Abstract
Objective:
There is disagreement regarding the value of the α/β ratio for prostate cancer. Androgen deprivation therapy (ADT) may dominate the effects of dose fractionation on prostate-specific antigen (PSA) response and confound estimates of the α/β ratio. We estimate this ratio from combined data on external beam radiation therapy (EBRT) and brachytherapy (BT)-treated patients, providing a range of doses per fraction, while accounting for the effects of ADT.
Methods:
We analyse data on 289 patients with local prostate cancer treated with EBRT (2 Gy per fraction) or EBRT plus one or two BT boosts of 10 Gy each. The timing of ADT was heterogeneous. We develop statistical models to estimate the α/β ratio based upon PSA measurements at 1 year as a surrogate for the surviving fraction of cancer cells as well as combined biochemical + clinical recurrence-free survival (bcRFS), controlling for ADT.
Results:
For the PSA-based end point, the α/β ratio estimate is 7.7 Gy [95% confidence interval (CI): 4.1 to 12.5]. Based on the bcRFS end point, the estimate is 18.0 Gy (95% CI: 8.2 to ∞).
Conclusion:
Our model-based estimates of the α/β ratio, which account for the effects of ADT and other important confounders, are higher than some previous estimates.
Advances in knowledge:
Although dose inhomogeneities and other limitations may limit the scope of our findings, the data suggest caution regarding the assumptions of the α/β ratio for prostate cancer in some clinical environments.
INTRODUCTION
Following the work of Brenner and Hall,1 several reports,2–7 including large, multicentre studies, have suggested that the α/β ratio for prostate cancer is low and may be comparable with that of late responding normal tissues. From a clinical perspective, this has justified an increasing use of hypofractionation in teletherapy, brachytherapy (BT) or combined BT and teletherapy for prostate cancer.
A recent meta-analysis of tumour control probability from five trials on hypofractionation in external beam radiation therapy (EBRT) estimated the α/β ratio to be as small as −0.07 Gy [95% confidence interval (CI): −0.73 to 0.59] or as large as 1.93 Gy (95% CI: −0.27 to 4.14).2 The variation was based mostly on the incorporation of a time-dependent effect; the inclusion of a time factor in models for assessing the α/β ratio also increased estimates in previous studies.3,4 The meta-analysis was sensitive to one influential study by Arcangeli et al.8 The study initially demonstrated an improvement in tumour control from hypofractionation, but an update with longer follow-up showed that this improvement was smaller than first reported.9 Similarly, a randomized trial by Pollack et al10 did not demonstrate the expected benefit from hypofractionation. Had these more recent data been available in the meta-analysis by Vogelius and Bentzen,2 they may have further changed the estimates of α/β ratio.
Some have argued for a higher α/β ratio in prostate cancer and that a single number may not be adequate.11,12 For example, the ratio may vary with prognostic factors, e.g. be larger for poorly differentiated tumours.5 In a multi-institutional study, Williams et al6 reported that how the estimate may change when the actual average dose delivered to the prostate from high dose rate (HDR) BT exceeds the prescribed dose: the estimated α/β ratio increases from 2.6 Gy to 4.5 or 7.1 Gy, respectively, for 20% or 40% increases. Recent studies13–15 of cohorts including patients treated with HDR BT also point to higher α/β ratio estimates with more variability, and some studies6,16,17 have identified differences between risk groups. Cold spots in the BT dose distribution may contribute to lower-than-expected control rates, and the applicability of the linear–quadratic (LQ) model at high doses per fraction is therefore uncertain.7 Finally, some reports suggest that a low α/β ratio is unlikely to exist in the castrate environment and that androgen deprivation therapy (ADT) may limit the potential advantage of hypofractionated regimens.18 The effect of ADT, additive to radiotherapy (RT), is additional growth arrest and apoptosis of hormone-responsive cancer cells. Thus, cancer cells that survive combined RT and ADT may have more aggressive characteristics and higher proliferative potential than those that survive RT alone. This hypothesis may suggest different α/β ratios in castrate and non-castrate environments although a large study contradicts such inference.4
These ambiguities provide the rationale for this research. In this study, we present α/β estimates and CIs for prostate cancer derived from an institutional data set of patients treated with external beam radiotherapy alone (EBRT) or EBRT plus single or double BT boosts. Most patients also received ADT before, during or after RT. A novel contribution of this article is our explicit approach to accounting for heterogeneity in the outcomes due to ADT.
METHODS AND MATERIALS
Patients, treatment prescription
We retrospectively analysed 289 patients, ages 44–83 years, with pathologically confirmed T1-T2N0M0 adenocarcinoma of the prostate treated between March 2003 and July 2012. All patients underwent EBRT with 6–20 MV photons generated by a linear accelerator using three-dimensional CT/MRI-based conformal planning. Vacuum-forming mattresses and thermoplastic masks were used for patient immobilization during EBRT. The doses prescribed for gross tumour volume encompassed the prostate, and pelvic lymph nodes were included in clinical tumour volume in the majority of high-risk patients. A planning target volume (PTV) expansion of 10–15 mm was applied, except for the posterior expansion of 7–8 mm. EBRT was delivered in 2 Gy fractions for all but two patients, who received 1.8 Gy fractions, with the total number of fractions depending on whether a patient also received BT boosts (following paragraph). The total dose of EBRT was specified according to The International Commission on Radiation Units and Measurements Reports 50 and 62, such that the heterogeneity in dose delivery to the PTV was kept within 95% and 107% of isodoses. Follow-up visits were arranged every 3 months during the first year after EBRT and every 6 months thereafter. Each evaluation included a clinical examination, digital rectal examination and prostate-specific antigen (PSA) measurement.
Patients with good performance status and prostate suitable for transrectal ultrasound (TRUS)-guided implantation were offered BT. 100 patients received a single 10-Gy HDR BT boost, given before, during or after EBRT, depending on logistic arrangements. The clinical details for these patients were first described in Smolska-Ciszewska et al.19 After preliminary evidence revealed disappointing control rates among patients receiving a single BT boost, an additional 59 patients were offered and received 2 BT boosts of either 10 Gy (17 patients) or 10.5 Gy (42 patients) before EBRT. Patients with a history of transurethral resection or clinical stage T3 were not eligible for BT boosts, and individual preferences of patients were also considered at treatment prescription. For BT appliance, patients were placed in the dorsal lithotomic position under spinal anaesthesia. A catheter was used to fill the bladder with sterile water. 14 to 20 needles were implanted to the prostate under the TRUS guidance. Real-time TRUS reconstruction of the implant position was used in dose planning, and the entire prostate gland was defined as clinical tumour volume. The prescribed dose was specified to cover the 90% of isodose. HDR 192-Iridium sources were used for treatment. About 36% of patients received elective RT to the pelvic lymph nodes (range 44–50 Gy), whether or not BT boosts were administered. Table 1 summarizes patient characteristics, stratified by zero, one or two BT boosts. The median total radiation delivered through EBRT was 67 Gy (range 57–78 Gy) over 42 days (range 30–79 days); for patients receiving EBRT only, the median delivery was 74 Gy in 37 fractions over 52 days, and for patients receiving 1 (2) BT boosts, it was 64 (67) Gy in 27 (23) fractions over 39 (32) days.
Table 1.
Characteristics of 289 patients, stratified by whether a patient received only external beam radiation therapy (EBRT), one 10-Gy brachytherapy (BT) boost (EBRT + 1BT), or two 10- to 10.5-Gy BT boosts (EBRT + 2BT)
| Characteristic | All, n = 289 | EBRT, n = 130 | EBRT + 1BT, n = 100 | EBRT + 2BT, n = 59 |
|---|---|---|---|---|
| Total dose (Gy) | ||||
| Median | 67 | 74 | 64 | 67 |
| Range | 57–78 | 70–78 | 57–66 | 66–74 |
| Number of Fx EBRT | ||||
| Median | 27 | 37 | 27 | 23 |
| Range | 23–41 | 35–41 | 26–28 | 23–27 |
| Duration EBRT (days) | ||||
| Median | 42 | 52 | 39 | 32 |
| Range | 30–79 | 46–79 | 33–48 | 30–45 |
| Duration RT (days) | ||||
| Median | 50 | 52 | 41 | 44 |
| Range | 33–80 | 46–79 | 34–58 | 33–80 |
| Age (years) | ||||
| Median | 66 | 67 | 65 | 64 |
| Range | 44–83 | 51–80 | 49–83 | 44–78 |
| iPSA (ng ml−1) | ||||
| Median | 12.1 | 13.9 | 11.8 | 11.7 |
| Range | 0.3–80.7 | 0.3–64.0 | 0.6–56.6 | 1.5–80.7 |
| Clinical T-stage, n (%) | ||||
| T1b–T1c | 111 (38) | 62 (48) | 41 (41) | 8 (14) |
| T2a–T2c | 175 (61) | 67 (52) | 59 (59) | 49 (83) |
| Missing | 3 (1) | 1 (1) | 0 (0) | 2 (3) |
| NCCN risk, n (%) | ||||
| Low | 53 (18) | 30 (23) | 18 (18) | 5 (8) |
| Int. | 105 (36) | 47 (36) | 36 (36) | 22 (37) |
| High | 131 (45) | 53 (41) | 46 (46) | 32 (54) |
| Gleason total, n (%) | ||||
| 2–7 | 217 (75) | 95 (73) | 73 (73) | 49 (83) |
| 8–10 | 30 (10) | 13 (10) | 7 (7) | 10 (17) |
| Missing | 42 (15) | 22 (17) | 20 (20) | 0 (0) |
| Whole pelvis EBRT, n (%) | ||||
| Yes | 105 (36) | 44 (34) | 40 (40) | 21 (36) |
| No | 184 (64) | 86 (66) | 60 (60) | 38 (64) |
Fx, fraction; NCCN, National Comprehensive Cancer Network; RT, radiotherapy.
The dose fractionation of EBRT was 2.0 Gy for all but two patients, who received 1.8 Gy per fraction.
“iPSA” is the patient's largest prostate-specific antigen measurement before commencing RT and “NCCN risk” is the NCCN risk category.
Due to rounding, not all percentages add to 100%.
There were differences in terms of the administration and timing of ADT relative to RT, which are summarized in Table 2. 22 patients never received ADT (neither prior to nor within 1 year of finishing RT), 136 received neoadjuvant ADT which ended prior to finishing RT, 58 received some adjuvant or salvage ADT at some point within the first year of completing RT but stopped before 1 year's time and 73 were receiving some ADT 1 year after finishing RT. The starting date of ADT may have been prior to, during or after RT. Figure 1 plots each patient's PSA up to 48 months after finishing RT, stratified by the RT and ADT groups from Tables 1 and 2. In summary, there is heterogeneity between RT groups, not only with regard to ADT but also other important variables such as PSA levels at baseline and clinical T-stage. This will be accounted for in the analyses.
Table 2.
Characteristics of 289 patients, stratified by timing of androgen deprivation therapy (ADT): no ADT, ADT ending before radiotherapy (RT) (ADTEnd < RTEnd), ADT ending after RT but before 1 year post-RT (RTEnd < 1 year) or ADT continuing past 1 year post-RT (ADTEnd ≥ 1 year)
| Characteristic | All, n = 289 | No ADT, n = 22 | ADTEnd < RTEnd, n = 136 | ADTEnd < 1 year, n = 58 | ADTEnd ≥ 1 year, n = 73 |
|---|---|---|---|---|---|
| Total dose (Gy) | |||||
| Median | 67 | 67 | 66.5 | 67 | 73.8 |
| Range | 57–78 | 57–76 | 62–78 | 64–76 | 64–78 |
| Number of Fx EBRT | |||||
| Median | 27 | 27.5 | 27 | 27 | 37 |
| Range | 23–41 | 23–38 | 23–39 | 23–38 | 23–41 |
| Duration EBRT (days) | |||||
| Median | 42 | 43 | 41 | 40 | 51 |
| Range | 30–79 | 30–58 | 30–79 | 30–56 | 31–70 |
| Duration RT (days) | |||||
| Median | 50 | 50 | 49 | 50 | 51 |
| Range | 33–80 | 36–72 | 33–79 | 35–61 | 36–80 |
| Age (years) | |||||
| Median | 66 | 64 | 66 | 64.5 | 67 |
| Range | 44–83 | 52–76 | 49–83 | 44–80 | 53–79 |
| iPSA (ng ml−1) | |||||
| Median | 12.1 | 8.4 | 11.8 | 13.1 | 15.4 |
| Range | 0.3–80.7 | 0.6–61.1 | 0.3–71.3 | 2.3–80.7 | 2.4–64.0 |
| Clinical T-stage, n (%) | |||||
| T1b–T1c | 111 (38) | 10 (45) | 59 (43) | 18 (31) | 24 (33) |
| T2a–T2c | 175 (61) | 12 (55) | 76 (56) | 39 (67) | 48 (66) |
| Missing | 3 (1) | 0 (0) | 1 (1) | 1 (2) | 1 (1) |
| NCCN risk, n (%) | |||||
| Low | 53 (18) | 9 (41) | 28 (21) | 9 (16) | 7 (10) |
| Int. | 105 (36) | 5 (23) | 61 (45) | 15 (26) | 24 (33) |
| High | 131 (45) | 8 (36) | 47 (35) | 34 (59) | 42 (58) |
| Gleason total, n (%) | |||||
| 2–7 | 217 (75) | 18 (82) | 101 (74) | 48 (83) | 50 (68) |
| 8–10 | 30 (10) | 3 (14) | 9 (7) | 6 (10) | 12 (16) |
| Missing | 42 (15) | 1 (5) | 26 (19) | 4 (7) | 11 (15) |
| Whole pelvis EBRT, n (%) | |||||
| Yes | 105 (36) | 7 (32) | 46 (34) | 21 (36) | 31 (42) |
| No | 184 (64) | 15 (68) | 90 (66) | 37 (64) | 42 (58) |
ADTEnd, end of ADT; EBRT, external beam radiation therapy; Fx, fraction; NCCN, National Comprehensive Cancer Network; RTEnd, end of RT.
“iPSA” is the patient's largest prostate-specific antigen measurement before commencing RT, and “NCCN risk” is the National Comprehensive Cancer Network risk category.
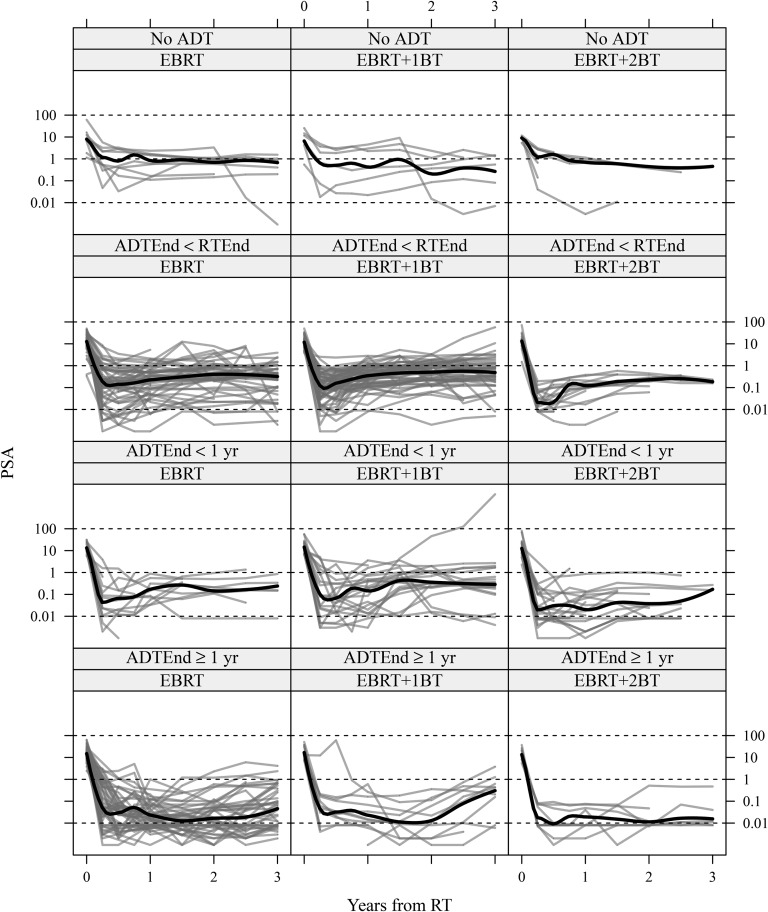
Figure 1.
Prostate-specific antigen (PSA) level over time from treatment, stratified by type of radiotherapy (RT) [external beam radiation therapy (EBRT) alone or EBRT plus 1 or 2 brachytherapy (BT) boosts] and timing of androgen deprivation therapy (ADT) (No ADT, ADT ending before RT, or ADT ending after RT but before 1 year post RT, or ADT continuing past 1 year post-RT). Locally smoothed average trajectories are superimposed in each panel. ADTEnd, end of ADT; RTEnd, end of RT.
End point for analysis
For our primary end point, we used the PSA value closest to 1 year after the end of RT; being the typical amount of time, it takes PSA to reach a stable low level after EBRT. 1 year was also chosen because the number of missing PSA values at that time was small relative to other time points. Since the focus of the analysis is on the effect of the fractionation schedule, using the cell survival LQ model, it is necessary to consider an early time that is most reflective of the direct cell-killing effect of the radiation. There were 278 patients with PSA measurements close to 1 year after RT. Out of the 278 patients, 233 had measurements at 1 year, 22 at 9 months, 21 at 6 months, 1 at 1.5 years and 1 at 2 years. Of the 11 remaining patients without PSA data close to 1 year, 2 had no indication of recurrence, and so we used the average of their 3-month and 3.5-year (or 4-year) PSA value. 3 patients were missing information on clinical T-stage, giving 277 patients with complete data.
As a secondary end point, we considered a combined biochemical/clinical recurrence-free survival (bcRFS), defined as either biochemical failure according to the Phoenix consensus criteria20 (PSA nadir + 2 ng ml−1) or clinically detected recurrence of the cancer. Figure 2 plots Kaplan–Meier estimates of the distribution of bcRFS across the four ADT groups.
Figure 2.
Kaplan–Meier estimates of the distribution of biochemical/clinical recurrence-free survival across the four androgen deprivation therapy (ADT) groups. ADTEnd, end of ADT; RT, radiotherapy; RTEnd, end of RT.
Statistical analysis
We directly incorporated the linear–quadratic model using a novel statistical approach that provides an estimate of the α/β ratio. The approach also directly accounts for and quantifies the effect of the large heterogeneity in PSA values at 1 year owing to the receipt and timing of ADT (Figure 1).
Androgen deprivation therapy groups
We constructed four distinct ADT groups. Let t denote the time, in years, since the end of RT for each patient and let j = 1, 2, 3, 4 index the ADT group, where each group is defined as follows:
(j = 1): has never received ADT (no ADT; n1 = 21)
(j = 2): the end of ADT (ADTEnd) is at t ≤ 0, i.e. the end of RT (RTEnd), and there is no ADT between 0 < t < 1 (ADTEnd < RTEnd; n2 = 131)
(j = 3): the end of ADT is between 0 < t < 1 (ADTEnd < 1 year; n3 = 55)
(j = 4): ADTEnd ≥ 1 year—(ADTEnd ≥ 1 year; n4 = 70).
Linear quadratic model
The α/β ratio measures the relative importance of the linear and quadratic terms in the LQ model. For fractionated radiation, the linear and quadratic terms are calculated by the sum of the fractionated doses and the sum of the squared fractionated doses, respectively. For patient i ∈ {1, …, nj} in group j ∈ {1, 2, 3, 4}, the linear and quadratic dose contributions are denoted by Dij = ∑kdoseijk and Sij = ∑kdose2ijk, where the sum is over both the EBRT and the BT portions of the regimen. The α/β ratio was assumed to be equal in all four ADT groups; however, the absolute effects of the linear and quadratic terms on PSA values were allowed to differ between the ADT groups, so that the relative impact on the outcome of ADT and radiation can differ between the four groups. For example, many patients receiving ADT had a very low PSA at 1 year (Figure 1), and the dose fractionation regime is expected to provide limited information about the α/β ratio for these patients. Nevertheless, there is some variation in the PSA values that may be explained by the dose and the fractionation schedule, which may provide some information about the α/β ratio.
For a given configuration of D and S, we can calculate the LQ equivalent total dose in 2 Gy fractions as a function of α/β,
| (1) |
thus allowing for a comparison of the different treatment regimens, had delivery been in 2 Gy fractions.
Statistical regression model
The outcome Yij in the regression model is log (PSA + 0.1) at 1 year after the end of RT for patient i ∈ {1, …, nj} in group j ∈ {1, 2, 3, 4}. The “+0.1” offset stabilizes the outcome when the measured PSA is nearly zero. The fitted model is
| (2) |
The Dijω + Sij part of the equation is how the LQ model is incorporated into the analysis, as Dijω + Sij is proportional to the α/β adjusted total dose. The parameters ω, µj and φj comprise the novel contribution of the model. The α/β ratio is given by ω, which is equal between the four ADT groups. Typical values of PSA at 1 year may differ between groups, which are allowed for by a group-specific intercept µj. The overall impact of the adjusted total dose may also differ between groups, indicated by the scaling parameter φj. Distributing φj into (Dijω + Sij), the linear and quadratic components of the model for each ADT group are αj ≡ φjω and βj ≡ φj, respectively.
We also adjust for the age of the patient (Ageij), the largest measured PSA value before the start of RT, [transformed as log (iPSAij + 0.1)], and whether or not the tumour stage is 1 [I (T-stageij = T1)]. Let Xij = [Ageij, log (iPSAij + 0.1), I (T-stageij = T1)]T denote the baseline covariates. The quantity, σεij, is assumed to be normally distributed with variance σ2, to account for measurement error. The parameters {γA, γP, γT} incorporate the effect of the baseline covariates. Gleason score, which was missing for 42 patients, was not included in the model, but we investigate its potential impact on our findings in the Discussion section.
The model explicitly accounts for whether or not a patient received 0, 1 or 2 BT boosts through each patient's value of Dij and Sij. The parameters in the model are γA, γP, γT, ω and {µj, φj}, which are estimated using non-linear least squares (implemented in the nls function in R).21 We calculated CIs using profile likelihood.
As a secondary analysis, we estimated the α/β ratio in an alternative model using bcRFS as the outcome. Patients who did not experience recurrence were censored at their last clinic visit. As before, the heterogeneity due to ADT renders less useful traditional methods for measuring α/β. We accounted for these differences in the following model. Defining λij (t) as the hazard for recurrence at time t, a proportional hazards model is given by
| (3) |
which decomposes λij (t) into a product of a non-parametric common hazard and a parametric component based on the covariates. For identifiability, we assume µ1 ≡ 1. As in model (2), ω is the α/β ratio, and the presence and timing of ADT is accounted for by µj, which modifies the common hazard λ0 (t), and φj, which allows for separate linear and quadratic effects but with a common ratio. To fit model (3), we maximized the profile partial likelihood over a grid of values of ω (≡α/β). We inverted the likelihood ratio test statistic to derive a CI for α/β.
RESULTS
The smoothed average trajectories of PSA in Figure 1 demonstrate the differences in patterns across the 12 panels and the need for our more flexible non-linear regression model that allows for heterogeneity between groups. Patients who never received ADT (first row) generally had more stable trajectories, both in the initial PSA drop and longer-term changes. Those who received neoadjuvant therapy before RT (second row) experienced a greater typical drop in PSA than patients who received none, but the slope of the typical trajectory is slightly positive. In the third row, patients who received planned adjuvant or salvage ADT experienced a greater-still drop in PSA, but levels often increased again. Finally, in the fourth row, patients who were receiving ADT 1 year after RT had generally smaller PSA levels. Except for the “No ADT” group, the average level of PSA in the EBRT + 2BT treated patients is slightly lower than in the EBRT and EBRT + 1BT patients. From Figure 2, the “ADTEnd ≥ 1 year” group experienced the longest response duration, and the “ADTEnd < RTEnd” group experienced the shortest response duration. However, in general, differences in bcRFS varied by no more than 0.10–0.15.
Table 3 gives estimates and 95% CIs for the parameters in model (2). A summary of the sensitivity of our findings to our modelling choices is given in the Discussion section. The α/β ratio, represented by ω, is estimated to be 7.7 Gy (95% CI: 4.1 to 12.5). The individual intercepts and linear and quadratic effects, respectively, µ, α and β, differ considerably, which suggests that these vary between the ADT groups, as expected. Fitting the alternate model (3) for the distribution of bcRFS, the estimate of α/β is higher: 18.0 Gy (95% CI: 8.2 to ∞). We also fit four models to each ADT group separately, giving respective α/β estimates of 29.9, 6.5 (95% CI: 2.9 to 10.5), 7.8 (95% CI: −10.1 to 44.1) and 10.7; CIs for Groups 1 and 4 were unbounded in both directions. From this, it is clear that most of the statistical information is drawn from Group 2, which is expected, given the sample sizes, and reasonable, based on prior discussion regarding the dominating effect of ADT. Also, none of the groups is incompatible with an α/β value of 7.7 Gy.
Table 3.
Estimates, standard errors (SE) and profile-based 95% confidence intervals (CIs) for parameters from model (2) relating PSA 1 year after finishing radiotherapy (RT) to the baseline covariates and dose fractionation
| Parameter description | Parameter label | Estimate (SE) | 95% CI |
|---|---|---|---|
| α/β ratio | ω | 7.7 (1.8) | 4.1 to 12.5 |
| Linear effect: no ADT | α1 | −0.024 (0.024) | −0.082 to 0.023 |
| Linear effect: ADTEnd < RTEnd | α2 | −0.055 (0.017) | −0.087 to −0.023 |
| Linear effect: ADTEnd < 1 year | α3 | −0.036 (0.016) | −0.075 to −0.008 |
| Linear effect: ADTEnd ≥ 1 year | α4 | −0.014 (0.015) | −0.050 to 0.016 |
| Quadratic effect: no ADT | β1 | −0.0031 (0.0031) | −0.0092 to 0.0031 |
| Quadratic effect: ADTEnd < RTEnd | β2 | −0.0071 (0.0018) | −0.0108 to −0.0034 |
| Quadratic effect: ADTEnd < 1 year | β3 | −0.0047 (0.0019) | −0.0084 to −0.0010 |
| Quadratic effect: ADTEnd ≥ 1 year | β4 | −0.0018 (0.0019) | −0.0056 to 0.0019 |
| Intercept: no ADT | µ1 | 2.5 (2.4) | −2.1 to 7.7 |
| Intercept: ADTEnd < RTEnd | µ2 | 4.8 (1.5) | 2.0 to 7.6 |
| Intercept: ADTEnd < 1 year | µ3 | 2.7 (1.6) | −0.3 to 6.1 |
| Intercept: ADTEnd ≥ 1 year | µ4 | 0.0 (1.5) | −3.0 to 3.3 |
| Common fixed effect: age | γA | −0.011 (0.007) | −0.026 to 0.002 |
| Common fixed effect: iPSA | γP | 0.072 (0.066) | −0.058 to 0.201 |
| Common fixed effect: T-stage | γT | 0.020 (0.101) | −0.179 to 0.220 |
ADT, androgen deprivation therapy; ADTEnd, end of ADT; RTEnd, end of RT.
The linear (αj ≡ φjω) and quadratic (βj = φj) components are allowed to differ between ADT groups but are constrained so as to have the same ratio (ω = αj/βj, j = 1, 2, 3, 4).
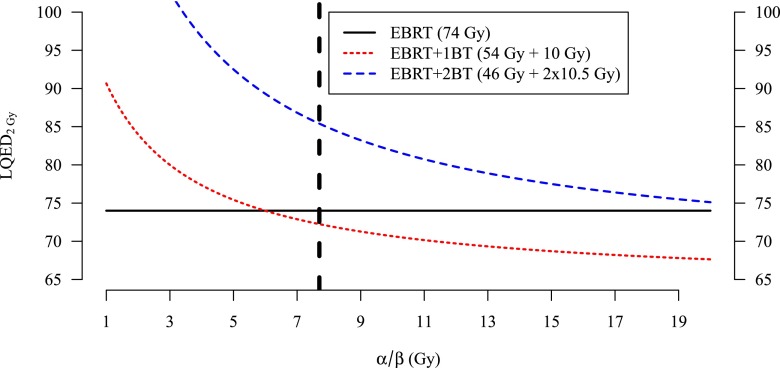
Figure 3 plots LQED2Gy (α/β) for typical values of D and S from the EBRT, EBRT + 1BT and EBRT + 2BT groups, with the value LQED2Gy(α/β = 7.7 Gy) = 85.4 Gy highlighted. LQED2Gy is largest in the EBRT + 2BT regimen and is so for all values of α/β < 23.5 Gy.
Figure 3.
Estimates of the linear–quadratic equivalent dose in 2 Gy fractions (LQED2 Gy) for three treatment regimens as a function of the α/β ratio. The vertical line denotes α/β = 7.7. BT, brachytherapy; EBRT, external beam radiation therapy.
DISCUSSION
Our models estimate the α/β ratio in prostate cancer to be 7.7 Gy (95% CI: 4.1 to 12.5) based on the PSA end point and 18.0 Gy (95% CI: 8.2 to ∞) based on the combined bcRFS end point. Using pure biochemical RFS as an end point, that is, ignoring clinical recurrence, the estimate is 11.7 Gy (not shown). Thus, although large, there is some dependence in the choice of end point. There is heterogeneity between patients, particularly with respect to ADT. Although our regression analysis and sensitivity analysis, described in the proceeding paragraphs, account for this heterogeneity the CI, (4.1 Gy, 12.5 Gy), is correspondingly wide, despite the wide range of doses per fraction that were used.
The data show, as expected, that the average value of PSA for those on ADT at 1 year (Group 4) is lower than the other groups and that the impact of the total dose and fraction size is much less in Group 4 (as the estimate of β4 is close to zero). Thus, this group is contributing less information than the other groups about the most appropriate value of α/β. This is also clear from analysing the four groups separately, which gives estimates of α/β (and CIs for Groups 2 and 3) of 29.9, 6.5 (95% CI: 2.9 to 10.5), 7.8 (95% CI: −10.1 to 44.1) and 10.7. The overlap in these CIs and the fact that the final estimate of α/β is contained within all provides some assurance that the assumption of the same α/β for all groups is reasonable and allows the analysis to utilize all data rather than discarding some of the ADT groups.
Heterogeneity in our data represents an important limitation of the present study. To evaluate as thoroughly as possible potential weaknesses in our data set and modelling strategy, we conducted four additional critical sensitivity analyses with respect to our primary model in Equation (2). First, Gleason score was excluded owing to being missing for 42 patients. However, because it is likely to be associated with PSA post RT, we investigated whether the α/β ratio is sensitive to this omission. We imputed the 42 missing Gleason scores 10 times and re-fit our model to these 10 imputed data sets, now also adjusting for Gleason score. The range of these imputation-based α/β ratios was 7.3–7.7 Gy, with a mean of 7.5 Gy. Second, we adjusted for total duration of RT (EBRT and BT), based on its reported effects on the α/β ratio estimate in Vogelius and Bentzen2 and estimated α/β = 6.8 Gy (95% CI: 2.3 to 12.1). Third, to account for the fact that the average delivered dose of HDR radiation via BT may exceed the PTV for the BT fractions owing to hot spots in the dose distribution, we increased by 20% the dose per fraction for the BT doses, as in Williams et al,6 finding an estimate of 17.0 Gy (95% CI: 8.8 to 31.4). Fourth, we used as an alternative end point PSA value closest to 2 years after the end of RT, obtaining α/β = 8.4 Gy (95% CI: 4.3 to 15.9). Each of these analyses is in accordance with our primary estimate of α/β = 7.7 Gy.
The use of a single BT boost was based on an assumption that LQED2Gy in EBRT + 1BT (54 Gy over 27 fractions plus 10 Gy boost) would be larger than that of EBRT (74 Gy over 37 fractions), assuming that the α/β ratio for prostate cancer was <6.0 Gy. However, for EBRT + 1BT, α/β = 7.7 Gy implies an LQED2Gy of 72.2 Gy, which is less than the LQED2Gy for EBRT of 74 Gy (Figure 3). By contrast, at this same α/β ratio, the implied LQED2Gy of 86 Gy for EBRT + 2BT is appreciably larger than that of either EBRT or EBRT + 1BT. This is consistent with the clinical decision to move from EBRT + 1BT to EBRT + 2BT.
Coherence with previous studies
Our findings of a larger α/β ratio disagree with those from several studies.1–7 However, as we discuss in the Introduction section here, there is sufficient uncertainty in the present literature so as not to rule out a large α/β ratio in all patient populations.
In a multicentre study encompassing 3145 patients treated with EBRT or EBRT + BT, Roberts et al7 observed that 5-year local control rates using BT alone or EBRT + BT given in 1–2 fractions were as much as 35% lower than predicted by the LQ model, assuming α/β = 1.42 Gy. Using our estimate, α/β = 7.7 Gy, which is based upon data from both EBRT alone and EBRT + 1/2BT patients, LQED2Gy values would have decreased, and predicted 5-year local control rates for patients receiving BT boosts would have correspondingly decreased, likely resulting in better agreement between predicted and observed responses in that article.
Our findings agree with those of a recent randomized clinical trial of 218 patients comparing EBRT (55 Gy over 20 fractions) to EBRT + 2BT (35.75 Gy over 13 fractions plus 2 fractions of 8.5 Gy) showed a significant improvement in bcRFS in the EBRT + 2BT arm, with a 31% reduction in the risk of recurrence.22 Based on a ratio of α/β = 1.5 Gy, the authors22 reported LQED2Gy estimates of 66.8 Gy and 92.0 Gy for EBRT and EBRT + 2BT, respectively. Using α/β = 7.7 Gy, LQED2Gy decreases to 59.3 and 66.9 Gy, respectively, and the EBRT + 2BT regimen still delivers a larger biological dose. Thus, the clinical outcome of that trial does not preclude relatively high α/β ratios for prostate cancer.
Another randomized clinical trial compared conventionally fractionated EBRT (76 Gy over 38 fractions) with hypofractionated EBRT (70.2 Gy over 26 fractions) in a cohort of 303 patients.10 The hypofractionated arm was planned to deliver an LQED2Gy of 84.2 Gy, assuming α/β = 1.5 Gy, and was expected to decrease disease failure rates by 15%. However, upon the trial's completion, patients receiving conventional fractionation had smaller, although not statistically significantly different, disease failure rates (21.4% vs 23.3%). Correspondence regarding the trial addressed the apparent discord between the trial's outcome and the increasing evidence in favour of hypofractionation.23–25 In fact, the observed near-isoeffectiveness of these two dose fractionations is less unexpected, given our estimate: α/β = 7.7 Gy yields an LQED2Gy of 75.3 Gy for the hypofractionated arm, slightly <76 Gy delivered conventionally.
Comparing our results to those of other adenocarcinomas, the α/β ratios for breast and rectal cancer are likely in the range of 4–5 Gy.26,27 On histological grounds, these may support our findings, although in clinical settings, cytotoxic responses to RT can be individually modified by concurrent use of hormonal treatment (prostate cancer, breast cancer) or chemotherapy (rectal cancer, breast cancer).
In summary, our approach, which incorporates patients who received one or two additional large fractions delivered via BT and also accounts for the use of ADT, estimates an α/β ratio of 7.7 Gy with 95% CI of (4.1 to 12.5 Gy). Although our conclusions are limited by the retrospective nature of our analysis, including non-randomized treatment selection, a relatively small sample size, and possible dose inhomogeneities, our estimate is larger than some previous estimates and suggests caution regarding the assumption of a small α/β ratio.
FUNDING
The work of PSB and JMGT was supported by the National Institutes of Health [P30 CA046592].
Contributor Information
Philip S Boonstra, Email: philb@umich.edu.
Jeremy MG Taylor, Email: jmgt@umich.edu.
Beata Smolska-Ciszewska, Email: bsmolska@io.gliwice.pl.
Katarzyna Behrendt, Email: kbehrendt@io.gliwice.pl.
Tomasz Dworzecki, Email: tdworzecki@io.gliwice.pl.
Marzena Gawkowska-Suwinska, Email: marzenags@io.gliwice.pl.
Brygida Bialas, Email: bbialas@io.gliwice.pl.
Rafal Suwinski, Email: rafals@io.gliwice.pl.
REFERENCES
- 1.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 1999; 43: 1095–101. doi: 10.1016/S0360-3016(98)00438-6 [DOI] [PubMed] [Google Scholar]
- 2.Vogelius IR, Bentzen SM. Meta-analysis of the alpha/beta ratio for prostate cancer in the presence of an overall time factor: bad news, good news, or no news? Int J Radiat Oncol Biol Phys 2013; 85: 89–94. doi: 10.1016/j.ijrobp.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang JZ, Guerrero M, Li XA. How low is the α/β ratio for prostate cancer? Int J Radiat Oncol Biol Phys 2003; 55: 194–203. doi: 10.1016/S0360-3016(02)03828-2 [DOI] [PubMed] [Google Scholar]
- 4.Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β= 1.4 (0.9–2.2) Gy. Int J Radiat Oncol Biol Phys 2012; 82: e17–24. [DOI] [PubMed] [Google Scholar]
- 5.Proust-Lima C, Taylor JM, Sécher S, Sandler H, Kestin L, Pickles T, et al. Confirmation of a low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys 2011; 79: 195–201. doi: 10.1016/j.ijrobp.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams SG, Taylor JM, Liu N, Tra Y, Duchesne GM, Kestin LL, et al. Use of individual fraction size data from 3756 patients to directly determine the α/β ratio of prostate cancer. Int J Radiat Oncol Biol Phys 2007; 68: 24–33. doi: 10.1016/j.ijrobp.2006.12.036 [DOI] [PubMed] [Google Scholar]
- 7.Roberts SA, Miralbell R, Zubizarreta EH, Fowler JF, Hendry JH. A modelled comparison of prostate cancer control rates after high-dose-rate brachyther- apy (3145 multicentre patients) combined with, or in contrast to, external- beam radiotherapy. Radiother Oncol 2014; 111: 114–19. doi: 10.1016/j.radonc.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 8.Arcangeli G, Saracino B, Gomellini S, Petrongari MG, Arcangeli S, Sentinelli S, et al. A prospective phase III randomized trial of hypofractionation versus conventional fractionation in patients with high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2010; 78: 11–18. doi: 10.1016/j.ijrobp.2009.07.1691 [DOI] [PubMed] [Google Scholar]
- 9.Arcangeli S, Strigari L, Gomellini S, Saracino B, Petrongari MG, Pinnaró P, et al. Updated results and patterns of failure in a randomized hypofractionation trial for high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012; 84: 1172–8. doi: 10.1016/j.ijrobp.2012.02.049 [DOI] [PubMed] [Google Scholar]
- 10.Pollack A, Walker G, Horwitz EM, Price R, Feigenberg S, Konski AA, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol 2013; 31: 3860–8. doi: 10.1200/JCO.2013.51.1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nahum AE, Movsas B, Horwitz EM, Stobbe CC, Chapman JD. Incorporating clinical measurements of hypoxia into tumor local control modeling of prostate cancer: implications for the α/β ratio. Int J Radiat Oncol Biol Phys 2003; 57: 391–401. doi: 10.1016/S0360-3016(03)00534-0 [DOI] [PubMed] [Google Scholar]
- 12.King CR, Mayo CS. Is the prostate α/β ratio of 1.5 from Brenner & Hall a modeling artifact? Int J Radiat Oncol Biol Phys 2000; 47: 536–9. doi: 10.1016/S0360-3016(00)00442-9 [DOI] [PubMed] [Google Scholar]
- 13.Afsharpour H, Walsh S, Collins Fekete CA, Vigneault E, Verhaegen F, Beaulieu L. On the sensitivity of α/β prediction to dose calculation methodology in prostate brachytherapy. Int J Radiat Oncol Biol Phys 2014; 88: 345–50. doi: 10.1016/j.ijrobp.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 14.Nickers P, Hermesse J, Deneufbourg JM, Vanbelle S, Lartigau E. Which α/β ratio and half-time of repair are useful for predicting outcomes in prostate cancer? Radiother Oncol 2010; 97: 462–6. doi: 10.1016/j.radonc.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 15.Shaffer R, Pickles T, Lee R, Moiseenko V. Deriving prostate alpha-beta ratio using carefully matched groups, long follow-up and the phoenix definition of biochemical failure. Int J Radiat Oncol Biol Phys 2011; 79: 1029–36. doi: 10.1016/j.ijrobp.2009.12.058 [DOI] [PubMed] [Google Scholar]
- 16.Bentzen SM, Ritter MA. The α/β ratio for prostate cancer: what is it, really? Radiother Oncol 2005; 76: 1–3. doi: 10.1016/j.radonc.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 17.Valdagni R, Nahum AE, Magnani T, Italia C, Lanceni A, Montanaro P, et al. Long-term biochemical control of prostate cancer after standard or hyper- fractionation: evidence for different outcomes between low–intermediate and high risk patients. Radiother Oncol 2011; 101: 454–9. doi: 10.1016/j.radonc.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 18.Williams SG, Pickles T, Kestin L, Buyyounouski M, Martinez A, Demanes J, et al. Androgen deprivation therapy plus hypofractionated prostate radiotherapy–are we castrating the radiobiological advantage? Int J Radiat Oncol Biol Phys 2008; 72: S73–4. doi: 10.1016/j.ijrobp.2008.06.933 [DOI] [Google Scholar]
- 19.Smolska-Ciszewska B, Miszczyk L, Białas B, Fijałkowski M, Plewicki G, Gawkowska-Suwińska M, et al. The effectiveness and side effects of conformal external beam radiotherapy combined with high-dose-rate brachytherapy boost compared to conformal external beam radiotherapy alone in patients with prostate cancer. Radiat Oncol 2015; 10: 60. doi: 10.1186/s13014-015-0366-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roach M, 3rd, Hanks G, Thames H, Jr, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006; 65: 965–74. doi: 10.1016/j.ijrobp.2006.04.029 [DOI] [PubMed] [Google Scholar]
- 21.R Core Team. R: a language and environment for statistical computing. Vienna, Austria; 2015. Available from: http://www.R-project.org/ [Google Scholar]
- 22.Hoskin PJ, Rojas AM, Bownes PJ, Lowe GJ, Ostler PJ, Bryant L. Randomised trial of external beam radiotherapy alone or combined with high- dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol 2012; 103: 217–22. doi: 10.1016/j.radonc.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 23.Brenner MJ, Kaplan ID. Is there any benefit from hypofractionation in external-beam irradiation for prostate cancer? J Clin Oncol 2014; 32: 1851–2. doi: 10.1200/JCO.2013.54.4247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogelius IR, Bentzen SM. Hypofractionated radiation therapy for prostate cancer: more food for thought from recent trial. J Clin Oncol 2014; 32: 1852–1853. doi: 10.1200/JCO.2013.54.8123 [DOI] [PubMed] [Google Scholar]
- 25.Pollack A, Walker G, Horwitz EM, Price R, Feigenberg S, Konski AA, et al. Reply to MJ Brenner, et al. and IR Vogelius et al. J Clin Oncol 2014; 32: 1853–1854. doi: 10.1200/JCO.2013.54.7729 [DOI] [PubMed] [Google Scholar]
- 26.The START Trialists’ Group, Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol 2008; 9: 331–41. doi: 10.1016/S1470-2045(08)70077-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suwinski R, Wzietek I, Tarnawski R, Namysl-Kaletka A, Kryj M, Chmielarz A, et al. Moderately low alpha/beta ratio for rectal cancer may best explain the outcome of three fractionation schedules of preoperative radiotherapy. Int J Radiat Oncol Biol Phys 2007; 69: 793–9. doi: 10.1016/j.ijrobp.2007.03.046 [DOI] [PubMed] [Google Scholar]