Abstract
Objective:
To evaluate the current guidelines as a model to predict malignancy and to determine further radiological predictors of malignancy in intraductal papillary mucinous neoplasms (IPMNs).
Methods:
384 patients who had undergone a pancreatic operation with the pathological diagnosis of IPMN as well as applicable pre-operative imaging (CT/MRI) were included in the study. Images were evaluated retrospectively in consensus by two radiologists, using a standardized checklist. Descriptive statistics, binary logistic regression and receiver operator curve analysis were performed to assess the International Consensus Guidelines and other radiological predictors of clinical malignancy (defined as carcinoma in situ and invasive carcinoma).
Results:
The best independent predictors of malignancy (n = 191) were solid components [odds ratio (OR) 3.98], parenchymal atrophy with main pancreatic duct dilation 5–9 mm (OR: 5.1) and common bile duct (CBD) dilation (OR: 31.26). >96% of all cases with CBD dilation were malignant IPMNs (positive-predictive value 96.4%; negative-predictive value 63.1%). Analysis of the current guidelines showed a diagnostic improvement with the addition of CBD dilation on determining the malignancy of IPMNs (sensitivity 82.2%/86.9%; specificity 72.7%/74.6%). Subanalysis of branch duct intraductal papillary mucinous neoplasms (BD-IPMNs; n = 168) also resulted in a diagnostic improvement with the addition of CBD dilation (sensitivity 28.6%/45.2%; specificity 92.9%/92.1%). The best independent predictors of malignancy for BD-IPMNs were parenchymal atrophy (OR: 4.00) and CBD dilation (OR: 29.3). Frequency analysis revealed that even small BD-IPMNs had already undergone malignant transformation (≤1 cm: 15%; 1–2 cm: 26%; 2–3 cm: 20%) with about 10% of those having a dilated bile duct.
Conclusion:
CBD dilation was a significant positive predictor of malignancy in IPMNs regardless of their size.
Advances in knowledge:
Introduction of CBD dilation as a radiological predictor for malignancy might increase the diagnostic accuracy of current imaging-based guidelines.
INTRODUCTION
Pancreatic intraductal papillary mucinous neoplasms (IPMNs) are mucin-producing tumours arising from cells either from the main duct or the branch ducts of the pancreas that have malignant potential.1,2 IPMNs can be classified according to the duct they arise from: these are branch duct intraductal papillary mucinous neoplasm (BD-IPMN), main duct intraductal papillary mucinous neoplasm (MD-IPMN) and mixed-type IPMN with main duct involvement having a higher malignant potential.3,4
Although many radiological predictors of malignancy have been studied, such as main pancreatic duct (MPD) dilation and presence of mural nodules, the radiological detection of malignancy in IPMNs still remains challenging.5,6 As most of these lesions are detected incidentally during imaging studies performed for unrelated reasons,7,8 imaging characteristics that are helpful for stratification of patients into resection or careful watching are necessary. Although all IPMNs have malignant potential, finding the right time for resection is crucial with benefits and risks being weighed up individually.9,10 This depends on the patients' co-morbidities, age and radiological characteristics of the lesion, e.g. an elderly patient with an IPMN defined by more benign radiological characteristics is an ideal candidate for further follow-up. The International Association of Pancreatology has issued consensus guidelines in 20063 and revised them in 2012,4 which could help the clinicians in deciding whether to operate a patient or to follow-up. The revision of 2012 defined radiological high-risk stigmata (MD-IPMN with MPD dilation >1 cm, mural nodules) vs worrisome features (BD-IPMN cyst size ≥3 cm, thickened cyst walls, MPD stenosis with distal pancreatic atrophy, adjacent lymphadenopathy) that can help clinicians on stratifying patients into operation and further follow-up with imaging. All of these parameters are under constant re-evaluation as more knowledge is gained through clinical experience. The size of BD-IPMNs is one criterion that is especially scrutinized. The original consensus guideline from 2006 recommended surgery in BD-IPMNs with a cyst size >3 cm but subsequent analyses have yielded relatively low rates of malignancy in these patients,11,12 which resulted in the downgrading of this characteristic into worrisome features in the 2012 iteration of the guidelines. It remains controversial in current literature13 if size is a helpful feature for stratification of patients into treatment by operation and further follow up by imaging.
The purpose of this study was to evaluate the current guidelines with regard to radiological features in order to predict malignancy and to determine further radiological markers of malignancy.
METHODS AND MATERIALS
Patient population and study design
Institutional review board approval was obtained for this study by our respective ethical committee. We included retrospectively all patients who had undergone pancreatic surgery at the University Hospital of Heidelburg, Germany, between March 2004 and July 2012 with the final pathological diagnosis of IPMN derived from a surgical database. All patients had applicable pre-operative imaging [CT and/or MRI with MR cholangiopancreatography (MRCP)].
The study population consisted of 384 patients (197 females and 187 males; age range 28–87 years, mean 64 years, standard deviation 10.3 years) who were analysed in this study.
Imaging
All included patients had at least one pre-operative CT (n = 270) scan with a non-enhanced and a contrast-enhanced phase (mandatory portal venous phase, optional an additional arterial phase) and/or an MRI examination (n = 217) with T1 weighted (pre-contrast and post-contrast) in axial orientation, T2 weighted in axial and coronal orientation and MRCP-sequences. In all patients, the time interval between imaging and operation did not exceed 12 weeks. Based on these pre-operative imaging studies, each patient was assigned a radiological diagnosis of MD-IPMN, BD-IPMN, mixed-type IPMN, pseudocyst or “other” (mostly cystic adenocarcinoma and chronic pancreatitis). For the differentiation of IPMN subtypes in BD- or MD-/mixed-type IPMNs, a cut-off value of 5 mm was defined for the MPD, which is in agreement with the current guidelines.4 All radiological criteria (Table 1) were evaluated retrospectively in consensus by two radiologists (AS, 5 years' experience in pancreas imaging, and MK, 12 years' experience in pancreas imaging), using a standardized checklist including: size, location, presence of a solid component, septum formation, vascular involvement (evaluated according to Klauss et al14), lymphadenopathy (defined as short-axis diameter of ≥1 cm15), parenchymal atrophy, common bile duct (CBD) dilation (defined as ≥8 mm or ≥1 cm in diameter post cholecystectomy16,17) and MPD diameter. Although the radiologists knew that they were presented images of histologically proven IPMNs, they were blinded for the histological type.
Table 1.
Radiological characteristics of all intraductal papillary mucinous neoplasms (IPMNs)
| Radiological characteristics | Malignant (n = 191) | Benign (n = 193) | pb | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| Invasive growth | 82 (42.9) | 17 (8.8) | <0.001 | 42.9 | 91.2 | 82.8 | 61.8 | 67.2 |
| Arterial infiltration | 19 (9.9) | 1 (0.5) | 0.003 | 9.9 | 99.5 | 95.0 | 52.8 | 54.9 |
| Central scarring | 7 (3.7) | 4 (2.0) | 0.350 | 3.7 | 97.5 | 63.6 | 50.7 | 51.0 |
| Venous infiltration | 58 (30.3) | 11 (5.7) | <0.001 | 30.4 | 94.3 | 84.1 | 57.8 | 62.5 |
| Calcification | 19 (9.9) | 5 (2.6) | 0.003 | 11 | 97 | 79.2 | 52.2 | 53.9 |
| Solid component | 104 (54.5) | 26 (13.5) | <0.001 | 54.5 | 86.5 | 80.0 | 65.8 | 70.6 |
| Lymphadenopathy | 40 (20.9) | 8 (4.1) | <0.001 | 20.9 | 95.9 | 83.3 | 55.1 | 58.6 |
| Bile duct dilation | 80 (41.9) | 3 (1.6) | <0.001 | 41.9 | 98.4 | 96.4 | 63.1 | 70.3 |
| Septum formation | 107 (56.0) | 74 (38.3) | 0.001 | 56 | 61.7 | 59.1 | 58.6 | 58.9 |
| Parenchymal atrophy | 107 (56.0) | 34 (17.6) | <0.001 | 56 | 82.4 | 75.9 | 65.4 | 69.2 |
| MD-IPMN | 33 (17.3) | 15 (7.8) | 0.006 | 17.3 | 19.2 | 68.8 | 53.0 | 55.0 |
| Mixed-type IPMN | 84 (44) | 45 (23.3) | <0.001 | 44 | 76.7 | 65.1 | 58.0 | 60.4 |
| Mixed or MD-IPMN | 117 (61.3) | 60 (31.1) | <0.001 | 68.9 | 61.3 | 66.1 | 64.3 | 65.1 |
| Location | ||||||||
| Head | 118 (61.8) | 138 (71.5) | 0.043 | 38.2 | 71.5 | 57.0 | 53.9 | 54.9 |
| Body | 32 (16.8) | 21 (10.9) | 0.095 | 16.8 | 89.1 | 60.4 | 52.0 | 53.1 |
| Tail | 33 (17.3) | 30 (15.5) | 0.647 | 17.3 | 84.5 | 5.0 | 16.0 | 51.0 |
| Throughout pancreas | 8 (4.2) | 1 (0.5) | 0.243 | 4.2 | 97.9 | 50.8 | 66.7 | 51.3 |
| MPD diametera,c (mm) | 8.12 (±1.03) | 4.41 (±0.52) | <0.001 | |||||
| Cyst sizea,c (mm) | 28.70 (±3.25) | 23.90 (±2.27) | 0.284 | |||||
| Age (years)a,d | 64.5 (±0.76) | 63.7 (±0.73) | 0.475 | |||||
| Gender | ||||||||
| Male | 100 (52.4) | 87 (45.1) | 0.154 | |||||
| Female | 91 (47.6) | 106 (54.9) | ||||||
MD-IPMN, main duct intraductal papillary mucinous neoplasm; MPD, main pancreatic duct; NPV, negative-predictive value; PPV, positive-predictive value.
Values in parentheses are percentages unless otherwise indicated.
Values are expressed in mean, standard deviations given in parentheses.
χ2 test.
Mann–Whitney U test.
Unpaired t test.
Histopathological analysis
All radiological variables included on the standardized checklist (Table 1) were analysed comparing them with the histopathological results derived from the pathological report. The lesions were histologically diagnosed as low-grade dysplasia (adenoma), moderate dysplasia (borderline), high-grade dysplasia (carcinoma in situ) or invasive carcinoma, according to the guidelines of the World Health Organization.1,18 The latter two of these histological diagnoses were considered to be clinically malignant.
The diagnosis was established by a pathologist (FB) experienced in pancreatic pathology based on the recommendations of the World Health Organization classification.
Statistical analyses
Descriptive statistics were calculated for all variables. For each continuous variable, normality was assessed by skewness, kurtosis and the Kolmogorov–Smirnov test.
Univariate analysis of variables that were normally distributed was carried out by the use of unpaired t-tests and a one-way analysis of variance. Variables that were not normally distributed were analysed by non-parametric equivalents including χ2 test for independence without Yates' continuity correction, Mann–Whitney U test and Kruskal–Wallis test for variance. The variables that obtained a p-value <0.2 with univariate analysis were subjected to a multistep multivariate binary logistic regression. Factors that had a p-value of <0.2 after the first step of multivariate analysis were subjected to a second-step analysis. Beyond the second step, variables that had p < 0.05 were subjected to further analysis, and other variables were removed from the model. This was continued until all remaining variables were significant at p < 0.05.
Receiver operator curves (ROCs) were used to identify whether the size of the tumour or MPD dilation had any trend in predictive values. Associations of radiological and pathological typing of IPMNs were analysed with χ2 test for independence and a non-parametric test for bivariate correlation.
Evaluation of the International Consensus Guidelines (ICG) was assessed with the calculation of each patient meeting one or more of the following radiological variables as defined by the 2006 and 2012 ICGs: radiologically diagnosed MD-IPMN or mixed-type IPMN or MPD ≥ 10 mm or the presence of a solid component (mural nodule) or BD-IPMN ≥30 mm in size (only valid for the ICG 2006). All patients were tested against these radiological criteria from the ICGs and compared with histopathologically proven malignancy of lesions (defined as carcinoma in situ and invasive carcinoma). In the second step, the radiological criterion of CBD dilation (the overall most important independent risk factor for malignancy, according to our results, that is not part of the radiological criteria of the ICGs) was added, and the results were compared with the unmodified versions based on the radiological characteristics of the ICGs.
The sensitivity, specificity and diagnostic accuracy were determined by the use of binary logistic regression, and a χ2 test for independence was used to compare the correlations between the different evaluations of the guidelines.
Subgroup analysis of BD-IPMNs was conducted to find predictors of malignancy specifically associated with this patient group, using the same statistical techniques as described in the previous paragraphs.
All analyses were conducted using SPSS® v. 21 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL), and statistical significance was set at a p-value of <0.05.
RESULTS
Patients
191 patients (49.7%) had a malignant IPMN according to the final pathological diagnosis. Among these lesions, 20.4% were classified as high-grade dysplasia (Cis; n = 39) and 79.6% as invasive carcinoma (n = 152). 17.3% of all these 191 malignant lesions had the main radiological diagnosis MD-IPMN (n = 33), 22% BD-IPMN (n = 42), 44% mixed-type IPMN (n = 84), 15.7% “other” (n = 30) and 1% were considered to represent a pseudocyst (n = 2). All patients had at least cystic components of the lesions.
Predictors of malignancy
Of all malignant lesions, 42.9% had invasive growth with 9.9% having an arterial infiltration and 30.4% showing a venous infiltration (most commonly the superior mesenteric vein). Solid components including mural nodules and thickened wall components were seen in 54.5% of these patients. Lymph node enlargement (>1-cm short axis) was present in 20.9% of cases. 41.9% of all malignant lesions were accompanied by dilation of the CBD (defined as ≥8 mm or ≥1 cm diameter in the case of prior cholecystectomy). More than 96% of all cases with a dilated CBD were malignant IPMNs [positive-predictive value (PPV) 96.4%; negative-predictive value 63.1%].
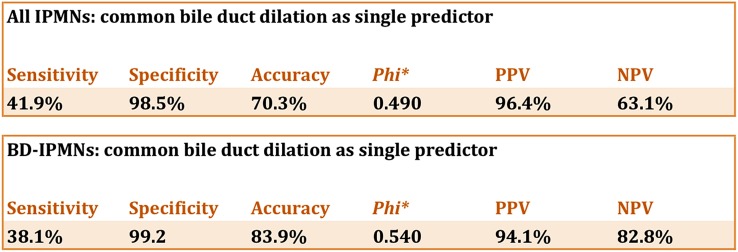
Univariate analysis showed that the most important single predictors of malignancy were CBD dilation (sensitivity 41.9%, specificity 98.5%; p < 0.001; Figures 1 and 2) and solid components (sensitivity 54.5%, specificity 86.5%; p < 0.001; Figure 3).
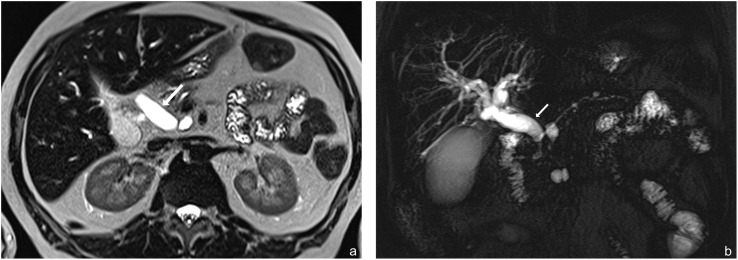
Figure 1.
(a, b) Examples of malignant branch duct intraductal papillary mucinous neoplasms with an associated dilated common bile duct (arrows). (a) Axial T2 haste and (b) MR cholangiopancreatography coronal images; note the non-dilated main pancreatic duct.
Figure 2.
Common bile duct dilation as a single predictor of malignancy in all intraductal papillary mucinous neoplasms (IPMNs) and in branch duct intraductal papillary mucinous neoplasms only. NPV, negative-predictive value; PPV, positive-predictive value.
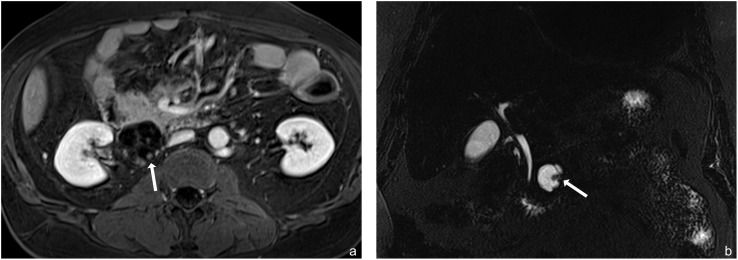
Figure 3.
(a, b) Examples of malignant intraductal papillary mucinous neoplasms with solid components (arrows). (a) Post-contrast T1 weighted axial and (b) MR cholangiopancreatography coronal images.
In total, only three benign IPMNs had a dilated CBD: one radiological BD-IPMN with 3-cm diameter and septum formation but without any other predictors of malignancy—this lesion was pathologically diagnosed as mixed-type IPMN adenoma; one radiological MD-IPMN with a size of 52 mm in the head of the pancreas with solid components, calcifications as well as MPD dilation of 14 mm and parenchymal atrophy that was pathologically diagnosed as mixed-type IPMN adenoma; one radiological mixed-type IPMN with multiple cysts in the head of the pancreas, solid components, septum formation and MPD dilation of 8 mm that was pathologically diagnosed as mixed-type IPMN with moderate dysplasia (borderline).
Using multistep multivariate binary logistic regression to take interactions of other variables into account showed that the statistically significant independent predictors of malignancy (Table 2) were the presence of a solid component [odds ratio (OR) 3.98; 95% confidence interval (CI), 2.2–7.1; p < 0.001], bile duct dilation (OR: 31.26; 95% CI: 9.2–105.8, p < 0.001), parenchymal atrophy (OR: 4.57; 95% CI: 2.6–7.9, p < 0.001), MPD diameter ≥1 cm (OR: 3.6; 95% CI: 1.6–8.1, p = 0.002), MPD diameter 5–9 mm (OR: 2.0; 95% CI: 1.1–3.7; p = 0.019) and an MPD diameter of 5–9 mm coupled with parenchymal atrophy (OR: 5.1; 95% CI: 1.6–8.1; p < 0.001).
Table 2.
Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for significant markers of malignancy for all intraductal papillary mucinous neoplasms (IPMNs) and branch duct intraductal papillary mucinous neoplasms (BD-IPMNs) only derived from multistep multivariate binary logistic regression
| Radiological characteristics | OR (95% CI) |
|---|---|
| All IPMN types | |
| CBD dilation | 31.26 (9.2–105.8) |
| MPD diameter 5–9 mm with parenchymal atrophy | 5.10 (1.6–8.1) |
| Parenchymal atrophy | 4.57 (2.6–7.9) |
| Solid component | 3.98 (2.2–7.1) |
| MPD diameter ≥1 cm | 3.60 (1.6–8.1) |
| MPD diameter 5–9 mm | 2.00 (1.1–3.7) |
| BD-IPMN | |
| CBD dilation | 29.30 (3.8–574.8) |
| Parenchymal atrophy | 4.00 (1.3–12.2) |
CBD, common bile duct; MPD, main pancreatic duct.
ROC analysis showed that there was no correlation between increasing size of the lesion and malignancy [area under the curve (AUC): 0.532; p = 0.284]. Analysis showed that an increasing MPD diameter was correlated with malignancy (AUC: 0.701; 95% CI, 0.648–0.754, p < 0.001) with a maximum sensitivity (61.8%) and specificity (73.1%) at a cut-off value of 6 mm (Youden index = 0.349).
Accuracy of guidelines
The present data show that a dilated bile duct is strongly associated with malignancy. The criterion “bile duct dilation” has been added to the current guidelines, and their sensitivity, specificity and diagnostic accuracy have been compared (Figure 4).
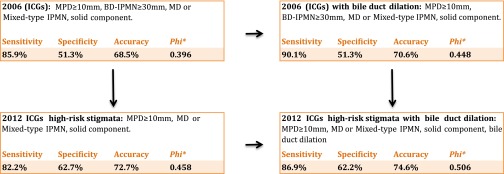
Figure 4.
Systematic approach to analysing the international consensus guidelines (ICG): the addition of bile duct dilation to the radiological criteria for malignancy yielded a significant improvement for the 2006 as well as the 2012 ICGs. *χ2 test with φ correlation coefficient. BD-IPMN, branch duct intraductal papillary mucinous neoplasms; IPMN, intraductal papillary mucinous neoplasms; MD, main duct; MPD, main pancreatic duct.
The model with the lowest diagnostic accuracy was the 2006 ICG3 (sensitivity of 85.9%, specificity of 51.3% and accuracy of 68.5%). The addition of a dilated bile duct (CBD dilation) to the 2006 guidelines yielded a sensitivity of 90.1% with an unchanged specificity of 51.3% (accuracy of 70.6%).
The 2012 ICG4 could improve on the 2006 guidelines with an increase of a diagnostic accuracy of 3.7% at the cost of sensitivity. Introducing CBD dilation to the 2012 ICG could increase the sensitivity without having a significant negative impact on the specificity (sensitivity of 86.9% vs 82.2%, specificity 62.2% vs 62.7% and accuracy of 74.6% vs 72.2%). This modified 2012 ICG had the highest φ correlation coefficient (φ 0.506) and accuracy (74.6%) of all analysed models.
Branch duct intraductal papillary mucinous neoplasm subanalysis
Univariate subanalysis of BD-IPMNs (Table 3) showed that the most reliable single predictor of malignancy was CBD dilation (sensitivity of 38.1%, specificity of 99.2%, accuracy 83.9%, φ 0.540; Figure 2). In fact, there was only one BD-IPMN with CBD dilation that was benign. The MPD diameter was of little help in differentiating benign and malignant BD-IPMNs although malignant lesions had a tendency of a more prominent MPD (benign BD-IPMNs: 2.39 ± 0.08 mm; malignant BD-IPMNs: 2.94 ± 0.22 mm; p = 0.048). Solid components were also significantly associated with malignancy using univariate logistic regression (sensitivity 28.6%, specificity 92.9%, accuracy 76.8%, φ 0.281). Multistep multivariate binary logistic regression showed that the independent predictors of malignancy (Table 2) were parenchymal atrophy (OR: 4.00; 95% CI: 1.3–12.2; p = 0.015) and CBD dilation (OR: 29.3; 95% CI: 3.8–574.8; p < 0.001). ROC analysis showed that there was no significant correlation between increasing size of the lesion (AUC: 0.525; p = 0.633) or increasing MPD diameter (AUC: 0.599; p = 0.055). There was no correlation between size and malignancy. Frequency analysis revealed that even small BD-IPMNs had already undergone malignant transformation (≤1 cm: 15%; 1–2 cm: 26%; 2–3 cm 20%) with about 10% of those having a dilated CBD. Analysing CBD dilation as an independent predictor for malignancy yields a PPV of 94.1% and a negative-predictive value of 82.8% with a sensitivity of 38.1% and a specificity of 99.2%.
Table 3.
Radiological characteristics of branch duct intraductal papillary mucinous neoplasms only
| Radiological characteristic | Malignant (n = 42) | Benign (n = 126) | pb | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| Invasive growth | 7 (16.7) | 6 (4.8) | 0.030 | 16.7 | 95.2 | 53.8 | 77.4 | 75.6 |
| Solid component | 12 (28.6) | 9 (7.1) | <0.001 | 28.6 | 92.9 | 57.1 | 79.6 | 76.8 |
| Bile duct dilation | 16 (38.1) | 1 (0.8) | <0.001 | 38.1 | 99.2 | 94.1 | 82.8 | 83.9 |
| Parenchymal atrophy | 11 (26.2) | 9 (7.1) | 0.001 | 26.2 | 92.9 | 55.0 | 79.1 | 76.2 |
| MPD diametera,c (mm) | 2.94 (±0.22) | 2.39 (±0.08) | 0.048 |
MPD, main pancreatic duct; NPV, negative-predictive value; PPV, positive-predictive value.
Values in parentheses are percentages unless otherwise indicated.
Values are expressed in mean, standard deviations given in parentheses.
χ2 test.
Mann–Whitney U test.
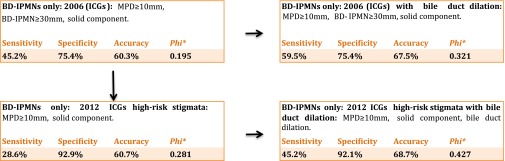
Analysing all BD-IPMNs (according to the radiological diagnosis) yielded the best model data with the 2012 ICG with the addition of CBD dilation (Figure 5). The 2012 ICG could detect 12 out of 42 malignant BD-IPMNs (28.6%), whereas the addition of CBD dilation to the 2012 ICG was able to detect 19 out of 42 malignant lesions (45.2%). Out of all 42 malignant BD-IPMNs, only 14 (33%) were ≥3 cm in diameter.
Figure 5.
Systematic approach to analysing the international consensus guidelines (ICG) for radiologically diagnosed branch duct intraductal papillary mucinous neoplasms (BD-IPMNs): the addition of bile duct dilation to the radiological criteria for malignancy yielded a significant improvement for the 2006 as well as the 2012 ICGs. *χ2 test with φ correlation coefficient. IPMN, intraductal papillary mucinous neoplasms; MPD, main pancreatic duct.
DISCUSSION
In this study, we show that among all analysed radiological features, a dilated CBD was found to be the best independent predictor of malignancy in IPMNs having a PPV of 96.4%. However, having a sensitivity of only 41.9%, this marker is not suitable for the exclusion of malignancy. Addition of CBD dilation to the ICGs has yielded a significant improvement for the detection of malignant IPMNs, without having a major negative impact on specificity, a fact that is especially important for the subgroup of BD-IPMNs. Factoring in a dilated CBD as a radiological criterion for the detection of malignancy could increase the detection rate by over 60% without a significant increase in false positives.
Subanalysis of all patients with CBD dilation has shown that the mechanism is not solely a result of direct compression of the bile duct by the neoplasm. A study by Maker et al19 has shown that viscous cyst fluid is significantly associated with highly dysplastic lesions. This study hypothesized that the increase of viscosity is the result of higher glycoprotein or mucin expression. Further studies analysing pancreatic fluid cysts have shown that many transmembrane proteins and mucinous columnar cells are sloughed with higher grades of dysplasia, which may also impact viscosity,20 a fact which is currently under evaluation for predicting malignant potential in cystic pancreatic neoplasms using diffusion-weighted imaging.21 Therefore, inference may be drawn that CBD dilation occurs owing to increased viscosity of the pancreatic fluid and the change of the fluid dynamics. Also, differences in compliance and elasticity of the MPD and CBD might also play a role and explain cases where there is a dilated CBD but not MPD dilation. This may be supported by the lack of correlation between size and cholestasis or MPD dilation. Another possible explanation could be the secondary formation of an adenocarcinoma beside the IPMN that causes CBD dilation.
It is therefore strongly recommended that other reasons for CBD dilation (such as stones or additional papillary neoplasms) should be excluded before ultimately choosing the appropriate treatment.
The importance of other well-known predictors of malignancy including MPD dilation, presence of solid components and parenchymal atrophy were confirmed in this study.22
However, it was an interesting finding, that although solid components are a significant predictor of malignancy in all IPMNs, they were only a significant predictor in the subgroup of BD-IPMNs using analysis of variance but lost their significance in multistep multivariate analysis, in contrast to parenchymal atrophy and a dilated bile duct. These seem to be the major criteria which radiologists should focus when reporting about BD-IPMN.
In univariate analysis, there was a significant tendency of more prominent MPD diameters in every IPMN subgroup, but no significant cut-off value in subgroup ROC analysis. Looking at all IPMNs, dilation of the MPD was an independent predictor of malignancy with the best cut-off value at 6 mm, according to ROC analysis—an MPD of 5–9 mm is already considered to be a “worrisome feature”,4 according to the current guidelines.
However, if these guidelines are taken into consideration, this is a finding of low clinical value because such a BD-IPMN with an MPD diameter of ≥6 mm would favour the diagnosis mixed-type IPMN instead of BD-IPMN that leads to a recommendation for resection by itself.3,4
A more interesting finding is that parenchymal atrophy in association with a dilated MPD is a significant co-finding with MPD dilation of 5–9 mm coupled with parenchymal atrophy having an even higher OR of malignancy in IPMNs than MPD dilation of ≥1 cm regardless of atrophy.
Size of the lesions is also of little help to detect the malignant lesions as there is no significant correlation between a lesions' size and its dignity, a finding that is especially interesting for the subgroup of BD-IPMNs, as the 2006 ICG recommended a resection of lesions ≥3 cm. A cut-off value for a lesion's size regarding its malignant potential could not be found, so size is of no help for the prediction of malignancy.23
The 2006 ICG3 were very clear in giving advice based on radiological data recommending resection or not. This has been convoluted with the release of the ICG of 20124 by the introduction of “worrisome features” vs “high-risk stigmata” without a clear-cut guideline whether to resect the tumour. We therefore defined the “high-risk” stigmata of the 2012 ICG as indication for resection, whereas further follow-up was deemed sufficient in case of “worrisome features”.
The 2012 ICG could improve on the 2006 guidelines with an increase of diagnostic accuracy, a finding which has been reported in previous literature.24
In contrast to some literature,25,26 there was neither a gender preference for IPMNs nor a significant difference between age and gender for malignant and benign lesions.
One limitation of the study is that it was designed for the evaluation of radiological predictors of malignancy only. As this study was conducted to increase the radiologist's reporting on IPMNs as an independent tool for clinical decision-making, clinical findings or laboratory values were not taken into consideration.
Another limitation is that only patients who have been resected were included. This introduces the risk of spectrum bias—suspicious imaging findings might be overrepresented in this patient group, compared with the patients who remain under watch and wait—although only 49.7% of the resected specimens had a malignant histology. CBD dilation might be overrepresented in the operated study group (owing to a higher probability of these patients to be clinically suspicious), but the high association of this feature with malignancy in these lesions was a surprising result. Nevertheless, these results should be confirmed with a prospective study including non-operated patients with IPMN.
While all patients had pre-operative contrast-enhanced imaging that was considered of good quality, protocols and scanners changed over the period of the study.
In conclusion, we showed that CBD dilation is the most important radiological feature to predict malignancy in IPMNs. The diameter of a BD-IPMN did not correlate with the likelihood of malignancy, and parenchymal atrophy was a significant predictor of malignancy, especially if combined with MPD dilation.
Acknowledgments
ACKNOWLEDGMENTS
The authors are grateful to Daniel Saure, MSc, for provision of statistical consultation.
Contributor Information
Albert Strauss, Email: Albert.Strauss@med.uni-heidelberg.de, Albert.Strauss@googlemail.com.
Matthew Birdsey, Email: matt.birdsey@gmail.com.
Stefan Fritz, Email: stefan.fritz@med.uni-heidelberg.de.
Bogata D Schwarz-Bundy, Email: bogata.bundy@med.uni-heidelberg.de.
Frank Bergmann, Email: frank.bergmann@med.uni-heidelberg.de.
Thilo Hackert, Email: thilo.hackert@med.uni-heidelberg.de.
Hans-Ullrich Kauczor, Email: Hans-Ulrich.Kauczor@med.uni-heidelberg.de.
Lars Grenacher, Email: l.grenacher@diagnostik-muenchen.de.
Miriam Klauss, Email: Miriam.Klauss@med.uni-heidelberg.de.
REFERENCES
- 1.Klöppel G, Solcia E, Longnecker D, Capella C, Sobin L. Histological typing of tumors of the exocrine pancreas. 2nd edn. Berlin, Germany: Springer; 1996. [Google Scholar]
- 2.Lim JH, Lee G, Oh YL. Radiologic spectrum of intraductal papillary mucinous tumor of the pancreas. Radiographics 2001; 21: 323–37; discussion 337–40. doi: 10.1148/radiographics.21.2.g01mr01323 [DOI] [PubMed] [Google Scholar]
- 3.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006; 6: 17–32. doi: 10.1159/000090023 [DOI] [PubMed] [Google Scholar]
- 4.Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012; 12: 183–97. doi: 10.1016/j.pan.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 5.Nagai K, Doi R, Kida A, Kami K, Kawaguchi Y, Ito T, et al. Intraductal papillary mucinous neoplasms of the pancreas: clinicopathologic characteristics and long-term follow-up after resection. World J Surg 2008; 32: 271–8; discussion 279–80. doi: 10.1007/s00268-007-9281-2 [DOI] [PubMed] [Google Scholar]
- 6.Mimura T, Masuda A, Matsumoto I, Shiomi H, Yoshida S, Sugimoto M, et al. Predictors of malignant intraductal papillary mucinous neoplasm of the pancreas. J Clin Gastroenterol 2010; 44: e224–9. doi: 10.1097/MCG.0b013e3181d8fb91 [DOI] [PubMed] [Google Scholar]
- 7.Correa-Gallego C, Ferrone CR, Thayer SP, Wargo JA, Warshaw AL, Fernández-Del Castillo C. Incidental pancreatic cysts: do we really know what we are watching? Pancreatology 2010; 10: 144–50. doi: 10.1159/000243733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg 2003; 138: 427–3; discussion 433–4. doi: 10.1001/archsurg.138.4.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kent TS, Jr CMV, Callery MP. Intraductal papillary mucinous neoplasm and the pancreatic incidentaloma. World J Gastrointest Surg 2010; 2: 319–23. doi: 10.4240/wjgs.v2.i10.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho C-K, Kleeff J, Friess H, Büchler MW. Complications of pancreatic surgery. HPB (Oxford) 2005; 7: 99–108. doi: 10.1080/13651820510028936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadakari Y, Ienaga J, Kobayashi K, Miyasaka Y, Takahata S, Nakamura M, et al. Cyst size indicates malignant transformation in branch duct intraductal papillary mucinous neoplasm of the pancreas without mural nodules. Pancreas 2010; 39: 232–6. doi: 10.1097/MPA.0b013e3181bab60e [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M. Controversies in the management of pancreatic IPMN. Nat Rev Gastroenterol Hepatol 2011; 8: 56–60. doi: 10.1038/nrgastro.2010.193 [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M. International consensus on the management of intraductal papillary mucinous neoplasm of the pancreas. Ann Transl Med 2015; 3: 286. doi: 10.3978/j.issn.2305-5839.2015.11.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klauss M, Mohr A, von Tengg-Kobligk H, Friess H, Singer R, Seidensticker P, et al. A new invasion score for determining the resectability of pancreatic carcinomas with contrast-enhanced multidetector computed tomography. Pancreatology 2008; 8: 204–10. doi: 10.1159/000128557 [DOI] [PubMed] [Google Scholar]
- 15.Ganeshalingam S, Koh D-M. Nodal staging. Cancer Imaging 2009; 9: 104–11. doi: 10.1102/1470-7330.2009.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjaminov F, Leichtman G, Naftali T, Half EE, Konikoff FM. Effects of age and cholecystectomy on common bile duct diameter as measured by endoscopic ultrasonography. Surg Endosc 2013; 27: 303–7. doi: 10.1007/s00464-012-2445-7 [DOI] [PubMed] [Google Scholar]
- 17.Perret RS, Sloop GD, Borne JA. Common bile duct measurements in an elderly population. J Ultrasound Med 2000; 19: 727–30; quiz 731. [DOI] [PubMed] [Google Scholar]
- 18.Lüttges J. What’s new? The 2010 WHO classification for tumours of the pancreas. [In German.] Pathologe 2011; 32(Suppl. 2): 332–6. doi: 10.1007/s00292-011-1515-2 [DOI] [PubMed] [Google Scholar]
- 19.Maker AV, Katabi N, Gonen M, DeMatteo RP, D'Angelica MI, Fong Y, et al. Pancreatic cyst fluid and serum mucin levels predict dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Ann Surg Oncol 2011; 18: 199–206. doi: 10.1245/s10434-010-1225-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boot C. A review of pancreatic cyst fluid analysis in the differential diagnosis of pancreatic cyst lesions. Ann Clin Biochem 2014; 51: 151–66. doi: 10.1177/0004563213503819 [DOI] [PubMed] [Google Scholar]
- 21.Kang KM, Lee JM, Yoon JH, Kiefer B, Han JK, Choi BI. Intravoxel incoherent motion diffusion-weighted MR imaging for characterization of focal pancreatic lesions. Radiology 2014; 270: 444–53. doi: 10.1148/radiol.13122712 [DOI] [PubMed] [Google Scholar]
- 22.Kim KW, Park SH, Pyo J, Yoon SH, Byun JH, Lee MG, et al. Imaging features to distinguish malignant and benign branch-duct type intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Ann Surg 2014; 259: 72–81. doi: 10.1097/SLA.0b013e31829385f7 [DOI] [PubMed] [Google Scholar]
- 23.Fritz S, Klauss M, Bergmann F, Hackert T, Hartwig W, Strobel O, et al. Small (Sendai negative) branch-duct IPMNs: not harmless. Ann Surg 2012; 256: 313–20. doi: 10.1097/SLA.0b013e31825d355f [DOI] [PubMed] [Google Scholar]
- 24.Goh BK, Thng CH, Tan DM, Low AS, Wong JS, Cheow PC, et al. Evaluation of the Sendai and 2012 International Consensus Guidelines based on cross-sectional imaging findings performed for the initial triage of mucinous cystic lesions of the pancreas: a single institution experience with 114 surgically treated patients. Am J Surg 2014; 208: 202–9. doi: 10.1016/j.amjsurg.2013.09.031 [DOI] [PubMed] [Google Scholar]
- 25.Klöppel G. Clinicopathologic view of intraductal papillary-mucinous tumor of the pancreas. Hepatogastroenterology 1998; 45: 1981–5. [PubMed] [Google Scholar]
- 26.Sugiura H, Kondo S, Islam HK, Ito K, Ono K, Morikawa T, et al. Clinicopathologic features and outcomes of intraductal papillary-mucinous tumors of the pancreas. Hepatogastroenterology 2002; 49: 263–7. [PubMed] [Google Scholar]