospital for overnight observation is an alternative strategy, but the vast majority of patients are discharged without requiring further treatment, making this an expensive option from the point of view of healthcare provision.34 NICE issued guidelines in June 2003 (updated January 2014) recommending that CT replace SXR and observation/admission. The introduction of these rules increased the number of CT performed by up to four times the previous rate in one study, reduced the SXRs performed to an almost negligible number and in some cases halved the admission rate (9–4%). The authors of one multicentre study calculated that this saved £290 per 100 patients with head injury. In the past 15 years, guidelines on minor head injury imaging have been published by several different institutions and countries. Smits et al36 examined the sensitivity and specificity of three sets of guidelines: the Dutch guidelines, the NICE 2003 guidelines (based on the Canadian CT head rule) and the European Federation of Neurological Societies. While the European Federation guidelines ensured a sensitivity of 100% in identifying patients with clinically relevant traumatic findings at CT, their specificity was low. The Dutch guidelines would have missed almost 25% of patients requiring neurosurgery. They concluded that NICE guidelines achieve the best trade-off on specificity (44%) and sensitivity (94%) for identifying requirement for neurosurgical intervention. The 2014 NICE guidelines on head injury in children are reproduced in Table 2. In the case of this virtual patient, CT Head Injury care is recommended based on NICE recommendations of 2014 1.4.10. NICE also issues guidelines on information for parents and carers. Those patients who have abnormal CT scan, or who have a Glasgow Coma Scale <15 despite normal imaging require further in-hospital care, which should be under the management of a consultant-led team trained in Head35 Injury care and include regular nursing observations.
Table 2.
2014 NICE guidelines on paediatric head injury37
| Recommendation 1.4.9 |
| For children who have sustained a head injury and have any of the following risk factors, perform a CT head scan within 1 h of the risk factor being identified |
| Suspicion of non-accidental injury |
| Post-traumatic seizure but no history of epilepsy |
| On initial emergency department assessment, GCS <14, or for children under 1 year GCS (paediatric) <15 |
| At 2 h after the injury, GCS <15 |
| Suspected open or depressed skull fracture or tense fontanelle |
| Any sign of basal skull fracture (haemotympanum, “panda” eyes, cerebrospinal fluid leakage from the ear or nose, Battle's sign) |
| Focal neurological deficit |
| For children below 1 year of age, presence of bruise, swelling or laceration of >5 cm on the head |
| Recommendation 1.4.10 |
| For children who have sustained a head injury and have more than one of the following risk factors (and none of those in Recommendation 1.4.9), perform a CT head scan within 1 h of the risk factors being identified |
| Loss of consciousness lasting more than 5 min (witnessed) |
| Abnormal drowsiness |
| Three or more discrete episodes of vomiting |
| Dangerous mechanism of injury (high-speed road traffic accident either as pedestrian, cyclist or vehicle occupant, fall from a height of >3 m, high-speed injury from a projectile or other object) |
| Amnesia (antegrade or retrograde) lasting more than 5 min4 |
| Recommendation 1.4.11 |
| Children who have sustained a head injury and have only one of the risk factors in Recommendation 1.4.10 (and none of those in Recommendation 1.4.9) should be observed for a minimum of 4 h after the head injury. If, during observation, any of the risk factors mentioned below are identified, perform a CT head scan within 1 h |
| GCS l <15 |
| Further vomiting |
| A further episode of abnormal drowsiness |
GCS, Glasgow Coma Scale.
CONCLUSION
The increasing use of advanced imaging for ED patients is being driven by multiple factors and shows no sign of levelling off. This trend has risk–benefit implications for individual patients as well as an impact on other patients, staff and hospital infrastructures. In an idealized future hospital, advanced imaging would be available in the ED and would include both CT and dedicated MRI scanners available 24/7 with the workforce to support them. Healthcare planners worldwide need to respond to burgeoning advanced imaging activity and ideally to anticipate future demands in order to improve the patient experience further. In doing so the ability of each country's healthcare system to meet the inevitable increased costs must be realistically assessed and borne in mind during healthcare planning.
Contributor Information
Elizabeth A Dick, Email: e.dick@imperial.ac.uk, elizabethdick2010@gmail.com, Elizabeth.Dick@imperial.nhs.uk, e.dick@ic.ac.uk.
Dinesh Varma, Email: d.varma@alfred.org.au.
Elika Kashef, Email: elika.kashef@imperial.nhs.uk.
John Curtis, Email: jmcurtis@liverpool.ac.uk.
REFERENCES
- 1.Korley FK, Pham JC, Kirsch TD. Use of advanced radiology during visits to US emergency departments for injury-related conditions, 1998–2007. JAMA 2010; 304: 1465–71. doi: 10.1001/jama.2010.1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larson DB, Johnson LW, Schnell BM, Salisbury SR, Forman HP. National trends in CT use in the emergency department: 1995–2007. Radiology 2010; 258: 164–73. doi: 10.1148/radiol.10100640 [DOI] [PubMed] [Google Scholar]
- 3.Raby N. Magnetic resonance imaging of suspected scaphoid fractures using a low field dedicated extremity MR system. Clin Radiol 2001; 56: 316–20. doi: 10.1053/crad.2000.0657 [DOI] [PubMed] [Google Scholar]
- 4.Stoker J, van Randen A, Laméris W, Boermeester MA. Imaging patients with acute abdominal pain. Radiology 2009; 253: 31–46. doi: 10.1148/radiol.2531090302 [DOI] [PubMed] [Google Scholar]
- 5.Rao PM, Rhea JT, Novelline RA, Mostafavi AA, McCabe CJ. Effect of computed tomography of the appendix on treatment of patients and use of hospital resources. N Engl J Med 1998; 338: 141–6. doi: 10.1056/NEJM199801153380301 [DOI] [PubMed] [Google Scholar]
- 6.Fazel R, Krumholz HM, Wang Y, Ross JS, Chen J, Ting HH, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med 2009; 361: 849–57. doi: 10.1056/NEJMoa0901249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tien HC, Tremblay LN, Rizoli SB, Gelberg J, Spencer F, Caldwell C, et al. Radiation exposure from diagnostic imaging in severely injured trauma patients. J Trauma 2007; 62: 151–6. doi: 10.1097/TA.0b013e31802d9700 [DOI] [PubMed] [Google Scholar]
- 8.Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med 2007; 357: 2277–84. doi: 10.1056/NEJMra072149 [DOI] [PubMed] [Google Scholar]
- 9.Tsushima Y, Yamada S, Aoki J, Motojima T, Endo K. Effect of contrast-enhanced computed tomography on diagnosis and management of acute abdomen in adults. Clin Radiol 2002; 57: 507–13. doi: 10.1053/crad.2001.0925 [DOI] [PubMed] [Google Scholar]
- 10.MacKersie AB, Lane MJ, Gerhardt RT, Claypool HA, Keenan S, Katz DS, et al. Nontraumatic acute abdominal pain: unenhanced helical CT compared with three-view acute abdominal series. Radiology 2005; 237: 114–22. doi: 10.1148/radiol.2371040066 [DOI] [PubMed] [Google Scholar]
- 11.Lameris W, van Randen A, van Es HW, van Heesewijk JP, van Ramshorst B, Bouma WH, et al. ; OPTIMA study group. Imaging strategies for detection of urgent conditions in patients with acute abdominal pain: diagnostic accuracy study. BMJ 2009; 338: b2431. doi: 10.1136/bmj.b2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purysko AS, Remer EM, Filho HM, Bittencourt LK, Lima RV, Racy DJ. Beyond appendicitis: common and uncommon gastrointestinal causes of right lower quadrant abdominal pain at multidetector CT. Radiographics 2011; 31: 927–47. doi: 10.1148/rg.314105065 [DOI] [PubMed] [Google Scholar]
- 13.Dhillon S, Halligan S, Goh V, Matravers P, Chambers A, Remedios D. The therapeutic impact of abdominal ultrasound in patients with acute abdominal symptoms. Clin Radiol 2002; 57: 268–71. doi: 10.1053/crad.2001.0862 [DOI] [PubMed] [Google Scholar]
- 14.Sala E, Watson CJ, Beadsmoore C, Groot-Wassink T, Fanshawe TR, Smith JC, et al. A randomized, controlled trial of routine early abdominal computed tomography in patients presenting with non-specific acute abdominal pain. Clin Radiol 2007; 62: 961–9. doi: 10.1016/j.crad.2007.01.030 [DOI] [PubMed] [Google Scholar]
- 15.Coursey CA, Nelson RC, Patel MB, Cochran C, Dodd LG, Delong DM, et al. Making the diagnosis of acute appendicitis: do more preoperative CT scans mean fewer negative appendectomies? A 10-year study. Radiology 2010; 254: 460–8. doi: 10.1148/radiol.09082298 [DOI] [PubMed] [Google Scholar]
- 16.Birnbaum BA, Jeffrey RB, Jr. CT and sonographic evaluation of acute right lower quadrant abdominal pain. AJR Am J Roentgenol 1998; 170: 361–71. doi: 10.2214/ajr.170.2.9456947 [DOI] [PubMed] [Google Scholar]
- 17.Allemann F, Cassina P, Röthlin M, Largiadèr F. Ultrasound scans done by surgeons for patients with acute abdominal pain: a prospective study. Eur J Surg 1999; 165: 966–70. doi: 10.1080/110241599750008099 [DOI] [PubMed] [Google Scholar]
- 18.Gaitini D, Beck-Razi N, Mor-Yosef D, Fischer D, Ben Itzhak O, Krausz MM, et al. Diagnosing acute appendicitis in adults: accuracy of color Doppler sonography and MDCT compared with surgery and clinical follow-up. AJR Am J Roentgenol 2008; 190: 1300–6. doi: 10.2214/AJR.07.2955 [DOI] [PubMed] [Google Scholar]
- 19.Ganguli S, Raptopoulos V, Komlos F, Siewert B, Kruskal JB. Right lower quadrant pain: value of the nonvisualized appendix in patients at multidetector CT. Radiology 2006; 241: 175–80. doi: 10.1148/radiol.2411050191 [DOI] [PubMed] [Google Scholar]
- 20.Lee CC, Golub R, Singer AJ, Cantu R, Jr, Levinson H. Routine versus selective abdominal computed tomography scan in the evaluation of right lower quadrant pain: a randomized controlled trial. Acad Emerg Med 2007; 14: 117–22. doi: 10.1111/j.1553-2712.2007.tb01754.x [DOI] [PubMed] [Google Scholar]
- 21.Perez J, Barone JE, Wilbanks TO, Jorgensson D, Corvo PR. Liberal use of computed tomography scanning does not improve diagnostic accuracy in appendicitis. Am J Surg 2003; 185: 194–7. doi: 10.1016/S0002-9610(02)01364-8 [DOI] [PubMed] [Google Scholar]
- 22.Giuliano V, Giuliano C, Pinto F, Scaglione M. CT method for visualization of the appendix using a fixed oral dosage of diatrizoate–clinical experience in 525 cases. Emerg Radiol 2005; 11: 281–5. doi: 10.1007/s10140-005-0414-3 [DOI] [PubMed] [Google Scholar]
- 23.Long SS, Long C, Lai H, Macura KJ. Imaging strategies for right lower quadrant pain in pregnancy. AJR Am J Roentgenol 2011; 196: 4–12. doi: 10.2214/ajr.196.5_supplement.00a4 [DOI] [PubMed] [Google Scholar]
- 24.Pedrosa I, Levine D, Eyvazzadeh AD, Siewert B, Ngo L, Rofsky NM. MR imaging evaluation of acute appendicitis in pregnancy. Radiology 2006; 238: 891–9. doi: 10.1148/radiol.2383050146 [DOI] [PubMed] [Google Scholar]
- 25.Aubé C, Delorme B, Yzet T, Burtin P, Lebigot J, Pessaux P, et al. MR cholangiopancreatography versus endoscopic sonography in suspected common bile duct lithiasis: a prospective, comparative study. AJR Am J Roentgenol 2005; 184: 55–62. [DOI] [PubMed] [Google Scholar]
- 26.Adamek HE, Albert J, Weitz M, Breer H, Schilling D, Riemann JF. A prospective evaluation of magnetic resonance cholangiopancreatography in patients with suspected bile duct obstruction. Gut 1998; 43: 680–3. doi: 10.1136/gut.43.5.680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006; 64: 248–54. doi: 10.1016/j.gie.2005.12.038 [DOI] [PubMed] [Google Scholar]
- 28.Lubovsky O, Liebergall M, Mattan Y, Weil Y, Mosheiff R. Early diagnosis of occult hip fractures MRI versus CT scan. Injury 2005; 36: 788–92. doi: 10.1016/j.injury.2005.01.024 [DOI] [PubMed] [Google Scholar]
- 29.Hossain M, Barwick C, Sinha AK, Andrew JG. Is magnetic resonance imaging (MRI) necessary to exclude occult hip fracture? Injury 2007; 38: 1204–8. doi: 10.1016/j.injury.2007.04.023 [DOI] [PubMed] [Google Scholar]
- 30.Cabarrus MC, Ambekar A, Lu Y, Link TM. MRI and CT of insufficiency fractures of the pelvis and the proximal femur. AJR Am J Roentgenol 2008; 191: 995–1001. doi: 10.2214/AJR.07.3714 [DOI] [PubMed] [Google Scholar]
- 31.Jenkins PJ, Slade K, Huntley JS, Robinson CM. A comparative analysis of the accuracy, diagnostic uncertainty and cost of imaging modalities in suspected scaphoid fractures. Injury 2008; 39: 768–74. doi: 10.1016/j.injury.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 32.Dorsay TA, Major NM, Helms CA. Cost-effectiveness of immediate MR imaging versus traditional follow-up for revealing radiographically occult scaphoid fractures. AJR Am J Roentgenol 2001; 177: 1257–63. doi: 10.2214/ajr.177.6.1771257 [DOI] [PubMed] [Google Scholar]
- 33.Memarsadeghi M, Breitenseher MJ, Schaefer-Prokop C, Weber M, Aldrian S, Gäbler C, et al. Occult scaphoid fractures: comparison of multidetector CT and MR imaging–initial experience. Radiology 2006; 240: 169–76. doi: 10.1148/radiol.2401050412 [DOI] [PubMed] [Google Scholar]
- 34.Hassan Z, Smith M, Littlewood S, Bouamra O, Hughes D, Biggin C, et al. Head injuries: a study evaluating the impact of the NICE head injury guidelines. Emerg Med J 2005; 22: 845–9. doi: 10.1136/emj.2004.021717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.NICE. Head injury: triage, assessment, investigation and early management of head injury in children, young people and adults. Available from: https://www.nice.org.uk/guidance/cg176 [PubMed] [Google Scholar]
- 36.Smits M, Dippel DW, de Haan GG, Dekker HM, Vos PE, Kool DR, et al. Minor head injury: guidelines for the use of CT–a multicenter validation study. Radiology 2007; 245: 831–8. doi: 10.1148/radiol.2452061509 [DOI] [PubMed] [Google Scholar]
- 37.National Institute for Health and Care Excellence. Head injury: assessment and early management. Available from: http://www.nice.org.uk/guidance/cg176/chapter/1-recommendations