Abstract
Cardiac CT has developed into a robust clinical tool during the past 15 years. Of the fields in which the potential of cardiac CT has raised more interest is chest pain in acute settings. In fact, the possibility to exclude with high reliability obstructive coronary artery disease (CAD) in patients at low-to-intermediate risk is of great interest both from the clinical standpoint and from the management standpoint. Several other modalities, with or without imaging, have been used during the past decades in the settings of new onset chest pain or in acute chest pain for both diagnostic and prognostic assessment of CAD. Each one has advantages and disadvantages. Most imaging modalities also focus on inducible ischaemia to guide referral to invasive coronary angiography. The advent of cardiac CT has introduced a new practice diagnostic paradigm, being the most accurate non-invasive method for identification and exclusion of CAD. Furthermore, the detection of subclinical CAD and plaque imaging offer the opportunity to improve risk stratification. Moreover, recent advances of the latest generation CT scanners allow combining both anatomical and functional imaging by stress myocardial perfusion. The role of cardiac CT in acute settings is already important and will become progressively more important in the coming years.
INTRODUCTION
Evaluating acute-onset chest pain (ACP) in the emergency department (ED) remains one of the most common and challenging clinical problems, accounting for 7 million visits annually in the USA and is the second most frequent cause of ED visits in adults, with related healthcare costs of $13 to $15 billion.1,2 Causes of ACP may include a wide spectrum of pathologies ranging from ischaemic cardiac diseases, non-ischaemic pathologies or non-cardiac conditions. However, only a minority of these patients require urgent treatment for a potentially life-threatening condition such as acute coronary syndrome (ACS), pulmonary embolism (PE), aneurysm rupture or acute aortic dissection (AD).
In patients with ACS, the sudden clinical manifestation of coronary artery disease (CAD) may present as sudden coronary death, ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI) or unstable angina (UA). Whereas STEMI and NSTEMI are defined by the presence of myocardial necrosis (and cardiac biomarker elevation), UA is defined as chest pain syndrome due to ischaemia without the presence of myocardial necrosis.3 In this scenario, timely triage and rapid management decisions are crucial, as they affect prognosis. Epidemiologic data indicate that ACS has a prevalence of 3 per 1000 inhabitants with higher 30-day mortality among patients with STEMI with respect to patients with NSTEMI (7% vs 3–5%, respectively).3 However, at 1 year, this difference is no longer present (12% vs 13%, respectively), and at long term, 4-year follow-up, patients with NSTEMI have a worse prognosis than patients with STEMI with a twofold difference in mortality owing to the higher risk profile including older age and more comorbid conditions.3 Patients with ACP in emergency units are typically at low risk of ACS and only 17% of patients finally met criteria for acute myocardial infarction (AMI) or UA.4 However, despite modern algorithms, they represent a diagnostic dilemma, considering that in 4% of cases, they are mistakenly discharged, resulting in a twofold higher risk of cardiac death compared with patients who are hospitalized.4 This also has important medicolegal implications, accounting for 20% of all medical malpractice claims.5 Thus, in the USA, a precautionary principle of “rule-out ACS” is adopted and, as a consequence, nearly 80% of the patients with ACP even at low-to-intermediate risk are admitted to acute-care hospital, representing a major burden to the USA health system.5
Different modalities have been proposed in the work-up of suspected ACS to assist in both diagnosing and ruling out. Accordingly, the use of advanced medical imaging for ED visits related to chest pain increased dramatically during the last decade (up to 367%).6 In particular, owing to the rapid technological improvements, cardiac CT has become the most accurate tool for the non-invasive evaluation of CAD.7 Current evidences derived from the first multicentre trials suggest that cardiac CT may be safely used for ruling out ACS in patients with low-to-intermediate risk.1,8 Furthermore, more recent advances in cardiac CT imaging enable integrated evaluation of anatomic and functional significance of CAD in a single examination.9 Moreover, cardiac CT plays a central role in the diagnosis, risk stratification and management of aortic diseases and other vascular conditions as examples for important differential diagnoses in the acute setting.10,11
The purpose of this review was to summarize the current standard of care (SOC) in managing patients with chest pain with acute pain onset and suspected ACS and evaluate all available evidence and future perspectives regarding the clinical role of cardiac CT in the setting of ACP syndromes in the ED.
CURRENT STANDARD OF CARE FOR ACUTE CHEST PAIN
Risk stratification
Identifying patients with ACS who require hospitalization and urgent treatment within the very large proportion with ACP is a diagnostic challenge, especially in individuals without clear symptoms or non-diagnostic electrocardiographic (ECG) features.
The first-line diagnostic tools in the assessment of suspected ACS combine detailed clinical history, acute 12-lead ECG findings and measurement of biochemical cardiac markers. Typically, repeated biomarker measurements and ECG monitoring are required over the next hours (≈12-h stay) to safely rule out ACS.
Cardiac troponin, a specific marker of cardiomyocyte injury, plays a central role in the diagnostic process and accurate risk stratification and makes it possible to differentiate NSTEMI from UA.3 In the era of high-sensitive troponin T or I assays, AMI can now be detected more frequently and earlier in patients presenting with ACP. Optimized fast-track protocols for ruling out AMI allow limiting sampling to the point of presentation and a 3-h control.3 Furthermore, cardiac troponin is a powerful, independent marker of adverse outcome.12 However, troponin elevation may occur in other non-ischaemic conditions, such as renal impairment, tachyarrhythmias, AD or PE.3
Among several prediction models for risk stratification of patients in the ED presenting with ACP syndrome, the GRACE (Global Registry of Acute Coronary Events) and TIMI (thrombolysis in myocardial infarction) risk scores are established for predicting short-term ischaemic risk.13,14 Guidelines recommend the use of the GRACE score (GRACE 2.0 risk score calculator, http://www.gracescore.org/WebSite/default.aspx?ReturnUrl=%2f), which includes several variables (age, systolic blood pressure, heart rate, serum creatinine, Killip class of heart failure at presentation, cardiac arrest at admission, elevated cardiac biomarkers and ST deviation) and has a better discriminatory performance, providing a direct estimation of in-hospital, 6-month, 1-year and 3-year mortality or the combined risk of death or AMI at 1 year.3,13 However, current guidelines also recognize the more simple seven-point TIMI score for UA/NSTEMI (http://www.timi.org/index.php?page=calculators), which includes clinical and medical history, CAD risk factors, ECG modification and serum cardiac enzyme levels. TIMI scores can be rapidly determined to predict the composite of death or (re)infarction or recurrent severe ischaemia requiring revascularization within 14 days of event.3,14 However, TIMI application to patients at low risk resulted in an inferior discriminative power compared with the GRACE score, which may be related to the non-inclusion of haemodynamic indicators.15,16
Role of non-invasive functional imaging
Patients with suspected UA/NSTEMI should be observed in interdisciplinary EDs or chest pain units until the diagnosis of AMI or UA is confirmed or ruled out.3 In this setting, different functional non-invasive modalities, with or without cardiac imaging, have been implemented as confirmatory test to guide triage decisions and aid in treatment selection.15
Among these, over the last two to three decades, echocardiography (echo) and myocardial perfusion imaging (MPI) with radionuclide single-photon emission CT (SPECT) have been the most widely used imaging modalities for ACP evaluation. Stress (exercise or pharmacological) imaging is preferable over exercise ECG owing to the higher diagnostic accuracy.3 Other major reasons for selecting an imaging stress test rather than exercise ECG are baseline ECG alterations and patients' inability to exercise.15 There are two approaches to detect stress-induced ischaemia: wall-motion analysis during the administration of an inotropic agent (e.g. dobutamine), which causes an adrenergic response; or perfusion analysis during administration of a coronary vasodilator agent (e.g. adenosine, dipyridamole or regadenoson) which determines a 3.5–4-fold increase in myocardial blood flow and a transmural redistribution of perfusion (coronary steal phenomenon).17,18
Over the last 10 years, cardiac MRI (CMRI) has emerged as a powerful functional imaging technique for the assessment and differential diagnosis of a wide spectrum of cardiac diseases associated with ACP presentation.3 However, major limitations of the widespread used technique are scanning availability, the relatively long examination time and logistical difficulties in the setting of ACP.
In patients who are stable and free of chest pain for several hours, with no ischaemic signs at 12-lead ECG and with serial negative cardiac troponin, a functional stress testing (preferably with imaging) may be performed during the period of observation or shortly after discharge. That is a Class I indication (Level of Evidence: A) according to the European Society of Cardiology (ESC) guidelines for the management of ACSs in patients presenting without persistent ST-segment elevation.3
The optimal imaging strategy is determined not only by the diagnostic performance but also by practical aspects such as local practice and availability, expertise with imaging techniques, medical facilities and individual patient characteristics.19 Furthermore, stress/rest imaging modalities are usually not widely available on a 24/7 basis in hospitals, resulting in longer diagnostic work-up with impact on healthcare costs.3 Advantages and disadvantages of functional imaging with echo, SPECT and MRI are reported in Table 1.
Table 1.
Advantages and limits of functional imaging: echocardiography (echo), single-photon emission CT (SPECT) and MRI
| Techniques | Advantages | Limits |
|---|---|---|
| Echo | • Safe | • Poor sensitivity |
| • Relatively modest cost | • High degree of operator dependence | |
| • Availability, portability, ease of performance | • Poor acoustic window in at least 10% of cases | |
| • Provides structural and functional data | • Artefacts | |
| • Provides important prognostic information | ||
| SPECT | • High SE and SP for detection of ischaemia | • High cost |
| • Transmural infarct | • Radiation exposure | |
| • Allows assessment of LV function | • Time consuming | |
| • Provides important prognostic information | • Logistical issue | |
| • FP findings (photon attenuation artefacts) | ||
| • FN results (possible balanced ischaemia not detectable by semi-quantitative analysis) | ||
| MRI | • Safe | • High cost |
| • Highest SE and SP for detection of ischaemia | • Time consuming | |
| • Allows assessment of LV function | • Limited availability | |
| • Detects subendocardial infarct (transmurality) | • Important logistic requirements | |
| • Differentiates new from old infarct (T2w imaging) | • Heart rate and respiratory motion artefacts | |
| • Detects UA without necrosis (T2w imaging) | ||
| • Provides important prognostic information |
FN, false negative; FP, false positive; LV, left ventricular; SE, sensitivity, SP, specificity; T2w, T2 weighted; UA, unstable angina.
Echocardiography
Echo is the first-line readily available imaging modality that is indicated in the majority of clinical scenarios associated with cardiac emergencies.19
Echo can assess the left ventricular (LV) systolic function and, in experienced hands, rapidly detect transient segmental hypokinesia or akinesia encountered during acute onset of different types of myocardial injury (ischaemia, stunning, hibernation or necrosis).20 According to the temporal sequence of the ischaemic cascade, regional wall-motion abnormalities (RWMA) appear after considerable flow reduction, earlier with respect to ECG ischaemic alterations. The detection of RWMA may be aided by administration of microbubble contrast agents that enhance delineation of endocardial borders.3,20 Contrast-enhanced echo may also allow evaluation of regional myocardial perfusion and viability, which may improve the sensitivity of the technique, although limited data are available in patients presenting with ACP.3,15,19,20 Furthermore, deformation imaging of the left ventricle (strain and strain rate) is a potentially useful technique to reveal subtle wall-motion abnormalities and improve the prognostics value of the technique.3,20,21 Numerous factors may affect the sensitivity in detecting acute ischaemia, including ischaemic myocardium size, timing of the study in relation to the clinical presentation, protocols and technology.15 Furthermore, a sizeable involvement by ischaemia or infarction of the transmural myocardial thickness (>20%)22 or of the left ventricle circumference (>12%)23 is required for echo in order to detect RWMA. Moreover, echo provides no information on the age of RWMA. These factors account for the wide variability in negative-predictive values (NPVs) (57–98%) and positive-predictive values (PPVs) (31–100%) of resting echo for ischaemia/AMI detection at the index visit, as shown by a review of 9 studies including a total of 955 patients.24 The lowest PPV of only 31% was observed among a population at low risk, in which the cardiac event rate was 17%.24 In all of these studies, the patients who had false-negative echo findings had NSTEMI or UA, reflecting the limited sensitivity of the technique in such a population.24
However, echo by providing both functional and structural data can help in detecting alternative source of ACP, such as pericardial disease, valvular heart disease, cardiomyopathies, acute AD or right ventricular dilatation, suggestive of acute PE.3,20 According to the ESC guidelines, “echocardiography is recommended to evaluate regional and global LV function and to rule in or rule out differential diagnoses” (Class I indication; Level of Evidence: C).3
Both exercise and pharmacological (dobutamine infusion with addition of atropine if necessary or high-dose dipyridamole and atropine) stress echo have been shown to be safe and accurate when performed in the acute setting.20 Pharmacological stress echo has been shown to have an excellent NPV for obstructive CAD of approximately 97%,25 providing short-term and long-term prognostic information comparable with SPECT in the triage of patients with ACP.15,20,25 Furthermore, dobutamine stress echo is more cost effective than exercise ECG testing.20,26 Moreover, contrast stress echo may predict cardiac events in patients with significant cardiac risk factors presenting with ACP and with suspected ACS, but non-diagnostic ECG and negative 12-h troponin.27 In this population at low risk, a negative stress myocardial contrast echo predicts an excellent outcome.27
The major limitation of echo is its subjective nature of assessment and intrinsic technical limitations of the technique, such as artefacts and insufficient transthoracic access, which may lead to uninterpretable results in >10% of cases.19 As a general rule for all imaging modalities in acute cardiovascular condition, guidelines advocate an advanced level of skill and expertise in imaging interpretation in order to avoid misdiagnosis.20
The most relevant (n > 300) studies evaluating the discriminatory power of stress echo in the acute setting are reported in Table 2.
Table 2.
Diagnostic accuracies of stress echocardiography in patients with low-risk, troponin-negative acute chest pain with non-diagnostic electrocardiogram
| Study | Number of patients | Stressor | Outcome | Follow-up (months) | SE | SP | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| Bholasingh et al28 | 377 | Dobutamine | MACE, UA | 6 | 36 | 95 | 31 | 96 |
| Bedetti et al29 | 552 | Dipyridamole or dobutamine | Cardiac death, AMI | 13 | 87 | 98 | 78 | 99 |
| Conti et al25 | 503 | Exercise | MACE, UA, CAG stenosis ≥50% | 6 | 85 | 95 | 81 | 97 |
AMI, acute myocardial infarction; CAG, invasive coronary angiography; MACE, major cardiac events including cardiac death, acute myocardial infarction and revascularization; NPV, negative-predictive value; PPV, positive-predictive value; SE, sensitivity; SP, specificity; UA, unstable angina.
Nuclear imaging
MPI using SPECT in the evaluation of ACP syndrome was first described more than 35 years ago using the thallium 201 planar imaging30 and subsequently incorporated in most comprehensive strategies for the triage of patients with suspected ACS.31 Nowadays, the thallium 201 has been largely replaced by technetium Tc 99m-based perfusion agents, which lead to higher quality images (because of less attenuation, scatter and blurring).19 Another advantage of Tc 99m-based agents is the significantly lower radiation dose owing to the shorter half-life.32 In addition to ischaemia detection, SPECT imaging by the implementation of ECG-gated acquisition allows the simultaneous assessment of RWMA and provides a quantitative determination of global systolic function.19,32
Numerous studies evaluating the discriminatory power of radionuclide SPECT have shown that a normal rest scan confers an excellent short-term prognosis, with reported sensitivities for the detection of AMI of >90% (provided that imaging is performed <6 h after onset of pain) and specificities in the range of 50–80%.19,32 In one of the few large randomized, prospective multicentre trials including 2475 patients, Udelson et al33 have shown that incorporating resting SPECT imaging into the ED SOC resulted in a significant reduction in hospitalization (from 52% to 42%), without any increase in the risk for subsequent cardiac events at 30 days. Rest SPECT imaging has several limitations. A perfusion defect can indicate a new or old infarct, and recognition of AMI requires assessment with sensitive cardiac biomarkers. Furthermore, a normal rest SPECT does not rule out concomitant CAD without rest ischaemia, for which stress imaging would be required.34 Large observational cohorts involving thousands of patients over the last two decades have demonstrated the high predictive accuracy of stress SPECT for cardiac events in ED.19 A combined 1-day rest/stress or 2-day stress/rest imaging strategy may further enhance the detection of myocardial ischaemia or viability, while a normal study is associated with excellent outcome.3,34,35 Owing to the high NPV, the annual cardiac event rate after a normal stress SPECT examination is extremely low <1% and comparable with that of stress echo (0.45% vs 0.54%, respectively).36 A large observational study of 805 patients evaluated for ACS has shown that the stress SPECT scan performed better than the resting nuclear scan in detecting a composite of cardiac events (AMI, revascularization, stenosis >70% not amenable to revascularization, life-threatening complication or cardiac death) at 30-day follow-up, with a significant improvement in both sensitivity (from 71 to 97%) and specificity (from 73 to 88%).37 It should be remembered, however, that the number of false positives provided for AMI might be high, and the positive-predictive power therefore might be very low.
Among the limits of SPECT imaging there are the low temporal resolution and the relatively low spatial resolution. The standard image matrix of tomographic SPECT images is 64 × 64 pixels, with the pixel size of 5–7 mm; therefore, small, subendocardial areas of ischaemic myocardium (3–5% of the left ventricle) may not be detected.15,32,38 These areas may be clinically relevant, considering that small infarcts associated with NSTEMI have a long-term morbidity and mortality comparable with that of larger infarcts.38–40
Finally, the limited availability of SPECT for off-hour imaging is a potential logistical issue.
Large studies (n > 300) assessing the discriminatory power of rest and stress SPECT in the evaluation of ACP are reported in Table 3.
Table 3.
Diagnostic accuracies of rest and stress single-photon emission CT in patients with low-risk, troponin-negative acute chest pain with non-diagnostic electrocardiogram
| Study | Number of patients | Stressor | Outcome | Follow-up | SE | SP | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| Kontos et al41 | 532 | Rest | AMI | Index | 93 | 71 | 15 | 99 |
| Tatum et al31 | 438 | Rest | AMI | 30 days | 100 | 78 | 7 | 100 |
| Fesmire et al37 | 805 | Exercise, dipy or dob | MACE, CAG stenosis ≥70% | 30 days | 97 | 88 | NA | NA |
| Lim et al34 | 377 | Dipy or dob | MACE, CAG stenosis ≥70% | 30 days | 85 | 93 | 35 | 99 |
| 1 year | 78 | 94 | 46 | 99 | ||||
| Conti et al25 | 503 | Exercise | MACE, UA, CAG stenosis ≥50% | 6 months | 86 | 90 | 67 | 97 |
| Conti et al42 | 798 | Exercise | MACE, UA, CAG stenosis ≥50% | 12 months | 90 | 85 | 52–74 | 98 |
AMI, acute myocardial infarction; CAG, invasive coronary angiography; dipy, dipyridamole; dob, dobutamine; MACE, major cardiac events including cardiac death, acute myocardial infarction and revascularization; NA, non-assessable; NPV, negative-predictive value; PPV, positive-predictive value; SE, sensitivity; SP, specificity; UA, unstable angina.
MRI
CMRI is the most comprehensive and versatile functional imaging technique. By using a multiparametric protocol, MRI enables assessment of cardiomyopathies, cardiac function, myocardial perfusion, viability and pericardial and valvular disease during a single imaging session, with a strong impact on patient management.43,44 The in-plane spatial resolution of perfusion MRI (up to 1–2 mm) is superior to that of SPECT, particularly for the detection of subendocardial ischaemia.43 According to two large prospective randomized studies, the MR-IMPACT (Magnetic Imaging for Myocardial Perfusion Assessment in Coronary Artery Disease Trial; n = 234; 18 European and USA centres) and CE-MARC (Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease; n = 752; 2 UK centres) trials, stress perfusion MRI performed better than SPECT for diagnosis of obstructive CAD.45,46 This superiority was also shown for multivessel disease CAD.45,46 According to a recent meta-analysis (n = 17901) evaluating the diagnostic performance of different MPI modalities, MRI performance was superior to SPECT and yielded a similar diagnostic accuracy as positron emission tomography, with a poll sensitivity of 89% and specificity of 76%.47
Furthermore, the excellent contrast resolution and the submillimetre spatial resolution of late gadolinium enhancement (LGE) sequence allow for detection of microinfarcts as small as <1 g of mass.43 Although subtle, these subendocardial infarcts are clinically relevant for long-term prognosis in patients with NSTEMI.38–40 A wide spectrum of other tissue injuries in the context of AMI may be precisely detected, such as microvascular obstruction and intramyocardial haemorrhage that may negatively influence the future remodelling of the left ventricle after revascularization.43
Moreover, by means of T2 weighted imaging, MRI can detect tissue oedema, the hallmark of acute myocardial injury, which correlates with the area at risk in the context of AMI.43 Moreover, oedema may be identified in the absence of gross irreversible injury by LGE in patients with UA without troponin elevation;48 this may give MRI a unique advantage over functional imaging modalities for detecting and localizing acute ischaemic alterations even in patients who are troponin negative.
A number of relatively small single-centre studies have evaluated the accuracy of acute rest CMRI for ED management of patients with chest pain. In a prospective study of 161 patients, sensitivity and specificity for identification of NSTEMI and UA were 84% and 85%, respectively.49 A study by Cury et al50 demonstrated that incorporating T2 weighted imaging into the protocol to differentiate acute from chronic wall-motion abnormalities improved the overall accuracy, in particular with improvement in the specificity and PPV (from 84 to 96% and from 58 to 85%, respectively), while maintaining high the sensitivity (85%) and NPV (96%). By adding stress MRI to the evaluation, another study found a sensitivity and specificity for identification of CAD of 96% and 83%, respectively, using adenosine stress perfusion and LGE.51 More recently, a negative stress MRI in patients presenting at the ED with chest pain was shown to have excellent short-term and mid-term prognosis, in particular no patients with a negative adenosine MRI scan (115 patients out of total 135 patients) had subsequent cardiac events at 1-year follow-up.52 Finally, some studies have shown that stress MRI has the ability to reduce cardiac-related costs of medical care during the index visit and over the first year subsequent to discharge, without an observed increase in major cardiac events.53,54
Moreover, from 7 to 15% of patients presenting with ACS had unobstructed coronaries on urgent invasive or non-invasive angiography representing a clinical dilemma, as the underlying diagnosis is variable and often unclear.55 In this scenario, CMRI may play a consistent role in differential diagnoses of important mimickers of ACS. By using a multiparametric protocol, MRI may be particularly useful in determining the diagnosis and differentiate ischaemic from non-ischaemic causes, thereby guiding patient management. These include in most of the cases (>95%) myocarditis, embolic/spontaneous recanalization myocardial infarction and Tako-Tsubo cardiomyopathy.3,55 Since some abnormalities such as myocardial oedema/inflammation may be reversible and resolve with time, MRI scan performed at the time of acute presentation is more sensitive for diagnosis.
MRI of coronary arteries remains inferior to cardiac CT in coronary stenosis evaluation and is not routinely performed in emergency condition, because it is time consuming and neither universally available nor feasible for all patients.43 MRI is very susceptible to motion artefacts related to irregular heart rhythm and erratic respiratory patterns that can occur while evaluating patients who are uncooperative; these factors may affect the diagnostic accuracy.
There are major limitations to the clinical routine implementation of MRI at many hospitals, namely the logistic challenge of providing 24-h availability of MRI to accommodate emergency studies, limited availability of infrastructure needed to perform the relatively complex CMRI examinations and lack of widespread expertise in CMRI.
CARDIAC CT
Technical aspects of cardiac CT
Over the last 15 years, cardiac CT has evolved rapidly and dramatically. Currently, the minimal standard for robust comprehensive cardiac imaging is a 64-detector CT. Several technical advances have led to a quantum leap in temporal and spatial resolution, with a progressive reduction in imaging scan time, volume of contrast media and radiation exposure way below the dose of conventional coronary angiography. State-of-the-art CT systems allow for a whole-heart acquisition in a single heartbeat using a wide-detector CT scanner (256 or 320 slices) with a Z-axis coverage of up to 16 cm or by using the prospectively ECG-triggered high-pitch spiral acquisition of the dual-source CT system.56
The implementation into routine clinical practice of prospective ECG gating has resulted in a consistent reduction of the radiation dose as low as 2–3 mSv, approaching 1 mSv or less in selected patients with the prospectively ECG-triggered high-pitch spiral acquisition.56,57 The high temporal resolution of the second-generation (75 ms) and third-generation (66 ms) dual-source CT scanner allows for adequate image quality even in patients with high heart rate, high variability of heart rate or arrhythmia.56,57 Recent technical developments including new detector material and integrated circuit detector, low kV (70–80 KV) protocol, new iterative reconstruction and motion-correction algorithms represent yet another milestone for substantial dose reduction, while the CT image quality may be further improved.56–59
Diagnostic performance and prognostic value of cardiac CT
Cardiac CT has emerged as the mainstay diagnostic tool for non-invasive anatomical assessment of coronary atherosclerosis.
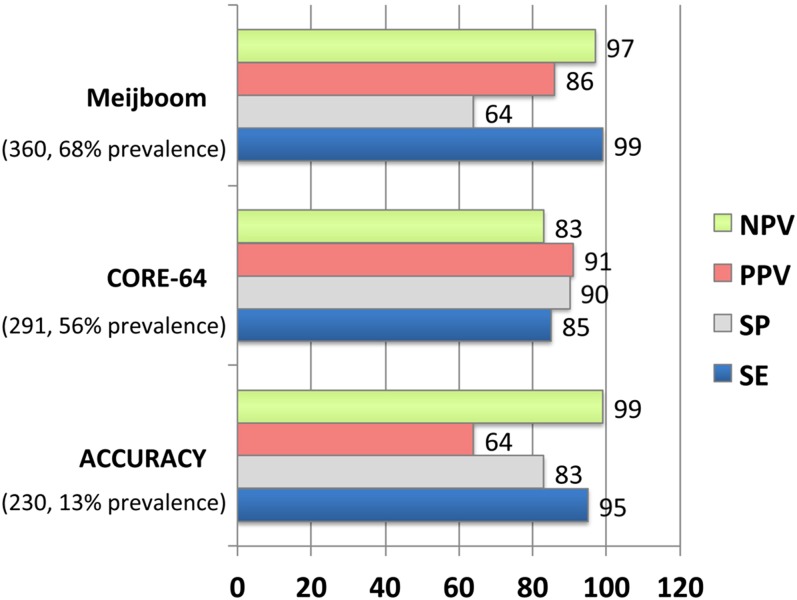
Several hundreds of single-centre studies and three large prospective randomized trials with cohort sizes from 230 to 360 patients have evaluated the diagnostic accuracy of cardiac CT as compared with the gold standard of invasive coronary angiography (Figure 1).60–62 The strength of cardiac CT is its high sensitivity superior to other imaging techniques (98–100%).7 According to the Bayesian theorem and the high NPV (99–100%) of cardiac CT, the consensus is to consider the use of cardiac CT mainly in populations with low-to-intermediate probability or after an inconclusive functional test owing to its excellent ability to rule out CAD.7,63 Important diagnostic and prognostic clinical studies in various patient populations have contributed to the robustness of the method and its implementation into daily clinical practice. In the large, prospective, multicentre CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes International Multicentre) registry (n = 23854), which included numerous clinical sites within North America, Europe and Asia, increasing extent, severity and location of CAD predicted adverse outcomes independently from clinical variables.64 Moreover, overwhelming evidence supports the excellent mid-term and long-term prognosis after a negative cardiac CT examination, with an average annualized rate of major cardiac events as low as 0.21%.65,66 This emphasizes a clinical value of cardiac CT for identification of patients with absence of coronary atherosclerotic plaques in whom no further additional testing and/or therapy is necessary or indicated.
Figure 1.
Prospective multicentre trials evaluating the diagnostic accuracy of cardiac CT for detection of obstructive coronary artery disease (CAD). Note the uniformly high sensitivity (SE) and negative-predictive value (NPV). Meijboom et al60 Diagnostic Accuracy of 64-Slice CT Coronary Angiography; ACCURACY,61 Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography; CORE-64,62 Coronary Artery Evaluation Using 64-Row Multidetector CT Angiography; PPV, positive-predictive value; SP, specificity.
This new era of cardiac CT has led to a new paradigm in clinical decision pathways. Given the logistic limitations of functional (stress) testing and the relatively low prevalence of CAD in the setting of ACP syndrome, direct, non-invasive assessment of coronary atherosclerosis and rapid ruling out of obstructive CAD by cardiac CT appears to be an appealing diagnostic alternative for early triage of ACS.
Cardiac CT in the emergency department
Several observation studies that included >3000 patients have evaluated the safety, effectiveness and diagnostic accuracy of 64-slice cardiac CT for triage of patients at the ED with ACP, some of which are reported in Table 4.
Table 4.
Most relevant single-centre studies evaluating the performance of cardiac CT in the triage of patients with acute chest pain
| Study | Number of patients | Risk | Inclusion criteria | ACS definition (rate) | Outcome (last follow-up) | CT criteria | SE | SP | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|
| Gallagher et al67 | 85 | Low risk (Reilly/Goldman criteria) | Negative troponin; normal ECG | AMI, UA + CAG >70% (8%) | Cardiac death or ACS (30 days) | Stenosis >50% CS>400 |
86 | 92 | 50 | 99 |
| Beigel et al68 | 340 | High risk excluded | Negative troponin; non-ischaemic ECG | CAG significant stenosis (4.4%) | MACE (5 months) | Stenosis >50% | 100 | 97 | 65 | 100 |
| Rubinshtein et al69 | 58 | Intermediate risk | Negative troponin; normal ECG | CAG ≥50% or positive troponins or positive stress test (34%) | MACE (15 months) | Stenosis ≥50% | 100 | 92 | 87 | 100 |
| ROMICAT I70 | 368 | Low risk | Negative troponin; non-ischaemic ECG | AMI, UA (8.4%) | MACE (6 months) | Plaque | 100 | 54 | 17 | 100 |
| Stenosis >50% | 77 | 87 | 35 | 98 | ||||||
| Johnson et al71 | 109 | Any risk | Negative troponin; non-ischaemic ECG | CAG >50% (14%) | CAG (6 months) | Stenosis >50% (per segment) | 100 | 99 | 79 | 100 |
| Dedic et al72 | 111 | Any risk | Troponine ≤0.15 μg l−1 | AMI, UA (17%) | AMI or revascularization (3 months) | Calcium | 89 | 41 | 24 | 95 |
| Any plaque | 100 | 40 | 26 | 100 | ||||||
| Stenosis>50% | 89 | 79 | 47 | 97 |
ACS, acute coronary syndrome; AMI, acute myocardial infarction; CAG, invasive coronary angiography; CS, calcium score; ECG, electrocardiogram; MACE, major adverse cardiac events including cardiac deaths, acute myocardial infarction, unstable angina and revascularization; NPV, negative-predictive value; PPV, positive-predictive value; SE, sensitivity; SP, specificity; UA, unstable angina.
Gallagher et al67 found that cardiac CT had superior diagnostic accuracy as compared with SPECT. A first large single-centre study, the ROMICAT (rule-out myocardial infarction/ischaemia using computer-assisted tomography) I trial, included 368 patients with negative initial biomarkers and non-ischaemic ECG and low-to-intermediate likelihood of ACS, of whom 31 patients were ultimately diagnosed with ACS. 50% of patients with ACP were free of CAD by cardiac CT and approximately one-fifth of patients were shown to have obstructive CAD. The main conclusion from this study was that the absence of plaque on cardiac CT safely excludes ACS (sensitivity 100%). The study showed an excellent NPV (98%) of significant stenosis for exclusion of ACS, but a low PPV (35%), since approximately 50% of patients with obstructive CAD or inconclusive assessment at cardiac CT were finally diagnosed with ACS.70 Furthermore, the limited sensitivity owing to a number of patients who were false negative (77%, n = 7 of 31) may be explained by the limited accuracy of cardiac CT to detect stenosis in small-calibre vessels (<2 mm); rupture or thrombosis in subcritical CAD or microvascular disease.70 However, the excellent NPV (>98%) was uniformly demonstrated in most of the series evaluating population with ACP. A study including nearly 600 patients at ED with low risk (TIMI risk score 0–2), of whom 84% patients were discharge based on a negative cardiac CT, likewise demonstrated a NPV of 100% for adverse events within 30 days.73 However, there are limits of its generalizability owing to the low prevalence of disease, as only seven patients were found to have CAD according to invasive coronary angiography and/or a positive stress test, and no patients died or had AMI during follow-up.73
According to a recent meta-analysis of prospective studies published in 2015 comparing the diagnostic accuracy of coronary cardiac CT with functional imaging modalities (n = 7800) for the assessment of ACP in ED setting, cardiac CT performed better that stress echo and SPECT in predicting significant CAD at invasive coronary angiography or the later presence of major adverse clinical outcomes.74 Cardiac CT demonstrated a pool sensitivity of 95%, specificity of 99%, PPV of 84% and NPV of 99%.74
Randomized controlled trials evaluating the effectiveness of cardiac CT in the emergency department
At present, five randomized controlled trials (RCTs) have evaluated the safety and economic impact of cardiac CT compared with the usual care for patients presenting to the ED with suspicion of ACS (Table 5). The first of these by Goldstein et al75 published in 2007 became the basis for the subsequent multicentre CT-STAT (coronary CT angiography for systematic triage of patients with acute chest pain to treatment) trial,76 comparing cardiac CT with SPECT. The ACRIN-PA77 (American College of Radiology Imaging Network and Pennsylvania Department of Health) and ROMICAT II78 (Rule Out Myocardial Ischemia/Infarction Using Computer Assisted Tomography) trials compared cardiac CT with the SOC, whereas exercise ECG was used for comparison with cardiac CT in the CT-COMPARE (CT coronary angiography compared with exercise electrocardiography) trial.79 Collectively, these trials recruited more than 3000 patients in academic US centres (n = 1–16)75–78 and more than 500 patients in a large tertiary academic Australian hospital, the Prince Charles Hospital in Brisbane.79 Patients were at low-to-intermediate risk (ROMICAT II and CT-COMPARE trials) or at low risk of ACS (Goldstein et al, ACRIN-PA and CT-STAT). For all the studies, the management of the patient's condition and the decision regarding admission or discharge after diagnostic testing were at the discretion of the treating clinician, thereby reflecting real-world practice in a large healthcare system. Accordingly, in the population at low risk, there were no deaths in any of the studies and a minimal number of AMI or UA at the index visit and at short-term follow-up (30 days or 6 months). CT-COMPARE trial reported two deaths in the CT arm and one death in the exercise ECG arm that resulted from unrelated causes at 12-month follow-up.
Table 5.
Randomized controlled trials evaluating clinical outcomes of early cardiac CT in the emergency department (ED) for the evaluation of patients with suspected acute coronary syndrome (ACS)
| Trial | Sites | Risk score | Number of patients (randomization) | Randomization | LOS (h) | ED discharge | ACS rate | MACE (30 days) | MACE (6 months) | MACE (12 months) | ED Cost ($) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Goldstein et al75 | 1 | Low risk | 197 (1 : 1) | CTA vs SPECT | 3.4 vs 15a,b | 89% vs 97%c | 5.1% | NA | 0% | NA | 1586 vs 1872a (USD) |
| CT-STAT76 | 16 | TIMI 0–4 | 699 (1 : 1) | CT vs SPECT | 2.9 vs 6.2a,b | 73% vs 81%c | 1.7% | NA | 0.8% vs 0.4%d | NA | 2137 vs 3458a (USD) |
| ACRIN-PA77 | 5 | TIMI 0–2 | 1370 (2 : 1) | CTA vs SOC | 18 vs 24.8a | 50% vs 23%a | 3.5% | 1.1 % vs 1.1% | NA | NA | NA |
| ROMICAT II78 | 9 | Low–intermediate risk | 985 (1 : 1) | CTA vs SOC | 23.2 vs 30.8a | 47% vs 12%a | 7.5% | 0.4% vs 1.2% | NA | NA | 2101 vs 2566a (USD) |
| CT-COMPARE79 | 1 | Low–intermediate risk | 562 (1 : 1) | CTA vs Ex-ECG | 13.5 vs 20.7a | 90% vs 89% | 4.2% | 0% | NA | 0.9% vs 0.4% | 2193 vs 2704a (AUD) |
ACRIN-PA, American College of Radiology Imaging Network and Pennsylvania Department of Health; AUD, Australian dollar; CT-COMPARE, CT coronary angiography compared with exercise electrocardiography; CT-STAT, coronary CT angiography for systematic triage of patients with acute chest pain to treatment; Ex-ECG, exercise stress electrocardiography; LOS, length of stay; MACE, major adverse cardiovascular events, defined as cardiac death, myocardial infarction or unstable angina; NA, non-assessable; ROMICAT, Rule-Out Myocardial Infarction/Ischaemia Using Computer Assisted Tomography; SOC, standard of care; SPECT, single-photon emission CT; TIMI, thrombolysis in myocardial infarction; USD, United States dollar.
p<0.001.
Goldstein et al and CT-STAT reported time to diagnosis instead of LOS.
p<0.05.
MACE rate included late revascularization in patients with normal index test.
The Goldstein et al and CT-STAT trials have shown that the cardiac CT arm allows a more rapid diagnosis compared with SPECT, with comparable outcomes with the two approaches.75,76 In the ACRIP-PA and the ROMICAT II trials, the CT-based strategy was associated with an increased rate of discharge from the ED of approximately 50% (vs 23% and 12% of patients in the SOC group, respectively), with a reduced overall length of stay (LOS) and without significant differences in major adverse cardiovascular events at 30-day follow-up.77,78 No adverse clinical outcome was observed in the ACRIN-PA study, whereas only two major cardiac events (1 AMI and 1 UA) were documented in the ROMICAT II trial. Interestingly, in both of these patients, cardiac CT established clinically significant CAD during the index hospitalization, but both patients had negative stress tests and were initially treated medically.78
A meta-analysis has recently been carried out to consolidate the findings of these four USA RCTs.80 According to the summary data, the CT-based strategy was associated with a significant reduction in LOS (ranging from 25 to 27%),77,78 and time to diagnosis (ranging from 44 to 77%).75,76,78 Three studies reported cost savings in the cardiac CT group, with a significant reduction from 15 to 38% of ED costs;75,76,78 however, the ROMICAT II trial reported no difference in the total cost of care at index hospitalization and follow-up.78 These studies demonstrated that the use of cardiac CT to triage ED chest pain is safe, consistently reduces LOS and reduces ED costs, but compared with traditional methods, is associated with slightly higher rates of invasive coronary angiography (8.4% vs 6.3%; p = 0.03) and revascularizations (4.6% vs 2.6%, p = 0.004).80 Also, the CT-COMPARE trial documented an increase of downstream testing (13.4% vs 7.5%; p = 0.02) and invasive coronary angiography procedures (9% vs 4.2%; p = 0.028) in the CT arm with respect to the exercise ECG strategy.79 However, in accordance with the USA trials, the CT-COMPARE trial reported a significant reduction by 34% of the LOS in the CT arm (13.5 h vs exercise ECG 19.7 h; p< 0.001), which drove a 20% reduction in hospital costs.79 Similarly, time to discharge from the ED was also significantly reduced (p< 0.0001). Furthermore, cardiac CT had improved diagnostic performance compared with exercise ECG, with a sensitivity of 100% (vs 83%), specificity of 94% (vs 91%) and area under the curve of 0.97 (vs 0.87, p = 0.2).79 According to other randomized trials, the study found a higher rate of detection of CAD in the CT arm.77–79
Further studies are warranted to assess the long-term prognostic impact regarding the quality of life and outcome of the cardiac CT strategy and cardiac CT-related invasive procedures compared with conventional care. Moreover, radiation exposure will be higher in the cardiac CT arm when SOC is based on exercise treadmill tests and stress echo, such as the case of the ROMICAT II and CT-COMPARE trials.78,79 Conversely, when the cardiac CT approach is compared with SPECT as SOC, like in the Goldstein et al and CT-STAT trials, both the radiation dose and cost will be lower.75,76 Fortunately, recent scanner innovations dramatically reduced exposure levels (to as low as 1–4 mSv for many patients).
In conclusion, these trials have demonstrated that a first approach with cardiac CT for patients at low and intermediate risk presenting to the ED with chest pain appears to be a safe and viable alternative to functional testing.
Prognosis of cardiac CT in the emergency department
Several studies have demonstrated the excellent outcome of a normal CT examination (absence of coronary plaques) at 2–5-year follow-up.81–83 The true impact can only be determined in prospective observational studies such as the ROMICAT I trial, given the blinded nature of the results of cardiac CT among both caregivers and patients. The ROMICAT trial indicated that the absence of CAD on CT provides a 2-year “warranty period” free of major cardiovascular events. In contrast, the presence of severe CAD is associated with the worst outcome (30.3% cumulative event rate), whereas those with mild or moderate CAD (≤50% stenosis) have intermediate outcomes (4.6% cumulative event rate).81 Interestingly, major adverse cardiac events developed in most of the patients (80%) during the first 30 days after presentation to the ED, suggesting that the strength of cardiac CT is in its diagnostic ability during the acute phase. Furthermore, evaluation of LV function at the index CT examination was shown to provide incremental prognostic value for long-term follow-up.81
Current recommendations and guidelines of cardiac CT in the emergency department
Since 2010 when cardiac CT received a rating of “appropriate” in patients at low-to-intermediate risk presenting with ACP at the ED,84 several guidelines by the ESC and American Heart Association/American College of Cardiology (AHA/ACC) as well as by other societies and organizations have discussed in recent years this application of cardiac CT.
According to the current AHA/ACC guidelines published in 2014, “in patients with possible ACS and a normal ECG, normal cardiac troponins, and no history of CAD, it is reasonable to initially perform (without serial ECGs and troponins) coronary CT angiography to assess coronary artery anatomy” (Class IIa recommendation, Level of Evidence: A).85 Accordingly, AHA/ACC guidelines state that “in low-risk patients with chest pain, coronary CT angiography can result in a more rapid, more cost-effective diagnosis than stress myocardial perfusion imaging” (p. e353, AHA/ACC Guideline of 2014).85
Similarly, recent ESC guidelines published in 2015 state that “MDCT coronary angiography should be considered as an alternative to invasive angiography to exclude ACS when there is a low to intermediate likelihood of CAD and when cardiac troponin and/or ECG are inconclusive”, (Class IIa recommendation, Level of Evidence: A).3
The use of cardiac CT to rule out ACS in patients at low-to-intermediate risk presenting with ACP is supported also by the Society of Cardiovascular CT guideline, which also provided recommendations on performance and interpretation of cardiac CT in the ED.1 Also, the NICE (National Institute for Health and Care Excellence) guideline on the management of chest pain of recent onset support the use of cardiac CT in patients at low pre-test probability of obstructive CAD (>10% but <30%) based on the algorithm provided in the guideline.86
Emerging applications and future outlook
A very modest PPV (35–65%) of cardiac CT in detecting ACS was emerged in some prospective observational studies, mainly driven by the low prevalence of ACS (5–20%) among the ambiguous and broad-based ED chest pain population.67,68,70,73 For that reason, different strategies have been implemented to improve the specificity and PPV of the cardiac CT examination; among these, the two most relevant are evaluation of plaque morphology, MPI and fractional flow reserve (FFR) derived from CT.
Plaque features associated with vulnerability
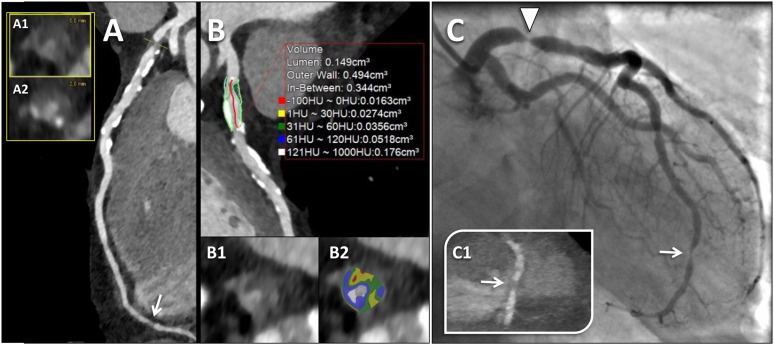
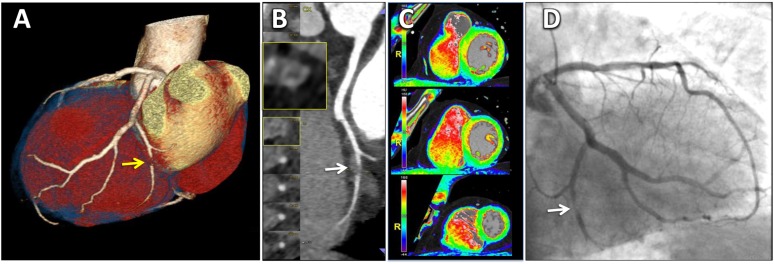
Most ACS are precipitated by luminal thrombi, which arise from three different plaque morphologies: rupture, erosion and calcified nodules.87 Of these, the most common substrate underlying ACS is the rupture of the vulnerable plaque (65–75%) that contained a necrotic core covered by a thin layer of fibrous cap.87 These lesions termed “thin-cap fibroatheroma” (TCFA), with a cap thickness of <65 μm, are considered to be the precursor lesions of plaque rupture.87 Also, plaque rupture and TCFAs are located predominantly in the proximal segment of the coronary tree, where the vessel calibre is larger, and thus the performance of cardiac CT is superior for plaque detection and analysis.87,88 The spatial resolution of current CT scanners (≈300 μm in plane) precludes the morphometric analysis of fibrous cap. However, according to observational and prognostic studies, some features associated with plaque vulnerability may be detected by cardiac CT. Large plaque volume, low CT attenuation (<30 and <60 Hounsfield unit), positive remodelling, eccentricity and spotty calcification are all associated with plaques vulnerable to rupture (Figure 2).88 Recent cardiac CT investigations have described a sign associated with high-risk atherosclerotic plaques, the “napkin-ring sign” (NRS). Plaques with a NRS have a CT-specific attenuation pattern with a central area of low CT attenuation (large necrotic core) and a ring-like plaque component of higher attenuation surrounding the central core (fibrous plaque tissue).88 This sign is highly specific for the presence of both advanced plaque and TFCA in histopathology (specificity: 98.9% and 94.1%, respectively).88 Also, in clinical investigations, the NRS had 96–100% specificity for the identification of TCFA or culprit ACS lesions (Figure 3).88
Figure 2.
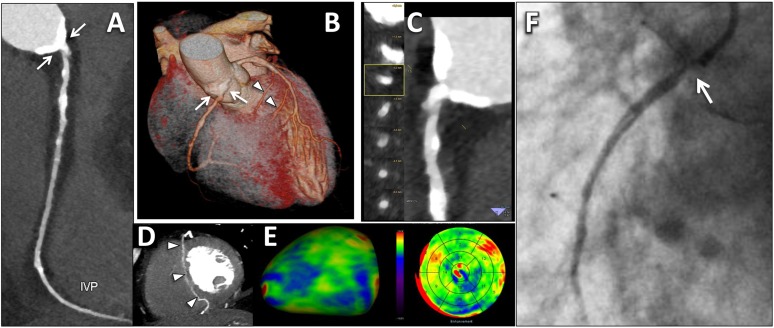
Vulnerable coronary plaque. Example of a 64-year old-male with acute chest pain, non-ischaemic electrocardiographic (ECG) alterations and mild elevation of high-sensitive troponin I (0.21 µg l−1). A prospectively ECG-triggered high-pitch spiral acquisition was performed. (a) Curved multiplanar reconstruction of the left anterior descending artery (LAD) with the corresponding orthogonal views (a1 and a2) delineates a plaque in the proximal segment causing a tight, critical stenosis (>90%), with features of vulnerability, including non-calcified plaque with a low-attenuation core and a ring-like plaque component with higher attenuation (napkin-ring sign), positive remodelling and spotty calcifications. A non-culprit non-calcified plaque with focal severe stenosis at the distal LAD was demonstrated too (arrow in a). (b) Quantitative analysis of the culprit plaque of the proximal LAD using a dedicated automated software. Plaque cross-section from Panel B (b1) and the corresponding image with colour overlay derived with adaptive threshold setting (b2) delineates a predominantly low-density component of the plaque [range 1–60 Hounsfield unit (HU), colour-coded in yellow and green] with small areas showing negative density values (colour-coded in red), consistent with a lipid-rich plaque. (c) Invasive coronary angiography confirmed the tight stenosis of the proximal LAD (arrowhead). Note the non-culprit critical stenosis of the distal apical segment of the LAD (arrow in c), with the corresponding CT image (arrow in panel c1). For colour image see online.
Figure 3.
A 58-year-old male with acute chest pain, normal electrocardiography and first troponin negative. Total calcium score was 79.5 (just above the 50° percentile according to age and sex). (a) Three-dimensional volume-rendering reconstruction showing a tight stenosis (>90%, arrow) of the posterolateral branch (PL) originating from the left circumflex artery (LCX). (b) Curved multiplanar reconstruction of the PL with the corresponding orthogonal views showing plaque with features of instability (arrow): non-calcified plaque with positive remodelling, large plaque volume with a hyperdense ring (napkin-ring sign). (c) A three-dimensional colour-coded reconstruction of myocardial perfusion in short-axis views showed absence of first-pass perfusion defects. (d) Invasive coronary angiography (ICA) confirmed the tight stenosis of the PL from the LCX (arrow). At the time of ICA, a mild rise of troponin was depicted (0.355 µg l−1).
In both stable and acute setting, a lack of coronary calcium (Agatston score=0) does not definitely exclude coronary stenosis and a large amount of calcium does not necessarily correlate with (high-grade) angiographic luminal stenosis or vulnerability of plaques. Accordingly, a recently study of ROMICAT II population, demonstrated that Agatston score=0 does not exclude ACS, although almost 50% of ACS developed in the minority of patients (7%) with high calcium score (>400).89
Another important concept is that in approximately 7–15% of all ACS events, no significant stenosis is detected by standard invasive angiogram corresponding to the event location.55 In this scenario, cardiac CT may play an important diagnostic role by identifying non-stenotic culprit lesion with the above morphological features of vulnerability, in the clinical contest of embolic/spontaneous recanalization myocardial infarction. According to a recent subanalysis of the ROMICAT II trial population, a score derived from high-risk plaque features was emerged to be an independent predictor of ACS during the index hospitalization and was incremental to clinical variable and presence of obstructive (≥50%) stenosis.90
Myocardial perfusion imaging and fractional flow reserve derived from CT
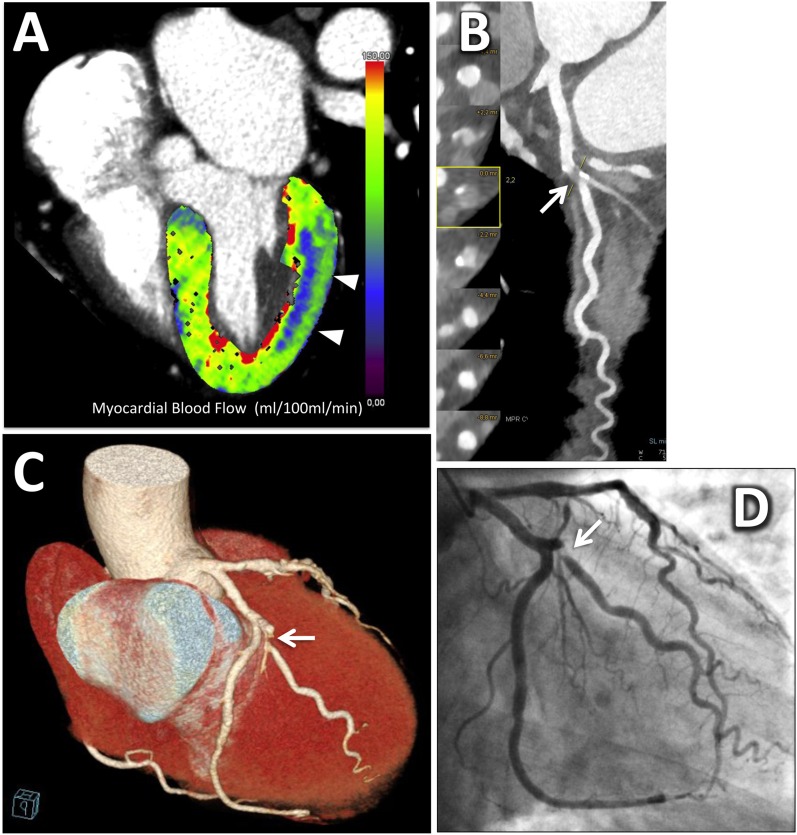
Although cardiac CT is able to detect CAD with a high degree of confidence (high sensitivity), it is, however, a poor predictor of myocardial ischaemia.91 The noteworthy recent technical developments of the latest generation CT scanners (≥64 slices) allow for static or dynamic perfusion imaging to be performed under rest/stress conditions.9 Thus, both functional and anatomical information are combined in the same examination, which is essential in state-of-the-art patient management.63 Static CT perfusion imaging refers to the assessment of myocardial perfusion acquired in a single, stationary phase during the arterial first pass of the iodinated contrast agent; this phase is generally used for the evaluation of both coronary artery morphology and myocardial perfusion at rest. Conversely, dynamic CT myocardial perfusion, first validated in animal studies, relies on the acquisition of multiple, consecutive phases, as the contrast bolus transits the myocardium and enables the calculation of quantitative haemodynamic parameters such as the myocardial blood flow (ml/100 ml min−1) derived from the computation of the time–attenuation curve9 (Figure 4).
Figure 4.
A 52-year-old male with acute chest pain, negative electrocardiography (ECG) and normal troponins level. After serial negative ECG and troponins, at 18 h from the admission, a dynamic stress CT myocardial perfusion scan with the second-generation dual-source CT system using the shuttle mode was performed, followed by the coronary CT angiographic acquisition under resting condition (stress/rest protocol). (a) A slightly oblique four-chamber view of the three-dimensional left ventricular reconstruction of the myocardial blood flow (MBF) derived from the dynamic stress acquisition showing a large subendocardial perfusion defect of the lateral wall, colour-coded in blue (arrowheads). According to a colour scale, the normally perfused myocardium is represented by green and yellow colours. The MBF of the ischaemic myocardium was significantly lower than that of the remote myocardium (54 ± 15 ml/100 ml min−1 vs 123 ± 21 ml/100 ml min−1, respectively). (b) Curved multiplanar reconstruction and the corresponding cross-section views demonstrated a critical stenosis (>90%) at the origin of the main obtuse marginal (OM) branch (arrow). Note the mixed component of the plaque, with positive remodelling, large non-calcified plaque volume and spotty calcifications. (c) Three-dimensional volume-rendering reconstruction demonstrating the critical stenosis of the OM (arrow) with vessel distribution matching the perfusion defect. (d) Invasive coronary angiography confirmed the critical stenosis (>90%) at the origin of the OM (arrow). For colour image see online.
According to recent prospective multicentre trials (the CORE320 and cross-over study of regadenoson), CT perfusion imaging substantially increased the specificity and overall accuracy to identify flow-limiting stenosis, as determined by invasive coronary angiography and SPECT.92–94 CT perfusion may improve the overall diagnostic accuracy of cardiac CT, particularly in the interpretation of intermediate (30–70%) stenosis,95 in the evaluation of stent patency,96 and of diffuse atherosclerotic involvement with densely calcified plaques97 (Figure 5).
Figure 5.
A 64-year-old female presenting at the emergency department with acute epigastric pain and dyspnoea. The electro-cardiographic (ECG) was normal and first troponins were negative. Calcium score was 973 (above the 90° percentile according to age and sex). A prospectively ECG-triggered high-pitch spiral acquisition was performed. (a) Curved multiplanar reconstruction of the right coronary artery (RCA) showing a large, circumferential aorto-ostial calcified plaque (arrows). (b) Three-dimensional volume-rendering reconstruction delineates a right-dominant coronary system. Note the large calcified aorto-ostial plaque of the RCA (arrows) and hypertrophic septal branches from the left anterior descending artery (LAD) (arrowheads). (c) Curved multiplanar reconstruction of the RCA with the corresponding cross-sections at the level of the aorto-ostial plaque. Note the heavy circumferential calcification, which masks the coronary lumen and makes it difficult to measure the degree of stenosis. (d) Maximum-intensity-projection reconstruction in the short-axis view showing collateral connections of the relevant septal branches of the LAD and IVP through the interventricular septum (arrowheads). (e) Inferior view of the three-dimensional left ventricular reconstruction of myocardial perfusion showing resting first-pass hypoperfusion of the inferior wall colour-coded in blue (left). The polar map using a 17-segment myocardial model also confirmed the large perfusion defects of the inferior wall (right). According to the resting myocardial perfusion, a critical aorto-ostial stenosis of the RCA was reported. A second high-sensitive troponin I measurement was mildly elevated (0.76 µg l−1). (f) Invasive coronary angiography confirmed the tight, subocclusive aorto-ostial stenosis (>90%) of the RCA (arrow), with slow distal run-off. IVP, interventricular posterior branch. For colour image see online.
A recent meta-analysis of Takx et al98 showed that CT perfusion has a similar performance to MRI and positron emission tomography and performed better than SPECT and echo in detecting haemodynamically significant CAD as compared with FFR. Another meta-analysis including 22 studies (n = 1507) indicated that CT perfusion imaging (both static and dynamic) compared with reference standards (including invasive coronary angiography, SPECT and MRI) provides good sensitivity and specificity, ranging from 75 to 89% and 78–95%, respectively.99
CT MPI is one of the most emerging applications of cardiac CT, with growing research evidence supporting its potential role. Strengths of CT perfusion are the higher, submillimetre spatial resolution with respect to SPECT, which permits the detection of small, subendocardial perfusion defects, in addition to the accuracy for detecting signs of old scar (myocardial thinning, calcifications or lipomatous metaplasia).100 A first evidence of CT perfusion applied in the evaluation of patients with ACP (TIMI risk score <4) presenting at the ED has demonstrated that rest CT perfusion was highly accurate in the detection of perfusion defects, with a sensitivity of 92% and specificity of 95% as compared with SPECT.101 Adding CT perfusion information to the cardiac CT data resulted in better PPV with reduced rates of false positives (Figure 5).101 Accordingly, in a subanalysis of the ROMICAT I trial, the sensitivity for ACS increased from 77% for obstructive CAD to 90% with addition of rest CT perfusion.102 Finally, a first recent prognostic study in a large cohort of patients (nearly 400 patients) showed that the extent and severity of rest myocardial hypoperfusion in addition to signs of necrotic myocardium as detected by CT prior to invasive coronary evaluation and revascularization is significantly and independently related to long-term (median 50 months) outcome in patients with NSTEMI.100 Interestingly, 63% of patients without rest perfusion defects finally required revascularization, indicating that CT perfusion at rest could not reliably exclude obstructive CAD. Indeed, it is well known that almost all cases with significantly reduced blood flow at rest show stenosis above 90%.17
Important prerequisites of CT perfusion are careful optimization of the CT imaging scans (“rest-only”, “stress-first” or “rest-first” acquisitions; kilovoltage; contrast media injection; reconstruction parameters; and manage radiation dose) and standardization of image interpretation that are still under investigation.9 Thus, this novel technique still has to be validated in larger settings as well as in real-world clinical practice.
FFR measured during invasive coronary angiography is the gold standard for lesion-specific coronary revascularization decisions in patients with stable CAD.63 Recently, a method using computational fluid dynamics to calculate non-invasive FFR derived from cardiac CT image data sets at rest (FFR-CT) has been developed.103 Even though FFR-CT technology is a relatively recent development, three prospective multicentre trials the DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained via Noninvasive Fractional FLOW Reserve), DEFACTO (Determination of Fractional Flow Reserve by Anatomic Computed Tomographic Anglography) and NXT (Analysis of Coronary Blood Flow using CT Angiography: Next Steps) trials have demonstrated encouraging results.103 Using invasive FFR as gold standard (cut-off for FFR ≤0.80), FFR-CT led to an improvement of cardiac CT accuracy, with a marked increase in specificity.103 Currently, the main shortcoming of the method is the necessity of the off-site analysis by computer-modelling software housed at HeartFlow, Inc.'s headquarters in Redwood City, California.103 The development and diffusion of new software algorithms that allow a fast in-house analysis could prove useful in the future implementation of FFR-CT in clinical practice.
Costs and resource utilization of cardiac CT in the emergency department
Four RCTs have shown that acute imaging with cardiac CT reduces ED costs (from 15 to 38%);75,76,78,79 however, it is still less clear whether the use of cardiac CT in the ED reduces total in-hospital costs. As demonstrated by the meta-analysis of Hulten et al,80 cardiac CT may slightly increase the rates of downstream invasive coronary angiography and revascularization (of about 2%), which in turn may affect cost. Accordingly, in the ROMICAT II study, the reduction in ED cost due to faster time to diagnosis was offset by the downstream index hospital costs of higher invasive procedures.78 Conversely, in the CT-COMPARE trial, despite cardiac CT having higher odds of downstream testing (odds ratio 2.0, 95% confidence interval: 1.1, 3.8; p = 0.02), 30-day per-patient costs, inclusive of invasive coronary angiography and interventions, were significantly lower for the CT arm compared with the exercise ECG approach ($AUD 2193 vs $AUD 2704; p < 0.001).79
However, whether this CT-driven approach translates into a better outcome as compared with the standard care is still to be determined by future prognostic studies. In addition, longer term follow-up may be needed to identify other differences in downstream test utilization between cardiac CT and usual care.
Recently, a cost analysis derived from the ROMICAT I trial has shown that cardiac CT may be a cost-saving imaging tool in subjects with ACP who have a low (<30%) prevalence of potentially obstructive CAD.104
In a comprehensive cost-effectiveness model analysis, a two-step diagnostic strategy of cardiac CT followed by SPECT for intermediate/indeterminate stenosis seems to be cost saving and more effective than the first stress imaging approach such as ECG, SPECT and echo for the assessment of population with ACP at low risk of CAD (prevalence of 2–30%).105
In conclusion, cardiac CT would be more cost effective in appropriately selected patients at low risk.
Moreover, the initial CT strategy may result in a higher recognition of mild-to-intermediate CAD, especially when applied in a broader patient risk profile, with a significant role in individual risk stratification. Secondary prevention with lifestyle modification and medication initiation or intensification with aspirin and statins might be started, with the ultimate goal to improve outcomes at long-term follow-up. The potential for cardiac CT to tailor medical therapy even beyond current preventive therapy guidelines has recently been addressed by an observation study among the population in ROMICAT I.106
Thus, it is possible that in the future, the final healthcare costs driven by subsequent hospitalizations, procedures and interventions might be consistently reduced by this CT-based prevention strategy.
Prerequisites and logistic challenges of cardiac CT in the emergency department
Actually, 64-slice CT technology is the minimum standard required for applying cardiac CT in the setting of patients with ACP at the ED.1 Although cardiac CT may be performed in most of the cases within the first 2–3 h from arrival to the ED, this approach has several logistic limitations. First, it is challenging to provide a 24/7 service, owing to lack of availability of appropriately trained personnel (technologists and physicians). Typically, cardiac CT is available only during normal business hours, between 7:00 am and 7:00 pm72 or between 8:00 am and 22.00 pm including weekends in the best scenario.79 Furthermore, adequate patient preparation in a quiet and comfortable room, with beta blocker and nitrate administration, is essential to enhance diagnostic accuracy. This may be challenging in a busy environment such as that of the ED with its particular special and challenging conditions, especially if only one CT scanner is available to triage patients.
Moreover, cardiac CT has known limitations such as high or irregular heart rate, severe calcifications (high calcium score) and elevated body mass index, some of which may be substantially overcome by latest CT scanner improvement. Indeed, cardiac CT may be not suitable for all comers, and adequate patient selection is an important prerequisite. However, patient selection can be challenging in the large proportion of patients at low risk presenting with ACP; in those cases, an indiscriminate use of cardiac CT may lead to X-ray overexposure, becoming also time consuming with potentially increasing ED costs.
Furthermore, in the era of high-sensitive troponins, an accelerated 2-h diagnostic protocol based on short-interval troponin tests and ACS risk score could identify those patients at lower risk suitable for early discharge, without the need of imaging.107
Finally, although the results of the first randomized trials are encouraging, it remains open whether these data can be reproduced in the diagnostic work-up of ACP in Europe, where care pathways may be different.
Extracoronary causes of acute chest pain
CT is the most comprehensive technique for the non-invasive evaluation of cardiothoracic structures in patients presenting with ACP at the emergency room. Advantages are the wide availability, high speed of acquisition and high spatial resolution (≤0.3 mm), which allows evaluating even small vascular structures.108 Several observational studies have evaluated the utility of a “double rule-out” or “triple rule-out” protocol for assessing aortic, pulmonary or cardiac sources of chest pain in the same examination (Figure 6).108 However, there is no clear benefit of these more complex approaches with respect to dedicated standard protocols. Several studies have demonstrated a comparable image quality, but at the cost of significantly higher radiation and contrast doses associated with these approaches.108,109 Furthermore, a systematic low prevalence of acute extracoronary (pulmonary or aortic) conditions (<1%) has emerged from these studies.108,109 In conclusion, although it may be of value in selected patients, the routine use of a “triple rule-out” protocol is not currently recommended.108,109
Figure 6.
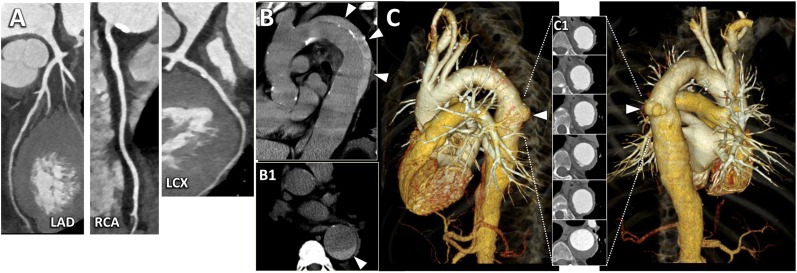
A 60-year-old male with acute onset of chest pain radiating to the back and dyspnoea. Non-specific repolarization alterations were documented at electrocardiography (ECG). Initial troponin level was normal. A triple-rule-out examination using a thoracoabdominal prospectively ECG-triggered high-pitch spiral acquisition with a dedicated contrast media injection protocol was performed. (a) CT angiography acquisition demonstrated a normal left anterior descending artery (LAD) and only mild atherosclerosis (stenosis <30%) at the proximal segment of the left circumflex artery (LCX) and right coronary artery (RCA). (b) In the left parasagittal plane, pre-contrast acquisition revealed a Type-B intramural haematoma (arrowheads) involving the descending thoracic aorta. The corresponding cross-section view delineated the semi-circumferential extension of the intramural haematoma (arrowhead in Panel b1). (c) Three-dimensional volume-rendering reconstructions of the thoracic vascular structures in the left lateral (left) and posterior (right) views, revealed an ectatic descending thoracic aorta with an ulcer-like projection at the middle level (arrowheads). Note the corresponding cross-section images showing the ulcer-like projection and the intramural haematoma (Panel c1) and the normally perfused pulmonary system.
Cardiac CT during cardiopulmonary resuscitation
In patients requiring cardiopulmonary resuscitation (CPR), the cause of precipitating pathology is frequently unclear. However, in most cases (60–80% cases), the underlying aetiology is a cardiovascular condition such as ACS, arrhythmia, PE, stroke, aneurysmal haemorrhage or pericardial constriction.110 Few case reports and initial case series have demonstrated the clinical feasibility of CT during CPR using manual chest compression or automated chest-compression devices.111,112 The CPR is typically temporarily interrupted during the short imaging acquisition time; therefore, artefacts related to breathing or cardiac contraction are avoided.110 The delay of acquisition after contrast media injection needs to be adapted to the prolonged circulation time, typically by doubling the standard delays.110 Under these special conditions, coronary arteries may be evaluated and an acute coronary occlusion causing an out-of-hospital cardiac arrest may be detected.111 Similarly, acute PE may be identified and systemic thrombolysis or mechanical thromboaspiration rapidly performed.112 In this scenario, when other methods such as diagnostic findings or medical history fail to identify the cause of the life-threatening underlying conditions, CT with its high diagnostic sensitivity may be a fast valuable tool for either targeted treatment or the decision to terminate efforts.110
CONCLUSION
The introduction of coronary CT in the evaluation of patients with ACP with suspected ACS has opened new paradigms in comprehension and management of this population presenting at the ED. The incorporation of cardiac CT in the initial diagnostic approach for patients with ACP at the ED is safe, accurate and reduces avoidable hospitalizations in patients at low-to-intermediate risk for ACS. Furthermore, this strategy seems to be cost effective in the low-risk category, although limited data and relatively incomplete cost analyses have been conducted. Moreover, cardiac CT provides excellent outcomes in patients directly discharged from the ED.
Novel emerging applications in cardiac imaging have been implemented, improving the overall diagnostic accuracy of the technique. Coronary artery plaque imaging by CT may aid in the detection of the culprit lesion, provide direct measurement of coronary atherosclerotic burden and allow greater individualization of preventive therapies.
Finally, CT myocardial perfusion may improve the detection of obstructive stenosis requiring revascularization by individuation of lesion-specific ischaemia, evaluate the physiological significance of non-obstructive (intermediate) stenosis and assist in further risk stratification beyond that obtained by pure anatomic imaging.
Contributor Information
Erica Maffei, Email: ericamaffei@gmail.com.
Sara Seitun, Email: saraseitun@yahoo.com.
Andrea I Guaricci, Email: andrea.guaricci@gmail.com.
Filippo Cademartiri, Email: filippocademartiri@gmail.com.
REFERENCES
- 1.Raff GL, Chinnaiyan KM, Cury RC, Garcia MT, Hecht HS, Hollander JE, et al. ; Society of Cardiovascular Computed Tomography Guidelines Committee. SCCT guidelines on the use of coronary computed tomographic angiography for patients presenting with acute chest pain to the emergency department: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2014; 8: 254–71. doi: 10.1016/j.jcct.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 2.National hospital ambulatory medical care survey: 2010 emergency department summary tables. In: 2010. [Google Scholar]
- 3.Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 267–315. doi: 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- 4.Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 2000; 342: 1163–70. doi: 10.1056/NEJM200004203421603 [DOI] [PubMed] [Google Scholar]
- 5.Pernès JM, Dupouy P, Labbé R, Sotirov Y, Pongas D, Mansour H, et al. Management of acute chest pain: a major role for coronary CT angiography. Diagn Interv Imaging 2015; 96: 1105–12. doi: 10.1016/j.diii.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 6.Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999-2008. NCHS Data Brief 2010; (43): 1–8. [PubMed] [Google Scholar]
- 7.Yang L, Zhou T, Zhang R, Xu L, Peng Z, Ding J, et al. Meta-analysis: diagnostic accuracy of coronary CT angiography with prospective ECG gating based on step-and-shoot, Flash and volume modes for detection of coronary artery disease. Eur Radiol 2014; 24: 2345–52. doi: 10.1007/s00330-014-3221-y [DOI] [PubMed] [Google Scholar]
- 8.Cademartiri F, Maffei E. CT coronary angiography in low-risk, acute chest pain. Nat Rev Cardiol 2012; 9: 615–6. doi: 10.1038/nrcardio.2012.130 [DOI] [PubMed] [Google Scholar]
- 9.Rossi A, Merkus D, Klotz E, Mollet N, de Feyter PJ, Krestin GP. Stress myocardial perfusion: imaging with multidetector CT. Radiology 2014; 270: 25–46. doi: 10.1148/radiol.13112739 [DOI] [PubMed] [Google Scholar]
- 10.Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. ; ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014; 35: 2873–926. doi: 10.1093/eurheartj/ehu281 [DOI] [PubMed] [Google Scholar]
- 11.Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al. ; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35: 3033–69, 3069a-3069k. doi: 10.1093/eurheartj/ehu283 [DOI] [PubMed] [Google Scholar]
- 12.Ohman EM, Armstrong PW, Christenson RH, Granger CB, Katus HA, Hamm CW, et al. Cardiac troponin T levels for risk stratification in acute myocardial ischemia. GUSTO IIA Investigators. N Engl J Med 1996; 335: 1333–41. doi: 10.1056/NEJM199610313351801 [DOI] [PubMed] [Google Scholar]
- 13.Stracke S, Dörr O, Heidt MC, Gündüz D, Neuhof C, Parahuleva M, et al. GRACE risk score as predictor of in-hospital mortality in patients with chest pain. Clin Res Cardiol 2010; 99: 627–31. doi: 10.1007/s00392-010-0160-8 [DOI] [PubMed] [Google Scholar]
- 14.Pollack CV, Jr, Sites FD, Shofer FS, Sease KL, Hollander JE. Application of the TIMI risk score for unstable angina and non-ST elevation acute coronary syndrome to an unselected emergency department chest pain population. Acad Emerg Med 2006; 13: 13–8. doi: 10.1111/j.1553-2712.2006.tb00978.x [DOI] [PubMed] [Google Scholar]
- 15.Amsterdam EA, Kirk JD, Bluemke DA, Diercks D, Farkouh ME, Garvey JL, et al. ; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation 2010; 122: 1756–76. doi: 10.1161/CIR.0b013e3181ec61df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bawamia B, Mehran R, Qiu W, Kunadian V. Risk scores in acute coronary syndrome and percutaneous coronary intervention: a review. Am Heart J 2013; 165: 441–50. doi: 10.1016/j.ahj.2012.12.020 [DOI] [PubMed] [Google Scholar]
- 17.Salerno M, Beller GA. Noninvasive assessment of myocardial perfusion. Circ Cardiovasc Imaging 2009; 2: 412–24. doi: 10.1161/CIRCIMAGING.109.854893 [DOI] [PubMed] [Google Scholar]
- 18.Al Jaroudi W, Iskandrian AE. Regadenoson: a new myocardial stress agent. J Am Coll Cardiol 2009; 54: 1123–30. doi: 10.1016/j.jacc.2009.04.089 [DOI] [PubMed] [Google Scholar]
- 19.Dedic A, Genders TS, Nieman K, Hunink MG. Imaging strategies for acute chest pain in the emergency department. AJR Am J Roentgenol 2013; 200: W26–38. doi: 10.2214/AJR.11.8296 [DOI] [PubMed] [Google Scholar]
- 20.Lancellotti P, Price S, Edvardsen T, Cosyns B, Neskovic AN, Dulgheru R, et al. The use of echocardiography in acute cardiovascular care: recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care 2015; 4: 3–5. doi: 10.1177/2048872614568073 [DOI] [PubMed] [Google Scholar]
- 21.Eek C, Grenne B, Brunvand H, Aakhus S, Endresen K, Smiseth OA, et al. Strain echocardiography predicts acute coronary occlusion in patients with non-ST-segment elevation acute coronary syndrome. Eur J Echocardiogr 2010; 11: 501–8. doi: 10.1093/ejechocard/jeq008 [DOI] [PubMed] [Google Scholar]
- 22.Lieberman AN, Weiss JL, Jugdutt BI, Becker LC, Bulkley BH, Garrison JG, et al. Two-dimensional echocardiography and infarct size: relationship of regional wall motion and thickening to the extent of myocardial infarction in the dog. Circulation 1981; 63: 739–46. doi: 10.1161/01.CIR.63.4.739 [DOI] [PubMed] [Google Scholar]
- 23.Kaul S. Echocardiography in coronary artery disease. Curr Probl Cardiol 1990; 15: 233–98. doi: 10.1016/0146-2806(90)90012-F [DOI] [PubMed] [Google Scholar]
- 24.Lewis WR. Echocardiography in the evaluation of patients in chest pain units. Cardiol Clin 2005; 23: 531–9. doi: 10.1016/j.ccl.2005.08.009 [DOI] [PubMed] [Google Scholar]
- 25.Conti A, Sammicheli L, Gallini C, Costanzo EN, Antoniucci D, Barletta G. Assessment of patients with low-risk chest pain in the emergency department: head-to-head comparison of exercise stress echocardiography and exercise myocardial SPECT. Am Heart J 2005; 149: 894–901. doi: 10.1016/j.ahj.2004.09.048 [DOI] [PubMed] [Google Scholar]
- 26.Nucifora G, Badano LP, Sarraf-Zadegan N, Karavidas A, Trocino G, Scaffidi G, et al. Comparison of early dobutamine stress echocardiography and exercise electrocardiographic testing for management of patients presenting to the emergency department with chest pain. Am J Cardiol 2007; 100: 1068–73. doi: 10.1016/j.amjcard.2007.05.027 [DOI] [PubMed] [Google Scholar]
- 27.Gaibazzi N, Squeri A, Reverberi C, Molinaro S, Lorenzoni V, Sartorio D, et al. Contrast stress-echocardiography predicts cardiac events in patients with suspected acute coronary syndrome but nondiagnostic electrocardiogram and normal 12-hour troponin. J Am Soc Echocardiogr 2011; 24: 1333–41. doi: 10.1016/j.echo.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 28.Bholasingh R, Cornel JH, Kamp O, van Straalen JP, Sanders GT, Tijssen JG, et al. Prognostic value of predischarge dobutamine stress echocardiography in chest pain patients with a negative cardiac troponin T. J Am Coll Cardiol 2003; 41: 596–602. doi: 10.1016/S0735-1097(02)02897-8 [DOI] [PubMed] [Google Scholar]
- 29.Bedetti G, Pasanisi EM, Tintori G, Fonseca L, Tresoldi S, Minneci C, et al. Stress echo in chest pain unit: the SPEED trial. Int J Cardiol 2005; 102: 461–7. doi: 10.1016/j.ijcard.2004.05.058 [DOI] [PubMed] [Google Scholar]
- 30.Wackers FJ, Lie KI, Liem KL, Sokole EB, Samson G, van der Schoot J, et al. Potential value of thallium-201 scintigraphy as a means of selecting patients for the coronary care unit. Br Heart J 1979; 41: 111–7. doi: 10.1136/hrt.41.1.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tatum JL, Jesse RL, Kontos MC, Nicholson CS, Schmidt KL, Roberts CS, et al. Comprehensive strategy for the evaluation and triage of the chest pain patient. Ann Emerg Med 1997; 29: 116–25. doi: 10.1016/S0196-0644(97)70317-2 [DOI] [PubMed] [Google Scholar]
- 32.Verberne HJ, Acampa W, Anagnostopoulos C, Ballinger J, Bengel F, De Bondt P, et al. 2015 updated EANM procedural guidelines for radionuclide myocardial perfusion imaging with SPECT and SPECT/CT. Updated 25 October 2015. Available from: http://eanm.org/publications/guidelines/2015_07_EANM_FINAL_myocardial_perfusion_guideline.pdf [DOI] [PMC free article] [PubMed]
- 33.Udelson JE, Beshansky JR, Ballin DS, Feldman JA, Griffith JL, Handler J, et al. Myocardial perfusion imaging for evaluation and triage of patients with suspected acute cardiac ischemia: a randomized controlled trial. JAMA 2002; 288: 2693–700. doi: 10.1001/jama.288.21.2693 [DOI] [PubMed] [Google Scholar]
- 34.Lim SH, Anantharaman V, Sundram F, Chan ES, Ang ES, Yo SL, et al. Stress myocardial perfusion imaging for the evaluation and triage of chest pain in the emergency department: a randomized controlled trial. J Nucl Cardiol 2013; 20: 1002–12. doi: 10.1007/s12350-013-9736-9 [DOI] [PubMed] [Google Scholar]
- 35.Nabi F, Chang SM, Xu J, Gigliotti E, Mahmarian JJ. Assessing risk in acute chest pain: the value of stress myocardial perfusion imaging in patients admitted through the emergency department. J Nucl Cardiol 2012; 19: 233–43. doi: 10.1007/s12350-011-9484-7 [DOI] [PubMed] [Google Scholar]
- 36.Metz LD, Beattie M, Hom R, Redberg RF, Grady D, Fleischmann KE. The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: a meta-analysis. J Am Coll Cardiol 2007; 49: 227–37. doi: 10.1016/j.jacc.2006.08.048 [DOI] [PubMed] [Google Scholar]
- 37.Fesmire FM, Hughes AD, Stout PK, Wojcik JF, Wharton DR. Selective dual nuclear scanning in low-risk patients with chest pain to reliably identify and exclude acute coronary syndromes. Ann Emerg Med 2001; 38: 207–15. doi: 10.1067/mem.2001.116594 [DOI] [PubMed] [Google Scholar]
- 38.Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 2003; 361: 374–9. doi: 10.1016/S0140-6736(03)12389-6 [DOI] [PubMed] [Google Scholar]
- 39.Berger CJ, Murabito JM, Evans JC, Anderson KM, Levy D. Prognosis after first myocardial infarction: comparison of Q-wave and non-Q-wave myocardial infarction in the Framingham Heart Study. JAMA 1992; 268: 1545–51. doi: 10.1001/jama.1992.03490120059029 [DOI] [PubMed] [Google Scholar]
- 40.Behar S, Haim M, Hod H, Kornowski R, Reicher-Reiss H, Zion M, et al. Long-term prognosis of patients after a Q wave compared with a non-Q wave first acute myocardial infarction: data from the SPRINT registry. Eur Heart J 1996; 17: 1532–7. doi: 10.1093/oxfordjournals.eurheartj.a014717 [DOI] [PubMed] [Google Scholar]
- 41.Kontos MC, Jesse RL, Schmidt KL, Ornato JP, Tatum JL. Value of acute rest sestamibi perfusion imaging for evaluation of patients admitted to the emergency department with chest pain. J Am Coll Cardiol 1997; 30: 976–82. [DOI] [PubMed] [Google Scholar]
- 42.Conti A, Vanni S, Sammicheli L, Raveggi S, Camaiti A, Pieralli F, et al. Yield of nuclear scan strategy in chest pain unit evaluation of special populations. Nucl Med Commun 2008; 29: 1106–12. doi: 10.1097/MNM.0b013e328310b361 [DOI] [PubMed] [Google Scholar]
- 43.Schwitter J, Arai AE. Assessment of cardiac ischaemia and viability: role of cardiovascular magnetic resonance. Eur Heart J 2011; 32: 799–809. doi: 10.1093/eurheartj/ehq481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruder O, Wagner A, Lombardi M, Schwitter J, van Rossum A, Pilz G, et al. European Cardiovascular Magnetic Resonance (EuroCMR) registry–multi national results from 57 centers in 15 countries. J Cardiovasc Magn Reson 2013; 15: 9. doi: 10.1186/1532-429X-15-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwitter J, Wacker CM, van Rossum AC, Lombardi M, Al-Saadi N, Ahlstrom H, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J 2008; 29: 480–9. doi: 10.1093/eurheartj/ehm617 [DOI] [PubMed] [Google Scholar]
- 46.Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 2012; 379: 453–60. doi: 10.1016/S0140-6736(11)61335-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E, et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol 2012; 59: 1719–28. doi: 10.1016/j.jacc.2011.12.040 [DOI] [PubMed] [Google Scholar]
- 48.Raman SV, Simonetti OP, Winner MW, 3rd, Dickerson JA, He X, Mazzaferri EL, Jr, et al. Cardiac magnetic resonance with edema imaging identifies myocardium at risk and predicts worse outcome in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol 2010; 55: 2480–8. doi: 10.1016/j.jacc.2010.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwong RY, Schussheim AE, Rekhraj S, Aletras AH, Geller N, Davis J, et al. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation 2003; 107: 531–7. doi: 10.1161/01.CIR.0000047527.11221.29 [DOI] [PubMed] [Google Scholar]
- 50.Cury RC, Shash K, Nagurney JT, Rosito G, Shapiro MD, Nomura CH, et al. Cardiac magnetic resonance with T2-weighted imaging improves detection of patients with acute coronary syndrome in the emergency department. Circulation 2008; 118: 837–44. doi: 10.1161/CIRCULATIONAHA.107.740597 [DOI] [PubMed] [Google Scholar]
- 51.Plein S, Greenwood JP, Ridgway JP, Cranny G, Ball SG, Sivananthan MU. Assessment of non-ST-segment elevation acute coronary syndromes with cardiac magnetic resonance imaging. J Am Coll Cardiol 2004; 44: 2173–81. doi: 10.1016/j.jacc.2004.08.056 [DOI] [PubMed] [Google Scholar]
- 52.Ingkanisorn WP, Kwong RY, Bohme NS, Geller NL, Rhoads KL, Dyke CK, et al. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol 2006; 47: 1427–32. doi: 10.1016/j.jacc.2005.11.059 [DOI] [PubMed] [Google Scholar]
- 53.Miller CD, Hwang W, Hoekstra JW, Case D, Lefebvre C, Blumstein H, et al. Stress cardiac magnetic resonance imaging with observation unit care reduces cost for patients with emergent chest pain: a randomized trial. Ann Emerg Med 2010; 56: 209–19 e2. doi: 10.1016/j.annemergmed.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller CD, Hwang W, Case D, Hoekstra JW, Lefebvre C, Blumstein H, et al. Stress CMR imaging observation unit in the emergency department reduces 1-year medical care costs in patients with acute chest pain: a randomized study for comparison with inpatient care. JACC Cardiovasc Imaging 2011; 4: 862–70. doi: 10.1016/j.jcmg.2011.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dastidar AG, Rodrigues JC, Ahmed N, Baritussio A, Bucciarelli-Ducci C. The role of cardiac MRI in patients with troponin-positive chest pain and unobstructed coronary arteries. Curr Cardiovasc Imaging Rep 2015; 8: 28. doi: 10.1007/s12410-015-9345-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maffei E, Martini C, De Crescenzo S, Arcadi T, Clemente A, Capuano E, et al. Low dose CT of the heart: a quantum leap into a new era of cardiovascular imaging [. In Italian.] Radiol Med 2010; 115: 1179–207. doi: 10.1007/s11547-010-0566-4 [DOI] [PubMed] [Google Scholar]
- 57.Morsbach F, Gordic S, Desbiolles L, Husarik D, Frauenfelder T, Schmidt B, et al. Performance of turbo high-pitch dual-source CT for coronary CT angiography: first ex vivo and patient experience. Eur Radiol 2014; 24: 1889–95. doi: 10.1007/s00330-014-3209-7 [DOI] [PubMed] [Google Scholar]
- 58.Deseive S, Chen MY, Korosoglou G, Leipsic J, Martuscelli E, Carrascosa P, et al. Prospective randomized trial on radiation dose estimates of CT angiography applying iterative image reconstruction: The PROTECTION V Study. JACC Cardiovasc Imaging 2015; 8: 888–96. doi: 10.1016/j.jcmg.2015.02.024 [DOI] [PubMed] [Google Scholar]
- 59.Andreini D, Pontone G, Mushtaq S, Bertella E, Conte E, Segurini C, et al. Low-dose CT coronary angiography with a novel IntraCycle motion-correction algorithm in patients with high heart rate or heart rate variability. Eur Heart J Cardiovasc Imaging 2015; 16: 1093–100. doi: 10.1093/ehjci/jev033 [DOI] [PubMed] [Google Scholar]
- 60.Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008; 52: 2135–44. doi: 10.1016/j.jacc.2008.08.058 [DOI] [PubMed] [Google Scholar]
- 61.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008; 52: 1724–32. doi: 10.1016/j.jacc.2008.07.031 [DOI] [PubMed] [Google Scholar]
- 62.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008; 359: 2324–36. doi: 10.1056/NEJMoa0806576 [DOI] [PubMed] [Google Scholar]
- 63.Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013; 34: 2949–3003. doi: 10.1093/eurheartj/eht296 [DOI] [PubMed] [Google Scholar]
- 64.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, et al. ; CONFIRM Investigators. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 2011; 58: 849–60. doi: 10.1016/j.jacc.2011.02.074 [DOI] [PubMed] [Google Scholar]
- 65.Pontone G, Andreini D, Bartorelli AL, Bertella E, Cortinovis S, Mushtaq S, et al. A long-term prognostic value of CT angiography and exercise ECG in patients with suspected CAD. JACC Cardiovasc Imaging 2013; 6: 641–50. doi: 10.1016/j.jcmg.2013.01.015 [DOI] [PubMed] [Google Scholar]
- 66.Jiang B, Wang J, Lv X, Cai W. Prognostic value of cardiac computed tomography angiography in patients with suspected coronary artery disease: a meta-analysis. Cardiology 2014; 128: 304–12. doi: 10.1159/000360131 [DOI] [PubMed] [Google Scholar]
- 67.Gallagher MJ, Ross MA, Raff GL, Goldstein JA, O’Neill WW, O’Neil B. The diagnostic accuracy of 64-slice computed tomography coronary angiography compared with stress nuclear imaging in emergency department low-risk chest pain patients. Ann Emerg Med 2007; 49: 125–36. doi: 10.1016/j.annemergmed.2006.06.043 [DOI] [PubMed] [Google Scholar]
- 68.Beigel R, Oieru D, Goitein O, Chouraqui P, Konen E, Shamiss A, et al. Usefulness of routine use of multidetector coronary computed tomography in the “fast track” evaluation of patients with acute chest pain. Am J Cardiol 2009; 103: 1481–6. doi: 10.1016/j.amjcard.2009.02.009 [DOI] [PubMed] [Google Scholar]
- 69.Rubinshtein R, Halon DA, Gaspar T, Jaffe R, Karkabi B, Flugelman MY, et al. Usefulness of 64-slice cardiac computed tomographic angiography for diagnosing acute coronary syndromes and predicting clinical outcome in emergency department patients with chest pain of uncertain origin. Circulation 2007; 115: 1762–8. doi: 10.1161/CIRCULATIONAHA.106.618389 [DOI] [PubMed] [Google Scholar]
- 70.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol 2009; 53: 1642–50. doi: 10.1016/j.jacc.2009.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson TR, Nikolaou K, Becker A, Leber AW, Rist C, Wintersperger BJ, et al. Dual-source CT for chest pain assessment. Eur Radiol 2008; 18: 773–80. doi: 10.1007/s00330-007-0803-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dedic A, Ten Kate GJ, Neefjes LA, Rossi A, Dharampal A, Rood PP, et al. Coronary CT angiography outperforms calcium imaging in the triage of acute coronary syndrome. Int J Cardiol 2013; 167: 1597–602. doi: 10.1016/j.ijcard.2012.04.099 [DOI] [PubMed] [Google Scholar]
- 73.Hollander JE, Chang AM, Shofer FS, McCusker CM, Baxt WG, Litt HI. Coronary computed tomographic angiography for rapid discharge of low-risk patients with potential acute coronary syndromes. Ann Emerg Med 2009; 53: 295–304. doi: 10.1016/j.annemergmed.2008.09.025 [DOI] [PubMed] [Google Scholar]
- 74.Romero J, Husain SA, Holmes AA, Kelesidis I, Chavez P, Mojadidi MK, et al. Non-invasive assessment of low risk acute chest pain in the emergency department: a comparative meta-analysis of prospective studies. Int J Cardiol 2015; 187: 565–80. doi: 10.1016/j.ijcard.2015.01.032 [DOI] [PubMed] [Google Scholar]
- 75.Goldstein JA, Gallagher MJ, O'Neill WW, Ross MA, O'Neil BJ, Raff GL. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol 2007; 49: 863–71. doi: 10.1016/j.jacc.2006.08.064 [DOI] [PubMed] [Google Scholar]
- 76.Goldstein JA, Chinnaiyan KM, Abidov A, Achenbach S, Berman DS, Hayes SW, et al. ; CT-STAT Investigators. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol 2011; 58: 1414–22. doi: 10.1016/j.jacc.2011.03.068 [DOI] [PubMed] [Google Scholar]
- 77.Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med 2012; 366: 1393–403. doi: 10.1056/NEJMoa1201163 [DOI] [PubMed] [Google Scholar]
- 78.Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, et al. ; ROMICAT-II Investigators. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012; 367: 299–308. doi: 10.1056/NEJMoa1201161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamilton-Craig C, Fifoot A, Hansen M, Pincus M, Chan J, Walters DL, et al. Diagnostic performance and cost of CT angiography versus stress ECG–a randomized prospective study of suspected acute coronary syndrome chest pain in the emergency department (CT-COMPARE). Int J Cardiol 2014; 177: 867–73. doi: 10.1016/j.ijcard.2014.10.090 [DOI] [PubMed] [Google Scholar]
- 80.Hulten E, Pickett C, Bittencourt MS, Villines TC, Petrillo S, Di Carli MF, et al. Outcomes after coronary computed tomography angiography in the emergency department: a systematic review and meta-analysis of randomized, controlled trials. J Am Coll Cardiol 2013; 61: 880–92. doi: 10.1016/j.jacc.2012.11.061 [DOI] [PubMed] [Google Scholar]
- 81.Schlett CL, Banerji D, Siegel E, Bamberg F, Lehman SJ, Ferencik M, et al. Prognostic value of CT angiography for major adverse cardiac events in patients with acute chest pain from the emergency department: 2-year outcomes of the ROMICAT trial. JACC Cardiovasc Imaging 2011; 4: 481–91. doi: 10.1016/j.jcmg.2010.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sozzi FB, Civaia F, Rossi P, Robillon JF, Rusek S, Berthier F, et al. Long-term follow-up of patients with first-time chest pain having 64-slice computed tomography. Am J Cardiol 2011; 107: 516–21. doi: 10.1016/j.amjcard.2010.10.006 [DOI] [PubMed] [Google Scholar]
- 83.Nasis A, Meredith IT, Sud PS, Cameron JD, Troupis JM, Seneviratne SK. Long-term outcome after CT angiography in patients with possible acute coronary syndrome. Radiology 2014; 272: 674–82. doi: 10.1148/radiol.14132680 [DOI] [PubMed] [Google Scholar]
- 84.Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P; et al. American College of Cardiology Foundation Appropriate Use Criteria Task Force; Society of Cardiovascular Computed Tomography; American College of Radiology; American Heart Association; American Society of Echocardiography; American Society of Nuclear Cardiology; NorthAmerican Society for Cardiovascular Imaging; Society for Cardiovascular Angiography and Interventions; Society for Cardiovascular Magnetic Resonance, ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol 2010; 56: 1864–94. doi: 10.1016/j.jacc.2010.07.005 [DOI] [PubMed] [Google Scholar]
- 85.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, et al. ; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons; American Association for Clinical Chemistry. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64: e139–228. doi: 10.1016/j.jacc.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 86.National Clinical Guideline Centre for Acute and Chronic Conditions (UK). Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin [. Internet.] London, UK. NICE Clinical Guideline Centre for Acute and Chronic Conditions; 2010. [Google Scholar]
- 87.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006; 47(Suppl. 8): C13–8. doi: 10.1016/j.jacc.2005.10.065 [DOI] [PubMed] [Google Scholar]
- 88.Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol 2014; 11: 390–402. doi: 10.1038/nrcardio.2014.60 [DOI] [PubMed] [Google Scholar]
- 89.Pursnani A, Chou ET, Zakroysky P, Deaño RC, Mamuya WS, Woodard PK, et al. Use of coronary artery calcium scanning beyond coronary computed tomographic angiography in the emergency department evaluation for acute chest pain: the ROMICAT II trial. Circ Cardiovasc Imaging 2015; 8: e002225. doi: 10.1161/CIRCIMAGING.114.002225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ferencik M, Mayrhofer T, Puchner SB, Lu MT, Maurovich-Horvat P, Liu T, et al. Computed tomography-based high-risk coronary plaque score to predict acute coronary syndrome among patients with acute chest pain—results from the ROMICAT II trial. J Cardiovasc Comput Tomogr 2015; 9: 538–45. doi: 10.1016/j.jcct.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Werkhoven JM, Schuijf JD, Gaemperli O, Jukema JW, Boersma E, Wijns W, et al. Prognostic value of multislice computed tomography and gated single-photon emission computed tomography in patients with suspected coronary artery disease. J Am Coll Cardiol 2009; 53: 623–32. doi: 10.1016/j.jacc.2008.10.043 [DOI] [PubMed] [Google Scholar]
- 92.Rochitte CE, George RT, Chen MY, Arbab-Zadeh A, Dewey M, Miller JM, et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. Eur Heart J 2014; 35: 1120–30. doi: 10.1093/eurheartj/eht488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Magalhães TA, Kishi S, George RT, Arbab-Zadeh A, Vavere AL, Cox C, et al. Combined coronary angiography and myocardial perfusion by computed tomography in the identification of flow-limiting stenosis—The CORE320 study: an integrated analysis of CT coronary angiography and myocardial perfusion. J Cardiovasc Comput Tomogr 2015; 9: 438–45. doi: 10.1016/j.jcct.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cury RC, Kitt TM, Feaheny K, Blankstein R, Ghoshhajra BB, Budoff MJ, et al. A randomized, multicenter, multivendor study of myocardial perfusion imaging with regadenoson CT perfusion vs single photon emission CT. J Cardiovasc Comput Tomogr 2015; 9: 103–12 e1-2. doi: 10.1016/j.jcct.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 95.Rossi A, Dharampal A, Wragg A, Davies LC, van Geuns RJ, Anagnostopoulos C, et al. Diagnostic performance of hyperaemic myocardial blood flow index obtained by dynamic computed tomography: does it predict functionally significant coronary lesions? Eur Heart J Cardiovasc Imaging 2014; 15: 85–94. doi: 10.1093/ehjci/jet133 [DOI] [PubMed] [Google Scholar]
- 96.Rief M, Zimmermann E, Stenzel F, Martus P, Stangl K, Greupner J, et al. Computed tomography angiography and myocardial computed tomography perfusion in patients with coronary stents: prospective intraindividual comparison with conventional coronary angiography. J Am Coll Cardiol 2013; 62: 1476–85. doi: 10.1016/j.jacc.2013.03.088 [DOI] [PubMed] [Google Scholar]
- 97.Sharma RK, Arbab-Zadeh A, Kishi S, Chen MY, Magalhães TA, George RT, et al. Incremental diagnostic accuracy of computed tomography myocardial perfusion imaging over coronary angiography stratified by pre-test probability of coronary artery disease and severity of coronary artery calcification: The CORE320 study. Int J Cardiol 2015; 201: 570–7. doi: 10.1016/j.ijcard.2015.05.110 [DOI] [PubMed] [Google Scholar]
- 98.Takx RA, Blomberg BA, El Aidi H, Habets J, de Jong PA, Nagel E, et al. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging 2015; 8: e002666. doi: 10.1161/CIRCIMAGING.114.002666 [DOI] [PubMed] [Google Scholar]
- 99.Pelgrim GJ, Dorrius M, Xie X, den Dekker MA, Schoepf UJ, Henzler T, et al. The dream of a one-stop-shop: meta-analysis on myocardial perfusion CT. Eur J Radiol 2015; 84: 2411–20. doi: 10.1016/j.ejrad.2014.12.032 [DOI] [PubMed] [Google Scholar]
- 100.Kühl JT, Linde JJ, Køber L, Kelbæk H, Kofoed KF. The transmural extent and severity of myocardial hypoperfusion predicts long-term outcome in NSTEMI: an MDCT Study. JACC Cardiovasc Imaging 2015; 8: 684–94. doi: 10.1016/j.jcmg.2015.01.022 [DOI] [PubMed] [Google Scholar]
- 101.Feuchtner GM, Plank F, Pena C, Battle J, Min J, Leipsic J, et al. Evaluation of myocardial CT perfusion in patients presenting with acute chest pain to the emergency department: comparison with SPECT-myocardial perfusion imaging. Heart 2012; 98: 1510–7. doi: 10.1136/heartjnl-2012-302531 [DOI] [PubMed] [Google Scholar]
- 102.Pursnani A, Lee AM, Mayrhofer T, Ahmed W, Uthamalingam S, Ferencik M, et al. Early resting myocardial computed tomography perfusion for the detection of acute coronary syndrome in patients with coronary artery disease. Circ Cardiovasc Imaging 2015; 8: e002404. doi: 10.1161/CIRCIMAGING.114.002404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hulten E, Ahmadi A, Blankstein R. CT assessment of myocardial perfusion and fractional flow reserve. Prog Cardiovasc Dis 2015; 57: 623–31. doi: 10.1016/j.pcad.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 104.Hulten E, Goehler A, Bittencourt MS, Bamberg F, Schlett CL, Truong QA, et al. Cost and resource utilization associated with use of computed tomography to evaluate chest pain in the emergency department: the Rule Out Myocardial Infarction using Computer Assisted Tomography (ROMICAT) study. Circ Cardiovasc Qual Outcomes 2013; 6: 514–24. doi: 10.1161/CIRCOUTCOMES.113.000244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Priest VL, Scuffham PA, Hachamovitch R, Marwick TH. Cost-effectiveness of coronary computed tomography and cardiac stress imaging in the emergency department: a decision analytic model comparing diagnostic strategies for chest pain in patients at low risk of acute coronary syndromes. JACC Cardiovasc Imaging 2011; 4: 549–56. doi: 10.1016/j.jcmg.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 106.Pursnani A, Schlett CL, Mayrhofer T, Celeng C, Zakroysky P, Bamberg F, et al. Potential for coronary CT angiography to tailor medical therapy beyond preventive guideline-based recommendations: insights from the ROMICAT I trial. J Cardiovasc Comput Tomogr 2015; 9: 193–201. doi: 10.1016/j.jcct.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Than M, Aldous S, Lord SJ, Goodacre S, Frampton CM, Troughton R, et al. A 2-hour diagnostic protocol for possible cardiac chest pain in the emergency department: a randomized clinical trial. JAMA Intern Med 2014; 174: 51–8. doi: 10.1001/jamainternmed.2013.11362 [DOI] [PubMed] [Google Scholar]
- 108.Ayaram D, Bellolio MF, Murad MH, Laack TA, Sadosty AT, Erwin PJ, et al. Triple rule-out computed tomographic angiography for chest pain: a diagnostic systematic review and meta-analysis. Acad Emerg Med 2013; 20: 861–71. doi: 10.1111/acem.12210 [DOI] [PubMed] [Google Scholar]
- 109.Burris AC, II, Boura JA, Raff GL, Chinnaiyan KM. Triple rule out versus coronary CT angiography in patients with acute chest pain: results from the ACIC consortium. JACC Cardiovasc Imaging 2015; 8: 817–25. doi: 10.1016/j.jcmg.2015.02.023 [DOI] [PubMed] [Google Scholar]
- 110.Wirth S, Körner M, Treitl M, Linsenmaier U, Leidel BA, Jaschkowitz T, et al. Computed tomography during cardiopulmonary resuscitation using automated chest compression devices–an initial study. Eur Radiol 2009; 19: 1857–66. doi: 10.1007/s00330-009-1359-9 [DOI] [PubMed] [Google Scholar]
- 111.Leidel BA, Kunzelmann M, Bitterling H, Reichle F, Wirth S, Kanz KG. Computer tomography showing left coronary artery occlusion in a patient having manual chest compressions. Resuscitation 2009; 80: 295–6. doi: 10.1016/j.resuscitation.2008.11.003 [DOI] [PubMed] [Google Scholar]
- 112.Schubert EC, Kanz KG, Linsenmaier U, Bogner V, Wirth S, Angstwurm M. Use of computed tomography and mechanical CPR in cardiac arrest to confirm pulmonary embolism: a case study. CJEM 2016; 18: 66–9. doi: 10.1017/cem.2015.3 [DOI] [PubMed] [Google Scholar]