Abstract
Objective:
To evaluate the prognostic role of both interim fluorine-18 fludeoxyglucose positron emission tomography (i-18F-FDG-PET) and end-of-chemotherapy fluorine-18 fludeoxyglucose positron emission tomography (eoc-18F-FDG-PET) in patients with early-stage Hodgkin lymphoma (HL).
Methods:
We screened 257 patients with early-stage HL treated with combined modality therapy between March 2003 and July 2011. All were staged using fluorine-18 fludeoxyglucose positron emission tomography (18F-FDG-PET) before chemotherapy and after two doxorubicin, bleomycin, vinblastine and dacarbazine cycles (i-18F-FDG-PET); 165 patients were also evaluated by 18F-FDG-PET at the end of chemotherapy (eoc-18F-FDG-PET).
Results:
After revision, 85% of patients were negative for i-18F-FDG-PET and 15% were positive. After eoc-18F-FDG-PET revision, 23 patients had a positive scan. The median follow-up was 56 months. The 5-year overall survival (OS) and progression-free survival (PFS) for the whole cohort were 97.5% and 95.6%, respectively. For i-18F-FDG-PET-negative and i-18F-FDG-PET-positive patients, the 5-year PFS rates were 98% and 84%, respectively; for eoc-18F-FDG-PET-negative and eoc-18F-FDG-PET-positive patients, the 5-year PFS rates were 97% and 78%, respectively. Combining the i-18F-FDG-PET and eoc-18F-FDG-PET results, the 5-year PFS were 97%, 100% and 82% in negative/negative, positive/negative and positive/positive groups, respectively. The 5-year OS rates were 98% and 83% in eoc-18F-FDG-PET-negative and eoc-18F-FDG-PET-positive patients, respectively; the 5-year OS was 98%, 100% and 83% in negative/negative, positive/negative and positive/positive groups, respectively.
Conclusion:
This study provides additional information on the prognostic role of i-18F-FDG-PET and eoc-18F-FDG-PET in early-stage HL. As data are accumulating and the clinical scenario is rapidly evolving, we might need to rethink the use of 18F-FDG-PET as a prognostic marker for early-stage HL in the near future.
Advances in knowledge:
This study provides additional information on the prognostic role of i-18F-FDG-PET and eoc-18F-FDG-PET in early-stage HL. On the basis of the present data, we may suggest to use eoc-18F-FDG-PET as a strong prognostic marker, especially for patients with i-18F-FDG-PET positivity.
INTRODUCTION
The combination of brief chemotherapy and radiotherapy (RT) has shown excellent results in early-stage Hodgkin lymphoma (HL), with cure rates >90%.1 As the risk of late treatment-related toxicity is still a matter of debate, over the past two decades, a great effort has been made to tailor treatment strategies on the basis of prognostic factors. Studies on advanced stages of HL showed that early interim fluorine-18 fludeoxyglucose positron emission tomography (i-18F-FDG-PET) is the most powerful tool to predict clinical outcome.2–4 Recent retrospective data suggest that i-18F-FDG-PET is prognostic also for patients with early-stage HL undergoing combined modality therapy.5–8 In two large co-operative positron emission tomography (PET)-response–adapted prospective trials,9,10 i-18F-FDG-PET negative-predictive value reached 90%. Although the evidence in favour of the use of i-18F-FDG-PET in early stages is increasing, the role of fluorine-18 fludeoxyglucose positron emission tomography (18F-FDG-PET) at the end of chemotherapy (eoc) before RT [i.e. after four doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD) cycles in most cases] has been poorly investigated. Preliminary studies on eoc-18F-FDG-PET reported a negative-predictive value for 2-year progression-free survival (PFS) of 94–100% for patients who received ABVD,11,12 with a positive-predictive value of 91–92%. Interestingly, Barnes et al12 in retrospective series of 96 patients found no difference in PFS according to i-18F-FDG-PET, whereas eoc-18F-FDG-PET resulted a strong prognostic factor. The ability of both i-18F-FDG-PET and eoc-18F-FDG-PET to predict prognosis is, however, influenced by multiple factors, including (a) timing in relation to chemotherapy, (b) image interpretation, (c) disease stage, (d) bulky disease, (e) chemotherapy scheme and (f) radiation therapy administration. In order to reduce the risk of misinterpretation, the 2007 International Harmonization Project criteria, the 5-point Deauville criteria, the Lugano classification and the 2015 recommendations of the International Conference on Malignant Lymphoma imaging and clinical working groups reset the rules for response evaluation.13–15 The interpretation key proposed by International Conference on Malignant Lymphoma for both interim and end-of-treatment PET is the Deauville 5-point scale, using the classic visual assessment.15,16 A previous collaborative study was able to show that the majority of patients with a positive i-18F-FDG-PET after two ABVD cycles (Deauville scores 4–5) can still be cured by the continuation of chemotherapy followed by radiation.6
The aim of the present study was to analyse a group of patients with early-stage HL treated with combined modality that had both i-18F-FDG-PET and eoc-18F-FDG-PET information, allowing for a comparison of outcomes and prognostic role.
METHODS AND MATERIALS
In this multicentre retrospective study, we reviewed medical records of patients affected with early-stage HL diagnosed between March 2003 and July 2011, all treated with combined modality therapy in four haematology/radiation oncology departments. Full outcome data and i-18F-FDG-PET results of this population were already reported.6 For the present report, we retrieved patients who had both i-18F-FDG-PET (after two cycles, immediately before the third cycle) and eoc-18F-FDG-PET scans. All patients had biopsy-proven early-stage HL, classified as favourable and unfavourable according to the European Organisation for Research and Treatment of Cancer (EORTC) criteria, and were treated with an intention-to-treat chemoradiation programme, with 3–4 cycles of ABVD followed by involved field RT. All patients started chemotherapy with ABVD; the number of cycles and any subsequent chemotherapy modification, after both i-18F-FDG-PET and eoc-18F-FDG-PET, were at the discretion of the treating physicians; all patients received consolidation RT limited to the initial sites of disease presentation; and the radiation dose ranged between 20 and 40 Gy delivered in 1.8 or 2 Gy daily fractions, at the discretion of the treating radiation oncologist. Bulky disease was defined according to the European Organisation for Research and Treatment of Cancer criteria. All 18F-FDG-PET scans (i-18F-FDG-PET and eoc-18F-FDG-PET) were reviewed by local nuclear medicine physicians, blinded for clinical outcome, and independently re-interpreted according to the Deauville 5-point scoring system. Positive scans (PET+) were defined by a Deauville score ≥4 (i.e. uptake moderately more than liver uptake). The institutional review boards approved the study.
Statistical considerations
Categorical variables were reported as absolute and percentage frequency. To verify a change between the paired-data, i-18F-FDG-PET and final 18F-FDG-PET, the McNemar test was used. The ability to predict the final 18F-FDG-PET status from i-18F-FDG-PET was expressed as sensitivity (true-positive rate, proportion of final PET positive correctly identified from positive interim PET) and specificity (true-negative rate, proportion of negative final PET correctly identified from negative interim PET). PFS was defined at the time from the date of diagnosis to the date of last observation, progression, relapse or death from any cause. Overall survival (OS) was defined from the date of diagnosis to the date of last follow-up or death from any cause. Survival estimates were calculated using the Kaplan–Meier method, and the log-rank test was used to compare survival between different cohorts. Cox proportional hazard regression model was used to perform univariate and multivariate analyses. The effect of covariates was reported as hazard ratio (HR), with confidence interval at 95% (95% CI).
RESULTS
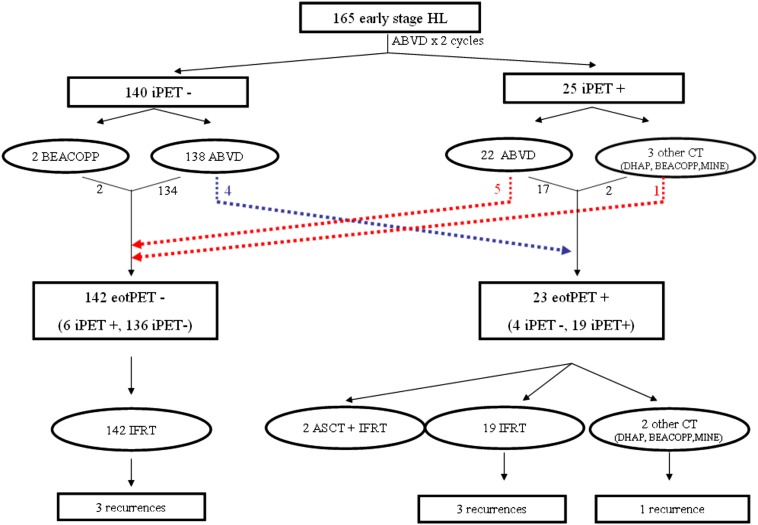
eoc-18F-FDG-PET and i-18F-FDG-PET information were available for 165 out of 257 screened patients. Pre-treatment and treatment characteristics of the whole cohort are described in detail in Table 1. After revision, 140 patients were i-18F-FDG-PET negative (Deauville scores 1–3), and 25 were i-18F-FDG-PET positive (Deauville scores 4–5). Among patients with a negative i-18F-FDG-PET scan, 138 continued with ABVD chemotherapy (4 total cycles) and only 2 patients received different chemotherapy regimens. Among positive patients, 3 were shifted to other chemotherapy regimens (DHAP, BEACOPP and MINE) plus RT, whereas 22 continued with ABVD and RT. After eoc-18F-FDG-PET revision, 23 patients had a positive scan (19 were also positive at i-18F-FDG-PET and 4 changed from negative to positive). Table 2 describes the concordance between i-18F-FDG-PET and eoc-18F-FDG-PET. Four patients turned out to be positive while being negative at interim scan, three with a Deauville score of 4 and one with a Deauville score of 5. Figure 1 describes in detail 18F-FDG-PET results and treatments received for the whole cohort.
Table 1.
Baseline demographic and clinical characteristics
| Characteristics | n | % |
|---|---|---|
| Total number | 165 | 100 |
| Age (years) (median) | 31 (19–67) | |
| Gender | ||
| Male | 72 | 44 |
| Female | 93 | 56 |
| Ann Arbor Stage | ||
| I | 2 | 1 |
| II | 163 | 99 |
| “B” symptoms | ||
| Yes | 34 | 21 |
| No | 131 | 79 |
| Bulky | ||
| Yes | 69 | 42 |
| No | 96 | 58 |
| Number of sites | ||
| 0–3 | 113 | 68 |
| ≥4 | 52 | 32 |
| Favourable | 49 | 30 |
| Unfavourable | 116 | 70 |
| Chemotherapy | ||
| ABVD | 161 | 97 |
| Other | 4 | 3 |
| Number of cycles | ||
| 3 | 16 | 10 |
| 4 | 127 | 77 |
| 5 | 3 | 2 |
| 6 | 19 | 11 |
| Interim PET | ||
| Positive | 25 | 15 |
| Negative | 140 | 80 |
| End-of-chemotherapy PET | ||
| Positive | 23 | 14 |
| Negative | 142 | 86 |
| RT dose (Gy) | ||
| ≤30.6 | 123 | 75 |
| >30.6 | 42 | 25 |
ABVD, adryamicin, bleomycin, vinblastine and dacarbazine; PET, positron emission tomography; RT, radiotherapy.
Table 2.
Distribution of interim fluorine-18 fludeoxyglucose positron emission tomography (i-18F-FDG-PET) and end-of-chemotherapy fluorine-18 fludeoxyglucose positron emission tomography (eoc-18F-FDG-PET) results
| i-18F-FDG-PET | eoc-18F-FDG-PET, n (%) |
Total | |
|---|---|---|---|
| Negative (−) | Positive (+) | ||
| Negative (−) | 136 (96) | 4 (17) | 140 (85) |
| Positive (+) | 6 (4) | 19 (83) | 25 (15) |
| Total | 142 | 23 | 165 |
Spearman test of correlation: 0.76 (p < 0.0001). 4/140 (2.8%) change from i-18F-FDG-PET negative to eoc-18F-FDG-PET positive; 6/25 (24%) change from i-18F-FDG-PET positive to eoc-18F-FDG-PET negative; 155/165 (94%) unchanged. McNemar's exact test, p = 0.754. Sensitivity, 83%; specificity, 96%; receiver–operating characteristic area, 89%; positive-predictive value, 76%; negative-predictive value, 97%.
Figure 1.
Treatment details and outcomes according to both interim fluorine-18 fludeoxyglucose positron emission tomography and end-of-chemotherapy fluorine-18 fludeoxyglucose positron emission tomography. ABVD, doxorubicin, bleomycin, vinblastine and dacarbazine; ASCT, autologous stem cell transplantation; eotPET, positron emission tomography response at the end of treatment; HL, Hodgkin lymphoma; IFRT, involved fields radiotherapy; iPET, positron emission tomography response at interim.
Patients were treated with a large RT dose spectrum. 42/165 (25.4%) patients received a total dose of ≥36 Gy and the remaining 123 (74.6%) received a total dose of <36 Gy. In the eoc-PET-positive group, 18 patients (18/23, 78%) received ≥36 Gy, while the remaining 5 patients received 30 Gy; all 3 relapsed patients received a total dose of ≥36 Gy.
After a median follow-up of 56 months (range 9–163 months), 158 patients were alive without evidence of disease, 1 alive with disease and 6 dead (4 due to disease and 2 due to other causes). Three out of four lymphoma-related deaths occurred in eoc-18F-FDG-PET-positive patients. Among the eoc-18F-FDG-PET-negative patients, three relapses occurred: one died of progressive disease and two obtained a second complete remission after salvage high-dose chemotherapy and autologous transplantation. The 5-year OS and disease-specific survival rate was 97.5% and 98.3%, respectively; 7 out of 165 (4.2%) patients had disease relapse/progression and the 5-year PFS for the whole cohort was 95.6%.
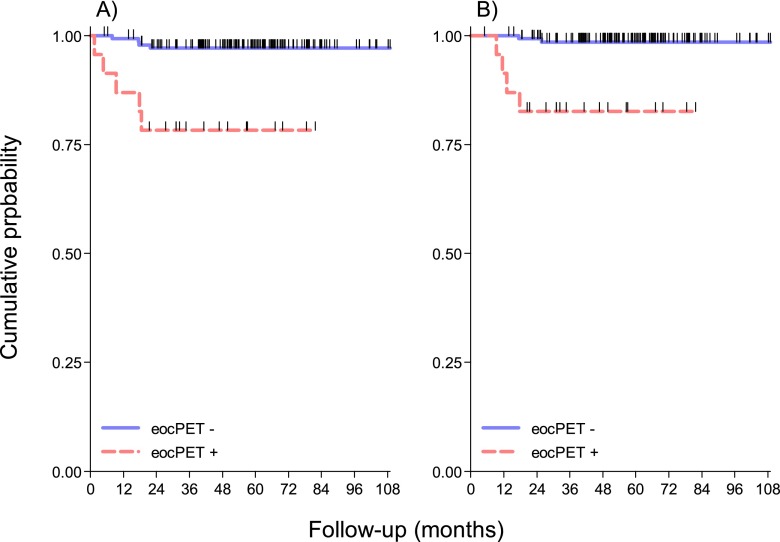
For i-18F-FDG-PET-negative and i-18F-FDG-PET-positive patients, the 5-year PFS rates were 98% and 84%, respectively (p = 0.0014); for eoc-18F-FDG-PET-negative and eoc-18F-FDG-PET-positive patients, the 5-year PFS rates were 97% and 78%, respectively (p < 0.0001). Combining interim and eoc results, the 5-year PFS rate was 97%, 100% and 82% in negative/negative, positive/negative and positive/positive groups, respectively (p < 0.0001). The 5-years OS rates were 98% and 83% in eoc-18F-FDG-PET negative and positive patients (p = 0.0001), respectively; 5-years OS was 98%, 100% and 83% in negative/negative, positive/negative and positive/positive groups, respectively (p < 0.00001). The Kaplan–Meier graphs displaying these findings are shown in Figures 1 and 2.
Figure 2.
(a) Kaplan–Meier projections of progression-free survival, stratified by end-of-chemotherapy fluorine-18 fludeoxyglucose positron emission tomography (eoc-18F-FDG-PET) positivity. Log-rank, p = 0.0001. (b) Kaplan–Meier projections of overall survival, stratified by eoc-18F-FDG-PET positivity. Log-rank p = 0.0001. eocPET, positron emission tomography response at the end of chemotherapy.
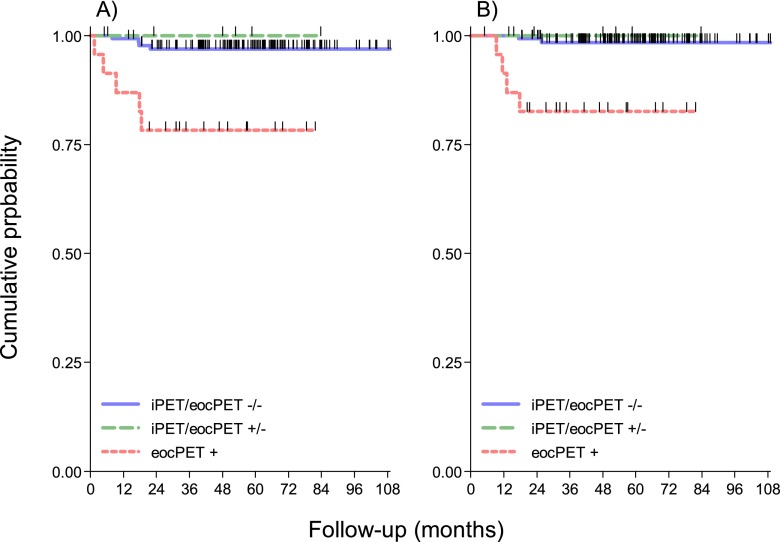
69 patients (69/165, 42%) had bulky disease at presentation; 58/69 (84%) were i-18F-FDG-PET negative (Deauville scores 1–3) and 11/69 (16%) were i-18F-FDG-PET positive (Deauville scores 4–5). After eoc-18F-FDG-PET revision, 10 patients were positive at scan (9 were also positive at i-18F-FDG-PET and 1 changed from negative to positive). In 3 out of 69 (4%) patients, the eoc-18F-FDG-PET result was changed and in 66/69 (96%) was unchanged. The correlation between i-18F-FDG-PET and eoc-18F-FDG-PET in bulky disease was 0.83 (Spearman's test of correlation p < 0.0001). Among patients with bulky disease, 5-year disease-free survival (DFS) and OS were 98% (95% CI 88–100%) and 60% (95% CI 25–83%) (p < 0.0001), and 98% (95% CI 88–100%) and 70% (95% CI 33–89%) (p = 0.0002) for eoc-18F-PET-negative and eoc-18F-PET-positive patients, respectively (Figure 3).
Figure 3.
(a) Kaplan–Meier projections of progression-free survival, stratified by positron emission tomography (PET) response at interim (iPET) or at the end of chemotherapy (eocPET). Log-rank p < 0.0001. (b) Kaplan–Meier projections of overall survival, stratified by fluorine-18 fludeoxyglucose (18F-FDG)-PET response during and after chemotherapy. Log-rank p = 0.00005.
At univariate analysis, B symptoms (p = 0.035), positive i-18F-FDG-PET (p = 0.014) and positive eoc-18F-FDG-PET (0.0001) resulted being associated with a worse OS; the same factors were associated with worse PFS (B symptoms p = 0.0114; positive i-18F-FDG-PET p < 0.0001; eoc-18F-FDG-PET p < 0.0001). Cox regression analysis adjusted by age, B-symptoms, stage, histology and chemotherapy cycles showed that positive eoc-18F-FDG-PET was the only significant predictor of disease recurrence (HR 8.30, 95% CI 1.87–36.9, p = 0.001) and worse OS (HR 11.9, 95% CI 2.07–68.2, p = 0.005). Comparing i-18F-FDG-PET and eoc-18F-FDG-PET results in terms of predictive value on PFS, a significant difference was detected, with integrated area under the curve (AUC) of 0.685 and 0.872 for i-18F-FDG-PET and eoc-18F-FDG-PET, respectively (Wilcoxon test, p = 0.003).
DISCUSSION
In this observational study, we aimed to explore the potential prognostic value of eoc-18F-FDG-PET in addition to early i-18F-FDG-PET in a cohort of 165 patients with early-stage HL who were treated with combined modality therapy. Our findings should be interpreted in light of previous observations on the prognostic role of i-18F-FDG-PET in early-stage HL.5–9 The retrospective nature, the limited number of events and the absence of a centralized PET review represent the major limitations of our study. However, the use of the Deauville score system limited the interobserver variability in imaging interpretation, and local nuclear medicine physicians performed a blinded review.
Moreover, we here provide novel findings on the combination of metabolic information obtained at different time points. To date, i-18F-FDG-PET is frequently used as a prognostic tool in patients treated with ABVD plus RT outside clinical trials. However, the use of this prognostic information is still a matter of debate. In the context of a more conservative strategy, without response adaptation, eoc-18F-FDG-PET may provide only additional prognostic information, without any evidence in support of a change in terms of deintensification or intensification at this step.
Some authors17 questioned the use of both i-18F-FDG-PET and eoc-18F-FDG-PET in this patient population, as the rate of positivity is low (around 15% among favourable and unfavourable patients together); the number of lymphoma-related deaths is small; and the use of i-18F-FDG-PET/eoc-18F-FDG-PET would only provide a little absolute risk difference between a large group of negative and a very small group of positive patients in terms of OS. However, results of previous studies,5–9 as well as the present analysis, suggest a more complex scenario: ABVD plus RT is able to cure approximately 80% of interim-PET-positive patients, but with an unacceptable rate of disease relapse and mortality for non-responders. eoc-18F-FDG-PET-positive patients received higher radiation doses, however, the potential impact of RT dose in this setting is unclear, and unfortunately, we cannot draw any conclusion on this issue from the present data.
Few other investigators also explored the prognostic significance of eoc 18F-FDG-PET positivity. Sher et al11 analyzed 73 patients with HL treated with ABVD and RT; 13 patients out of the 73 (16%) were eoc-18F-FDG-PET positive before RT. Nine of these patients (69%) achieved a complete remission after RT, while four (31%) relapsed. Among the PET-negative group (60 patients), 57 (97%) remained in continuous complete remission while 3 relapsed. All three patients who relapsed despite eoc-18F-FDG-PET negativity obtained a continuous complete remission with a second line therapy, and only one of four in the PET-positive group, with a consequent inferior OS. We cannot provide data on response to second-line chemotherapy for our cohort, but the failure rate was significantly higher and OS projections were inferior in our study as well.
The latter finding suggests the potential ability of eoc-18F-FDG-PET to select patients at a worse prognosis among the cohort of i-18F-FDG-PET positive, with a higher predictive value on PFS of eoc-18F-FDG-PET as showed by Wilcoxon test with an integrated area under the curve of 0.685 and 0.872 for i-18F-FDG-PET and eoc-18F-FDG-PET, respectively (p = 0.003). Besides, despite i-18F-FDG-PET may be an attractive surrogate tool for response, the rate of positive/negative patients (6/25, 24%) raises significant concerns on treatment intensification at this stage. Patients who were converted from positive into negative at the end of treatment experienced similar outcomes as their i-18F-FDG-PET-negative counterparts. Therefore eoc-18F-FDG-PET may be beneficial in reducing the proportion of patients receiving intensified treatment on the basis of i-18F-FDG-PET results, and either as a single measurement or in combination with i-18F-FDG-PET adds important prognostic information, being the most relevant prognostic factor at Cox regression analysis. The recently presented results of the H10 trial (PET-positive arms) might partially change this scenario. i-18F-FDG-PET-positive patients who received BEACOPP-esc (escalated cyclophosphamide, doxorubicin, etoposide, procarbazine, prednisone, vincristine, bleomycin) plus RT had clearly superior PFS rates if compared with the continuation of ABVD plus RT (5-year PFS rate of 91% vs 77%; p = 0.002).18 This huge difference in PFS questions the continuation of ABVD plus RT in favour of an intensified chemotherapy regimen followed by the same RT protocol. One may argue that a proportion of these patients could be overtreated (approximately 15% of the i-18F-FDG-PET-positive patients, according to the present study), but the expected global PFS is certainly higher. The information obtained by i-18F-FDG-PET and eoc-18F-FDG-PET could be therefore useless if the treatment strategy is unlikely to be changed, limiting its indications to patients with unfavourable presentations where early intensification is proposed if positive (as for H10 PET positive). Conversely, if i-18F-FDG-PET is planned and performed only in order to have prognostic information, the indication for a second eoc-18F-FDG-PET in the minority of i-18F-FDG-PET-positive patients (15%) may provide additional knowledge.
In this regard, the integration of new PET-based quantitative methods for measuring tumour burden, as for example total metabolic tumour volume or total lesion glycolysis,19 as well as other clinical and biological markers, are currently being developed for a better risk stratification and personalized therapy and will probably be used at the time of i-18F-FDG-PET response evaluation. In conclusion, this retrospective study provides additional information on the prognostic role of i-18F-FDG-PET and eoc-18F-FDG-PET in patients with early-stage HL treated with combined modality therapy. On the basis of the present data, we may suggest to use eoc 18F-FDG-PET as a strong prognostic marker especially for patients with i-18F-FDG-PET positivity. However, major uncertainties remain on the indication, interpretation criteria and exact role of 18F-FDG-PET in this setting. As data are accumulating and the clinical scenario is rapidly evolving, we might need to rethink the use of 18F-FDG-PET as a prognostic marker for early-stage HL in the near future.
Contributor Information
Patrizia Ciammella, Email: patrizia.ciammella@asmn.re.it.
Andrea Riccardo Filippi, Email: andreariccardo.filippi@unito.it.
Gabriele Simontacchi, Email: gabriele.simontacchi@unifi.it.
Michela Buglione, Email: buglione@med.unibs.it.
Barbara Botto, Email: bbotto@cittadellasalute.to.it.
Monica Mangoni, Email: monica.mangoni@unifi.it.
Cinzia Iotti, Email: cinzia.iotti@asmn.re.it.
Francesco Merli, Email: francesco.merli@asmn.re.it.
Luigi Marcheselli, Email: luigi.marcheselli@unimore.it.
Gianni Bisi, Email: gianni.bisi@unito.it.
Umberto Ricardi, Email: umberto.ricardi@unito.it.
Annibale Versari, Email: annibale.versari@asmn.re.it.
REFERENCES
- 1.Engert A, Plütschow A, Eich HT, Lohri A, Dörken B, Borchmann P, et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med 2010; 363: 640–52. doi: 10.1056/NEJMoa1000067 [DOI] [PubMed] [Google Scholar]
- 2.Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, et al. Early interim 2-[18F]fluoro-2-deoxy-d-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin's lymphoma: a report from a joint Italian–Danish study. J Clin Oncol 2007; 25: 3746–52. doi: 10.1200/JCO.2007.11.6525 [DOI] [PubMed] [Google Scholar]
- 3.Hutchings M, Loft A, Hansen M, Pedersen LM, Buhl T, Jurlander J, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood 2006; 107: 52–9. doi: 10.1182/blood-2005-06-2252 [DOI] [PubMed] [Google Scholar]
- 4.Zinzani PL, Tani M, Fanti S, Alinari L, Musuraca G, Marchi E, et al. Early positron emission tomography (PET) restaging: a predictive final response in Hodgkin's disease patients. Ann Oncol 2006; 17: 1296–300. doi: 10.1093/annonc/mdl122 [DOI] [PubMed] [Google Scholar]
- 5.Cerci JJ, Pracchia LF, Linardi CC, Pitella FA, Delbeke D, Izaki M, et al. 18F-FDG PET after 2 cycles of ABVD predicts event-free survival in early and advanced Hodgkin lymphoma. J Nucl Med 2010; 51: 1337–43. doi: 10.2967/jnumed.109.073197 [DOI] [PubMed] [Google Scholar]
- 6.Filippi AR, Botticella A, Bellò M, Botto B, Castiglione A, Gavarotti P, et al. Interim positron emission tomography and clinical outcome in patients with early stage Hodgkin lymphoma treated with combined modality therapy. Leuk Lymphoma 2013; 54: 1183–7. doi: 10.3109/10428194.2012.735667 [DOI] [PubMed] [Google Scholar]
- 7.Simontacchi G, Filippi AR, Ciammella P, Buglione M, Saieva C, Magrini SM, et al. Interim PET after two ABVD cycles in early-stage Hodgkin lymphoma: outcomes following the continuation of chemotherapy plus radiotherapy. Int J Radiat Oncol Biol Phys 2015; 92: 1077–83. doi: 10.1016/j.ijrobp.2015.04.021 [DOI] [PubMed] [Google Scholar]
- 8.Rigacci L, Puccini B, Zinzani PL, Biggi A, Castagnoli A, Merli F, et al. The prognostic value of positron emission tomography performed after two courses (INTERIM-PET) of standard therapy on treatment outcome in early stage Hodgkin lymphoma: a multicentric study by the fondazione italiana linfomi (FIL). Am J Hematol 2015; 90: 499–503. doi: 10.1002/ajh.23994 [DOI] [PubMed] [Google Scholar]
- 9.Raemaekers JM, André MP, Federico M, Girinsky T, Oumedaly R, Brusamolino E, et al. Omitting radiotherapy in early positron 607emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 2014; 32: 1188–94. doi: 10.1200/JCO.2013.51.9298 [DOI] [PubMed] [Google Scholar]
- 10.Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin's lymphoma. N Engl J Med 2015; 372: 1598–607. [DOI] [PubMed] [Google Scholar]
- 11.Sher DJ, Mauch PM, Van Den Abbeele A, LaCasce AS, Czerminski J, Ng AK. Prognostic significance of mid- and post-ABVD PET imaging in Hodgkin's lymphoma: the importance of involved-field radiotherapy. Ann Oncol 2009; 20: 1848–53. doi: 10.1093/annonc/mdp071 [DOI] [PubMed] [Google Scholar]
- 12.Barnes JA, LaCasce AS, Zukotynski K, Israel D, Feng Y, Neuberg D, et al. End-of-treatment but not interim PET scan predicts outcome in nonbulky limited-stage Hodgkin's lymphoma. Ann Oncol 2011; 22: 910–15. doi: 10.1093/annonc/mdq549 [DOI] [PubMed] [Google Scholar]
- 13.Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. ; Imaging Subcommittee of International Harmonization Project in Lymphoma. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol 2007; 25: 571–8. doi: 10.1200/JCO.2006.08.2305 [DOI] [PubMed] [Google Scholar]
- 14.Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymphoma 2009; 50: 1257–60. doi: 10.1080/10428190903040048 [DOI] [PubMed] [Google Scholar]
- 15.Gallamini A, Barrington SF, Biggi A, Chauvie S, Kostakoglu L, Gregianin M, et al. The predictive role of interim positron emission tomography for Hodgkin lymphoma treatment outcome is confirmed using the interpretation criteria of the Deauville five-point scale. Haematologica 2014; 99: 1107–13. doi: 10.3324/haematol.2013.103218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheson BD. Staging and response assessment in lymphomas: the new Lugano classification. Chin Clin Oncol 2015; 4: 5. doi: 10.3978/j.issn.2304-3865.2014.11.03 [DOI] [PubMed] [Google Scholar]
- 17.Adams HJA, Kwee TC. In regard to Simontacchi et al. Int J Radiat Oncol Biol Phys 2015; 93: 724–5. doi: 10.1016/j.ijrobp.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 18.Early FDG-PET adapted treatment improves the outcome of early FDG-PET-positive patients with Stages I/II Hodgkin lymphoma (HL): final results of the randomized intergroup EORTC/LYSA/FIL H10 Trial. Clin Adv Hematol Oncol 2015; 13: 16–17. [Google Scholar]
- 19.Kanoun S, Tal I, Berriolo-Riedinger A, Rossi C, Riedinger JM, Vrigneaud JM, et al. Influence of software tool and methodological aspects of total metabolic tumor volume calculation on baseline [18F]FDG PET to predict survival in Hodgkin lymphoma. PLoS One 2015; 16: e0140830. doi: 10.1371/journal.pone.0140830 [DOI] [PMC free article] [PubMed] [Google Scholar]