Abstract
Ischemic colitis (IC) is the most common vascular disorder of the gastrointestinal tract with a reported incidence of 6.1–44 cases/100,000 person years with confirmatory histopathology. However, the true incidence of IC poses some difficulty, and even vigilant clinicians with patients at high risk often miss the diagnosis, since clinical presentation is non-specific or could have a mild transient nature. Detection of IC results is crucial to plan the correct therapeutic approach and reduce the reported mortality rate (4–12%). Diagnosis of IC is based on a combination of clinical suspicion, radiological, endoscopic and histological findings. Some consider colonoscopy as a diagnostic test of choice; however, preparation is required and it is not without risk, above all in patients who are severely ill. There are two manifestations of vascular colonic insult: ischaemic and reperfusive. The first one occurs above all during ischaemic/non-occlusive mesenteric ischaemia; in this case, the colonic wall appears thinned with dilated lumen and fluid appears in the paracolic space. When reperfusion occurs, the large bowel wall appears thickened and stratified, because of subepithelial oedema and/or haemorrhage, with consequent lumen calibre reduction. Shaggy contour of the involved intestine and misty mesentery are associated with the pericolic fluid. The pericolic fluid results are a crucial finding for IC diagnosis since its evidence suggests the presence of an ongoing damage thus focusing the attention on other pathological aspects which could be otherwise misdiagnosed, such as thinned or thickened colonic wall. Moreover, the pericolic fluid may increase or decrease, depending on the evolution of the ischaemic damage, suggesting the decision of medical or surgical treatment. Radiologists should not forget the hypothesis of IC, being aware that multidetector CT could be sufficient to suggest the diagnosis of IC, allowing for early identification and grading definition, and in a short-term follow-up, discriminating patients who need urgent surgery from patients in whom medical treatment and follow-up can be proposed.
INTRODUCTION
Ischaemic colitis (IC) is the most common vascular disorder of the gastrointestinal tract1,2 and the second most frequent cause of lower gastrointestinal bleeding.3
IC is the consequence of an acute interruption or chronic decrease in the colonic blood supply,4 which results in ischaemic necrosis of variable severity.5,6
The incidence of IC is underestimated because it could be a misdiagnosed cause of acute abdomen; otherwise, it could have a mild transient nature, so clinical presentation can be non-specific and highly variable.7
Although up to 85% of cases of IC, when conservatively managed, improve within 1 or 2 days and IC resolves completely within 1 or 2 weeks, close to one-fifth of patients develop peritonitis or clinically deteriorate, requiring surgery. Surgery should be limited to the excision of the irreversibly necrotized intestine, as it can lead to bacteraemia and sepsis, colonic stricture, persistent abdominal pain and bloody diarrhoea and protein-losing enteropathy.7
That being so, in order to plan the correct therapeutic approach of IC, firstly, it is crucial to detect this condition early and, second, to discriminate patients needing urgent operative intervention from patients in whom follow-up can be proposed as an alternative to surgery.8
Aetiological factors in colonic ischaemia
IC is the consequence of an acute interruption or chronic decrease in the colonic blood supply,4 which may be either occlusive or non-occlusive in origin.3
Many medical conditions and medications can cause ischaemia by reducing blood flow to the colon.6
The most common cause of IC is low flow state9 and can be considered as a form of non-occlusive ischaemic disease (NOMI). In a minor percentage of cases, IC can be due to occlusive causes (37.5% in our series) such as inferior mesenteric artery (IMA) thrombosis, embolism or atherosclerosis.10
Vascular colonic anatomy
Two major arteries supply most of the blood to the colon: the superior mesenteric artery (SMA) which supplies blood to the ascending and transverse colon and the IMA which supplies blood to the descending and sigmoid colon. The internal iliac arteries supply the rectum.
The splenic flexure and sigmoid colon are the regions where the two circulations (SMA and IMA) meet each other (so-called watershed areas).
The differences in the mode of division of the IMA are numerous, but most are referable to a common pattern. The IMA arises from the abdominal aorta and proceeds towards the pelvis, giving off its first branch, the left colic artery. The latter ascends directly towards the splenic flexure. The sigmoid arteries exhibit two principal modes of origin. There is great variability in the mode of division of the sigmoid arteries, but the exact topographical pattern of these vessels is of small surgical importance.
The vessels of the large bowel differ from those of the small intestine in that they do not form so many arcades and have, alongside the mesenteric border of the colon, a marginal artery from which the vasa recti arise. While the vasa recti do not mutually anastomose, the marginal artery, first described by von Haller (1786)11 and later by Drummond (1913),12 is composed of the terminal portions of the branches from the major vascular arcades and therefore forms an important anastomosis between them: it can keep the left colon viable when the IMA is occluded or is ligated during rectosigmoidectomy.
The adequacy of the blood supply to the distal colon is dependent on the marginal artery. In the absence of arterial disease, the entire colon and rectum may be vascularized from branches of the SMA through the medium of this marginal vessel.13
Watershed areas
Watershed areas correspond to the splenic flexure, also known as Griffith's point, and sigmoid colon, or Sudeck's point. These regions are particularly vulnerable to ischaemic insults, being located between two different vascular systems.
At the splenic flexure, the link between SMA and IMA systems and the marginal artery of Drummond is occasionally tenuous and is absent in up to 5% of patients; a 1.2–2.8 cm2 area may be devoid of vasa recta.
Sudeck's point refers to the anastomosis between the sigmoid arteries and superior haemorroidary artery originating from IMA.
A third potential watershed area is the right colon, where the marginal vessel is poorly developed in up to 50% of people.14–16
Diagnostic imaging modalities
Different imaging methods have been used to diagnose IC, including plain radiography of the abdomen, barium enema (BE), ultrasound, CT, MRI and colonoscopy. All can suggest or support the diagnosis, but none have findings that are specific enough to make a definitive diagnosis, except when infarction has occurred.17
Plain radiography
Plain radiography may show suggestive signs of disease such as “thumbprinting”, which appears as rounded densities along the sides of a gas-filled distended colon, loss of haustration and dilation of the colonic loops; in one retrospective report, these findings were present in 21% of 41 patients with IC and no patient had the signs of advanced changes such as intramural gas “pneumatosis linearis”, portal venous gas and megacolon.18
Barium enema
BE has been reported to have a sensitivity approaching 80%.19 Today, it has a limited role in diagnosis and has been replaced by CT and colonoscopy, largely because of the latter's greater accuracy and ability to allow sampling of the mucosa for pathology.
Moreover, BE should not be performed in any patient with suspicion of gangrene, perforation or peritonitis.
Ultrasound
Although ultrasound is not the standard diagnostic tool for patients with suspected IC, it could be useful in suggesting the pathology by revealing some related findings: symmetric bowel wall thickening (100%), segmental involvement of the colon (80%), preservation of colon wall stratification (66%), altered pericolic fat (28%), free fluid (19%) and pneumatosis (1.7%).20
However, experience with ultrasonography in the setting of IC is very limited, and it is believed that this technique lacks specificity for bowel wall thickening and has a high false-negative rate.21
False-negative sonographic studies can be explained by several circumstances. In patients who have early ischaemia, imaging findings can be normal. IC with wall thinning can be missed on ultrasound, although this circumstance is more frequent in acute mesenteric ischaemia.21
CT
CT examination with i.v. contrast media is actually considered the main technique for the non-invasive diagnosis of IC and also in cases of acute abdomen of different and various origins, as it is readily available in the emergency department;22,23 it can suggest IC when it is unsuspected, can diagnose complications and exclude other illnesses.24
In case of abdominal pain, there are no pathognomonic CT findings of IC.25
IC is usually non-occlusive in nature, but CT (or CT angiography) can be used to identify whether or not vascular occlusions are present. With emerging technology, contrast-enhanced CT can sometimes assess the patency of the celiac, SMA and IMA without devoted CT angiography. Unfortunately, the IMA cannot be considerate a specific finding of IC, since it is occluded in almost 10% of patients over 60 years of age who are asymptomatic.26
Few data exist on the sensitivity and specificity of CT for assessing vascular occlusion in IC; a meta-analysis regarding sensitivity and specificity of CT in the diagnosis of mesenteric arterial embolism, mesenteric arterial thrombosis and mesenteric venous thrombosis reported a sensitivity and specificity of 93.3% and 95.9%, respectively.27
MRI
As recently proposed,28–31 MRI may be a substitute for invasive procedures in diagnosing and grading IC, allowing for the early identification of pathological findings and for the definition of the grade of ischaemic lesions.
This method has been formally studied in only a small number of patients with IC; findings are similar to those of CT and as with CT, such findings usually are not specific enough to make a definitive diagnosis.
The advantage of using MRI for the clinical management of IC is the possibility of performing a short-term follow-up without the employment of ionizing radiation or i.v. contrast material.
In particular, MRI can discriminate patients with urgent operative intervention from patients in whom follow-up can be proposed as an alternative to surgery.
Colonoscopy
Colonoscopy is often considered the diagnostic test of choice in establishing the diagnosis of IC, being the test with higher sensitivity for this pathology. It is possible to demonstrate mucosal changes and perform biopsies on them at the same time.
However, just after one colonoscopy, the diagnosis of IC cannot be confirmed unless mucosal gangrene is observed or infarction or ghost cells are evident at histopathology; thus, these histopathological findings are uncommon and of limited value for IC diagnosis.32
In case of severe IC, CT should be performed to evaluate the distribution of disease, whereas focused colonoscopy should be realized to confirm the nature of the CT abnormality. The endoscopic examination should be extended just till the distal limit of the damaged bowel.
Colonoscopy should not be avoided in case of acute peritonitis or evidence of irreversible ischaemic damage (i.e. gangrene and pneumatosis), and it could be performed without bowel preparation to prevent hypoperfusion caused by dehydrating cathartics; in addition, it is not available in all the emergency departments”.33
Focusing attention on both the diagnosis and follow-up of IC, this article aimed to describe the CT radiological findings, which are useful to recognize this entity in correlation with aetiology, occlusive and non-occlusive forms, to distinguish its phase, acute, subacute and chronic, and extension and to identify the presence of reperfusion characterizing its effectiveness.
Radiological diagnosis
CT imaging and technique
CT in acute abdomen is useful to exclude serious medical conditions other than IC e.g. diverticulitis, suggest the diagnosis of IC and reveal which areas of the colon are involved, as well as recognize its phase and presence of reperfusion.
CT images should be obtained with a spiral technique in a craniocaudal direction (from the base of the lungs to the pelvic brim) and with the patient in supine position.
Oral and rectal administration of contrast material is not recommended (or useful) for accurate CT examination and assessment of acute large bowel ischaemia, as it does not add any additional information for the final diagnosis.
Acquisition of both unenhanced and contrast-enhanced CT scans is always necessary, acquiring images in the late arterial phase (start delay 45–50 s) and in the portal venous phase (start delay 70–80 s) with an i.v. injection of 2 ml kg−1 of non-ionic contrast material followed by 40 ml of saline solution using a peristaltic semi-automated power injector (4 ml s−1 flow rate) with an 18-gauge needle in the antecubital vein.
Unenhanced CT is useful to not only identify reperfusive phenomena or intramural haemorrhage, appearing as high-attenuation areas of the large bowel wall, but also constitute a baseline Hounsfield unit value of the large bowel wall for the assessment of contrast enhancement.
After medical treatment, unenhanced CT could be useful to monitor the effectiveness of reperfusion, evaluate the reduction or increase of the retroperitoneal fluid as well as the large bowel calibre and to detect the onset of large bowel wall necrosis such as the presence of intestinal pneumatosis.33 On the other hand, thanks to contrast-enhanced CT, it is possible to evaluate the degree of large bowel wall enhancement, which becomes absent in the case of necrosis, and the presence of ischaemic signs on splanchnic organs. Contrast-enhanced scans should be realized with an i.v. injection of 2 ml kg−1 of non-ionic contrast material followed by 40 ml of saline solution using an automated power injector (3–4 ml s−1 flow rate), with an 18-gauge needle in the antecubital vein and should be performed in the late arterial phase (start delay 45–50 s) and in the portal venous phase (start delay 70–80 s). It is always recommended to visualize the CT images with a window setting for both the soft tissue [width: 300–350; level: 40–50), to detect the changes in the large bowel wall, splanchnic organs, mesentery and vessels, and lung parenchyma (width: 450–100; level: 100–0), to recognize the presence extraluminal gas.34
Radiological key points
In the diagnosis of IC, radiologists should indicate:
-
the type of damage, which could be:
◦ ischaemic
◦ reperfusive; and in this case, it should be indicated whether the reperfusion is effective or not
the location and extension of the damage
-
the phase of the damage, which could be:
◦ acute
◦ subacute
◦ chronic.
The types of damage
Arterial ischaemic damage
The colon is particularly susceptible to ischaemia, perhaps owing to its relatively low blood flow, its unique decrease in blood flow during periods of functional activity and its sensitivity to autonomic stimulation. The degree to which colonic blood flow must diminish before ischaemia results varies with the acuteness of the event, degree of pre-existing vascular collateralization and length of time the low flow state persists. As mentioned above, the interruption or decrease in the colonic blood supply may be either occlusive or non-occlusive in origin (NOMI). In occlusive forms, CT may demonstrate a thrombus or embolus in the corresponding mesenteric vessel.
When there is an interruption in the colonic blood supply without reperfusion, the colonic wall remains thin or “paper thin” and unenhanced, associated with dilation of the lumen.35 Thinning of the large bowel wall or “paper-thin wall” is caused by the volume loss of tissue and vessels in the large bowel wall and the loss of intestinal muscular tone owing to lack of blood flow. Pericolic fluid could be present and is located in the paracolic recess.9,20 The i.v. contrast media allow the evaluation of the large wall enhancement: it results large bowel wall hypodensity on enhanced CT, related to the absence of effective reperfusion due to the large bowel wall ischaemic injury. These findings are useful in diagnosing IC before reperfusion, being the only sign present beyond dilation only gas-filled and “paper-thin wall”. However, the latter could be misdiagnosed owing to the similarity with the physiological distension of the colon caused by the presence of intestinal gas. Vascular pneumatosis is due to large bowel infarction in case of advanced and irreversible damage as well as pneumoperitoneum/pneumoretroperitoneum or mesenteric pneumatosis. In case of NOMI, beyond intestinal damage, there is also a reduction of contrast enhancement of abdominal parenchyma, most commonly in the liver, spleen, kidneys and pancreas (Figure 1).
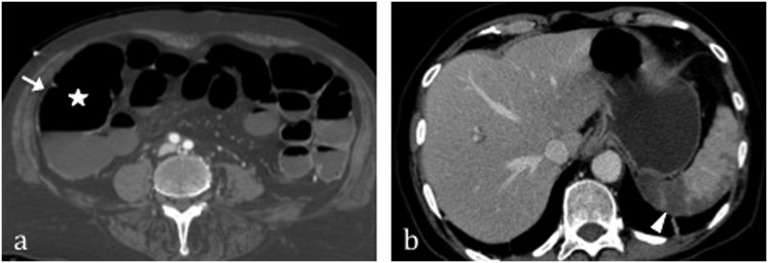
Figure 1.
Arterial ischaemic damage in a patient with heart failure and consequent non-occlusive mesenteric ischaemia without reperfusion. Note the “paper thin” and unenhanced large bowel wall (arrow) associated with dilation of the lumen (star) (a). (b) Splenic infarction is evident (arrowhead).
Reperfusive damage
Ischaemia, both occlusive or NOMI, may be followed by reperfusion injury and, for relatively brief periods of ischaemia, this combined injury may produce more damage than just reduction of blood flow without reperfusion. In case of IMA occlusion, reperfusion is always present if SMA is still viable. In this case, the blood supply to the left colon is restored from SMA, after a more or less long-lasting ischaemic period, which is dependent on the effectiveness of the anastomotic circles between IMA and SMA. The appearance of reperfused large bowel differs from the non-reperfused. It appears as thickening of the large bowel wall, usually resulting from subepithelial oedema and/or haemorrhage, with consequent lumen calibre reduction.
The unenhanced phase may detect any haemorrhagic phenomenon of the large bowel wall; mucosal hyperdensity from haemorrhagic phenomena may determine a typical feature: the “little rose” sign.36,37
In a thickened large bowel segment, a stratified enhancement pattern corresponding to the classic target sign is useful for excluding malignant conditions. This pattern is produced by alternating rings of enhancing mucosa with adjacent hypodense oedematous submucosa. Shaggy contour of the involved intestine and misty mesentery is associated with the pericolic fluid, which may increase or decrease, depending on the evolution of the ischaemic damage (Figure 2).
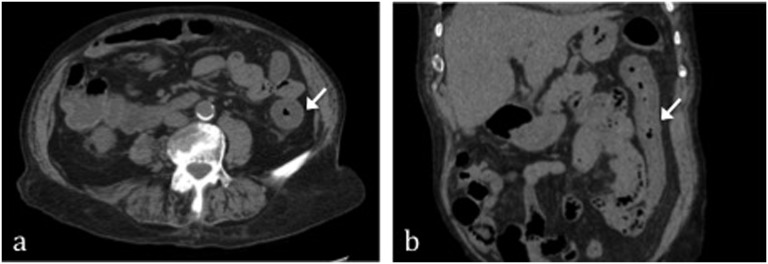
Figure 2.
Reperfusive damage after superior mesenteric artery occlusion in a patient with inferior mesenteric artery ligation for previous sigmoidectomy. On multidetector CT in the acute phase, it appears as thickening of the large bowel wall [arrows in (a) and (b)] with consequent lumen calibre reduction.
In the chronic phase, pericolic fluid is absent; in the injured colonic wall, fibrotic reaction develops leading to continuous mild and irregular circumferential thickening with gaping lumen,18,38 as well as the target sign, due to acute mucosal haemorrhagic and oedematous phenomena, is absent.
Location and extension
A segmental pattern is typical in case of occlusive IC, which can be explained by the vascular anatomy of the colon and rectum, where watershed-susceptible areas exist.
The left colon is affected most often (32.6%), followed by the distal colon (24.6%), right colon (25.2%) and entire colon (7.3%).39
No colonic region is spared from involvement in case of NOMI.40
Previous studies41 report that ischaemic changes are continuously and uniformly distributed along an entire vascular colonic territory, with a marked distinction between ischaemic and non-ischaemic tracts; this constitutes a differential diagnostic element with other pathologies having similar imaging features but different distributions, such as Crohn's disease or other inflammatory conditions.42
Phase of damage
Imaging allows determining the morphofunctional alterations associated with IC and allows estimating the timing of ischaemic damage.
In the acute phase, the main differential findings between occlusive IC and non-occlusive IC (NOMI) are represented by the paper-thin large bowel wall with dilated lumen in case of NOMI vs large bowel wall thickening and related signs, such as target sign, and lumen calibre reduction in case of IMA occlusion.
In the subacute phase, it is important to evaluate the effectiveness of reperfusion: in this sense, the changes in the large bowel wall thickness and in the pericolic fluid amount are useful parameters to be monitored. In case of effective reperfusion, large bowel wall thickening should decrease with progressive gaping of the lumen, while pericolic fluid should decrease (Figures 2 and 3).
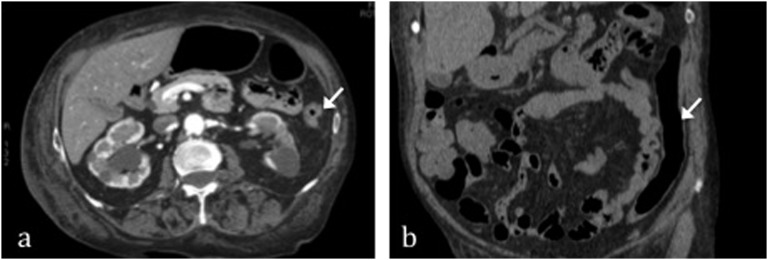
Figure 3.
The same patient as in Figure 2. 2 days after, thanks to blood supply restoration, CT shows a reduction of large bowel wall thickening and progressive gaping of lumen, arrows in (a) and (b).
In the chronic phase, rather than define the aetiology, it is important to assess the effects of the ischaemic injury, represented by non-uniform fibrosis of the large bowel wall (Figure 4).
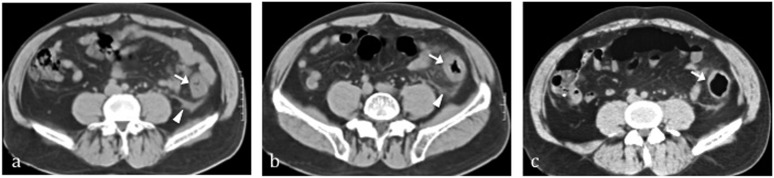
Figure 4.
Reperfusive damage from still available superior mesenteric artery in a patient with inferior mesenteric artery occlusion. In the acute phase (Day 1), (a) it appears as thickening of the large bowel wall on multidetector CT with consequent lumen calibre reduction and the typical feature of “little rose” sign (arrow). Shaggy contour of the involved intestine and misty mesentery is associated with the pericolic fluid (arrowhead). In the subacute phase (Day 3), (b) thanks to an effective reperfusion large bowel wall thickening decrease with progressive gaping of lumen (white arrow) while pericolic fluid decreases (arrowhead). The patient underwent new CT in the chronic phase, (c) showing fibrotic reaction that led to continuous mild and irregular circumferential thickening with gaping lumen (arrow).
The more difficult form of IC to be detected at imaging is NOMI before reperfusion, since only the paper-thin wall can be misdiagnosed like a normal colon; in this case, given the suspicion induced by medical history, particular attention should be paid to the presence of pericolic fluid as well as ischaemic phenomena of the parenchymal organs.39
Also, in the subacute phase, the thickened affected colonic wall could be misinterpreted as a normal tract with collapsed lumen instead of as pathology, whereas in chronic forms, the irregular thickening of the large bowel could be misdiagnosed, if the patient's clinical history is unknown.
Radiological finding classification
The radiological findings can be summarized in five main categories30,43,44 being variously combined, as described above, in relation to the pathophysiology of the developing anoxic process and to the timing of the examination:
- Vessel findings
- vascular occlusion—resulting as a defect or stop in the vessel lumen
- vascular pneumatosis—presence of gas within the mesenteric venules and portal venous system
- Peritoneal/retroperitoneal cavity findings
- free fluid—presence of fluid in the peritoneal and/or retroperitoneal cavity
- pneumoperitoneum/pneumoretroperitoneum—presence of gas within the peritoneal or retroperitoneal spaces
- Bowel wall findings
- bowel wall diameter—bowel-loop dilatation, defined as a large bowel diameter >8 cm
- wall thickness is considered normal when <3 mm, with distended bowel loops; in the absence of distension, the wall is considered pathological when its thickness is greater than approximately 1 cm
- wall enhancement is considered pathological in the presence of a double-halo or target configuration (two or three concentric rings) or when absent
- wall pneumatosis is defined as the presence of small air bubbles within the bowel wall.
- Mesentery findings
- pericolic streakiness—corresponds to shaggy contour of the involved intestine and misty mesentery
- mesenteric pneumatosis is defined as the presence of small air bubbles within the mesenteric fat.
- Parenchymal findings
- ischaemic injuries—signs of ischaemic damage involving the liver, kidneys, spleen and pancreas.
At CT scan, parenchymal infarctions could be depicted as a hypodense parenchymal area, at times triangular in shape, with sharp peripheral contours or as a rounded central or marginal area with irregular appearance and ill-defined shape. Moreover, CT could show intralesional gas not correlated to bacterial infection.
In the hyperacute phase, CT may show areas of mottled increased attenuation, representing areas of haemorrhagic infarct. In the acute–subacute phase, infarcts tend to become focal and progressively better demarcated. In the chronic phase, infarcts may disappear completely; but, more commonly, they reveal progressive volume loss caused by fibrotic contraction of the infarct.45,46
CONCLUSION
There are two different manifestations of vascular colonic insult: ischaemic and reperfusive. In the ischaemic damage, the colonic wall appears thinned with dilated lumen and fluid appears in the paracolic space. When reperfusion occurs, the large bowel wall appears thickened and stratified, because of subepithelial oedema and/or haemorrhage, with consequent lumen calibre reduction.
The pericolic fluid is a crucial finding for IC diagnosis since its evidence suggests the presence of ongoing damage thus focusing the attention on other pathological aspects which could be misdiagnosed: in fact, in case of ischaemic IC, the dilated thinned colonic wall could be misinterpreted as a physiological colonic distension caused by the presence of intestinal gas. In case of reperfusion, the amount of pericolic fluid may increase or decrease, depending on the evolution of the ischaemic damage, suggesting the decision of medical or surgical treatment.
Contributor Information
Daniela Berritto, Email: berritto.daniela@gmail.com.
Francesca Iacobellis, Email: francesca.iacobellis@libero.it.
Maria Antonietta Mazzei, Email: mamazzei@gmail.com.
Luca Volterrani, Email: volterrani@unisi.it.
Giuseppe Guglielmi, Email: giuseppe.guglielmi@unifg.it.
Luca Brunese, Email: luca.brunese@unimol.it.
Roberto Grassi, Email: roberto.grassi@unina2.it.
REFERENCES
- 1.Theodoropoulou A, Koutroubakis IE. Ischemic colitis: clinical practice in diagnosis and treatment. World J Gastroenterol 2008; 14: 7302–8. doi: 10.3748/wjg.14.7302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgins PD, Davis KJ, Laine L. Systematic review: the epidemiology of ischaemic colitis. Aliment Pharmacol Ther 2004; 19: 729–38. doi: 10.1111/j.1365-2036.2004.01903.x [DOI] [PubMed] [Google Scholar]
- 3.Paterno F, Longo WE. The etiology and pathogenesis of vascular disorders of the intestine. Radiol Clin North Am 2008; 46: 877–85. doi: 10.1016/j.rcl.2008.06.005 [DOI] [PubMed] [Google Scholar]
- 4.Westgeest HM, Akol H, Schreuder TC. Pure naratriptan-induced ischemic colitis: a case report. Turk J Gastroenterol 2010; 21: 42–4. doi: 10.4318/tjg.2010.0047 [DOI] [PubMed] [Google Scholar]
- 5.Stamatakos M, Douzinas E, Stefanaki C, Petropoulou C, Arampatzi H, Safioleas C, et al. Ischemic colitis: surging waves of update. Tohoku J Exp Med 2009; 218: 83–92. doi: 10.1620/tjem.218.83 [DOI] [PubMed] [Google Scholar]
- 6.Baixauli J, Kiran RP, Delaney CP. Investigation and management of ischemic colitis. Cleve Clin J Med 2003; 70: 920–1. doi: 10.3949/ccjm.70.11.920 [DOI] [PubMed] [Google Scholar]
- 7.Elder K, Lashner BA, Al Solaiman F. Clinical approach to colonic ischemia. Cleve Clin J Med 2009; 76: 401–9. doi: 10.3949/ccjm.76a.08089 [DOI] [PubMed] [Google Scholar]
- 8.Reginelli A, Iacobellis F, Berritto D, Gagliardi G, Di Grezia G, Rossi M, et al. Mesenteric ischemia: the importance of differential diagnosis for the surgeon. BMC Surg 2013; 13 Suppl. 2: S51. doi: 10.1186/1471-2482-13-S2-S51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furukawa A, Kanasaki S, Kono N, Wakamiya M, Tanaka T, Takahashi M, et al. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol 2009; 192: 408–16. doi: 10.2214/AJR.08.1138 [DOI] [PubMed] [Google Scholar]
- 10.Siegelman SS, Sprayregen S, Boley SJ. Angiographic diagnosis of mesenteric arterial vasoconstriction. Radiology 1974; 112: 533–42. doi: 10.1148/112.3.533 [DOI] [PubMed] [Google Scholar]
- 11.Drummond H. Some points relating to the surgical anatomy of the arterial supply of the large intestine. Proc R Soc Med 1914; 7: 185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Haller A. First lines of physiology 2. Edinburgh, UK: Elliot; 1786. pp. 139. [Google Scholar]
- 13.Griffiths JD. Surgical anatomy of the blood supply of the distal colon. Ann R Coll Surg Engl 1956; 19: 241–256. [PMC free article] [PubMed] [Google Scholar]
- 14.Boley SJ, Brandt LJ, Veith FJ. Ischemic disorders of the intestines. Curr Probl Surg 1978; 15: 1–85. doi: 10.1016/S0011-3840(78)80018-5 [DOI] [PubMed] [Google Scholar]
- 15.Brandt LJ, Boley SJ. Colonic ischemia. Surg Clin North Am 1992; 72: 203–29. [DOI] [PubMed] [Google Scholar]
- 16.Bharucha AE, Tremaine WJ, Johnson CD, Batts KP. Ischemic proctosigmoiditis. Am J Gastroenterol 1996; 91: 2305–9. [PubMed] [Google Scholar]
- 17.Oliva IB, Davarpanah AH, Rybicki FJ, Desjardins B, Flamm SD, Francois CJ, et al. ACR Appropriateness Criteria® imaging of mesenteric ischemia. Abdom Imaging 2013; 38: 714–9. doi: 10.1007/s00261-012-9975-2 [DOI] [PubMed] [Google Scholar]
- 18.Wittenberg J, Athanasoulis CA, Williams LF, Jr, Paredes S, O'Sullivan P, Brown B. Ischemic colitis. Radiology and pathophysiology. Am J Roentgenol Radium Ther Nucl Med 1975; 123: 287–300. doi: 10.2214/ajr.123.2.287 [DOI] [PubMed] [Google Scholar]
- 19.Iida M, Matsui T, Fuchigami T, Iwashita A, Yao T, Fujishima M. Ischemic colitis: serial changes in double contrast barium enema examinations. Radiology 1986; 159: 337–41. doi: 10.1148/radiology.159.2.3961164 [DOI] [PubMed] [Google Scholar]
- 20.Ripollés T, Simó L, Martínez-Pérez MJ, Pastor MR, Igual A, López A. Sonographic findings in ischemic colitis in 58 patients. AJR Am J Roentgenol 2005; 184: 777–85. [DOI] [PubMed] [Google Scholar]
- 21.Taourel P, Aufort S, Merigeaud S, Doyon FC, Hoquet MD, Delabrousse E. Imaging of ischemic colitis. Radiol Clin North Am 2008; 46: 909–24. doi: 10.1016/j.rcl.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 22.Boley SJ, Schwartz S, Lash J, Sternhill V. Reversible vascular occlusion of the colon. Surg Gynecol Obstet 1963; 116: 53–60. [PubMed] [Google Scholar]
- 23.Reginelli A, Genovese E, Cappabianca S, Iacobellis F, Berritto D, Fonio P, et al. Intestinal ischemia: US-CT findings correlations. Crit Ultrasound J 2013; 5 Suppl. 1: S7. doi: 10.1186/2036-7902-5-S1-S7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scaglione M, Grassi R, Pinto A, Giovine S, Gagliardi N, Stavolo C, et al. Positive predictive value and negative predictive value of spiral CT in the diagnosis of closed loop obstruction complicated by intestinal ischemia. Radiol Med 2004; 107: 69–77. [PubMed] [Google Scholar]
- 25.Wolff JH, Rubin A, Potter JD, Lattimore W, Resnick MB, Murphy BL, et al. Clinical significance of colonoscopic findings associated with colonic thickening on computed tomography: is colonoscopy warranted when thickening is detected? J Clin Gastroenterol 2008; 42: 472–5. doi: 10.1097/MCG.0b013e31804c7065 [DOI] [PubMed] [Google Scholar]
- 26.Bailey JA, Jacobs DL, Bahadursingh A, Longo WE. Endovascular treatment of segmental ischemic colitis. Dig Dis Sci 2005; 50: 774–9. doi: 10.1007/s10620-005-2572-2 [DOI] [PubMed] [Google Scholar]
- 27.Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology 2010; 256: 93–101. doi: 10.1148/radiol.10091938 [DOI] [PubMed] [Google Scholar]
- 28.Iacobellis F, Berritto D, Somma F, Cavaliere C, Corona M, Cozzolino S, et al. Magnetic resonance imaging: a new tool for diagnosis of acute ischemic colitis? World J Gastroenterol 2012; 18: 1496–501. doi: 10.3748/wjg.v18.i13.1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berritto D, Somma F, Landi N, Cavaliere C, Corona M, Russo S, et al. Seven-Tesla micro-MRI in early detection of acute arterial ischaemia: evolution of findings in an in vivo rat model. Radiol Med 2011; 116: 829–41. doi: 10.1007/s11547-011-0676-7 [DOI] [PubMed] [Google Scholar]
- 30.Mazzei MA, Guerrini S, Cioffi Squitieri N, Imbriaco G, Chieca R, Civitelli S, et al. Magnetic resonance imaging: is there a role in clinical management for acute ischemic colitis? World J Gastroenterol 2013; 19: 1256–63. doi: 10.3748/wjg.v19.i8.1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grassi R, Cavaliere C, Cozzolino S, Mansi L, Cirillo S, Tedeschi G, et al. Small animal imaging facility: new perspectives for the radiologist. Radiol Med 2009; 114: 152–67. doi: 10.1007/s11547-008-0352-8 [DOI] [PubMed] [Google Scholar]
- 32.Montoro MA, Brandt LJ, Santolaria S, Gomollon F, Sánchez Puértolas B, Vera J, et al. Clinical patterns and outcomes of ischaemic colitis: results of the Working Group for the Study of Ischaemic Colitis in Spain (CIE study). Scand J Gastroenterol 2011; 46: 236–46. doi: 10.3109/00365521.2010.525794 [DOI] [PubMed] [Google Scholar]
- 33.Lassandro F, Mangoni de Santo Stefano ML, Porto AM, Grassi R, Scaglione M, Rotondo A. Intestinal pneumatosis in adults: diagnostic and prognostic value. Emerg Radiol 2010; 17: 361–5. doi: 10.1007/s10140-010-0868-9 [DOI] [PubMed] [Google Scholar]
- 34.Moschetta M, Stabile Ianora AA, Pedote P, Scardapane A, Angelelli G. Prognostic value of multidetector computed tomography in bowel infarction. Radiol Med 2009; 114: 780–91. doi: 10.1007/s11547-009-0422-6 [DOI] [PubMed] [Google Scholar]
- 35.Saba L, Berritto D, Iacobellis F, Scaglione M, Castaldo S, Cozzolino S, et al. Acute arterial mesenteric ischemia and reperfusion: macroscopic and MRI findings, preliminary report. World J Gastroenterol 2013; 19: 6825–33. doi: 10.3748/wjg.v19.i40.6825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romano S, Romano L, Grassi R. Multidetector row computed tomography findings from ischemia to infarction of the large bowel. Eur J Radiol 2007; 61: 433–41. [DOI] [PubMed] [Google Scholar]
- 37.Romano S, Lassandro F, Scaglione M, Romano L, Rotondo A, Grassi R. Ischemia and infarction of the small bowel and colon. spectrum of imaging findings. Abdom Imaging 2006; 31: 277–92. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am 1992; 72: 65–83. [DOI] [PubMed] [Google Scholar]
- 39.Brandt LJ, Feuerstadt P, Blaszka MC. Anatomic patterns, patient characteristics, and clinical outcomes in ischemic colitis: a study of 313 cases supported by histology. Am J Gastroenterol 2010; 105: 2245–52. doi: 10.1038/ajg.2010.217 [DOI] [PubMed] [Google Scholar]
- 40.Brandt LJ, Feuerstadt P, Longstreth GF, Boley SJ. Clinical guideline: epidemiology, risk factors, patterns of presentation, diagnosis, and management of colon ischemia (CI). Am J Gastroenterol 2015; 110: 18–44. doi: 10.1038/ajg.2014.395 [DOI] [PubMed] [Google Scholar]
- 41.Iacobellis F, Berritto D, Fleischmann D, Gagliardi G, Brillantino A, Mazzei MA, et al. CT findings in acute, subacute, and chronic ischemic colitis: suggestions for diagnosis. Biomed Res Int 2014; 2014: 895248. doi: 10.1155/2014/895248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glauser PM, Wermuth P, Cathomas G, Kuhnt E, Käser SA, Maurer CA. Ischemic colitis: clinical presentation, localization in relation to risk factors, and long-term results. World J Surg 2011; 35: 2549–54. doi: 10.1007/s00268-011-1205-5 [DOI] [PubMed] [Google Scholar]
- 43.Mazzei MA, Volterrani L. Nonocclusive mesenteric ischaemia: think about it. Radiol Med 2015; 120: 85–95. doi: 10.1007/s11547-014-0460-6 [DOI] [PubMed] [Google Scholar]
- 44.Berritto D, Crincoli R, Iacobellis F, Iasiello F, Pizza NL, Lassandro F, et al. Primary pneumatosis intestinalis of small bowel: a case of a rare disease. Case Rep Surg 2014; 2014: 350312. doi: 10.1155/2014/350312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giovine S, Pinto A, Crispano S, Lassandro F, Romano L. Retrospective study of 23 cases of hepatic infarction: CT findings and pathological correlations. Radiol Med 2006; 111: 11–21. doi: 10.1007/s11547-006-0002-y [DOI] [PubMed] [Google Scholar]
- 46.Rabushka LS, Kawashima A, Fishman EK. Imaging of the spleen: CT with supplemental MR examination. Radiographics 1994; 14: 307–32. doi: 10.1148/radiographics.14.2.8190956 [DOI] [PubMed] [Google Scholar]