Abstract
Objective:
To investigate whether MRI in emergency radiology can detect (a) additional trauma-related findings after minor head injury (MHI) or (b) structural, non-trauma-related intracranial lesions when trauma biomarker S-100B concentration is raised, or clinical symptoms are unexplained, or both.
Methods:
41 patients with MHI were included. Concentrations of S-100B in serum were measured and categorized using an established cut-off at 0.1 μg l−1. Intracerebral trauma-related as well as non-trauma-related chronic structural findings (atrophy, microangiopathy and chronic parenchymal defects) were assessed by cranial CT (CCT) and MRI by two independent radiologists (UL and LLG). All CCT and MRI results were compared with biomarker S-100B.
Results:
Compared with CCT, MRI detected 10 additional lesions. 5 patients had abnormal MRI with a total of 15 trauma-related lesions and showed elevated S-100B concentrations. Although sensitivity of S-100B was 100%, specificity was only 25%. Patients with structural brain lesions showed significantly higher S-100B serum levels (0.50 and 0.14 μg l−1, p = 0.01).
Conclusion:
Biomarker S-100B has proven its high negative-predictive value to rule out intracranial bleeding in patients after MHI even if MRI is used as imaging modality. Regarding the low specificity of S-100B, structural lesions of the brain parenchyma not related to the acute trauma may be associated with increased serum concentrations of protein S-100B.
Advances in knowledge:
Biomarker S-100B has a high negative-predictive value to rule out intracranial bleeding after MHI. Biomarker S-100B's low specificity may be associated with non-traumatic brain parenchyma lesions. MRI is superior to CCT in detecting subtle findings in neuroimaging after MHI. Biomarker S-100B can potentially reduce the large number of normal CCT studies after MHI.
INTRODUCTION
There are nearly 1.6 million traumatic brain injuries (TBIs) in the USA each year (incidence 538/100,000); in Europe, however, the incidence is far lower at 235/100,000 and neurotrauma remains the leading cause of death in patients under 45 years old.1–3 In the context of risk stratification after head injury, besides established clinical predictors such as headache, amnesia or loss of consciousness, objective and independent indicators have been recently investigated.4,5
Astroglial-derived protein S-100B is one of the most promising biomarkers for patients being clinically at high risk of developing intracranial complications after minor head injury (MHI). In addition to its role as a quantitative prognostic marker in severe head injury,6,7 several studies8,9 have proven a high test sensitivity and strong negative-predictive value (NPV), suggesting that it has the potential to reduce the large number of normal cranial CT (CCT) examinations after MHI by up to 30%. However, biomarker S-100B has an overall poor specificity. According to a recent meta-analysis, combined sensitivity can reach up to 94% [95% confidence interval (CI), 88–98%] and a combined specificity is only 44% (95% CI, 30–58%).10–12
Some reports have also shown raised concentrations of the biomarker in patients with non-traumatic parenchymal lesions such as infections13 and extracranial injuries.14 As MHI is very common and imaging is associated with significant costs and radiation exposure, an additional risk parameter could be of great value to stratify the patients at risk for TBI to undergo CCT. At the moment, S-100B is partially introduced into clinical practice. The American College of Emergency Physicians and the federal US Center for Disease Control and Prevention mentioned in their guidelines that S-100 can be useful as a screening test in MHI.5,15
Despite CCT being the first-line imaging modality of choice after MHI in emergency radiology, MRI is considered to be superior in the detection of subtle cortical contusions and small subdural haematomas within the first 24 h.16 It is also more sensitive for the detection of subacute or chronic subarachnoid haemorrhages17 and can provide evidence for diffuse axonal injuries after MHI in about 30% of the patients in whom CCT has failed to show an abnormality.18 Furthermore, MRI is the primary diagnostic tool for the assessment of non-acute cerebral pathologies because of its superior ability to visualize parenchymal alterations.
The purpose of this study was to evaluate the value and limitations of S-100B after MHI using MRI in emergency radiology. We investigated whether MRI can detect (a) additional trauma-related findings after MHI or (b) structural, non-trauma-related intracranial lesions when S-100B biomarker concentration is raised and CCT is normal or equivocal.
METHODS AND MATERIALS
Patients
The study was approved by the local ethics committee, and informed consent was obtained from all patients or from relatives if the patient was unconscious.
For this study, 41 patients with a history of minor head trauma (Glasgow Coma Scale on admission: 13–15) were examined using CCT and underwent MRI of the head within 48 h of admission. Separately, blood samples were obtained on admission for the measurement of S-100B concentration. Besides history of MHI, CCT imaging was indicated, if at least one of the following risk factors was present: loss of consciousness, post-traumatic amnesia, nausea, vomiting, severe headache, dizziness, vertigo, intoxication, treatment with anticoagulants and age over 60 years, following established clinical MHI guidelines. Those who are under the age of 18 years, pregnant females and patients with multiple injuries were excluded. Clinical indications for a subsequent MR examination within 48 h were defined as negative or equivocal CCT with discrepant neurological symptoms and/or increased concentration of S-100B (>0.1 μg l−1) in serum. Owing to intoxication, 17 patients could not give their consent to the measurement of S-100B concentrations during the first few hours. As the half-life period of S-100B in serum is about 2.2 h,19 the measured values in this group were not used for this study.
Some patients were also included simultaneously in a large multicentre study at 3 Level I trauma centres with a total of 1309 patients following a different study protocol.8 Limitations of this recruiting process are described in the Discussion section.
Cranial CT examination
After an initial neurological examination, a standard CCT (120 kV, 360 mA) was performed in an emergency radiology department to evaluate the brain parenchyma (3- to 5-mm slice thickness) and the skull using high-resolution bony reconstruction (1- to 2-mm slice thickness). All axial scans were read by two radiologists and written reports were provided. For this study, all CCTs were anonymized and reinterpreted by two trained independent and blinded radiologists with 7 and 12 years’ experience in emergency radiology who were unaware of the final diagnosis; controversial findings were read in consensus. All patterns of intracranial haemorrhage and skull fracture were considered to be trauma-related findings. The patients were grouped into CCT negative (CCT−, no abnormal findings) and CCT positive (CCT+, abnormal findings). If intracranial haemorrhage could not be excluded safely, the patient was also considered as “CCT positive” because of an equivocal CT finding deserving further evaluation.
MR examination
MRI of the cranium was taken according to a standard protocol using high-field 1.5-T MRI scanner (Siemens, Forchheim, Germany) with 40-mT quantum gradients, coronal including fluid-attenuated inversion recovery, haemosensitive axial T2*weighted gradient echo sequence, diffusion-weighted imaging, axial T1 weighted and axial and sagittal T2 weighted. Images were interpreted in the same way as the CT images. Again, patients were grouped into MRI negative (MRI−, no abnormality) and MRI positive (MRI+, at least one trauma-related finding). In addition, the following non-trauma-related, but structural intracerebral findings were also assessed: cerebral atrophy, microangiopathic lesions and chronic parenchymal defects of the brain.
S-100B measurement
Blood samples were processed to serum and assayed with Elecsys® S-100, an electrochemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany); routine testing took 18 min. The lower detection limit is 0.005 μg l−1, and concentrations of up to 39 μg l−1 can be measured without dilution; results were reported as μg l−1. A cut-off value for diagnostic S-100B serum concentrations had been established at 0.1 μg l−1 in prior studies from the same institution (LMU University Munich, Germany) and more detailed information available in these cited publications.8,20 Patients with raised serum concentrations were grouped as S-100B positive (S-100B+) and concentrations below the cut-off were considered as S-100B negative (S-100B−).
Statistical analysis
To assess the significance of differences between groups, we used the SPSS® software v. 15.0 (IBM Corp., Armonk, NY; formerly SPSS Inc., Chicago, IL). Because personal and clinical data were not normally distributed, they have been reported as median and interquartile range (IQR). To compare different S-100B concentrations related to clinical and radiological findings, we used the non-parametric Mann–Whitney U test. Sensitivity, specificity, positive-predictive value (PPV) and NPV were calculated from contingency tables.
RESULTS
Demographics
Patient demographics are shown in Table 1.
Table 1.
Patient demographics
| Age, years, mean ± SD (min–max) | 54.6 ± 23.3 (20–89) |
| Gender | |
| Male | 21 (51.2) |
| Female | 20 (48.8) |
| GCS on admission | |
| 15 | 36 (87.8) |
| 14 | 4 (9.8) |
| 13 | 1 (2.4) |
| Mechanism of injury | |
| Fall | 25 (61.0) |
| Motor vehicle accident | 12 (29.3) |
| Other (e.g. assault, collision) | 4 (9.8) |
| Indication for MRI | |
| a) Unexplained clinical symptoms | 9 (22.0) |
| b) Elevated S-100B concentration | 26 (63.4) |
| c) Combination of (a) and (b) | 6 (14.6) |
| Outcome | |
| Admitted for observation | 11 (26.8) |
| Discharged | 30 (73.2) |
GCS, Glasgow Coma Scale; max, maximum; min, minimum; SD, standard deviation.
Data are number (%) of patients, unless otherwise stated.
Cranial CT and MRI results
Of 41 (100%) patients, 12 (29.3%, CCT+) patients were categorized as CCT positive with a total of 14 trauma-related lesions: contusions (n = 8); subarachnoid (n = 1), subdural (n = 2) and epidural (n = 1) haemorrhages; and fractures (n = 2).
5 (12.2%, MRI+) patients had abnormal MRI with a total of 15 trauma-related lesions: contusions (n = 7); subarachnoid (n = 5), subdural (n = 2) and epidural (n = 1) haemorrhages.
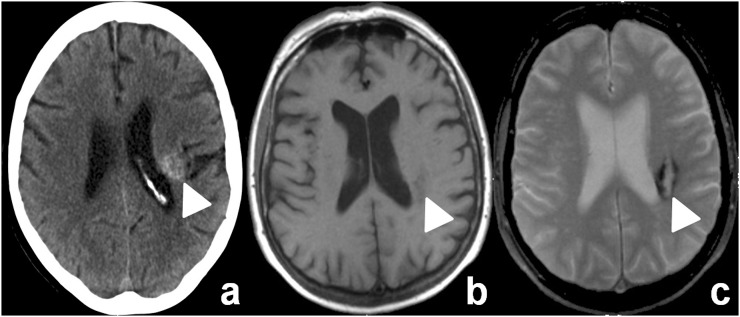
Five patients had trauma-related findings diagnosed by both CCT and MRI; another seven patients had positive or questionable CCT scans and lesions were not confirmed by MRI (Table 2). In the latter group, one haemorrhage suspected on CCT was then correctly diagnosed as cavernoma by MRI (Figure 1). The remaining six positive CCT were assessed as artefacts, in retrospect, because of the negative MRI scan. The rate of CCT scans that were supposed to be false-positive CCT findings was 17%.
Table 2.
Contingency table—sensitivity, specificity, positive-predictive value (PPV) and negative-predictive value (NPV) of cranial CT (CCT) compared with MRI
| CCT findings | MRI positive | MRI negative | NPV and PPV |
|---|---|---|---|
| CCT positive | 5 (12) | 7 (17) | PPV 42% |
| CCT negative | 0 (0) | 29 (71) | NPV 100% |
| Sensitivity 100% | Specificity 81% |
Data are number (%) of patients.
Figure 1.
MRI clarifying cranial CT (CCT) findings as cavernoma. A 69-year-old female after a fall. CCT (a) shows an atypical intracranial haemorrhage (arrowhead). MRI (b, T1 weighted; c, T2*weighted) detected a cavernoma (arrowheads).
Compared with CCT, MRI detected 10 additional lesions: 6 contusions and 4 subarachnoid haemorrhages. However, none of both skull fractures was detected (Table 3 and Figure 2).
Table 3.
Patient characteristics, S-100B categories and cranial CT (CCT) as well MRI findings of selected cases of the study group
| Patient number | Age (years) | Gender | GCS on admission | Mechanism of injury | Risk factors | S-100B | CCT findings (n) | MRI findings (n) |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | Female | 15 | Fall | LOC, nausea, severe headache, anticoagulants | Elevated | Contusion (1), SDH (1) | Contusions (2), SDH (1) |
| 2 | 26 | Female | 15 | MVA | LOC, PTA, nausea, severe headache, dizziness | Elevated | Fracture (1) | Contusions (2), SAH (3) |
| 3 | 42 | Male | 15 | Fall | Intoxication | Elevated | SAH (1) | SAH (1), contusions (2) |
| 4 | 22 | Male | 15 | Fall | LOC, PTA | Elevated | Fracture (1), EDH (1) | EDH (1) |
| 5 | 73 | Female | 15 | MVA | LOC, PTA, severe headache, age over 60 years | Elevated | SDH (1) | SDH (1), contusions (1), SAH (1) |
| 6 | 80 | Female | 15 | Fall | Dizziness, anticoagulants, age over 60 years | Elevated | Contusion (1) | Cavernoma |
| 7 | 54 | Female | 15 | Fall | LOC, PTA, dizziness, intoxication | Elevated | Contusion (1) | Negative |
| 8 | 33 | Male | 14 | Fall | LOC, PTA, intoxication | Elevated | Contusion (1) | Negative |
| 9 | 56 | Male | 15 | MVA | PTA, LOC | Elevated | Contusion (1) | Negative |
| 10 | 44 | Male | 15 | MVA | LOC, severe headache | Not elevated | Contusion (1) | Negative |
| 11 | 39 | Male | 15 | MVA | Severe headache, dizziness | Not elevated | Contusion (1) | Negative |
| 12 | 44 | Male | 15 | MVA | LOC, severe headache | Not elevated | Contusion (1) | Negative |
EDH, epidural haemorrhage; GCS, Glasgow Coma Scale; LOC, loss of consciousness; MVA, motor vehicle accident; PTA, post-traumatic amnesia; SAH, subarachnoid haemorrhage; SDH, subdural haemorrhage.
Elevated S-100B: concentrations >0.1 μg l−1.
Cases 1–5 are patients with positive MRI findings. MRI shows additional trauma-related lesions compared with CCT (additional findings appear in bold type). Skull fractures were not diagnosed by MRI. Case 6 represents an example where MRI clearly distinguished a cavernoma from a suspected contusion. Cases 7–9 are patients with false-positive CCT findings in patients with elevated S-100B concentrations. Cases 10–12 are patients without elevated S-100B concentrations where the initial CCT was assessed as pathologic with suspicion of intracerebral contusions; MRI was able to rule out intracranial trauma, and therefore, these findings were interpreted as artefacts, in retrospect.
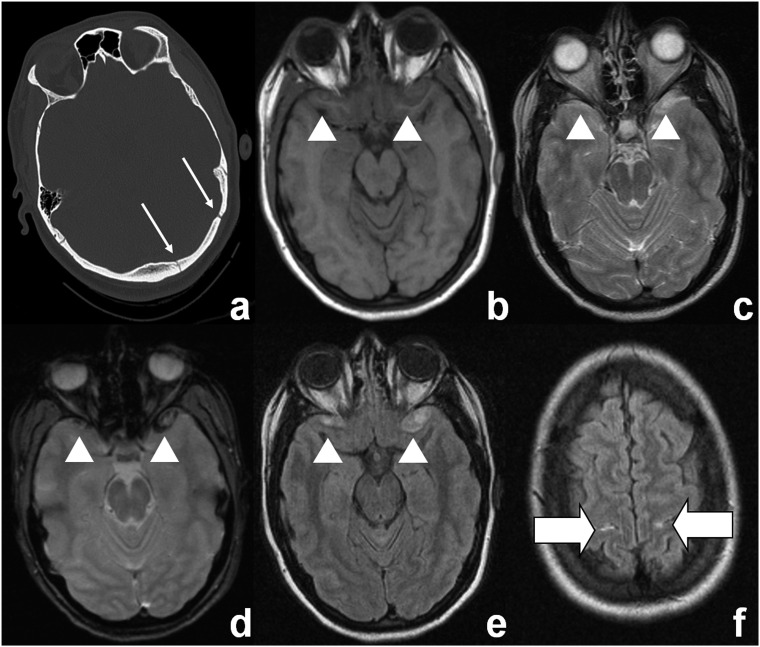
Figure 2.
MRI detects additional intracranial bleeding. A 26-year-old female after motor vehicle accident with elevated S-100B serum concentration (0.924 μg l−1). CCT (a) shows a fracture of the occipital bone (thin arrows), but no intracranial bleeding. MRI (b, T1 weighted; c, T2 weighted; d, T2*weighted; e and f, fluid-attenuated inversion recovery) shows front temporal contusions (arrowheads) as well as subarachnoid haemorrhage (thick arrows in f).
Measurement of S-100B concentrations
41 (100%) blood samples were obtained; 1 patient with elevated S-100B concentration was excluded from the detailed analysis because of the enormously high value of 19.82 μg l−1, which we put down to a technical failure.
The median concentration of the remaining 40 samples was 0.35 μg l−1 (IQR 0.12–0.65 μg l−1; minimum 0.04 μg l−1; maximum 2.39 μg l−1). 32 (78%, S-100B+) patients showed an increased concentration in serum: median concentration 0.47 μg l−1, IQR 0.27–0.81 μg l−1. By contrast, only nine (22%) patients were within the reference range: median concentration 0.06 μg l−1, IQR 0.04–0.07 μg l−1.
S-100B concentrations and trauma-related findings on cranial CT and MRI
In 27 of 32 (84%) patients with high S-100B concentrations, the results were false positives with regard to negative MRI, serving as the gold standard; 4 of the 27 also had a false positive CCT. All patients in whom the S-100B concentration was within the reference range (n = 9) had MRI that showed no abnormality. In five patients with positive MR, S-100B concentrations were abnormally raised. On the basis of a contingency table, sensitivity, specificity, positive-predictive value and NPV were estimated (Table 4).
Table 4.
Contingency table—S-100B concentrations and MRI findings
| S-100B findings | MRI positive | MRI negative | PPV and NPV |
|---|---|---|---|
| S-100B positive | 5 (12) | 27 (66) | PPV 16% |
| S-100B negative | 0 (0) | 9 (22) | NPV 100% |
| Sensitivity 100% | Specificity 25% |
NPV, negative-predictive value; ppv positive-predictive value.
Data are number (%) of patients.
However, there was no significant difference between the median concentration in patients with positive MR scans and in those without (0.33 μg l−1, IQR 0.24–0.67 μg l−1; and 0.35 μg l−1, IQR 0.10–0.65 μg l−1).
Also remarkable was the fact that 3 out of 41 patients had S-100B concentrations within the reference range despite CCT being categorized as positive. As these cases did not show any trauma-related finding on MRI, they were assessed as artefacts, in retrospect (Table 3).
S-100B concentrations and non-traumatic lesions on MRI
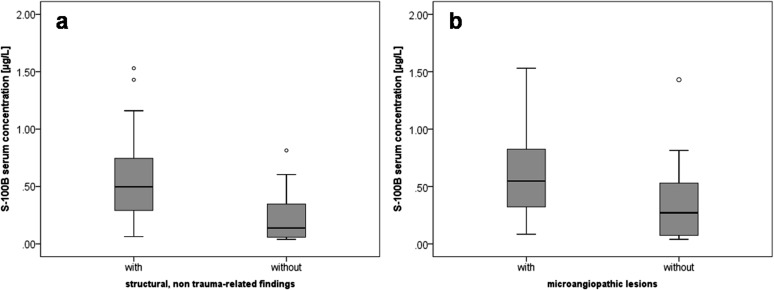
To assess the low test specificity, S-100B was also related to non-traumatic lesions on MRI: significantly higher concentrations were measured in patients with cerebral atrophy than in younger or healthy patients with no signs of cerebral atrophy (0.47 and 0.22 μg l−1; p = 0.02) (Table 5). Microangiopathic lesions (0.55 and 0.27 μg l−1; p = 0.06) and chronic parenchymal defects of the brain (0.50 and 0.27 μg l−1; p = 0.09) showed again higher concentrations of S-100B, but not significantly so. In addition, a subgroup analysis of patients without intracranial bleeding (CCT as well as MRI) was performed: significantly higher S-100B serum concentrations were found in patients with different types of chronic structural, non-trauma-related findings (0.50 and 0.14 μg l−1; p = 0.01) and in patients with microangiopathic lesions (0.55 and 0.27 μg l−1; p = 0.038) (Figure 3).
Table 5.
Median serum concentrations related to non-traumatic intracranial findings
| Non-traumatic findings | Patients with non-traumatic lesions |
Patients without non-traumatic lesions |
p-value | ||
|---|---|---|---|---|---|
| n (%) | S-100B concentration (μg l−1) | n (%) | S-100B concentration (μg l−1) | ||
| Chronic parenchymal defect (s.p. infarction or trauma) | 12 (30.0) | 0.50 (0.31–0.70) | 28 (70.0) | 0.27 (0.08–0.62) | 0.09 |
| Brain atrophy | 17 (42.5) | 0.47 (0.29–0.84) | 23 (57.5) | 0.22 (0.07–0.53) | 0.02 |
| Microangiopathy | 12 (30.0) | 0.55 (0.32–0.83) | 28 (70.0) | 0.27 (0.08–0.57) | 0.06 |
s.p., status post concentrations are given as median (interquartile range). Mann–Whitney U test was used to compare different S-100B concentrations. The cut-off value of serum concentrations was 0.1 μg l−1.
Figure 3.
Box plots: S-100B serum concentrations in a subgroup of patients without intracranial bleeding. Significantly increased S-100B concentrations were found in patients with different types of structural, non-trauma-related chronic intracranial findings, such as cerebral atrophy, microangiopathic lesions and chronic parenchymal defects (a). In a more detailed analysis, patients with microangiopathic lesions have shown again relevant higher S-100B concentrations (b). Circles indicate artifacts.
DISCUSSION
Neurotrauma is the leading cause of death and disability under the age of 45 years, and studies8,21–23 have shown that CCT can detect all relevant neurosurgical lesions independent of the grading of severity of injury. Due to its wide availability and diagnostic impact, diagnosis by CCT is the standard. The availability of MRI in emergency radiology has increased since the 1990s, and its use after MHI has shown that it is more sensitive than CCT in the detection of many post-traumatic conditions.16,18,24–26
Our results show, as other published data, the diagnostic superiority of MRI in emergent neuroimaging. Of 15 (100%) post-traumatic parenchymal lesions found with MRI, CCT showed only 5 (33%) post-traumatic parenchymal lesions (Figure 2). Data comparing the diagnostic accuracy of MRI and CCT are scarce because a definite reference standard is missing. A study by Orrison et al27 analysed 107 patients retrospectively, and overall sensitivities of CCT and MRI were 63% and 96%, respectively. The ability of MRI to detect contusions, shear injuries and subdural and epidural haematomas was considerably higher. Despite the recent progress in MRI techniques, bone injuries remain a domain of CCT;27,28 in this study, two skull fractures were detected only by CCT.
In our study, only 5 (42%) of 12 positive CT examinations could be confirmed by MRI; 6 (50%) were false positives and 1 was identified as cavernous angioma (Figure 1). This is because the high specificity of MRI leads to better visualization of brain parenchyma. Ahlhelm et al29 suggested that up to half of the cavernomas identified by MRI are not detected by CCT.
The other six false-positive CCT findings were supposed to be artefacts that were misinterpreted as questionable subtle bleedings. Published data about the specificity of CCT and MRI, respectively, for the detection of contusions are rare. However, MRI has proven its superior sensitivity for the detection of small contusions and microhaemorrhages; for most other extra-axial lesions, the sensitivity of both modalities is shown to be comparable.30
As far as the impact of S-100B as a predictive biochemical trauma marker after head trauma is concerned, our results from an emergency radiology department are in agreement with those of other authors,8,11 although we found now for the first time the significant difference in concentrations between the MRI that did and did not show non-traumatic parenchyma lesions. However, this MRI study confirms previous studies based on CT scanning: S-100B in serum has a high sensitivity and a high NPV for trauma-related intracranial lesions after MHI. A recent meta-analysis including >2000 patients showed a combined sensitivity of 94 % (95% CI, 88–98%) and a combined specificity of 44% (95% CI, 30–58%) with diagnostic odds ratio of 10.3 (95% CI, 4.2–24.9%).10 Muller et al31 concluded in a consensus paper that the biomarker can triage patients to CCT, again with a high NPV and decrease the large number of normal CCT and set free capacities of the emergency rooms.
Townend and Ingebrigtsen could define in a meta-analysis an S-100B cut-off at 2.5 μg l−1, which is related to dependent disability and post-concussion syndrome and is also a specific test for this. Patients above the cut-off are at high risk for disability after MHI.32
The specificity of S-100B was again low in this study. Our results showed for the first time that there was a difference between patients with chronic brain atrophy and those without. We therefore hypothesize that structural defects caused by older or chronic infarction or post-traumatic lesions or microangiopathy could explain, to some extent, the low specificity. However, further research in a larger study population is needed for confirmation. These results, at the time, confirm, however, possible limitations of a trauma biomarker S-100B in clinical practice.
The usefulness of biomarker S-100B in patients with CCT-negative MHI is based on its high sensitivity (100%) and high NPV, allowing for a safe rule out of significant cerebral injuries, if biomarker S-100B is normal. This can include early discharge from the hospital after suspected MHI and no further need for imaging in this group of patients.
A clear interdisciplinary recommendation of the use of S-100B in clinical practice is still missing in Europe with some national societies being in favour, however lack of statements.33,34 The American College of Emergency Physicians and the federal US Center for Disease Control and Prevention mentioned in their recent guidelines that S-100B can be useful as a screening test in minor head trauma.15
A notable literature review of 270 pages describes in a health technology assessment again the potential of S-100B biomarker for being a screening test in minor head trauma. Automated laboratory test systems are available in the market and well-established and in use in many trauma hospitals.35
The value of S-100B as a predictor of severity and outcome in patients with acute ischaemic stroke has been investigated by several studies before.36,37 To the best of our knowledge, data about the value of S-100B in the context of chronic post-ischaemic findings are rare. The results of our study might indicate that chronic structural alterations of the human brain, such as microangiopathic lesions and chronic structural post-ischaemic findings, tended to be associated with higher S-100B concentrations in serum. Because this study was conducted within a clinical setting, the exact pathophysiological mechanism remains unclear, as it is in many other conditions38 and is also a limitation. However, Chang et al39 suggested a relationship between chronic gliosis and neuroprotein S-100B using an animal model. This might support our findings.
Our results might also indicate that patients with brain atrophy show higher serum concentrations of S-100B. The average age of 55 years in our study population has to be discussed in front of this background; however, neuroprotein S-100B is by the majority so far considered as an age-independent biomarker.40 Petzold et al41 have found an association between brain atrophy in Alzheimer's disease and increased levels of S-100B in the cerebrospinal fluid. Apart from that, elevated levels of neuroprotein S-100B were also found in other neurodegenerative diseases.42 Unden et al13 showed that cerebral infectious diseases have also had a considerable influence on the S-100B concentration in serum.
Our study was primarily designed for patients presenting to an emergency department of a trauma centre, so that a comprehensive evaluation of the neurobehavioral status is still missing. A limitation of this study is the small sample size. The main reasons of screen failure were missing consent to the entire study, recruiting dropouts or logistic issues as the availability of our MRI scanner for emergency imaging and off-hour activation, as it was not always possible to arrange an appointment within 48 h. In addition, some patients with normal CCT were not willing to undergo subsequent MRI.
The MRI protocol used in this study was established in clinical routine to rule out TBI and was not altered for this study. In recent years, susceptibility-weighted imaging and diffusion tensor imaging (DTI) have received a lot of attention but are little used in the clinical setting of TBI. Chastain et al43 have shown that fluid-attenuated inversion recovery and T1 weighted sequences were able to correlate with outcome after MHI, while the optimal use of susceptibility-weighted imaging was unclear. By contrast, DTI has been discussed as a potential MRI marker for neurological outcome after MHI.44 Most of the DTI studies were however conducted during the subacute stage after trauma. It can be hypothesized that DTI can detect further lesions, which explain further the low specificity of S-100B measurement.
In conclusion, S-100B has proven its high sensitivity to rule out intracranial bleeding in patients after MHI, even if MRI is used as imaging modality. In regard to its low specificity, structural lesions of the brain parenchyma not related to trauma may be associated with increased serum concentrations of protein S-100B. However, this topic requires further research. In clinical practice, CCT is the undisputable first-line imaging tool in the acute setting. However, MRI has confirmed its value as a sophisticated imaging tool after head injury, particularly to complete investigations when CCT findings are unclear and the clinical presentation of the patient remains doubtful.
Contributor Information
Ulrich Linsenmaier, Email: ulrich.linsenmaier@gmx.de.
Stefan Wirth, Email: stefan.wirth@med.lmu.de.
Karl-Georg Kanz, Email: Karl-Georg.Kanz@mri.tum.de.
Lucas L Geyer, Email: lucas.geyer@med.lmu.de.
REFERENCES
- 1.Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil 2006; 21: 544–8. [DOI] [PubMed] [Google Scholar]
- 2.Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir (Wien) 2006; 148: 255–68; discussion 68. doi: 10.1007/s00701-005-0651-y [DOI] [PubMed] [Google Scholar]
- 3.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006; 367: 1747–57. [DOI] [PubMed] [Google Scholar]
- 4.Metting Z, Wilczak N, Rodiger LA, Schaaf JM, van der Naalt J. GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology 2012; 78: 1428–33. doi: 10.1212/WNL.0b013e318253d5c7 [DOI] [PubMed] [Google Scholar]
- 5.Stiell IG, Clement CM, Rowe BH, Schull MJ, Brison R, Cass D, et al. Comparison of the Canadian CT head rule and the New Orleans criteria in patients with minor head injury. JAMA 2005; 294: 1511–18. doi: 10.1001/jama.294.12.1511 [DOI] [PubMed] [Google Scholar]
- 6.Bohmer AE, Oses JP, Schmidt AP, Peron CS, Krebs CL, Oppitz PP, et al. Neuron-specific enolase, S100B, and glial fibrillary acidic protein levels as outcome predictors in patients with severe traumatic brain injury. Neurosurgery 2011; 68: 1624–30; discussion 30–1. [DOI] [PubMed] [Google Scholar]
- 7.Vos PE, Lamers KJ, Hendriks JC, van Haaren M, Beems T, Zimmerman C, et al. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology 2004; 62: 1303–10. doi: 10.1212/01.WNL.0000120550.00643.DC [DOI] [PubMed] [Google Scholar]
- 8.Biberthaler P, Linsenmaier U, Pfeifer KJ, Kroetz M, Mussack T, Kanz KG, et al. Serum S-100B concentration provides additional information for the indication of computed tomography in patients after minor head injury: a prospective multicenter study. Shock 2006; 25: 446–53. doi: 10.1097/01.shk.0000209534.61058.35 [DOI] [PubMed] [Google Scholar]
- 9.Springborg JB, Unden J, Ingebrigtsen T, Romner B. Brain injury marker S100B can reduce the use of computer tomography in minor head injuries—secondary publication. [In Danish.] Ugeskr Laeger 2009; 171: 978–81. [PubMed] [Google Scholar]
- 10.Leidel BA, Bogner V, Zock M, Kanz KG. Serological determination of protein S100B. Significance in emergency diagnosis of adults with mild craniocerebral trauma–meta-analysis. [In German.] Unfallchirurg 2012; 115: 903–12. doi: 10.1007/s00113-010-1946-x [DOI] [PubMed] [Google Scholar]
- 11.Unden J, Romner B. A new objective method for CT triage after minor head injury—serum S100B. Scand J Clin Lab Invest 2009; 69: 13–17. [DOI] [PubMed] [Google Scholar]
- 12.Unden J, Romner B. Can low serum levels of S100B predict normal CT findings after minor head injury in adults?: an evidence-based review and meta-analysis. J Head Trauma Rehabil 2010; 25: 228–40. [DOI] [PubMed] [Google Scholar]
- 13.Unden J, Christensson B, Bellner J, Alling C, Romner B. Serum S100B levels in patients with cerebral and extracerebral infectious disease. Scand J Infect Dis 2004; 36: 10–13. [DOI] [PubMed] [Google Scholar]
- 14.Korfias S, Stranjalis G, Psachoulia C, Vasiliadis C, Pitaridis M, Boviatsis E, et al. Slight and short-lasting increase of serum S-100B protein in extra-cranial trauma. Brain Inj 2006; 20: 867–72. doi: 10.1080/02699050600832395 [DOI] [PubMed] [Google Scholar]
- 15.Jagoda AS, Bazarian JJ, Bruns JJ, Jr, Cantrill SV, Gean AD, Howard PK, et al. Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann Emerg Med 2008; 52: 714–48. doi: 10.1016/j.annemergmed.2008.08.021 [DOI] [PubMed] [Google Scholar]
- 16.Doezema D, King JN, Tandberg D, Espinosa MC, Orrison WW. Magnetic resonance imaging in minor head injury. Ann Emerg Med 1991; 20: 1281–5. doi: 10.1016/S0196-0644(05)81065-0 [DOI] [PubMed] [Google Scholar]
- 17.Noguchi K, Ogawa T, Inugami A, Toyoshima H, Okudera T, Uemura K. MR of acute subarachnoid hemorrhage: a preliminary report of fluid-attenuated inversion-recovery pulse sequences. AJNR Am J Neuroradiol 1994; 15: 1940–3. [PMC free article] [PubMed] [Google Scholar]
- 18.Mittl RL, Grossman RI, Hiehle JF, Hurst RW, Kauder DR, Gennarelli TA, et al. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. AJNR Am J Neuroradiol 1994; 15: 1583–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Blomquist S, Johnsson P, Luhrs C, Malmkvist G, Solem JO, Alling C, et al. The appearance of S-100 protein in serum during and immediately after cardiopulmonary bypass surgery: a possible marker for cerebral injury. J Cardiothorac Vasc Anesth 1997; 11: 699–703. doi: 10.1016/S1053-0770(97)90160-9 [DOI] [PubMed] [Google Scholar]
- 20.Oh EJ, Kim YM, Jegal DW, Kahng J, Park YJ, Han K. Diagnostic value of Elecsys S100 as a marker of acute brain injury in the emergency department. J Clin Lab Anal 2007; 21: 387–92. doi: 10.1002/jcla.20201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PM. Indications for computed tomography in patients with minor head injury. N Engl J Med 2000; 343: 100–5. doi: 10.1056/NEJM200007133430204 [DOI] [PubMed] [Google Scholar]
- 22.Nagy KK, Joseph KT, Krosner SM, Roberts RR, Leslie CL, Dufty K, et al. The utility of head computed tomography after minimal head injury. J Trauma 1999; 46: 268–70. doi: 10.1097/00005373-199902000-00012 [DOI] [PubMed] [Google Scholar]
- 23.Stiell IG, Hebert PC, Wells GA, Vandemheen KL, Tang AS, Higginson LA, et al. Vasopressin versus epinephrine for inhospital cardiac arrest: a randomised controlled trial. Lancet 2001; 358: 105–9. doi: 10.1016/S0140-6736(01)05328-4 [DOI] [PubMed] [Google Scholar]
- 24.Gentry LR, Godersky JC, Thompson B, Dunn VD. Prospective comparative study of intermediate-field MR and CT in the evaluation of closed head trauma. AJR Am J Roentgenol 1988; 150: 673–82. doi: 10.2214/ajr.150.3.673 [DOI] [PubMed] [Google Scholar]
- 25.Jenkins A, Teasdale G, Hadley MD, Macpherson P, Rowan JO. Brain lesions detected by magnetic resonance imaging in mild and severe head injuries. Lancet 1986; 2: 445–6. doi: 10.1016/S0140-6736(86)92145-8 [DOI] [PubMed] [Google Scholar]
- 26.Yokota H, Kurokawa A, Otsuka T, Kobayashi S, Nakazawa S. Significance of magnetic resonance imaging in acute head injury. J Trauma 1991; 31: 351–7. doi: 10.1097/00005373-199103000-00007 [DOI] [PubMed] [Google Scholar]
- 27.Orrison WW, Gentry LR, Stimac GK, Tarrel RM, Espinosa MC, Cobb LC. Blinded comparison of cranial CT and MR in closed head injury evaluation. AJNR Am J Neuroradiol 1994; 15: 351–6. [PMC free article] [PubMed] [Google Scholar]
- 28.Wiesmann M, Brückmann H. Bildgebende Diagnostik akuter Schädel-Hirn-Verletzungen. [In German.] Radiologe 1998; 38: 645–58. doi: 10.1007/s001170050405 [DOI] [PubMed] [Google Scholar]
- 29.Ahlhelm F, Hagen T, Schulte-Altedorneburg G, Grunwald I, Reith W, Roth C. Cavernous malformations. [In German.] Radiologe 2007; 47: 863–7. doi: 10.1007/s00117-007-1546-0 [DOI] [PubMed] [Google Scholar]
- 30.Lee H, Wintermark M, Gean AD, Ghajar J, Manley GT, Mukherjee P. Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. J Neurotrauma 2008; 25: 1049–56. doi: 10.1089/neu.2008.0566 [DOI] [PubMed] [Google Scholar]
- 31.Muller B, Evangelopoulos DS, Bias K, Wildisen A, Zimmermann H, Exadaktylos AK. Can S-100B serum protein help to save cranial CT resources in a peripheral trauma centre? A study and consensus paper. Emerg Med J 2011; 28: 938–40. doi: 10.1136/emj.2010.095372 [DOI] [PubMed] [Google Scholar]
- 32.Townend W, Ingebrigtsen T. Head injury outcome prediction: a role for protein S-100B? Injury 2006; 37: 1098–108. doi: 10.1016/j.injury.2006.07.014 [DOI] [PubMed] [Google Scholar]
- 33.Neurochirurgie DGf. Leitlinie Schadel-Hirn-Trauma im Erwachsenenalter. 2015. Available from: http://wwwleitliniennet
- 34.Neurologie DGf. Leitlinien für Diagnostik und Therapie in der Neurologie—Leichtes Schädel-Hirn-Trauma. 2012. Available from: http://wwwleitliniennet
- 35.Pandor A, Goodacre S, Harnan S, Holmes M, Pickering A, Fitzgerald P, et al. Diagnostic management strategies for adults and children with minor head injury: a systematic review and an economic evaluation. Health Technol Assess 2011; 15: 1–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nash DL, Bellolio MF, Stead LG. S100 as a marker of acute brain ischemia: a systematic review. Neurocrit Care 2008; 8: 301–7. doi: 10.1007/s12028-007-9019-x [DOI] [PubMed] [Google Scholar]
- 37.Ahmad O, Wardlaw J, Whiteley WN. Correlation of levels of neuronal and glial markers with radiological measures of infarct volume in ischaemic stroke: a systematic review. Cerebrovasc Dis 2012; 33: 47–54. doi: 10.1159/000332810 [DOI] [PubMed] [Google Scholar]
- 38.Korfias S, Stranjalis G, Papadimitriou A, Psachoulia C, Daskalakis G, Antsaklis A, et al. Serum S-100B protein as a biochemical marker of brain injury: a review of current concepts. Curr Med Chem 2006; 13: 3719–31. doi: 10.2174/092986706779026129 [DOI] [PubMed] [Google Scholar]
- 39.Chang MS, Ariah LM, Marks A, Azmitia EC. Chronic gliosis induced by loss of S-100B: knockout mice have enhanced GFAP-immunoreactivity but blunted response to a serotonin challenge. Brain Res 2005; 1031: 1–9. doi: 10.1016/j.brainres.2004.07.043 [DOI] [PubMed] [Google Scholar]
- 40.Wiesmann M, Missler U, Gottmann D, Gehring S. Plasma S-100b protein concentration in healthy adults is age- and sex-independent. Clin Chem 1998; 44: 1056–8. [PubMed] [Google Scholar]
- 41.Petzold A, Jenkins R, Watt HC, Green AJ, Thompson EJ, Keir G, et al. Cerebrospinal fluid S100B correlates with brain atrophy in Alzheimer's disease. Neurosci Lett 2003; 336: 167–70. doi: 10.1016/S0304-3940(02)01257-0 [DOI] [PubMed] [Google Scholar]
- 42.Yardan T, Erenler AK, Baydin A, Aydin K, Cokluk C. Usefulness of S100B protein in neurological disorders. J Pak Med Assoc 2011; 61: 276–81. [PubMed] [Google Scholar]
- 43.Chastain CA, Oyoyo UE, Zipperman M, Joo E, Ashwal S, Shutter LA, et al. Predicting outcomes of traumatic brain injury by imaging modality and injury distribution. J Neurotrauma 2009; 26: 1183–96. doi: 10.1089/neu.2008.0650 [DOI] [PubMed] [Google Scholar]
- 44.Bigler ED, Bazarian JJ. Diffusion tensor imaging: a biomarker for mild traumatic brain injury? Neurology 2010; 74: 626–7. doi: 10.1212/WNL.0b013e3181d3e43a [DOI] [PubMed] [Google Scholar]