Abstract
Objective:
To evaluate the relationship between vasovagal-related stress on positron emission tomography (PET)/CT and adrenal fludeoxyglucose (FDG) uptake.
Methods:
We reviewed the medical records of 1358 consecutive patients who underwent FDG PET/CT examinations and selected those who presented with vasovagal-related symptoms and acute hypotension immediately before FDG injection (vasovagal reflex group). Patients who underwent FDG PET/CT examinations on the same days as the vasovagal reflex group without new complaints or any adrenal lesion were used as controls. We evaluated adrenal FDG uptake visually and by means of adrenal maximum standardized uptake value (SUVmax) and adrenal/liver (A/L) SUVmax ratio. Next, we reviewed the FDG PET/CT images of the same 1358 patients and selected the cases presenting with bilateral avid FDG uptake.
Results:
4 patients were included in the vasovagal reflex group, and all of them showed bilateral avid adrenal FDG uptake visually, while 19 patients in the control group did not. The mean adrenal SUVmax and the mean A/L SUVmax ratio were significantly higher in the vasovagal reflex group than in the control group (p < 0.001). 10 (0.74%) patients, including 4 patients from the vasovagal reflex group, showed bilateral avid FDG uptake with normal adrenal configuration on CT.
Conclusion:
Vasovagal-related stress immediately before FDG injection may increase bilateral adrenal FDG uptake.
Advances in knowledge:
Vasovagal-related stress may be included in the differential diagnosis of the cause of bilateral avid adrenal FDG uptake.
INTRODUCTION
Previous studies have reported the utility of fludeoxyglucose (FDG) positron emission tomography (PET)/CT to characterize adrenal diseases, especially to distinguish benign from malignant adrenal disease.1–3 FDG uptake is usually higher in malignant adrenal tumours than in benign adrenal tumours, but some adenomas are also known to show avid FDG uptake.2,3 We encountered a female patient who presented with pallor and hypotension immediately before FDG injection and bilateral avid adrenal FDG uptake despite normal adrenal configuration on CT. We speculated that the avid uptake may be due to adrenal activation caused by vasovagal-related stress. However, vasovagal-related stress-induced adrenal avid FDG uptake has never been reported as a full article.
The purpose of this study was to examine whether adrenal FDG uptake increases under vasovagal-related stress conditions. First, we compared the adrenal FDG uptake between patients with vasovagal-related symptoms immediately before FDG injection and those without such symptoms. Next, we examined the frequency of bilateral avid adrenal FDG uptake with normal adrenal CT configuration on FDG PET/CT examinations.
METHODS AND MATERIALS
Our institutional review board approved this retrospective study (Approval No. 433), and the need to obtain informed consent for this study was waived.
Patients
This retrospective study population consisted of 1358 consecutive patients who underwent FDG PET/CT for known or unknown malignant disorders at our institution from April 2012 to March 2013.
A board-certified radiologist (MJ) reviewed the medical records of those patients and selected the patients presenting with new complaints or symptoms at FDG PET/CT examinations. In our institution, all patients had been closely monitored by nurses throughout the examination, and any changes in their mental or physical conditions had been recorded. Blood pressures were not measured routinely, but were measured when a sudden symptomatic change appeared. When a patient presented with severe acute hypotension (<90 mmHg systolic) immediately after venous puncture of a catheter for FDG injection without hypoglycemia and recovered promptly, we considered the cause of hypotension to be vasovagal reflex. The patients presenting with both acute symptoms (cold sweat, pallor, nausea, dizziness, headache etc.) and acute hypotension due to vasovagal reflex immediately before FDG injection were included in the vasovagal reflex group. The patients who underwent FDG PET/CT examinations on the same days as the vasovagal reflex group without any adrenal lesion or acute new symptoms were used as controls, excluding the potential bias in FDG samples, climate or other environments.
Positron emission tomography/CT imaging
Venipunctures for FDG administration were performed by nurses using 22- or 24-gauge peripheral i.v. catheters (Introcan Safety®; B. Brown Medical Industries, Malaysia). All patients fasted for at least 5 h before the intravenous injection of 1.5–5.5 MBq kg−1 (average, 3.7 MBq kg−1) of FDG (FDG scan; Nihon Medi-Physics, Tokyo, Japan). PET/CT examinations started 1 h after FDG injection, and data were acquired from the head to the toe or thigh during shallow breathing using a GE Discovery™ 600M scanner (GE Medical System, Milwaukee, WI). CT data for attenuation correction and anatomic coregistration were acquired with the following parameters: 120 kVp, automatic tube current (15–100 mA depending on the patient's total body mass), 0.6 s per CT rotation and 3.75-mm slice thickness. PET emission data were obtained 1 h after FDG injection, for 2.5 min at each bed position, using the three-dimensional high-sensitivity mode with an axial field of view of 60 cm. The images were reconstructed at 3.3-mm section thickness. The images were displayed for visual interpretation in three orthogonal projections and with whole-body maximum intensity projection.
Image analysis
Transaxial PET/CT and coregistered PET/CT slice images of 3.3-mm thickness were displayed and interpreted on a workstation (AW VolumeShare 4; GE Medical System).
First, the PET/CT images of the vasovagal reflex and control groups were reviewed visually by two independent radiologists with 4 and 9 years’ (YN and MN) experience in PET diagnosis, who were blinded to clinical information and patient conditions. The images were presented to the readers in a randomized order. The adrenal uptake was evaluated as non-avid, if the adrenal uptake was equal to or less than the hepatic uptake, and avid, if the adrenal uptake was more than the hepatic uptake. The maximum standardized uptake value (SUVmax) of each adrenal gland and the liver were recorded by a radiologist with 5 years’ experience (MJ) in PET diagnosis. A rectangular three-dimensional volume of interest (VOI) to encompass the entire adrenal gland was drawn. The location of the SUVmax within the adrenal was confirmed by viewing the fused images. The adrenal VOI was adjusted to exclude other adjacent structures such as the liver and kidney. The two-dimensional (2D) rectangular region of interest was set over the adrenal slice image that contained the most intense FDG uptake when it was difficult to put the adrenal VOI because the adrenal gland was in contact with its adjacent structures. The ratio of the adrenal SUVmax to the mean liver SUVmax (A/L SUVmax ratio) was also calculated. The mean liver SUVmax was calculated from three measurements of the liver by drawing 11-cm3 rectangular VOIs centred within the right, median and left of the liver. Care was taken to exclude any areas with non-homogeneity or abnormalities such as a tumour, gallbladder and fissure of ligamentum teres.
Second, two radiologists with 5 and 12 years’ (MJ and MYN) experience in PET diagnosis reviewed the FDG PET/CT images of the same 1358 patients in consensus without any clinical information and selected the cases with bilateral avid adrenal FDG uptake. In cases with bilateral avid adrenal FDG uptake, adrenal CT images were also reviewed. The SUVmax of each adrenal gland and A/L SUVmax ratio were also calculated for these cases in the same manner as for the vasovagal reflex and control groups.
Statistical analysis
The kappa (κ) statistic was used to evaluate interobserver agreement on image interpretation. The Mann–Whitney U test was used to explore the differences in adrenal uptake indexes between the vasovagal reflex and control groups, in which average values of the bilateral adrenal glands were used. The Kruskal–Wallis test was used to explore the differences in quantitative variables among the groups, in which average values of the bilateral adrenal glands were used. The post hoc paired comparisons after the Kruskal–Wallis test were calculated. A mean value was expressed with a standard deviation. Two-tailed p-values of >0.05 were considered statistically significant. The statistical program SPSS® statistics 22 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL) was used.
RESULTS
All patients were able to complete the FDG PET/CT examinations. 8 (0.59%) of the 1358 patients had presented new complaints or symptoms at FDG PET/CT examination. Among them, four female patients (mean age, 41 years; age range, 28–54 years) showed acute hypotension immediately before FDG injection when they had venous puncture and were included in the vasovagal reflex group. The characteristics of the vasovagal reflex group patients are summarized in Table 1. 19 patients without an adrenal lesion (13 males and 6 females; mean age, 62 years; age range, 33–84 years) were included in the control group. At FDG injection, the mean plasma glucose level of patients in the vasovagal reflex and control groups were 95 mg dl−1 (range, 84–106 mg dl−1) and 112 mg dl−1 (range, 86–193 mg dl−1), respectively (p = 0.097).
Table 1.
Characteristics of patients in the vasovagal reflex group
| Patient | Age (years)/sex | Background disease | Timing of symptom manifestation | Initial main symptom | Blood pressure (mmHg) | CT findings |
|
|---|---|---|---|---|---|---|---|
| Right | Left | ||||||
| 1 | 28/F | Post-operative thyroid cancer | 5 min before FDG injection | Pallor | 72/46 | Normal | Normal |
| 2 | 52/F | Hepatic angiomyolipoma | 10 min before FDG injection | Cold sweat | 60/– | Normal | Normal |
| 3 | 31/F | Uveitis | 11 min before FDG injection | Nausea | 50/– | Normal | Normal |
| 4 | 54/F | Malignant schwannoma | 12 min before FDG injection | Cold sweat | 69/47 | Normal | Normal |
F, female; FDG, fludeoxyglucose.
In visual assessment, the interpretation between the two readers completely accorded (κ = 1.00). All bilateral adrenal glands in the vasovagal reflex group showed avid FDG uptake, whereas no adrenal glands in the control group showed avid FDG uptake. All patients in the vasovagal reflex group showed normal bilateral adrenal configuration on CT.4
Bilateral adrenal SUVmax and A/L SUVmax ratio in the vasovagal reflex and control groups are shown in Figures 1 and 2, respectively. Both the mean adrenal SUVmax and mean A/L SUVmax ratio were significantly higher in the vasovagal reflex group than in the control group (p < 0.001 for both). The FDG PET/CT images of two cases in the vasovagal reflex group and one case in the control group are shown in Figures 3 and 4, respectively.
Figure 1.
Comparison of bilateral adrenal maximum standardized uptake value (SUVmax) between the vasovagal reflex and control groups. A significant difference is seen between two groups (p < 0.001). The number of patients at each point is shown in parenthesis.
Figure 2.
Comparison of bilateral adrenal/liver (A/L) maximum standardized uptake value (SUVmax) ratio between the vasovagal reflex and control groups. A significant difference is seen between two groups (p < 0.001). The number of patients at each point is shown in parenthesis.
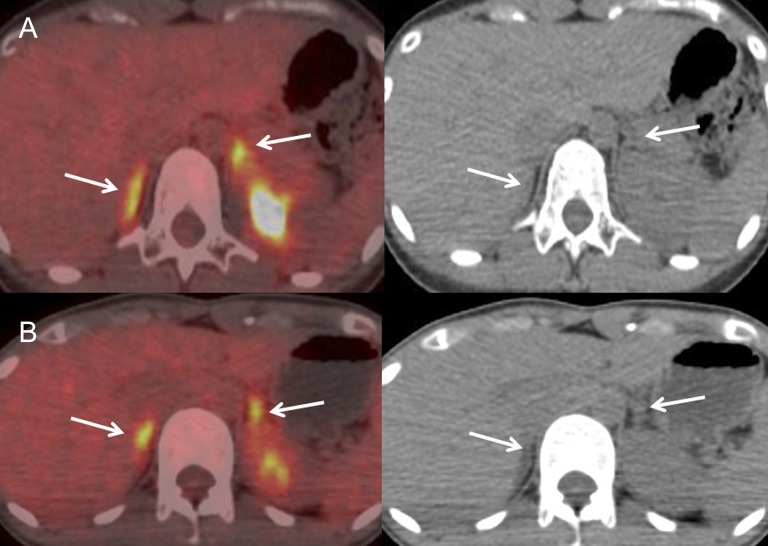
Figure 3.
Fused fludeoxyglucose (FDG) positron emission tomography/CT images and CT images of two patients in the vasovagal reflex group. Adrenals are shown by arrows. A 28-year-old female (Patient 1 in Table 1) looked pale, and her blood pressure decreased to 72/46 mmHg immediately after securing an intravenous line for FDG injection. She recovered after resting for several minutes, and FDG was injected 5 min later. Bilateral avid adrenal FDG uptake is shown [maximum standardized uptake value (SUVmax): right, 5.5, left, 6.0; adrenal/liver (A/L) SUVmax ratio: right, 2.4, left, 2.6], whereas adrenal CT configuration is normal (a). A 31-year-old female (Patient 3 in Table 1) complained of nausea 3 min after securing an intravenous line for FDG injection and then she had convulsions for several seconds, and her systolic blood pressure decreased to 50 mmHg. She recovered after resting for several minutes, and FDG was injected 11 min later. Bilateral avid adrenal FDG uptake is shown (SUVmax: right, 5.7, left, 5.2; A/L SUVmax ratio: right, 2.2, left, 2.0), whereas adrenal CT configuration is normal (b).
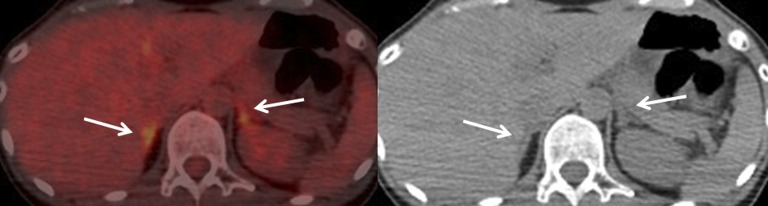
Figure 4.
Fused fludeoxyglucose (FDG) positron emission tomography/CT image and CT image of a 44-year-old female with cervical cancer in the control group. Adrenals are shown by arrows. Bilateral adrenal FDG uptake is almost equal to the hepatic uptake [maximum standardized uptake value (SUVmax): right, 2.0, left, 2.4; A/L SUVmax ratio: right, 0.8, left, 1.0] and adrenal CT configuration is normal.
By reviewing the FDG PET/CT images of the 1358 consecutive patients, 15 patients with bilateral avid adrenal FDG uptake were identified. Among them, five patients had bilateral adrenal masses or swollen adrenals on CT (abnormal adrenal configuration group). The remaining 10 (0.74%) patients had bilateral normal adrenal configuration on CT, among whom 4 (0.30%) patients belonged to the vasovagal reflex group, but the other 6 (0.44%) patients did not show any vasovagal-related symptoms with acute hypotension on FDG PET/CT examinations (unknown-cause group) (Table 2, Figure 5). In the abnormal adrenal configuration group, three patients had clinically diagnosed bilateral adrenal metastases from lung cancer because they had histopathologically proven primary lung cancer and multiple metastases in other organs; one patient had bilateral pheochromocytomas that were histopathologically diagnosed by operation, and one patient had clinically diagnosed hyperplasia caused by an adrenocorticotropic hormone-producing tumour from unknown origin.
Table 2.
Characteristics and imaging findings of patients who showed bilateral adrenal avid fludeoxyglucose uptake
| Group | Patient | Age (years)/sex | Background disease (histopathological type) | Adrenal lesion | CT findings |
|
|---|---|---|---|---|---|---|
| Right | Left | |||||
| Abnormal adrenal configuration | 1 | 70/M | Lung cancer (NSCLC) | Bilateral metastases | Irregularly swollen | Irregularly swollen |
| 2 | 70/M | Lung cancer (unknown) | Bilateral metastases | A 1.5-cm mass | A 2-cm mass | |
| 3 | 57/M | Lung cancer (SCLC) | Bilateral metastases | Irregularly swollen | Irregularly swollen | |
| 4 | 16/M | Pheochromocytoma | Bilateral pheochromocytomas | A 5.5-cm mass | A 4.4-cm mass | |
| 5 | 72/M | ACTH-producing tumour from unknown origin | Bilateral hyperplasia | Diffusely swollen | Diffusely swollen | |
| Unknown cause | 6 | 83/M | Lung cancer (SCC) | Unknown | Normal | Normal |
| 7 | 67/M | Oesophageal cancer (SCC) | Unknown | Normal | Normal | |
| 8 | 49/F | Uterine cervical cancer (SCC) | Unknown | Normal | Normal | |
| 9 | 72/M | Usual interstitial pneumonia | Unknown | Normal | Normal | |
| 10 | 70/F | Vasculitis syndrome | Unknown | Normal | Normal | |
| 11 | 44/F | Pancreatic neuroendocrine tumour | Unknown | Normal | Normal | |
| Vasovagal reflex | 12–15 | Same patients as Patients 1–4 in the vasovagal reflex group described in Table 1 | ||||
ACTH, adrenocorticotropic hormone; F, female; M, male; NSCLC, non-small-cell lung carcinoma; SCLC, small-cell lung carcinoma; SCC, squamous-cell carcinoma.
Figure 5.
Fused fludeoxyglucose (FDG) positron emission tomography (PET)/CT and CT images of a 44-year-old female (Patient 11 in Table 2) with pancreatic neuroendocrine tumour in the unknown-cause group. Adrenals are shown by arrows. She complained of headache and nausea after she entered the FDG PET/CT laboratory. Blood pressure was 104/78 mmHg. FDG was injected 30 min later. Bilateral adrenal avid FDG uptake is shown [maximum standardized uptake value (SUVmax): right, 4.1, left, 3.8; adrenal/liver SUVmax ratio: right, 1.4, left, 1.3), whereas adrenal CT configuration is normal. This normal configuration was not changed on the contrast-enhanced CT 3 years later.
The bilateral adrenal SUVmax and A/L SUVmax ratio in the abnormal adrenal configuration, vasovagal reflex and unknown-cause groups are shown in Figure 6 and Figure 7, respectively. The mean adrenal SUVmax and mean A/L SUVmax ratio were higher in the abnormal adrenal configuration group than in the vasovagal reflex group and unknown-cause group. Both of them were significantly higher in the abnormal adrenal configuration group than in the unknown-cause group (p = 0.005 and p = 0.021, respectively).
Figure 6.
Comparison of bilateral adrenal maximum standardized uptake value (SUVmax) among abnormal adrenal configuration, vasovagal reflex and unknown-cause groups. A significant difference is seen between the abnormal adrenal configuration and the unknown-cause groups (p = 0.005). The number of patients at each point is shown in parenthesis. n.s., not significant.
Figure 7.
Comparison of bilateral adrenal/liver (A/L) maximum standardized uptake value (SUVmax) ratio among abnormal adrenal configuration, vasovagal reflex and unknown-cause groups. A significant difference is seen between the abnormal adrenal configuration and the unknown-cause groups (p = 0.021). The number of patients at each point is shown in parenthesis. n.s., not significant.
DISCUSSION
The adrenal gland is a multifunctional organ that produces steroid hormones and catecholamines that are essential for life. The major hormones secreted by the adrenal cortex are aldosterone, cortisol and dehydroepiandrosterone sulfate, and the adrenal medulla secretes catecholamines. Stress has been shown to increase the secretion of epinephrine and cortisol.5,6
In our study, four patients presented with vasovagal reflex symptoms evidenced by severe hypotension (Table 1) and they all showed bilateral avid adrenal FDG uptake. It is known that pain and/or anxiety by medical procedures cause a vasovagal reflex.7,8 Thus, pain and/or anxiety caused by venous catheterization might have caused the vasovagal reflex in the four patients in our study. Catecholamines are known to be important mediators of cardiovascular responses, and it is generally believed that their secretion is increased in anxiety.9 Chosy and Graham9 noted that urinary catecholamine levels were substantially higher prior to syncope in subjects who fainted than in control subjects, and Banditt et al10 and Sra et al11 reported that plasma epinephrine levels increased significantly immediately before the onset of the vasovagal syncope. Medullary catecholamine products serve as first responders to stress by acting within seconds (cortisol takes 20 min).6 Therefore, although we did not measure the adrenal hormones, the bilateral avid adrenal FDG uptake may be related to adrenomedullary activation. Our results indicated that adrenal FDG uptake after vasovagal reflex occur rapidly, while it is unknown that how long the adrenal activation continues. In our study, however, bilateral avid adrenal uptake occurred by FDG injection at 5–12 min after vasovagal reflex symptoms appeared in the four patients with vasovagal reflex.
In general, tumour FDG activity greater than liver activity is considered to be suggestive of malignancy.1,3 Recent articles have proposed discrete thresholds of FDG avidity that are highly sensitive for adrenal metastases. Brady et al2 reported that combined PET/CT with mean attenuation >10 HU and SUVmax >3.1 had 97.3% sensitivity and 86.2% specificity. Watanabe et al12 reported that an adrenal SUVmax >3.9 had 96% sensitivity and 82% specificity, and A/L SUV ratio >1.37 had 96% sensitivity and 100% specificity. In our study, six of eight adrenal glands in the vasovagal-reflex group presented with A/L SUVmax ratio >1.37. Thus, an avid adrenal uptake induced by stress can be high enough to be misdiagnosed as malignancy.
Differential diagnoses for bilateral avid adrenal FDG uptake other than metastasis include malignant lymphoma,13 pheochromocytoma,14 adrenal hyperplasia causing Cushing's syndrome,15 adrenal tuberculosis,16 adrenal histoplasmosis,17 adrenal haemorrhage18 and adrenal myelolipoma.19 However, these diseases except mild hyperplasia may exhibit clear morphological abnormalities. Therefore, it appears relatively easy to differentiate the stress-induced avid adrenal uptake from other avid adrenal FDG uptake lesions mentioned above on the basis of its normal adrenal configuration on CT. Thus, it is necessary to confirm the adrenal configuration on CT and the patient condition before the FDG injection when we encounter bilateral adrenal avid FDG uptake.
Bagheri et al20 reported that 1 of 20 patients with normal adrenal glands presented with bilateral avid adrenal FDG uptake that was greater than the hepatic uptake, with no clinical findings suggesting adrenal hyperplasia or Cushing's disease. They did not report the patient condition before and during the examination. In our study, 0.74% (10/1358) of patients showed bilateral avid adrenal FDG uptake with normal adrenal configuration on CT. 4 of 10 cases appeared to be caused by vasovagal-related stress, which was recognized by subjective and objective new symptoms. But, the causes were unknown in the remaining six cases (unknown-cause group), including three patients with cancer, one patient with dyspnoea due to interstitial pneumonia one patient with continuing fever due to vasculitis and one patient with pancreatic neuroendocrine tumour. The patient with vasculitis (Patient 10 in Table 2) received the second FDG PET/CT examination 7 months later, which revealed bilateral non-avid adrenal FDG uptake. The patient with neuroendocrine tumour complained of a sudden headache and nausea without hypotension 30 minutes before FDG injection. Thus, we speculate that other than vasovagal-related stress, some form of acute and/or chronic severe stress before FDG injection may be associated with bilateral avid adrenal FDG uptake. Leboulleux et al21 reported transient avid adrenal FDG uptake in the remaining normal adrenal gland after adrenalectomy for adrenocortical carcinoma was observed under 1, ortho-1, para’-dichloro-diphenyl-dichloroethane treatment. There is some possibility that unknown concomitant drugs may cause adrenal FDG uptake.
In our study, there were three patients who presented new complaints or symptoms (i.e. claustrophobia, sweat or vomiting due to anxiety and feeling oppression) during FDG PET/CT examination without hypotension after FDG injection, and all of them presented with bilateral non-avid adrenal FDG uptake.
There are a few reports evaluating the normal range of adrenal FDG uptake.20,22,23 Kim et al22 retrospectively analyzed FDG PET/CT images 1 h after FDG injection and reported normal adrenal SUVmax for the healthy group (right, 1.66 ± 2.1; left, 1.86 ± 0.3) and the lung cancer group (right, 1.79 ± 0.30; left, 1.90 ± 0.37). The normal adrenal SUVmax in our control group (right, 2.3 ± 0.3; left, 2.4 ± 0.4) was somewhat higher than that observed in the above report. We speculate that the slightly different normal adrenal SUVmax values may be mainly due to the differences in PET/CT apparatuses and methods of data processing between the individual studies.
The limitations of our retrospective study are as follows: a follow-up imaging study was not performed for every patient who presented with bilateral avid adrenal FDG uptake with normal adrenal configuration on CT. Of the four vasovagal reflex patients (Table 1), one patient with post-operative thyroid cancer underwent a follow-up CT study 3 years later and showed bilateral normal adrenal configuration to confirm no adrenal metastasis, two patients had no malignancy and the last patient (Patient 4; malignant schwannoma) died 4 months after the FDG PET/CT examination, although her bilateral adrenals showed normal configuration on CT 1 month after the FDG PET/CT examination. However, for patients with malignancy, the possibility of small-sized bilateral metastases cannot be excluded. Pagani24 reported that thin-needle biopsies of normal adrenal glands by CT criteria in patients with small-cell lung carcinoma revealed that 4 (17%) of 24 patients were positive for metastases and 1 (4%) patient had bilateral adrenal metastases. All the four patients with vasovagal-related stress and bilateral avid adrenal FDG uptake were females and relatively younger. It is remained to be clarified whether the vulnerability to stress which induces bilateral avid adrenal FDG uptake may differ or not in sex and age. We acknowledge that it is difficult to draw robust conclusions in this study because there are only four cases with vasovagal reflex episodes. Further studies with larger study population are expected.
CONCLUSION
Although rare, vasovagal-related stress immediately before FDG injection may be one of the causes for bilateral avid adrenal uptake with normal adrenal configuration on FDG PET/CT.
Acknowledgments
ACKNOWLEDGMENTS
We would like to thank Dr Chihaya Koriyama for her assistance with the statistical analysis. We also thank the staff at our hospital and our secretaries for their contributions to this study. No potential conflicts of interest were disclosed.
Contributor Information
Megumi Jinguji, Email: megu@m.kufm.kagoshima-u.ac.jp, jinmegu@gmail.com.
Masatoyo Nakajo, Email: toyo.nakajo@dolphin.ocn.ne.jp.
Masayuki Nakajo, Email: nakajo@m.kufm.kagoshima-u.ac.jp.
Yoshiaki Nakabeppu, Email: yoshi@m3.kufm.kagoshima-u.ac.jp.
Takashi Yoshiura, Email: yoshiura@m3.kufm.kagoshima-u.ac.jp.
REFERENCES
- 1.Boland GW, Dwamena BA, Sangwaiya MJ, Goehler AG, Blake MA, Hahn PF, et al. Characterization of adrenal masses by using FDG PET: A systematic review and meta-analysis of diagnostic test performance. Radiology 2011; 259: 117–26. doi: 10.1148/radiol.11100569 [DOI] [PubMed] [Google Scholar]
- 2.Brady MJ, Thomas J, Wong TZ, Franklin KM, Ho LM, Paulson EK. Adrenal nodules at FDG PET/CT in patients known to have or suspected of having lung cancer: a proposal for an efficient diagnostic algorithm. Radiology 2009; 250: 523–30. doi: 10.1148/radiol.2502080219 [DOI] [PubMed] [Google Scholar]
- 3.Chong S, Lee KS, Kim HY, Kim YK, Kim BT, Chung MJ, et al. Integrated PET-CT for the characterization of adrenal gland lesions in cancer patients: diagnostic efficacy and interpretation pitfalls. Radiographics 2006; 26: 1811–25. doi: 10.1148/rg.266065057 [DOI] [PubMed] [Google Scholar]
- 4.Montagne JP, Kressel HY, Korobkin M, Moss AA. Computed tomography of the normal adrenal glands. AJR Am J Roentgenol 1978; 130: 963–6. doi: 10.2214/ajr.130.5.963 [DOI] [PubMed] [Google Scholar]
- 5.White BA. The adrenal gland. In: Koeppen BM. and Stanton BA, eds. Berne & Levy physiology 6th edn, updated edn. Philadelphia, PA: Mosby; 2010. pp. 738–57. [Google Scholar]
- 6.Mitchell TC, Meikle AW. Adrenal function. In: Bishop ML, Fody EP, Schoeff LE. Clinical chemistry: techniques, principles, correlations. 7th edn. Philadelphia, PA: Lippincott, Williams, and Wilkins; 2013. pp. 453–71. [Google Scholar]
- 7.Deacon B, Abramowitz J. Fear of needles and vasovagal reactions among phlebotomy patients. J Anxiety Disord 2006; 20: 946–60. doi: 10.1016/j.janxdis.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 8.Ditto B, Gilchrist PT, Holly CD. Fear-related predictors of vasovagal symptoms during blood donation: it’s in the blood. J Behav Med 2012; 35: 393–9. doi: 10.1007/s10865-011-9366-0 [DOI] [PubMed] [Google Scholar]
- 9.Chosy JJ, Graham DT. Catecholamines in vasovagal fainting. J Psychosom Res 1965; 9: 189–94. doi: 10.1016/0022-3999(65)90032-2 [DOI] [PubMed] [Google Scholar]
- 10.Benditt D, Ermis C, Padanilam B, Samniah N, Sakaguchi S. Catecholamine response during haemodynamically stable upright posture in individuals with and without tilt-table induced vasovagal syncope. Europace 2003; 5: 65–70. doi: 10.1053/eupc.2002.0271 [DOI] [PubMed] [Google Scholar]
- 11.Sra JS, Murthy V, Natale A, Jazayeri MR, Dhala A, Deshpande S, et al. Circulatory and cattecholamine changes during head-up tilt testing in neurocardiogenic (vasovagal) syncope. Am J Cardiol 1994; 73: 33–7. doi: 10.1016/0002-9149(94)90723-4 [DOI] [PubMed] [Google Scholar]
- 12.Watanabe H, Kanematsu M, Goshima S, Kondo H, Kawada H, Noda Y, et al. Adrenal-to-liver SUV ratio is the best parameter for differentiation of adrenal metastases from adenomas using 18F -FDG PET/CT. Ann Nucl Med 2013; 27: 648–53. doi: 10.1007/s12149-013-0730-8 [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Sun H, Gao S, Bai R. Primary bilateral adrenal lymphoma: 2 case reports. J Comput Assist Tomogr 2006; 30: 791–3. doi: 10.1097/01.rct.0000216112.15564.0c [DOI] [PubMed] [Google Scholar]
- 14.Taïeb D, Sebag F, Barlier A, Tessonnier L, Palazzo FF, Morange I, et al. 18F -FDG avidity of pheochromocytomas and paragangliomas: a new molecular imaging signature? J Nucl Med 2009; 50: 711–7. doi: 10.2967/jnumed.108.060731 [DOI] [PubMed] [Google Scholar]
- 15.Basu S, Nair N. 18F -FDG uptake in bilateral adrenal hyperplasia causing Cushing’s syndrome. Eur J Nucl Med Mol Imaging 2005; 32: 384. doi: 10.1007/s00259-004-1629-3 [DOI] [PubMed] [Google Scholar]
- 16.Li YJ, Cai L, Sun HR, Gao S, Bai RJ. Increased FDG uptake in bilateral adrenal tuberculosis appearing like malignancy. Clin Nucl Med 2008; 33: 191–2. doi: 10.1097/RLU.0b013e318162ddb3 [DOI] [PubMed] [Google Scholar]
- 17.Umeoka S, Koyama T, Saga T, Higashi T, Ito N, Kamoto T, et al. High 18F -fluorodeoxyglocose uptake in adrenal histoplasmosis; a case report. Eur Radiol 2005; 15: 2483–6. doi: 10.1007/s00330-005-2683-3 [DOI] [PubMed] [Google Scholar]
- 18.Repko BM, Tulchinsky M. Increased F-18 FDG uptake in resolving atraumatic bilateral adrenal hemorrhage (hematoma) on PET/CT. Clin Nucl Med 2008; 33: 651–3. doi: 10.1097/RLU.0b013e3181813179 [DOI] [PubMed] [Google Scholar]
- 19.Castinetti F, Verschueren A, Cassagneau P, Brue T, Sebag F, Daniel L, et al. Adrenal myelolipoma: an unusual cause of bilateral highly 18F-FDG-avidrenal masses. J Clin Endocrinol Metab 2012; 97: 2577–8. doi: 10.1210/jc.2012-1713 [DOI] [PubMed] [Google Scholar]
- 20.Bagheri B, Maurer AH, Cone L, Doss M, Adler L. Characterization of the normal adrenal gland with 18F -FDG PET/CT. J Nucl Med 2004; 45: 1340–3. [PubMed] [Google Scholar]
- 21.Leboulleux S, Deandreis D, Escourrou C, Ghuzlan AA, Bridals F, Aupérin A, et al. Fluorodesoxyglucose uptake in the remaining adrenal glands during the follow-up of patients with adrenocortical carsinoma: do not consider it as malignancy. Eur J Endocrinol 2011; 164: 89–94. doi: 10.1530/EJE-10-0666 [DOI] [PubMed] [Google Scholar]
- 22.Kim BS, Lee JD, Kang WJ. Differentiation of an adrenal mass in patients with non-small cell lung cancer by means of a normal range of adrenal standardized uptake values on FDG PET/CT. Ann Nucl Med 2015; 29: 276–83. doi: 10.1007/s12149-014-0937-3 [DOI] [PubMed] [Google Scholar]
- 23.Meier JM, Alavi A, Iruvuri S, Alzeair S, Parker R, Houseni M, et al. Assessment of age-related changes in abdominal organ structure and function with computed tomography and positron emission tomography. Semin Nucl Med 2007; 37: 154–72. doi: 10.1053/j.semnuclmed.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 24.Pagani JJ. Normal adrenal glands in small cell lung carsinoma: CT-guided biopsy. AJR Am J Roentgenol 1983; 140: 949–51. doi: 10.2214/ajr.140.5.949 [DOI] [PubMed] [Google Scholar]