Abstract
Baseline ultrasound is essential in the early assessment of patients with a huge haemoperitoneum undergoing an immediate abdominal surgery; nevertheless, even with a highly experienced operator, it is not sufficient to exclude parenchymal injuries. More recently, a new ultrasound technique using second generation contrast agents, named contrast-enhanced ultrasound (CEUS) has been developed. This technique allows all the vascular phase to be performed in real time, increasing ultrasound capability to detect parenchymal injuries, enhancing some qualitative findings, such as lesion extension, margins and its relationship with capsule and vessels. CEUS has been demonstrated to be almost as sensitive as contrast-enhanced CT in the detection of traumatic injuries in patients with low-energy isolated abdominal trauma, with levels of sensitivity and specificity up to 95%. Several studies demonstrated its ability to detect lesions occurring in the liver, spleen, pancreas and kidneys and also to recognize active bleeding as hyperechoic bands appearing as round or oval spots of variable size. Its role seems to be really relevant in paediatric patients, thus avoiding a routine exposure to ionizing radiation. Nevertheless, CEUS is strongly operator dependent, and it has some limitations, such as the cost of contrast media, lack of panoramicity, the difficulty to explore some deep regions and the poor ability to detect injuries to the urinary tract. On the other hand, it is timesaving, and it has several advantages, such as its portability, the safety of contrast agent, the lack to ionizing radiation exposure and therefore its repeatability, which allows follow-up of those traumas managed conservatively, especially in cases of fertile females and paediatric patients.
INTRODUCTION
Nowadays, trauma is the third leading cause of death in the first four decades of life, and it is often associated with permanent disability.1
Blunt abdominal trauma is a really frequent entity in patients with polytrauma, and since clinical examination does not always give sufficient information about the presence and the extension of solid organ injuries, it is mandatory to use imaging techniques as reliable as possible to have an accurate staging of the disease. Ultrasonography (US) is really valuable in the early assessment of a polytrauma, regardless of haemodynamic status; in fact, owing to its high sensitivity in the identification of intra-abdominal free fluid, it has largely replaced peritoneal lavage, becoming the first method used for this purpose, especially in haemodynamically unstable patients.2,3 This technique is known worldwide with the acronym FAST (focused assessment with sonography for trauma).
In fact, several studies have shown that its sensitivity for detection of intraperitoneal free fluid is excellent, ranging from 63% to 99%,4–6 so that nowadays there is a general consensus in stating that in a haemodynamically unstable patient with polytrauma at admission, the presence of a huge amount of free intraperitoneal fluid detected by ultrasound requires an emergency laparotomy. Its advantages consist of lack of ionizing radiations, its portability, the possibility to be performed at patient's bedside and its high sensibility in the detection of intraperitonal fluid,7 comparable with CT. Nevertheless, ultrasound has its own limitations, because it is strongly operator dependent and body-type’ patient dependent, and above all its accuracy in the detection of solid organ injury is very low, especially in the absence of abdominal free fluid,8 as demonstrated by Valentino et al9 who reported a sensitivity of 45.7% in the assessment of traumatic lesions.
Therefore, ultrasound is essential in the assessment of haemodynamically unstable patients with a huge haemoperitoneum undergoing an immediate abdominal surgery; nevertheless, even with a high experienced operator, it is not sufficient to exclude parenchymal injuries.
In the recent years, a new ultrasound technique using second generation ultrasound contrast agents (USCAs), named contrast-enhanced ultrasound (CEUS) has been developed.
This technique employs a software operating at low mechanical index able to analyse the resonance signals originated by second generation contrast agents without the destruction of bubbles, allowing to perform all the vascular phase in real time.10–12
These contrast agents, consisting of perfluorocarbon or sulphur hexafluoride, encapsulated by a very resistant phospholipid shell, are composed by stabilized gas microbubbles (1–7 micron), which are blood-pool agents with a non-linear reverberation. They remain intravascular and produce a non-linear harmonic response that can be separated from the tissue signal using contrast harmonic ultrasound.13,14
Sonovue® (Bracco, Milan, Italy) is the USCA currently used in Europe. It is a lyophilized powder to be suspended in water. Its preparation takes only a few seconds, and once in solution, it is immediately ready to be used.15 In addition, it does not require fasting or laboratory tests.
The contrast agent is administered with a quick bolus through an antecubital vein. The arterial phase starts after 10–20 s and proceeds up to 30–40 s. During the venous and late phase, the contrast agent is distributed to the whole capillary bed and the concentration slowly decreases until its excretion through the lungs. The venous and late phase lasts in the range of 2–6 min, varying in each abdominal parenchyma that is continuously scanned during each contrast phase. CEUS allows continuous depiction of the lesion in real time.
The low solubility of Sonovue®, associated with the high resistance of its shell to the mechanical effect of ultrasound beam, gives it a long duration, so that it is possible to explore in real time all the vascular phases. Contrast imaging requires a dedicated software, able to suppress the signals coming from stationary tissue improving contrast resolution, such as a real-time harmonic imaging scanning using dynamic low mechanical index,16 which allows to differentiate the signal between background tissue and gas-filled microbubbles, without bursting the last.17,18
The microbubbles emit harmonics at twice the insonation frequency by the reflection of ultrasound beam, then the transducer separates the fundamental frequency from the second harmonic using inverted phase pulse, and then acquires the signal.9,19
These contrast agents are routinely employed in most European as well as in many countries in Asia, and they recently obtained FDA approval for echocardiography.20 They differ from CT and MRI contrast agents because they are fundamentally intravascular (‘‘blood-pool’’) substances, lacking any interstitial spread, so that CEUS findings mainly overlap CT and MR ones during arterial phase imaging but not in the venous one, because CT and MR contrast media spread out into the extravascular space.
USCA is administered IV in two split doses of 2.4 ml each in order to evaluate the right and left upper quadrant separately; it is injected in an antecubital vein followed by 10 ml of saline water. The entire examination lasts for 4–6 min (Figure 1).
Figure 1.
Contrast-enhanced ultrasound (CEUS) protocol. CEUS is performed in sequence before exploring the kidneys, then the liver and finally the spleen.
USCAs are generally well tolerated with a rate of adverse reactions very low (about 0.014%).21–24 Some contraindications include severe cardiopulmonary conditions.25 They do not have any interactions with the thyroid gland, and they are not nephrotoxic, thus can be safely applied in case of acute or chronic renal failure because they have not a renal excretion.20
Their employment is not authorized on pregnant females and during breast feeding.
Few studies exist on the use of CEUS in childhood,26,27 because the ultrasound contrast agents are not licensed for paediatric use. However, a large survey study was carried out, which also included the use of product in intravenous route: responses suggest a favourable safety profile of this second generation ultrasound contrast agents in children.28
The solid organ parenchymal enhancement after intravenous administration of USCAs is different, depending on the differences in the organ vascularization. The kidneys have the most rapid, intense enhancement as a consequence of lacking glomerular filtration; the spleen shows a strong but persistent enhancement (up to 6 or 8 min); the liver and the pancreas behave intermediately, showing a mounting enhancement of their intensity. In the liver, because of the dual vascular supply, the hepatic phases after the arterial phase include the portal phase (40–120 s after contrast injection) and the sinusoidal (or late) phase (120–300 s after contrast injection).
CEUS findings are related to contrast media distribution, defined as homogeneous, heterogeneous or absent. A normal parenchyma appears as homogeneous and hyperechoic without any distortion of the echotexture, and the vascular structures are clearly recognizable.
A solid organ injury appears as a non-enhancing defect, sharply demarcated from the well enhanced and healthy tissue, especially during the venous phase29,30 (Figure 2). In particular, bruises show different aspects, because they can appear as simple and ill-defined oedematous areas to hypoechoic image with a reduced perfusion. Lacerations are recognizable as linear or branched hypoechoic bands, perpendicular to the organ capsule, and they can be associated with capsular discontinuity.
Figure 2.
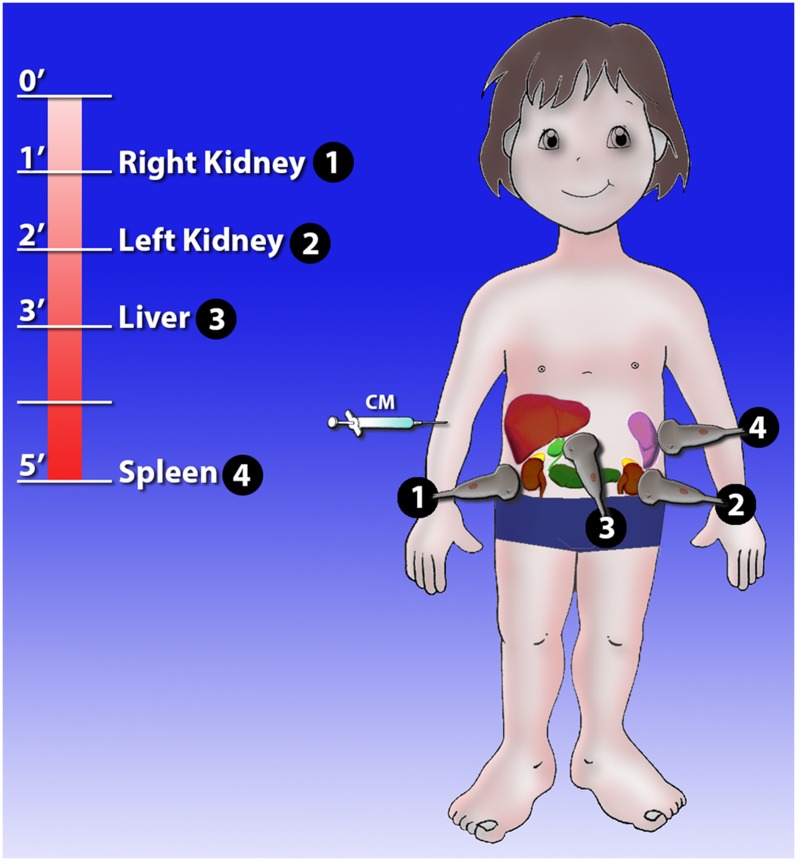
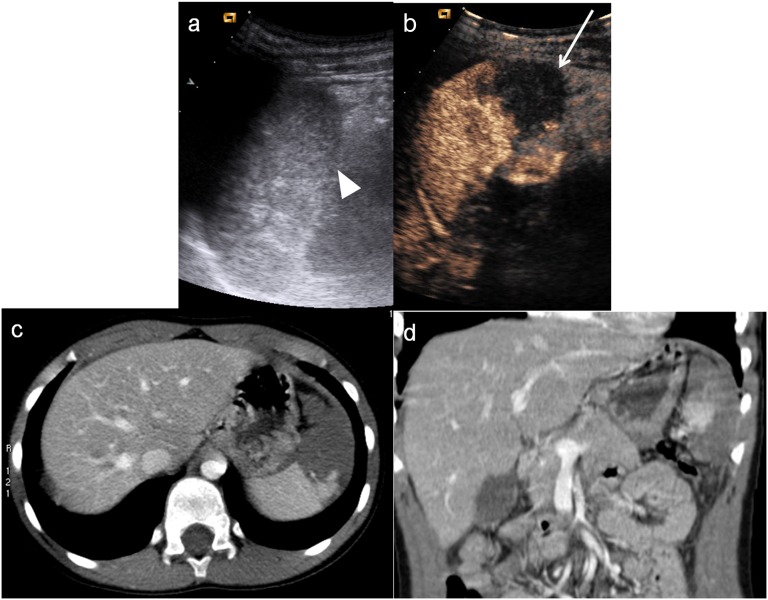
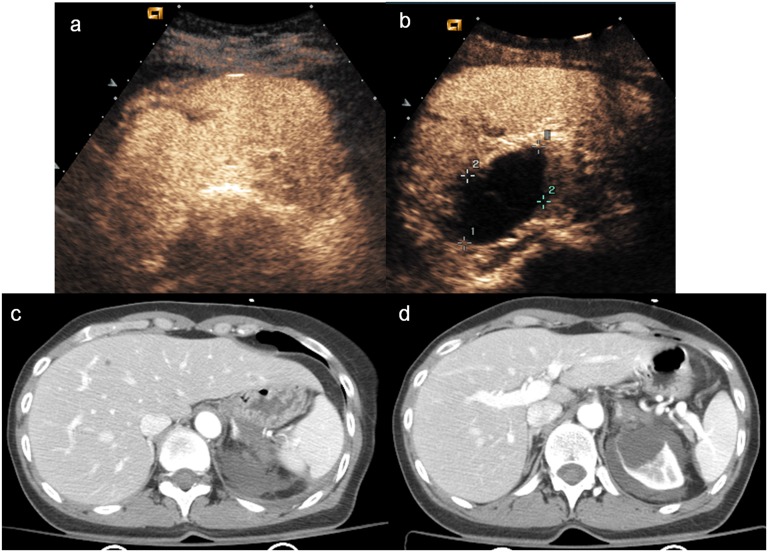
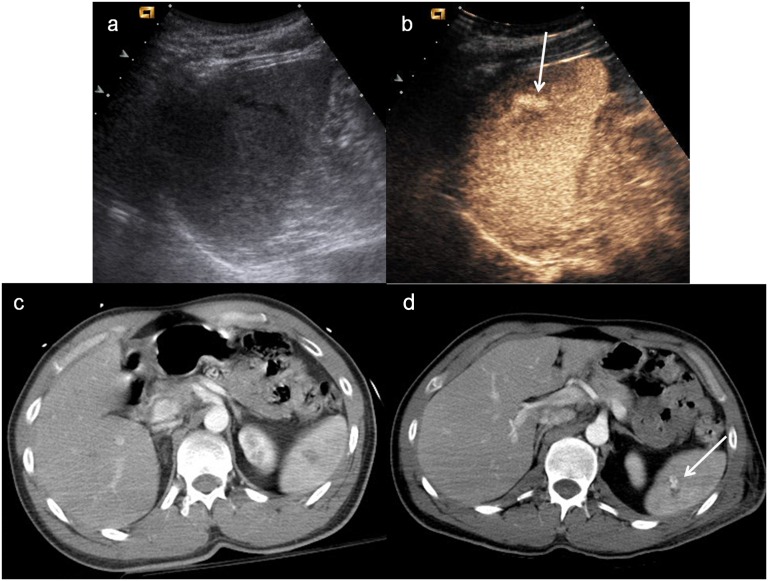
Child, hepatic injury. (a) Baseline ultrasonography shows a mild hyperechoic area in the liver parenchyma; (b) contrast-enhanced ultrasound (CEUS) demonstrates a well-defined hypoechoic lesion (white arrow); (c) axial scan and (d) coronal CT reconstruction confirm the hepatic lesion, corresponding in size and shape to that observed at CEUS.
The intraparenchymal haematoma appears as heterogeneous hypoechoic area with ill-defined contours with a poor definition of vascular structures; subcapsular haematoma is usually identified as a non-enhancing lenticular area surrounding parenchyma (Figure 3) in which it can be possible to recognize active extravasation of contrast media.31,32
Figure 3.
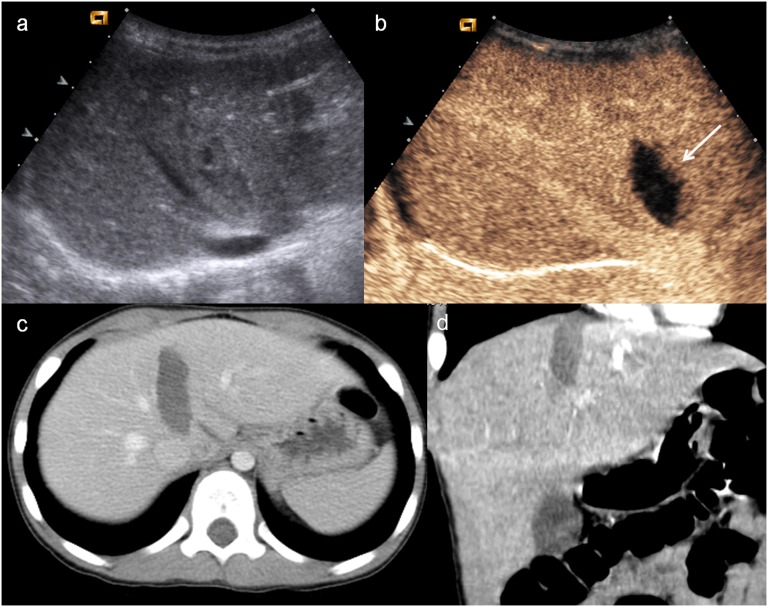
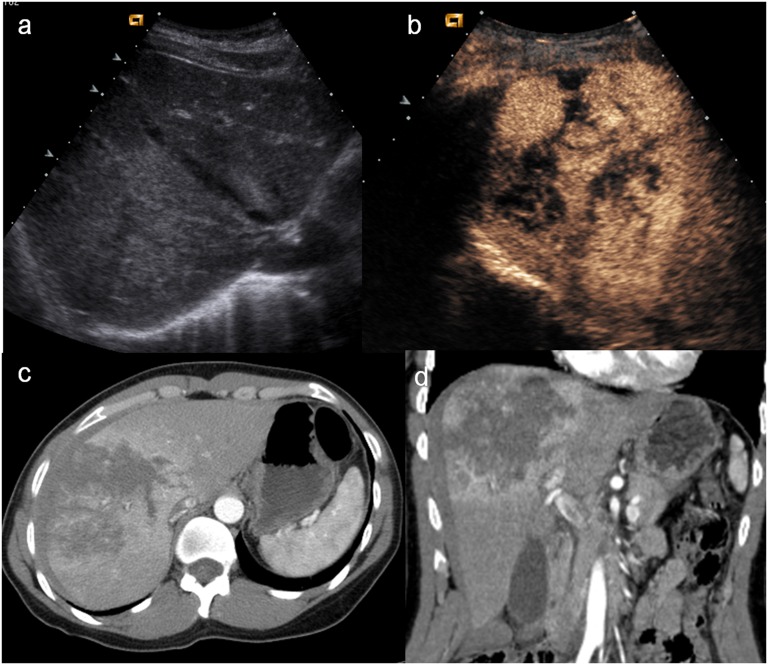
Child, renal injury. (a) Baseline ultrasonography shows echostructural inhomogeneity of the middle of the left kidney; (b) contrast-enhanced ultrasound (CEUS) demonstrates a subtle renal laceration (white arrow) and a subcapsular haematoma surrounding the kidney; (c) axial CT scan and (d) coronal reconstruction confirm the CEUS findings (arrowheads).
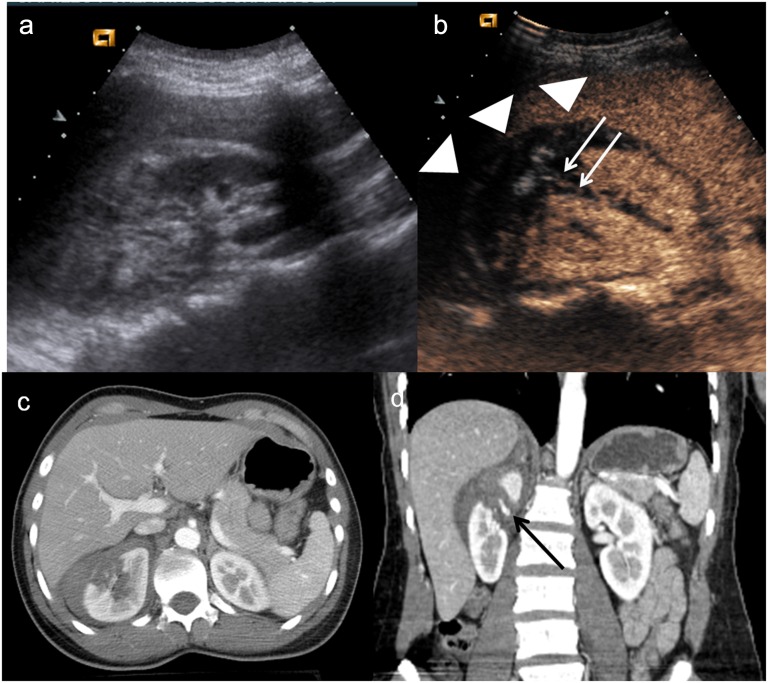
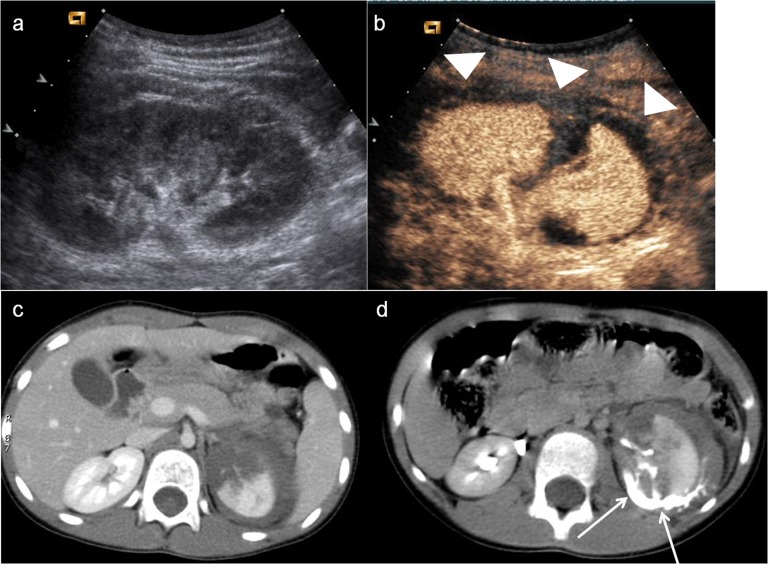
Active bleeding is visualized in the early stage as microbubbles extravasation within the peritoneal or retroperitoneal space (Figure 4). The absence of organ perfusion (Figure 5) is indicative of complete avulsion of the vascular pedicle.
Figure 4.
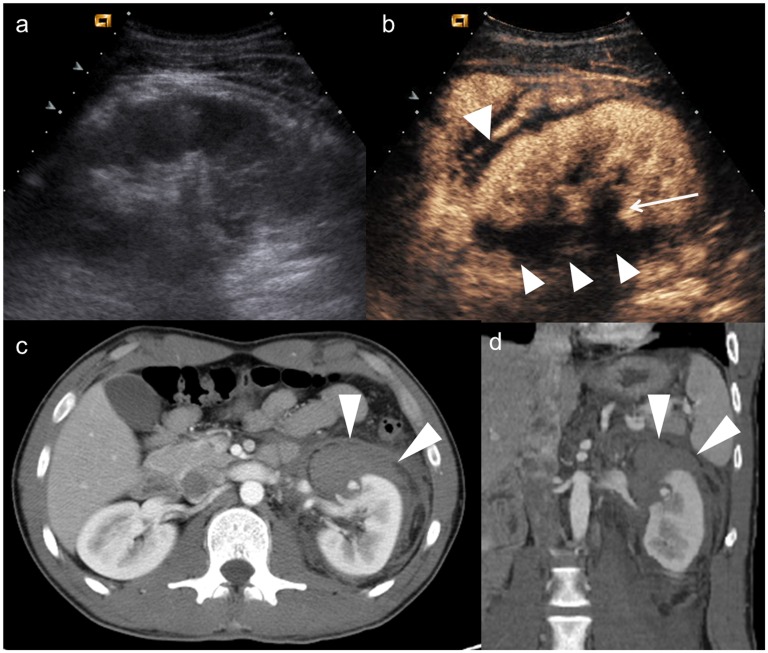
Child, hepatic injury. (a) Baseline ultrasonography shows only echostructural inhomogeneity on VII hepatic segment; (b–c) contrast-enhanced ultrasound demonstrates a laceration and parenchymal haematoma (arrowheads) involving the hepatic capsule, with hyperechoic bubbles indicating active bleeding (white arrow); (d) axial CT scan and (e) coronal reconstruction confirm the involvement of hepatic capsule and the active bleeding in peritoneal cavity.
Figure 5.
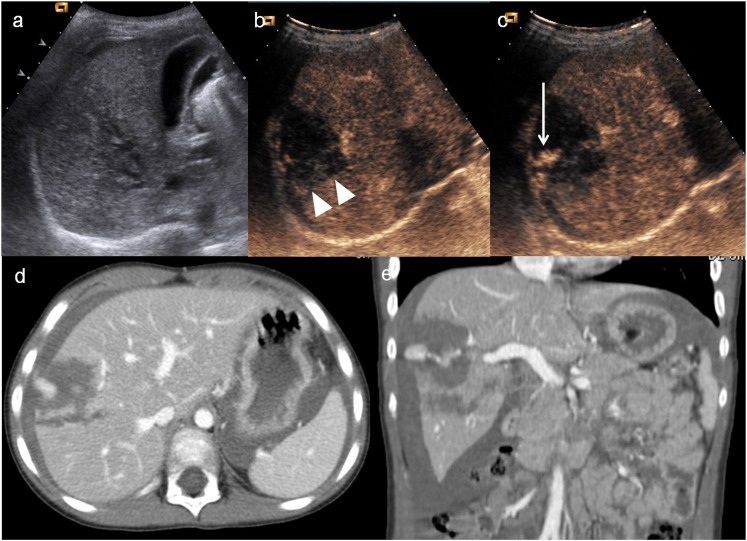
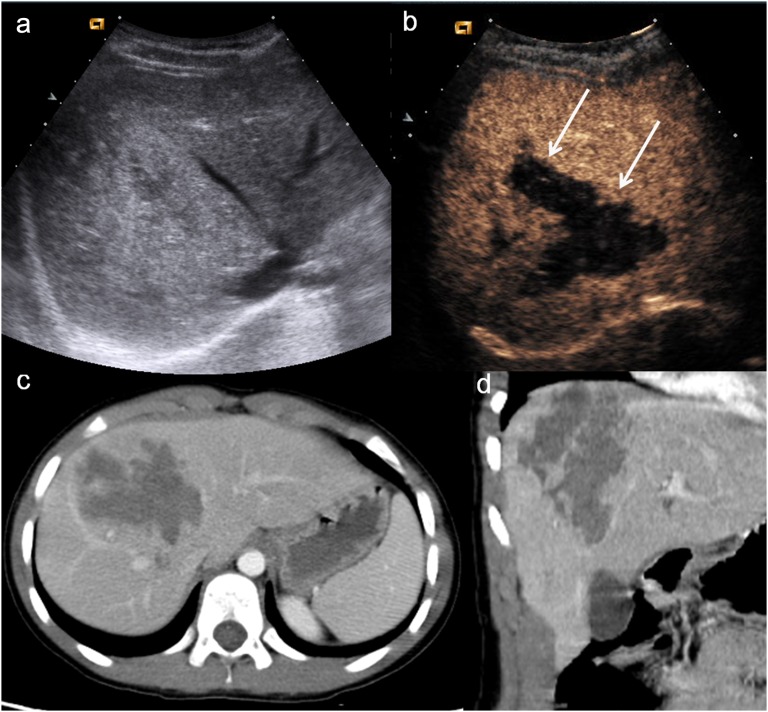
Child, splenic trauma. (a) Baseline ultrasonography shows swelling and echostructural inhomogeneity of the lower pole of the spleen (arrowhead); (b) contrast-enhanced ultrasound shows a large triangular-shaped hypoechoic area at the lower pole of the spleen (white arrow), corresponding to an area of devascularization; (c) axial CT scan and (d) coronal view confirm the triangular-shaped not vascularized area.
For a trauma protocol, CEUS follows conventional ultrasound in the assessment of solid organ injuries. It can be performed using contrast pulse sequencing or using pulse inversion harmonic and energy-modulated technique at low acoustic power. The focus is set to the deeper aspect of the organ with a potential traumatic injury.20
TRAUMATIC LESIONS
Liver
Hepatic injuries appear as areas of absent or reduced perfusion, better recognizable during the venous phase, which lasts about 2 min;33,34 in fact, in the late phase (lasting until the complete microbubbles clearance from the liver parenchyma, occurring at 4–6 min), the image deteriorates very quickly and all the parenchymal defects become indistinguishable. The complete exploration of the liver can be limited by its wide surface and by areas not easily accessible such as hepatic dome and lateral segments, especially in uncooperative patients because of interposition of ribs, stomach and intestines. Moreover, those injuries of severe entities are not easily detected in the acute phase owing to their echogenicity with respect to the healthy tissue; so that CT remains the standard of reference for hepatic lesions due to high-energy trauma.
Some studies reported the high value of CEUS in the staging of hepatic lesions following mild isolated abdominal trauma, such as demonstrated in our previous work33 where the accuracy of CEUS was compared with conventional ultrasound and spiral CT. 203 patients with isolated abdominal trauma were examined with baseline US and CEUS and a spiral CT as the standard of reference, evaluating the number of lesions, capsular involvement, the size and their sonographic pattern.
In this study, CEUS correctly identified all of the lesions detected by conventional ultrasound, recognizing confluent lesions measuring up to 3 cm in diameter in two patients, missed on conventional ultrasound, and in other four patients, it identified one traumatic lesion not visible with basal ultrasound. CEUS was able to recognize capsular involvement in 14 patients, and in 3 of these patients, the involvement had not been correctly identified on conventional ultrasound (Figure 6). At CEUS, the aspect of hepatic injury was of a strongly hypoechoic area against the healthy tissue with an increased echogenicity. With respect to conventional ultrasound, the lesions appeared with better defined margins and larger size because of a better definition of peripheral components (Figure 7).
Figure 6.
Young females, hepatic injury. (a) Baseline ultrasonography, irregular hyperechoic area in the right lobe. Capsular involvement is not demonstrated; (b) contrast-enhanced ultrasound shows multiple lacerations in the right hepatic lobe, involving the capsular surface; (c) axial CT scan and (d) coronal view confirm a huge irregular-shaped laceration and the capsular rupture.
Figure 7.
Males, hepatic injury. (a) Baseline ultrasonography shows a large hyperechoic area in the right lobe; (b) on contrast-enhanced ultrasound (CEUS), compared with conventional ultrasound, the lesion appears to have better defined margins (white arrows) because of a better definition of peripheral components; (c) axial CT scan and (d) coronal reconstruction show finding similar to CEUS.
Therefore, the employment of CEUS greatly improved the quantity and quality of findings with respect to conventional ultrasound, because the number of detected lesions increased as also the number of appreciable foci; there was also a high improvement in image quality which leads to a better definition of lesion size. In almost all the cases, the assessment of lesions identified by CEUS was similar to CT, allowing a precise grading of lesions, with a prognostic value equal to that of CT.
Spleen
This is the most frequent organ affected by abdominal trauma.35 As occurs in the liver, the evaluation of the spleen can be limited by the interposition of ribs and the splenic flexure, especially referring to the exploration of the upper pole and the subphrenic region (Figure 8). Its arterial phase starts at 12–20 s and it has a long duration, responsible for a peculiar irregular enhancement of the organ, called “zebra”, because of the movement of the contrast media within the red pulp and the white pulp. This feature makes difficult to recognize any tissue lesion. The venous phase, starting at 40–60 s after contrast-media injection, is the most reliable one for the detection of organ injury, because the healthy parenchyma appears as homogeneous enhancing tissue with a long duration (about 5–7 min).36 Catalano et al37 described the CEUS findings of the traumatized spleen. In our recent experience38 in which 256 patients with a history of low-energy blunt abdominal trauma were retrospectively evaluated, CEUS identified 34/35 splenic injuries with CT as the standard of reference; the false-negative result was due to a splenic lesion measuring <1 cm (which had not relevant consequences for patient management and prognosis) demonstrating that CEUS was accurate as CT in the detection and staging of traumatic splenic injuries (Figure 9).
Figure 8.
Young girl, splenic injury. (a) Baseline ultrasonography not clearly depicts the upper pole of the spleen; (b) contrast-enhanced ultrasound demonstrates a complete fracture of the subphrenic region of the spleen, involving the capsule (white arrow), which is confirmed on (c) axial CT scan and (d) coronal view.
Figure 9.
Females, splenic and renal injury. (a–b) Contrast-enhanced ultrasound shows a subtle lesion of splenic parenchyma, involving the capsule, which was not evident on baseline ultrasonography. A large cyst of renal upper pole is also depicted; (c–d) axial CT scan confirms the little lesion of the spleen and a subcapsular renal haematoma, due to the rupture of the upper pole cyst.
A disturbing factor affecting this technique performed on the spleen is the quick decrease of enhancement in splenic veins, because the spleen acts as an active filter of microbubbles, thus resulting in a mild perfusion of splenic vessels. This peculiarity may create a problem of differential diagnosis as the veins can be mistaken with lacerations, but the awareness of this circumstance can help to solve this problem. A reinjection of a second bolus of USCA can help when in doubt, as recommended by Valentino et al.34
Kidneys
CEUS often outperforms conventional ultrasound in the evaluation of renal trauma. In the kidney, the cortex usually enhances very soon and very intensively, while the pyramids enhance from the periphery to the centre in about 30 s.39 The optimal time period for kidney assessment is up to 2.5 min, because it is the most effective time period to detect renal injuries. The two kidneys have to be explorated separately with two different boluses; their exploration can sometimes be difficult because the left one, in particular, can be hidden by ribs and bowel gas.40
Renal traumatic injuries may appear as defects of vascularization in a normally perfused organ.
When a thrombosis or a renal artery tear occurs, they manifest as absence of parenchymal perfusion;41 focal contrast material extravasation suggests active haemorrhage (Figure 10).
Figure 10.
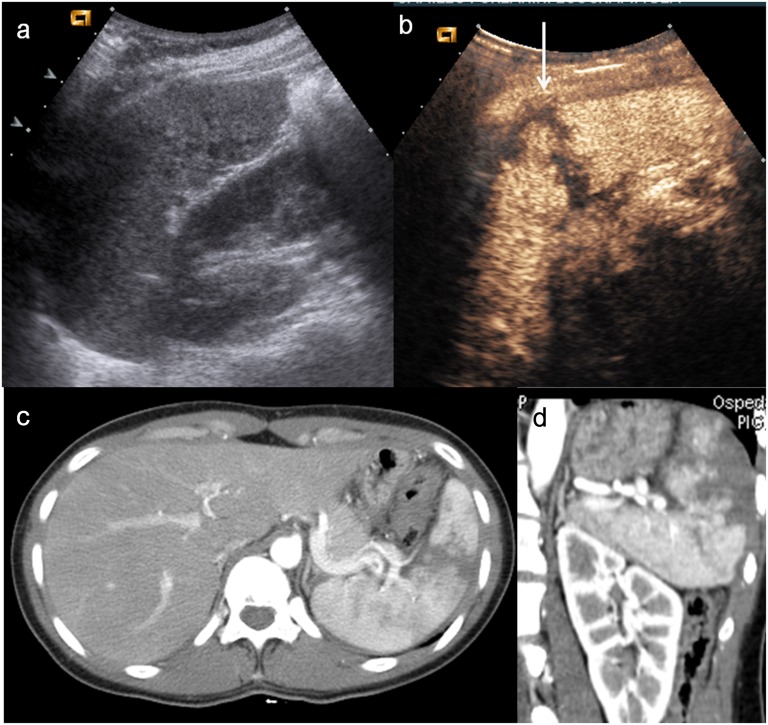
Child, renal trauma. (a) Baseline ultrasonography shows a mild hyperechoic area in the middle-third of the right kidney; (b) contrast-enhanced ultrasound demonstrates a renal fracture with an associated perirenal collection (arrowheads) and evidence of hyperechoic spots within it (white arrow), corresponding to active bleeding; (c–d) axial CT scan and coronal view confirm the renal fracture, the perirenal haematoma and active bleeding (black arrow).
Our experience on renal trauma42 showed that CEUS was able to correctly identify 28/28 renal parenchymal lesions, with or without perirenal or retroperitoneal haematoma and that MDCT confirmed all the cases positive at CEUS. One of the main concern of CEUS performed for renal injuries is its inability to detect lesions of collecting system because of a lack of microbubbles urinary excretion (Figure 11). Thus, CEUS is not recommended in case of suspicion of injury to the urinary tract, being contrast-enhanced CT (CE-CT) the gold standard for this purpose.38
Figure 11.
Child, renal trauma. (a) Baseline ultrasonography shows only a fine cortical parenchymal inhomogeneity of the left kidney with perirenal fluid collection; (b) contrast-enhanced ultrasound shows a large renal fracture and highlights very well the presence of perirenal fluid (arrowheads); (c) axial CT scan in the venous phase demonstrates the renal fracture and confirms the perirenal fluid; (d) axial CT scan in the late phase shows the iodinated urine leakage (white arrows) in the perirenal space (urinoma).
Pancreas
Pancreatic trauma is relatively uncommon, occurring in <2% of blunt abdominal trauma, but owing to its retroperitoneal location, mortality is quite high, ranging from 70% to 80% when there is also involvement of aorta, the superior mesenteric artery or vena cava.
Missed pancreatic lesions lead to complications in 20–30% of cases and mortality in about 20%; therefore, a prompt diagnosis is essential.
Since its retroperitoneal location, physical examination is not reliable; CT is the gold standard to evaluate any pancreatic injury because of the low sensitivity and specificity of ultrasound in post-traumatic pancreatic damage,35 useful to identify only peripancreatic fluid; nevertheless, CE-CT cannot be used at patient's bedside for an early diagnosis or at trauma scene. In a previously published report, a case of pancreatic lesion has been detected with CEUS43 in a 5-year-old boy suffering from pancreatic laceration.
More recently, LV et al44 have investigated the role of CEUS in the detection of pancreatic injury in a larger cohort of patients, with CE-CT as the standard of reference.
In this study, CEUS correctly identified 21/22 pancreatic lesions, appearing as anechoic or hypoechoic perfusion defects with blurred margins and capsular involvement; the detection rate of CEUS with CT as the reference standard was 95.5%. In addition, CEUS was also able to identify 6/21 pancreatic ductal injuries with respect to CE-CT which identified 7/22. By CEUS, it was possible to better evaluate the peripancreatic space with respect to conventional ultrasound, because of the difference in blood supply making possible to visualize with higher detail pancreatic and peripancreatic microcirculation perfusion. The authors highlighted the capability of CEUS to detect parenchymal and capsular injuries with excellent imaging characteristics and its portability, allowing its employment in emergency room and at patient's bedside. Moreover, CEUS is timely, with a room time <5 min and it can be performed without interrupting other physical examination or resuscitation procedures. The authors concluded declaring that CEUS should not be intended as a substitute for CT but a possibility to boost ultrasound role in the screening of pancreatic trauma (Figure 12).
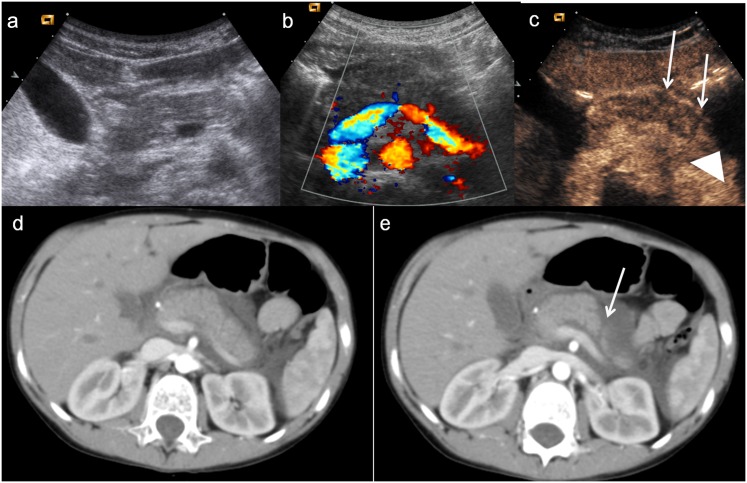
Figure 12.
Child, pancreatic injury. (a–b) Baseline ultrasonography with CD imaging does not show any pancreatic lesion. c) Contrast-enhanced ultrasound shows swelling of pancreatic body and allows to suspect subtle lesions of the pancreatic body and tail (white arrows), associated with pre-pancreatic fluid collection (arrowhead). (c–d) Axial CT scans confirm the pancreatic tail lesion (white arrow) with pre-pancreatic fluid collection.
Active bleeding
CEUS is able to demonstrate active contrast media extravasation as hyperechoic bands appearing as round or oval spot of variable size or as a fountain-like or serpentine-like hyperechoic jet.45–47 Because of a real-time scanning, CEUS can easily detect contrast extravasation immediately after vessel opacification.48 As Catalano et al demonstrated,49 CEUS correctly identified 20 cases of active bleeding, and among these, 9 had an available CE-CT correlation which confirmed the presence and location of bleeding; among the patients with a negative CEUS for active bleeding, CT confirmed these data. Both the techniques demonstrated to have 100% of sensitivity and specificity for active bleeding diagnosis.
The authors outline that active bleeding should be differentiable from calcifications, which appear unchanged over time and visible also on pre-contrast scans; from normal vessels, which have a different anatomy and a more regular course; from pseudoaneurysms and post-traumautic arteriovenous fistulas which appear quite similar to active bleeding (Figure 13), although this differential diagnosis is clinically less relevant because both the situations require further procedures such as surgery or embolization.
Figure 13.
Males, splenic injury. (a) Baseline ultrasonography performed 3 days after trauma shows a subtle hypoechoic rim in the splenic parenchyma; (b) contrast-enhanced ultrasound (CEUS) demonstrates a clear hyperechoic spot (white arrow), surrounded by hypoechoic area, which is due to an arteriovenous fistula; (c) axial CT scan performed immediately after trauma shows only a small contusion in the splenic parenchyma; (d) 3 days later, axial CT scan performed on the basis of CEUS findings confirms the arteriovenous fistula (white arrow).
Paediatric patients
As previously reported, CEUS is almost as sensitive as CT in the assessment of blunt abdominal trauma in adults, allowing to select those patients who need further diagnostic investigation and procedures. It seems to have the same relevant role also in paediatric population;50–52 in fact, as demonstrated by Valentino et al26 who compared the sensitivity and specificity of baseline ultrasound with that of CEUS in the detection of solid organ injuries in children with blunt abdominal trauma, with CT as the gold standard; in a cohort of 27 patients, CEUS showed 13 of 14 lesions in 12 patients with positive CT and no lesions in patients with negative CT, demonstrating that CEUS was accurate almost as CT in the detection of solid organ lesions. Our experience about this topic has shown to be quite similar;27 in fact, CEUS identified 67/67 patients with parenchymal lesions with respect to baseline ultrasound (26/67) with a diagnostic accuracy of 100%. Moreover, in some patients, CEUS identified also prognostic factors, such as parenchymal active bleeding in 8 cases (Figure 4) and partial devascularization in 1 case (Figure 5). MDCT confirmed all parenchymal lesions. Therefore, CEUS demonstrated to be more sensitive and accurate than baseline ultrasound and almost as sensitive as CT in the identification and characterization of solid organ lesions in blunt abdominal trauma.
Follow-up
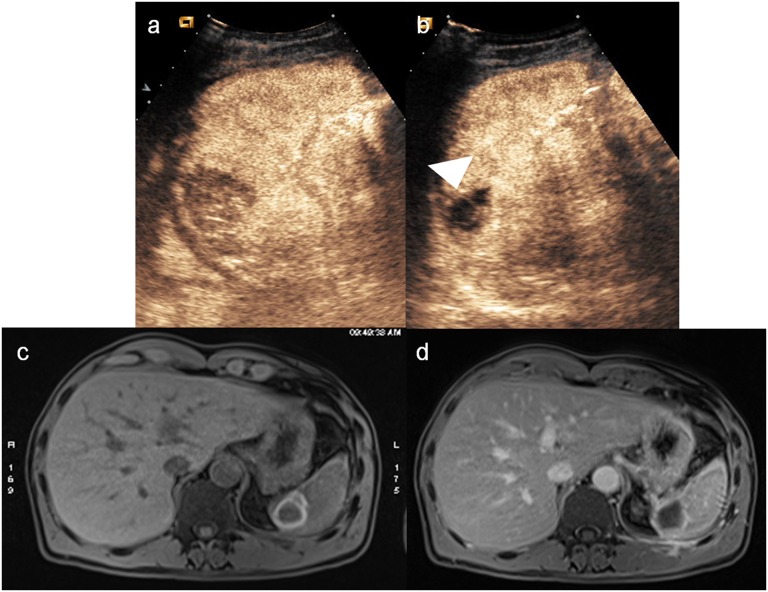
Less is known about CEUS feasibility in a follow-up setting. A study carried on by Manetta et al53 emphasized the role of CEUS in the follow-up of patients with minor hepatic or splenic injuries. They demonstrated that CEUS was as accurate as CT in the identification of lesion site and allowed to follow all the repair process until their complete resolution, highlighting that during the follow-up phase, the lack of urgency and the knowledge of the lesion site allows to perform a more detailed baseline examination (Figure 14). They conclude that, if CEUS is used in this way, it gains a detection power similar to CT and MR, allowing to avoid the use of CT, especially in young patients and in pregnant females.
Figure 14.
Males, splenic trauma treated by embolization. (a) Contrast-enhanced ultrasound (CEUS) performed immediately after the embolization shows an hypoechoic devascularized area at the upper pole of the spleen, in the site of embolization; (b) CEUS performed 2 months later shows the reduction in size of the splenic ischaemic area (arrowhead); (c–d) MRI performed at the same time of the second CEUS allows to better define the size and the extent of the devascularized area and provides a temporal stage of the lesion.
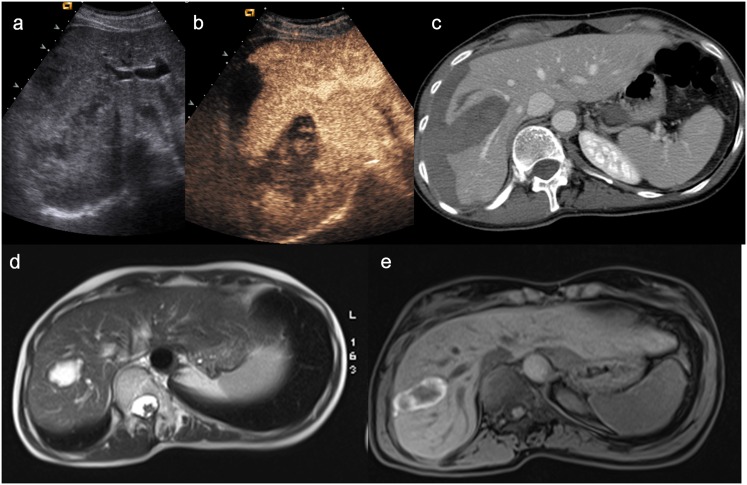
Nevertheless, our very recent experience, focused on the role of CEUS in the follow-up of patients with blunt abdominal trauma managed conservatively in comparison with MRI,54 showed CEUS limitations in some specific cases, such as the lack in monitoring urinary tract injuries and adrenal gland injuries. On the other hand, MRI demonstrated to be very effective in this way, because of the improved contrast and soft tissue resolution, allowing a temporal stage of lesions, and its ability to exclude all negative prognostic factors, such as late bleeding, rupture of the urinary tract or additional injuries (Figure 15). Its application is even more important considering that many patients with trauma are in childhood; for this reason, the use of this technique in the follow-up, which does not use ionizing radiation, makes it also extremely profitable compared to CT.
Figure 15.
(a) Baseline ultrasonography shows an inhomogeneous hypoechoic–hyperechoic parenchymal lesion of the liver and a subcapsular haematoma; (b) contrast-enhanced ultrasound (CEUS) allows to depict the hepatic laceration and the parenchymal and subcapsular haematoma well; (c) axial CT scan confirms the CEUS findings; (d–e) MRI, performed 4 months later, demonstrates the reduction in size of the lesion and the disappearance of subcapsular haematoma well.
DISCUSSION AND CONCLUSIONS
In a trauma setting, baseline ultrasound performed on victims with low-energy injuries is a valuable technique because of its rapidity and non-invasivity, it can be used at patient's bedside and does not interfere with the resuscitation procedures; in addition, it has a high sensitivity for the detection of free abdominal fluid.55
Nevertheless, it has a very poor accuracy for the assessment of parenchymal injuries, which depends on lesion location, ranging from 27% to 68.6%.56
The most used and sensitive imaging technique for high-energy injuries is CE-CT, because it provides not only a comprehensive assessment of the disease but it can also grade trauma severity and help to decide on the most appropriate patient management.57 Nevertheless, its location in the radiology department of many trauma centres makes this technique time consuming for technical and logistic reasons. In addition, other CT limitations include potential contrast agent allergy, nephrotoxicity and costs, besides the use of ionizing radiation, which is the main limitation in fertile females and in paediatric patients (children are at least four times more sensitive than adults to ionizing radiation, because of a longer life expectancy and a faster cell rate division).58
To overcome these limitations, CEUS can be approached as a valuable imaging method between baseline ultrasound and CT, because it provides additional data not achievable by conventional ultrasound imaging and can reduce radiation exposure. Moreover, it can be performed in some selected patients (paediatric patients and females of reproductive age). Its application on children is still considered off-label, and it is allowed only after the parents (or legal guardians) have been adequately informed and given their specific consent. As previously reported, recent studies have underlined the safety and the usefulness of CEUS in children26,27 and several international paediatric and radiological societies are pushing for its wider registration, including paediatric use.59
In a traumatic setting, CEUS outperforms conventional ultrasound for the detection of abdominal solid organ injuries; in particular, in haemodynamically stable patients, the use of contrast medium greatly improved the number of detected lesions but also the quality of findings, with a better definition of margins, extension and relationship with capsule and vessels.38,60
CEUS can suggest the presence of associated vascular injuries such as contrast medium extravasation, parenchyma infarction and vascular pedicle avulsion.49
Miele et al33 found an excellent correlation between the size of the traumatic injury (as shown at CT) and the related CEUS findings as for as hepatic traumatic injuries, advocating its employment as a first-line examination in mild blunt abdominal trauma and in paediatric patients, leaving CT as the gold standard for polytrauma and major trauma.
In particular, as for as paediatric population, Emery et al61 found that 34% of children with ultrasound negative examination had an intra-abdominal injury at CT, concluding that screening baseline ultrasound should be approached with caution if performed to depict blunt abdominal trauma. Our experience on this topic27 showed that CEUS in children is more sensitive and accurate than baseline ultrasound and almost as sensitive as CT in the identification and characterization of blunt abdominal trauma. These results are in agreement with the experience of Valentino et al26 who suggest that CEUS can be considered for the triage of haemodynamically stable children with a history of abdominal trauma.
Nevertheless, CEUS has some limitations that have to be borne in mind, such as the cost of contrast media, need for scanners with dedicated software, longer examination times, lack of panoramicity, the fact that it is strongly operator dependent and the fact that it does not allow a complete abdominal survey because of problems related to lesion location (such as pancreas behind bowel or stomach, aorta in obese patients, a fatty liver); but the largest limitation of CEUS, also according to our experiences, is the poor ability to detect active bleeding and injuries to the urinary tract.27,38
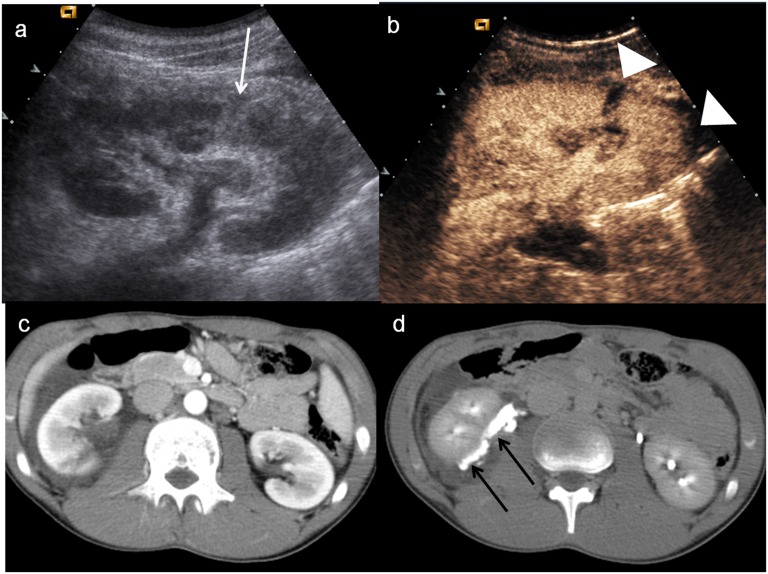
As for as the latter one, we have to remember that USCAs are intravascular and are unsuitable for demonstrating extravasation in the renal collecting system, also because they are characterized by lung excretion. Therefore, in case of traumatic gross haematuria, gross haematuria or microhaematuria following renal biopsy or lithotripsy performed during the previous 12 h, our findings suggest that CEUS could be very useful in the detection of the parenchymal lesion, but it cannot demonstrate the urinary tract lesion. For these reasons, in the management of renal trauma in stable patients with normal urinalysis baseline ultrasound is followed by CEUS, but in patients with a minor trauma and gross or microhaematuria or those who underwent invasive procedures in the previous 12 h, baseline ultrasound is always followed by CE-CT with delayed scans to study the urinary tract (Figure 16). This approach allows a better screening of disease and allows also to make considerable savings of both financial and staff resources.
Figure 16.
Child, renal trauma. (a) Baseline ultrasonography shows only an hyperechoic area at the lower pole of the right kidney (white arrow); (b) contrast-enhanced ultrasound demonstrates very well the incomplete renal fracture and a little amount of perirenal haematoma (arrowheads); (c) axial CT scan in venous phase shows the subtle renal laceration and the perirenal fluid collection; (d) axial CT scan in the late phase demonstrates the iodinated urine leakage (black arrows) due to the urinary tract lesion.
Regarding active bleeding, in traumatized patients, we need to detect active bleeding and to differentiate between parenchymal and vascular bleeding: in our series conducted on children,27 we were able to correctly identify only 50% of parenchymal bleeding and no cases of vascular bleeding. However, in these last cases, both ultrasound and CEUS identified massive haemoperitoneum which represents an indirect sign of severe abdominal organ injury, leading thus to CE-CT examination. A further survey we performed on adults38 disclosed CEUS inability to identify four cases of active bleeding.
Another significant limitation is that CEUS cannot detect direct signs of peritoneal bleeding related to intestine or mesentery injuries. However, it should be considered that these lesions occur more frequently in high-energy trauma rather than in the minor trauma and therefore in these patients CE-CT is mandatory.
Some recent research advocated the application of CEUS in the follow-up of patients with trauma conservatively managed until discharge,33,42,53,62,63 both in order to reduce unnecessary CT examinations and to overcome poorly visible traumatic injuries at conventional ultrasound, better revealed using USCAs. In fact, in our institution the assessment of every haemodynamically stable patient sustaining low-energy blunt abdominal trauma includes a baseline ultrasound and a CEUS examination to rule out abdominal traumatic injuries; those cases positive at CEUS undergo CE-CT to exclude any negative prognostic factors such as active bleeding or rupture or urinary tract, while those patients with traumatic lesions conservatively treated are monitored by CEUS and MRI in order to obtain a safe and expedite patient discharge.54
Contributor Information
Vittorio Miele, Email: vmiele@sirm.org.
Claudia Lucia Piccolo, Email: clapiccolo@libero.it.
Michele Galluzzo, Email: galluzzom@tiscali.it.
Stefania Ianniello, Email: stefianni66@gmail.com.
Barbara Sessa, Email: barbara.sessa@tiscali.it.
Margherita Trinci, Email: margherita.trinci@libero.it.
REFERENCES
- 1.van Beeck EF, van Roijen L, Mackenbach JP. Medical costs and economic production losses due to injuries in the Netherlands. J Trauma 1997; 42: 1116–23. doi: 10.1097/00005373-199706000-00023 [DOI] [PubMed] [Google Scholar]
- 2.Poletti PA, Wintermark M, Schnyder P, Becker CD. Traumatic injuries: role of imaging in the management of the polytrauma victim (conservative expectation). Eur Radiol 2002; 12: 969–78. doi: 10.1007/s00330-002-1353-y [DOI] [PubMed] [Google Scholar]
- 3.McKenney KL, Nuñez DB, Jr, McKenney MG, Asher J, Zelnick K, Shipshak D. Sonography as primary screening technique for blunt abdominal trauma: experience with 899 patients. AJR Am J Roentgenol 1998; 170: 979–85. doi: 10.2214/ajr.170.4.9580140 [DOI] [PubMed] [Google Scholar]
- 4.Brown MA, Sirlin CB, Hoyt DB, Casola G. Screening ultrasound in blunt abdominal trauma. J Intensive Care Med 2006; 18: 253–60. doi: 10.1177/0885066603256103 [DOI] [PubMed] [Google Scholar]
- 5.Branney SW, Wolfe RE, Moore EE, Albert NP, Heinig M, Mestek M, et al. Quantitative sensitivity of ultrasound in detecting free intraperitoneal fluid. J Trauma 1995; 39: 375–80. doi: 10.1097/00005373-199508000-00032 [DOI] [PubMed] [Google Scholar]
- 6.Paajnen H, Lahti P, Nordback I. Sensitivity of transabdominal ultrasonography in detection of intraperitoneal fluid in humans. Eur Radiol 1999; 9: 1423–5. [DOI] [PubMed] [Google Scholar]
- 7.Miele V, Andreoli C, Grassi R. The management of emergency radiology: key facts. Eur J Radiol 2006; 59: 311–14. doi: 10.1016/j.ejrad.2006.04.020 [DOI] [PubMed] [Google Scholar]
- 8.Chiu WC, Cushing BM, Rodriguez A, Ho SM, Mirvis SE, Shanmuganathan K, et al. Abdominal injuries without hemoperitoneum: a potential limitation focused abdominal sonography trauma (FAST). J Trauma 1997; 42: 617–23. [DOI] [PubMed] [Google Scholar]
- 9.Valentino M, Serra C, Zironi G, De Luca C, Pavlica P, Barozzi L. Blunt abdominal trauma: emergency contrast-enhanced sonography for detection of solid organ injuries. AJR Am J Roentgenol 2006; 186: 1361–7. doi: 10.2214/AJR.05.0027 [DOI] [PubMed] [Google Scholar]
- 10.Madsen HH. Ultrasound contrast: the most important innovation in ultrasound in recent decades. Acta Radiol 2008; 49: 247–8. doi: 10.1080/02841850801961647 [DOI] [PubMed] [Google Scholar]
- 11.Blomley MJ, Cooke JC, Unger EC, Monaghan MJ, Cosgrove DO. Microbubble contrast agents: a new era in ultrasound. BMJ 2001; 322: 1222–5. doi: 10.1136/bmj.322.7296.1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mihalik JE, Smith RS, Toevs CC, Putnam AT, Foster JE. The use of contrast-enhanced ultrasound for the evaluation of solid abdominal organ injury in patients with blunt abdominal trauma. J Trauma Acute Care Surg 2012; 73: 1100–5. doi: 10.1097/TA.0b013e31825a74b5 [DOI] [PubMed] [Google Scholar]
- 13.Thorelius L. Contrast-enhanced ultrasound for extrahepatic lesions: preliminary experience. Eur J Radiol 2004; 51: S31–8. doi: 10.1016/j.ejrad.2004.03.028 [DOI] [PubMed] [Google Scholar]
- 14.Uhlendorf V, Scholle FD, Reinhardt M. Acoustic behaviour of current ultrasound contrast agents. Ultrasonics 2000; 38: 81–6. doi: 10.1016/S0041-624X(99)00128-6 [DOI] [PubMed] [Google Scholar]
- 15.Morel DR, Schwieger I, Hohn L, Terrettaz J, Llull JB, Cornioley YA, et al. Human pharmacokinetics and safety evaluation of SonoVue, a new contrast agent for ultrasound imaging. Invest Radiol 2000; 35: 80–5. doi: 10.1097/00004424-200001000-00009 [DOI] [PubMed] [Google Scholar]
- 16.Bauer A, Solbiati L, Weissman N. Ultrasound imaging with SonoVue: low mechanical index real time imaging. Acad Radiol 2002; 9(Suppl. 2): S282–4. [DOI] [PubMed] [Google Scholar]
- 17.Solbiati L, Tonolini M, Cova L, Goldberg SN. The role of contrast-enhanced ultrasound in the detection of focal liver lesions. Eur Radiol 2001; 11(Suppl. 3): E15–26. [DOI] [PubMed] [Google Scholar]
- 18.Leen E, Angerson WJ, Yarmenitis S, Bongartz G, Blomley M, Del Maschio A, et al. Multi-centre clinical study evaluating the efficacy of SonoVue (BR1), a new ultrasound contrast agent in Doppler investigation of focal hepatic lesions. Eur J Radiol 2002; 41: 200–6. doi: 10.1016/S0720-048X(01)00457-0 [DOI] [PubMed] [Google Scholar]
- 19.Burns PN, Wilson SR, Simpson DH. Pulse inversion imaging of liver blood flow: improved method for characterizing focal masses with microbubble contrast. Invest Radiol 2000; 35: 58–71. doi: 10.1097/00004424-200001000-00007 [DOI] [PubMed] [Google Scholar]
- 20.Pinto F, Miele V, Scaglione M, Pinto A. The use of contrast-enhanced ultrasound in blunt abdominal trauma: advantages and limitations. Acta Radiol 2014; 55: 776–84. doi: 10.1177/0284185113505517 [DOI] [PubMed] [Google Scholar]
- 21.Piscaglia F, Bolondi L; Italian Society for Ultrasound in Medicine and Biology (SIUMB) Study Group on Ultrasound Contrast Agents. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol 2006; 32: 1369–75. doi: 10.1016/j.ultrasmedbio.2006.05.031 [DOI] [PubMed] [Google Scholar]
- 22.Farina R, Catalano O, Stavolo C, Sandomenico F, Petrillo A, Romano L. Emergency radiology. Radiol Med 2015; 120: 73–84. doi: 10.1007/s11547-014-0480-2 [DOI] [PubMed] [Google Scholar]
- 23.Bertolotto M, Catalano O. Contrast-enhanced ultrasound: past, present, and future. Ultrasound Clin 2009; 4: 339–67. doi: 10.1016/j.cult.2009.10.011 [DOI] [Google Scholar]
- 24.Catalano O, Aiati L, Barozzi L, Bokor D, De Marchi A, Faletti C, et al. CEUS in abdominal trauma: multicenter study. Abdom Imaging 2009; 34: 225–34. doi: 10.1007/s00261-008-9452-0 [DOI] [PubMed] [Google Scholar]
- 25.Bokor D, Chambers JB, Rees PJ, Mant TG, Luzzani F, Spinazzi A. Clinical safety of SonoVue, a new contrast agent for ultrasound imaging, in healthy volunteers and in patients with chronic obstructive pulmonary disease. Invest Radiol 2001; 36: 104–9. doi: 10.1097/00004424-200102000-00006 [DOI] [PubMed] [Google Scholar]
- 26.Valentino M, Serra C, Pavlica P, Labate AM, Lima M, Baroncini S, et al. Blunt abdominal trauma: diagnostic performance of contrast-enhanced US in children—initial experience. Radiology 2008; 246: 903–9. doi: 10.1148/radiol.2463070652 [DOI] [PubMed] [Google Scholar]
- 27.Menichini G, Sessa B, Trinci M, Galluzzo M, Miele V. Accuracy of contrast-enhanced ultrasound (CEUS) in the identification and characterization of traumatic solid organ lesions in children: a retrospective comparison with baseline US and CE-MDCT. Radiol Med 2015; 120: 989–1001. doi: 10.1007/s11547-015-0535-z [DOI] [PubMed] [Google Scholar]
- 28.Riccabona M. Application of a second-generation US contrast agent in infants and children–a European questionnaire-based survey. Pediatr Radiol 2012; 42: 1471–80. doi: 10.1007/s00247-012-2472-5 [DOI] [PubMed] [Google Scholar]
- 29.Thorelius L. Emergency real-time contrast-enhanced ultrasonography for detection of solid organ injuries. Eur Radiol 2007; 17(Suppl. 6): F107–11. [DOI] [PubMed] [Google Scholar]
- 30.Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 2012; 33: 33–59. doi: 10.1055/s-0031-1281676 [DOI] [PubMed] [Google Scholar]
- 31.Cokkinos D, Antypa E, Stefanidis K, Tserotas P, Kostaras V, Parlamenti A, et al. Contrast-enhanced ultrasound for imaging blunt abdominal trauma—indications, description of the technique and imaging review. Ultraschall Med 2012; 33: 60–7. doi: 10.1055/s-0031-1273442 [DOI] [PubMed] [Google Scholar]
- 32.Afaq A, Harvey C, Aldin Z, Leen E, Cosgrove D.Contrast-enhanced ultrasound in abdominal trauma. Eur J Emerg Med 2012; 19: 140–5. doi: 10.1097/MEJ.0b013e328348c980 [DOI] [PubMed] [Google Scholar]
- 33.Miele V, Buffa V, Stasolla A, Regine G, Atzori M, Ialongo P, et al. Contrast enhanced ultrasound with second generation contrast agent in traumatic liver lesions. [In Italian.] Radiol Med 2004; 108: 82–91. [PubMed] [Google Scholar]
- 34.Valentino M, Serra C, Pavlica P, Barozzi L. Contrast-enhanced ultrasound for blunt abdominal trauma. Seminars in Ultrasound CT MRI 2007; 28: 130–40. doi: 10.1053/j.sult.2007.01.008 [DOI] [PubMed] [Google Scholar]
- 35.Körner M, Krötz MM, Degenhart C, Pfeifer KJ, Reiser MF, Linsenmaier U. Current role of emergency US in patients with major trauma. Radiographics 2008; 28: 225–42. doi: 10.1148/rg.281075047 [DOI] [PubMed] [Google Scholar]
- 36.Chiavaroli R, Grima P, Tundo P. Characterization of nontraumatic focal splenic lesions using contrast-enhanced sonography. J Clin Ultrasound 2011; 39: 310–15. doi: 10.1002/jcu.20831 [DOI] [PubMed] [Google Scholar]
- 37.Catalano O, Lobianco R, Sandomenico F, Siani A. Splenic trauma: evaluation with contrast-specific sonography and a second-generation contrast medium: preliminary experience. J Ultrasound Med 2003; 22: 467–77. [DOI] [PubMed] [Google Scholar]
- 38.Sessa B, Trinci M, Ianniello S, Menichini G, Galluzzo M, Miele V. Blunt abdominal trauma: role of contrast enhanced ultrasound (CEUS) in the detection and staging of abdominal traumatic lesions compared to US and CE-MDCT. Radiol Med 2015; 120: 180–9. doi: 10.1007/s11547-014-0425-9 [DOI] [PubMed] [Google Scholar]
- 39.Cagini L, Gravante S, Malaspina CM, Cesarano E, Giganti M, Rebonato A, et al. Contrast enhanced ultrasound (CEUS) in blunt abdominal trauma. Crit Ultrasound J 2013; 5(Suppl. 1): S9. doi: 10.1186/2036-7902-5-S1-S9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McArthur C, Baxter GM. Current and potential renal applications of contrast-enhanced ultrasound. Clin Radiol 2012; 67: 909–22. doi: 10.1016/j.crad.2012.01.017 [DOI] [PubMed] [Google Scholar]
- 41.Regine G, Stasolla A, Miele V. Multidetector computed tomography of the renal arteries in vascular emergencies. Eur J Radiol 2007; 64: 83–91. doi: 10.1016/j.ejrad.2007.06.007 [DOI] [PubMed] [Google Scholar]
- 42.Regine G, Atzori M, Miele V, Buffa V, Galluzzo M, Luzietti M, et al. Second-generation sonographic contrast agents in the evaluation of renal trauma. [In Italian.] Radiol Med 2007; 112: 581–7. doi: 10.1007/s11547-007-0164-2 [DOI] [PubMed] [Google Scholar]
- 43.Valentino M, Galloni SS, Rimondi MR, Gentili A, Lima M, Barozzi L. Contrast-enhanced ultrasound in non-operative management of pancreatic injury in childhood. Pediatr Radiol 2006; 36: 558–60. doi: 10.1007/s00247-006-0157-7 [DOI] [PubMed] [Google Scholar]
- 44.Lv F, Tang J, Luo Y, Nie Y, Liang T, Jiao Z, et al. Emergency contrast-enhanced ultrasonography for pancreatic injuries in blunt abdominal trauma. Radiol Med 2014; 119: 920–7. doi: 10.1007/s11547-014-0410-3 [DOI] [PubMed] [Google Scholar]
- 45.Lv F, Tang J, Luo Y, Li Z, Meng X, Zhu Z, et al. Contrast-enhanced ultrasound imaging of active bleeding associated with hepatic and splenic trauma. Radiol Med 2011; 116: 1076–82. doi: 10.1007/s11547-011-0680-y [DOI] [PubMed] [Google Scholar]
- 46.Marmery H, Shanmuganatan K, Mirvis SE, Richard H, 3rd, Silker C, Miller LA, et al. Correlation of multidetector CT findings with splenic arteriography and surgery: prospective study in 392 patients. J Am Coll Surg 2008; 206: 685–93. doi: 10.1016/j.jamcollsurg.2007.11.024 [DOI] [PubMed] [Google Scholar]
- 47.Hamilton JD, Kumaravel M, Censullo ML, Cohen AM, Kievlan DS, West OC. Multidetector CT evaluation of active extravasation in blunt abdominal and pelvic trauma patients. Radiographics 2008; 28: 1603–16. doi: 10.1148/rg.286085522 [DOI] [PubMed] [Google Scholar]
- 48.Catalano O, Cusati B, Nunziata A, Siani A. Active abdominal bleeding: contrast-enhanced sonography. Abdom Imaging 2006; 31: 9–16. doi: 10.1007/s00261-005-0369-6 [DOI] [PubMed] [Google Scholar]
- 49.Catalano O, Sandomenico F, Raso MM, Siani A. Real time, contrast- enhanced sonography: a new tool for detecing active bleeding. J Trauma 2005; 59: 933–9. doi: 10.1097/01.ta.0000188129.91271.ab [DOI] [PubMed] [Google Scholar]
- 50.Oldenburg A, Hohmann J, Skrok J, Albrecht T. Imaging of paediatric splenic injury with contrast-enhanced ultrasonography. Pediatr Radiol 2004; 34: 351–4. doi: 10.1007/s00247-003-1092-5 [DOI] [PubMed] [Google Scholar]
- 51.Harkanyi Z. Potential applications of contrast-enhanced ultrasound in pediatric patients. Ultrasound Clin 2013; 8: 403–22. doi: 10.1016/j.cult.2013.04.002 [DOI] [Google Scholar]
- 52.Piskunowicz M, Kosiak W, Batko T. Intravenous application of second-generation ultrasound contrast agents in children: a review of the literature. Ultraschall Med 2012; 33: 135–40. doi: 10.1055/s-0031-1281936 [DOI] [PubMed] [Google Scholar]
- 53.Manetta R, Pistoia ML, Bultrini C, Stavroulis E, Di Cesare E, Masciocchi C. Ultrasound enhanced with sulphur-hexafluoride-filled microbubbles agent (SonoVue) in the follow-up of mild liver and spleen trauma. [In Italian.] Radiol Med 2009; 114: 771–9. doi: 10.1007/s11547-009-0406-6 [DOI] [PubMed] [Google Scholar]
- 54.Miele V, Piccolo CL, Sessa B, Trinci M, Galluzzo M. Comparison between MRI and CEUS in the follow-up of patients with blunt abdominal trauma managed conservatively. Radiol Med 2015. Epub ahead of print. doi: 10.1007/s11547-015-0578-1 [DOI] [PubMed] [Google Scholar]
- 55.Poletti PA, Kinkel K, Vermeulen B, Irmary F, Unger PF, Terrier F. Blunt abdominal trauma: shoud US be used to detect both free fluid and organ injuries? Radiology 2003; 227: 95–103. doi: 10.1148/radiol.2271020139 [DOI] [PubMed] [Google Scholar]
- 56.Poletti PA, Mirvis SE, Shanmuganathan K, Takada T, Killeen KL, Perlmutter D, et al. Blunt abdominal trauma patients: can organ injury be excluded without performing computed tomography? J Trauma 2004; 57: 1072–81. doi: 10.1097/01.TA.0000092680.73274.E1 [DOI] [PubMed] [Google Scholar]
- 57.Pinto F, Bode PJ, Tonerini M, Orsitto E. The role of the radiologist in the management of politrauma patients. Eur J Radiol 2006; 59: 315–16. doi: 10.1016/j.ejrad.2006.04.021 [DOI] [PubMed] [Google Scholar]
- 58.Strauss KJ, Kaste SC. The ALARA (as low as reasonably achievable) concept in pediatric interventional and fluoroscopic imaging: striving to keep radiation doses as low as possible during fluoroscopy of pediatric patients- a white paper executive summary. Pediatr Radiol 2006; 36(Suppl. 2): 110–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Darge K, Papadopoulou F, Ntoulia A, Bulas DI, Coley BD, Fordham LA, et al. Safety of contrast-enhanced ultrasound in children for non-cardiac applications: a review by the Society for Pediatric Radiology (SPR) and the International Contrast Ultrasound Society (ICUS). Pediatr Radiol 2013; 43: 1063–73. doi: 10.1007/s00247-013-2746-6 [DOI] [PubMed] [Google Scholar]
- 60.Lv F, Tang J, Ning Y, Zhou X, Luo Y, Liang T, et al. Effectiveness of contrast-enhanced ultrasound in the classification and emergency management of abdominal trauma. Eur Radiol 2014; 24: 2640–8. doi: 10.1007/s00330-014-3232-8 [DOI] [PubMed] [Google Scholar]
- 61.Emery K, McAneney CM, Racadio JM, Johnson ND, Evora DK, Garcia VF. Absent peritoneal fluid on screening trauma ultrasonography in children: a prospective comparison with computed tomography. J Pediatr Surg 2001; 36: 565–9. doi: 10.1053/jpsu.2001.22283 [DOI] [PubMed] [Google Scholar]
- 62.Baverstock R, Simons R, McLoughlin M. Severe blunt renal trauma: a 7-year retrospective review from a provincial trauma center. Can J Urol 2001; 8: 1372–6. [PubMed] [Google Scholar]
- 63.Margenthaler JA, Weber TR, Keller MS. Blunt renal trauma in children: experience with conservative management at a pediatric trauma center. J Trauma 2002; 52: 928–32. doi: 10.1097/00005373-200205000-00018 [DOI] [PubMed] [Google Scholar]