Abstract
In the setting of mass casualty incidents (MCIs), hospitals need to divert from normal routine to delivering the best possible care to the largest number of victims. This should be accomplished by activating an established hospital disaster management plan (DMP) known to all staff through prior training drills. Over the recent decades, imaging has increasingly been used to evaluate critically ill patients. It can also be used to increase the accuracy of triaging MCI victims, since overtriage (falsely higher triage category) and undertriage (falsely lower triage category) can severely impact resource availability and mortality rates, respectively. This article emphasizes the importance of including the radiology department in hospital preparations for a MCI and highlights factors expected to influence performance during hospital DMP activation including issues pertinent to effective simulation, such as establishing proper learning objectives. After-action reviews including performance evaluation and debriefing on issues are invaluable following simulation drills and DMP activation, in order to improve subsequent preparedness. Historically, most hospital DMPs have not adequately included radiology department operations, and they have not or to a little extent been integrated in the DMP activation simulation. This article aims to increase awareness of the need for radiology department engagement in order to increase radiology department preparedness for DMP activation after a MCI occurs.
INTRODUCTION
In a mass casualty incident (MCI), the number of casualties by definition overwhelms available resources. There is no specified number of victims to define a MCI, since the number of victims at which resources become overwhelmed depends on baseline capacity.1 In MCI events, the care paradigm shifts from the greatest good for each individual to the greatest good for the greatest number of victims, potentially resulting in focusing care only on the portion of the affected patients most likely to benefit.2,3 MCI events usually present a serious threat to the health of the community, disrupting normal services and requiring implementation of special procedures by paramedical services, primary care organizations and hospitals.4 Therefore, the World Health Organization also uses the definition of a MCI as an event resulting in a number of victims great enough to disrupt the normal course of emergency and health care services.5
MCIs can be of natural origin, such as tornados, flooding, earthquakes or pandemics, or can be man made, either unintentional or intentional. Examples of unintentional man-made MCIs are transportation accidents such as bus, plane or train crashes, factory explosions or gas leaks. Examples of intentional MCIs are acts of terrorism, such as mass shootings or detonation of explosives, which may release biologic, chemical or radioactive agents.3 The reported annual number of natural MCIs alone has quadrupled in the past three decades, with some suggesting that a disaster occurs somewhere in the world every day, stressing the need to be prepared.6,7 Some well-known examples on long lists of just the largest MCIs since 2000 are: the 9/11 Twin Tower attack (New York, 2001), the Indian Ocean tsunami (2004), the Madrid train bombings (2004), the London bombings (2005), the hurricane Katrina (New Orleans, 2005), the Christchurch (NZ) earthquake (2011), the Tōhoku earthquake and tsunami (Japan, 2011), the Hurricane Sandy (New York, 2012), the Rana Plaza collapse (Bangladesh, 2013), the Ebola outbreak (West Africa, 2014), the Mina stampede (Mecca 2015) and the Paris terrorist shootings (2015).
After the occurrence of a MCI, pre-hospital care on scene involves triaging victims into categories giving consideration to severity of injury, likelihood of survival and available resources. Those needing immediate medical attention in order to survive are coded T1 (tagged red), those needing further medical attention but who are able to wait are coded T2 (tagged yellow) and those with minor injuries, also called the “walking wounded”, are coded T3 (tagged green). Additional categories exist but are not universally used, such as T4 (tagged black) for those unlikely to survive or in need of too many resources. As soon as logistics allow, T1 (red) and T2 (yellow) victims are transported to hospitals in the region, where they undergo renewed triage, further evaluation and treatment as deemed necessary (Table 1).
Table 1.
Suggestions for imaging type and location per triage code
| Triage code | Medical attention | Imaging | Location |
|---|---|---|---|
| T1—red | Immediate | WBCT or angio-intervention, if needed | Radiology ED, angiosuite |
| T2—yellow | Urgent, able to wait | WBCT or ultrasound, radiography, selected CTa | Radiology ED |
| T3—green | Walking wounded | Selective imaging | Radiology department |
| T4—black | Unlikely to survive | No imaging |
Radiology ED, radiology facilities located in or next to the emergency department; WBCT, whole-body computer tomography.
Imaging strategy dependent on local facilities and capacity.
Highest rate of triage errors occur in T1 and T2 groups, the role for imaging is biggest in these groups.
Although MCIs vary greatly in type and are reasonably rare, they can occur anywhere at any given moment. The ability to manage a sudden influx of injured patients at any time relies on well-trained and drilled personnel.3 Delivery of care in response to MCI events differs in many respects from the routine care provided on a daily basis.1 Therefore, all hospitals should have a disaster management plan (DMP) in place to be activated after notification of a MCI occurrence. Hospitals should practice DMP execution, followed by debriefing on specific training goals and analysis of areas that can be improved. This not only improves disaster preparedness, but can improve daily emergency care as well.8
Imaging examinations including ultrasound, radiography (XR) and CT can be used to improve the accuracy of triage in MCIs. Imaging utilization has been reported as high as 93% of victims in one study in the military setting of three explosive MCIs in Iraq in 2008 and 72% of victims in a large civilian airplane crash in 2009 in the Netherlands, exceeding numbers previously reported in literature.9,10 Imaging providers must therefore be prepared to support treatment teams during DMP activation after MCIs. However, in the multinational, multi Level 1 trauma centre experience of the authors, radiology departments are underrepresented in—and in many cases excluded from—the DMPs of hospitals or associated drills.
This article aims to provide insight into the role of a radiology department in preparation for and during the aftermath of a MCI. What role does imaging have in the work-up of MCI victims? What does a radiologist need to know and do? How should imaging logistics be incorporated in preparedness planning? Do we need to amend our normal routine? How should radiology personnel be trained to reach maximal performance? These are the questions that will be answered below.
Sequence of events after a mass casualty incident
After a MCI occurs, local authorities typically institute a regional command centre to co-ordinate the logistics of health care provision to the victims. Hospital administration is notified of the MCI, at which point they activate the hospital DMP, or alternatively named standard operating procedure. The role of the hospital is pre-defined in agreement with the broader regional health care coalition. The initiation of the DMP activates a Disaster Command Committee (DCC) within the hospital, usually consisting of board directors, departmental chairs and high-level administrative personnel. It is of utmost importance to have radiology representation in this team. The DCC communicates with the regional command centre about the hospital capacity and expected numbers of incoming patients. DCC team members relay information to co-ordinating personnel in the active care areas, including the lead radiologist, the lead traumatologist and lead emergency physician. This is usually performed at set time intervals, e.g. every 30 min, to respond quickly to new information.
After DMP activation by the administration, the hospital prepares for a potential sudden influx of patients beyond the normal capacity, termed “surge”. During daytime, this will imply reorganizing scheduled care. Preparatory measures, especially during off hours, include calling in extra personnel, usually by automated means such as group pages or other messaging, which must be regularly checked to remain up to date. Key locations in the hospital are rapidly prepared for an influx of severely injured patients (generally meaning those coded “T1” or “tagged red”), involving preparation of the emergency department (ED), operating rooms (ORs) and intensive care unit, which often requires evacuation of current patients to other care locations. Adequate preparation and training is required to ensure that this process is efficient, regardless of the time of the day or day of the week.
It takes time to evaluate the situation at the scene of the MCI and to determine the exact nature and estimated number of victims. It is not uncommon for the official declaration of a MCI and hospital notification to take more than 30 min, even if the event has occurred in a densely populated area such as the city of London.11 As a result, hospital staff are often first aware of a disaster through electronic communication well before the MCI is officially reported to the hospital.12 Patients may start to arrive at the hospital ED before the ideal sequence as described has taken place. The closer the hospital is to the site of the MCI, the sooner self-referred patients or “walking wounded”, mostly with minor injuries, may arrive. These more stable patients often escape triage at the scene, and typically comprise the majority of patients, with T1-coded patients making up only 10–15%.1,12,13 For this reason, the hospital closest to the MCI often becomes the designated facility for the “walking wounded”, with the patients who underwent triage at the scene transported to other nearby hospitals. More remote hospitals have more lag time before receiving patients, time that should be used with maximal efficiency to prepare personnel and equipment.
The surge in patient volume after immediate onset MCIs such as explosions or accidents often peaks within 60–90 min, and most patients arrive within 2–4 h.13 So-called surge equilibrium occurs as soon as the response of staff, space and supplies match the needs of patients.14 This does not yet mean that the impact of the incident is over. The decision to scale down and deactivate the DMP is made by the DCC as soon as the hospital can resume normal function. However, after scaling down, hospital staff must be cautious not to let their guard down. In the first days following the acute surge, there is higher than normal demand on resources, including imaging studies for follow-up. Furthermore, it cannot be stressed enough how important it is to acquire feedback from all personnel involved, who should be thanked for their contribution and encouraged to disclose any issues that arose to the leadership.
CAPACITY ISSUES
Surge capacity refers to the ability to evaluate and care for an increased volume of patients that challenges or exceeds normal operating capacity.15 There is usually no way to observe surge capacity in “real life”, which means it must be calculated using known figures—such as OR and intensive care unit capacity, number of available ED bays and hospital beds etc.—and through the use of simulations and statistical models.8,15–17 Even if surge capacity is determined in a simulated disaster drill, it remains uncertain if the results would be applicable after a real MCI.
There is also a surge capacity for radiology.18 It is specific to each imaging modality and should be determined by conducting regular exercises and simulations. Assessment should include the number of examinations that can be performed simultaneously or in a given time period. Evaluation should include time needed for image transfer, time for image reading and reporting, time for patients to be transferred to and from radiology areas, the capacity of patient waiting areas and so on. Each imaging modality should undergo a throughput analysis to determine the maximum capacity, although it must be understood that throughput in a real situation depends on specific examinations, the ability of patients to co-operate and other factors that may be difficult to measure or account for.
One specific consideration for radiology services is the number of personnel needed to operate the imaging devices. Whenever possible, simultaneous use of more than one device is preferable to keep patient throughput at a high level. Particularly during after hours and on weekends, there is often a shortage of available hospital personnel. Additional personnel should thus be contacted and called in as soon as the hospital is aware of incoming MCI patients. Computerized automatic alarming systems are preferred to human telephone operators because they are able to send many calls in a short time period.19 The number of staff available directly influences radiology surge capacity in terms of how many different modalities and imaging devices can be operated simultaneously.
Imaging protocols need to be optimized and standardized to achieve maximal efficiency and shortest imaging examination durations. There have been some observational reports of the time needed for different imaging modalities in trauma patients.10,20 Depending on the imaging methods and algorithms chosen, significant time reduction can be achieved for imaging examinations. Imaging during MCI patient work-up needs to be straightforward, robust and as comprehensive as possible. Imaging protocols need to be devised to meet these requirements, especially for CT, which can be performed by scanning tests with dummies. Suggested amendments in imaging protocols for CT in MCI patients are described in the CT sections below.
ROLE OF IMAGING
Field triage and need to verify/correct triage categories
After a MCI, field triage takes place as described before by paramedics and other staff at the site using methods such as Simple triage and rapid treatment or similar algorithms.2,21–23 Although these algorithms are well established, a recent review reported only moderate accuracy.24 All existing algorithms do produce a considerable rate of undertriage (falsely lower category) and overtriage (falsely higher category).23,25,26 Frykberg27 analysed overtriage rates and mortality in several MCIs and found an almost linear positive correlation between the overtriage rate and mortality. Although undertriage results in poor individual outcome rates and mortality, overtriage blocks critical resources such as ORs and results in high overall mortality.
Self-evacuated and walk-in casualties who were not triaged at the scene must also be considered. In a report of the Boston Marathon bombing, the authors observed that >50% of all victims who arrived did not have a triage code badge.12 To avoid missing triage class assignments and reduce incorrect assignments that may have changed during transport, all MCI patients should be retriaged in the admission area.
Patient selection for imaging
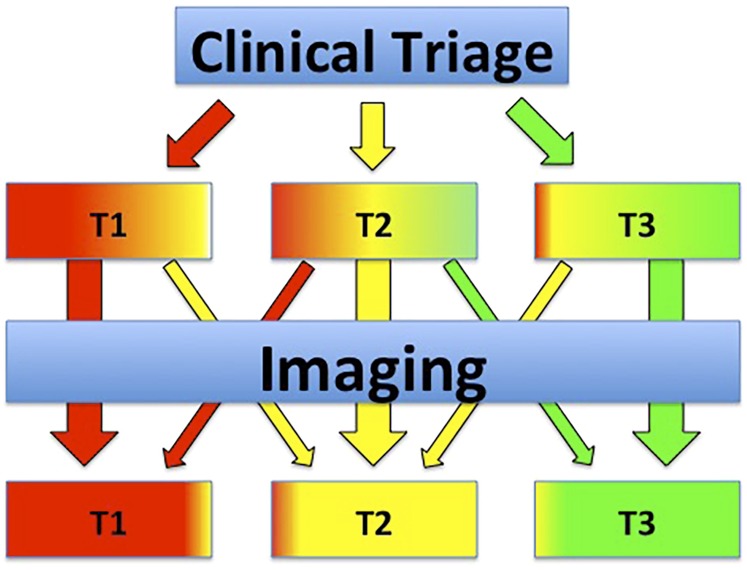
Before casualties are transported to radiology department facilities, they must undergo assessment by a triage team, if they have not yet been assigned a triage category. This is an absolute key point in maintaining availability of radiology (and other) resources for other more urgent patients.3 Victims triaged to category green (T3) should not undergo any imaging as long as more urgent patients are expected to arrive in the radiology department. Patients tagged yellow (T2) typically need at least some basic imaging to detect if they are undertriaged (correct to red, T1) or overtriaged (correct to green, T3). In patients tagged red, imaging should be limited if they need to be transferred quickly to the OR (pericardial effusion, large hemothorax etc.). If the patient is stable enough, whole-body CT may be performed to detect all possible fatal injuries with high accuracy. Some patients have injuries so severe that they cannot be saved during the surge period without blocking resources for other patients with a higher probability of survival.28 These cases must be discussed with the team leader; if the chance of survival is too low, no further intervention or imaging should be undertaken (Table 1).29 In essence, the role of imaging in MCI patients is to increase accurate triage, thereby reducing resource burden and mortality (Figure 1). Owing to limitations in capacity, imaging should be reserved for those patients in whom it will alter management and should be performed according to urgency.
Figure 1.
Schematic depiction of the role of imaging in the selected group of mass casualty incident patients needing imaging for urgent treatment decisions. The role of imaging in this patient subset is to increase the accuracy of triage to minimize resource burden and patient mortality. Increased homogeneity of the colour in the triage category boxes for T1, T2 and T3 resembles higher percentage of correctly coded patients after imaging, resulting in more proper resource allocation.
The chief radiologist in charge in the hospital after DMP activation should be integrated into the DCC team on the same level as the chiefs of surgery, emergency medicine and anaesthesiology to properly organize overall supply of personnel and equipment. There should be direct communication with the radiology team leaders in the care areas, who distribute the patients between the available imaging modalities. The lead radiologist must also be in contact with the technologists and must be aware of devices in use and those that remain free for incoming patients.
Ultrasound
There have been some reports of ultrasound use by paramedics during field triage using hand-held devices that indicate that ultrasound can be helpful to detect life-threatening conditions such as severe abdominal bleeding and pneumothorax and make triage categorization more accurate.30,31 On the other hand, ultrasound has some limitations as well as a very broad range of reported sensitivity and specificity for detecting free fluid and injuries in patients with major trauma.32 In a MCI simulation study looking for free abdominal fluid detected by Focused assessment with sonography in trauma (FAST, also known as focused abdominal sonography for trauma), the authors observed a false-positive (=overtriage) rate of 59% and a sensitivity of 67%.31 The average time needed for a FAST examination was about 2 min.
During in-hospital triage of incoming victims, FAST can be also used to confirm correct assignment of triage codes. All of the above-mentioned limitations apply in this scenario, but there is also a chance that ultrasound in the ED might form a significant bottleneck because most EDs have only a limited number of available ultrasound machines. Ultrasound is inappropriate for injuries to other body regions in a DMP-activated setting because of the time needed for special procedures such as Doppler and vascular examinations. Nonetheless, when used, images of identified injuries can be printed and attached to triage cards for documentation.
Radiography
XR is reported to be the imaging modality most frequently ordered in MCI patients, with chest radiographs, spine and extremities being the most common.33–35 Although XR is available at any hospital with a dedicated ED, the maximum capacity of patients that can be examined simultaneously may be limited by the number of rooms, devices (such as X-ray generators, plate readers or printers) and technical staff. Brunner et al34 reported that the duration for XR examinations after the Boston bombing took twice as long as the 12-month average before that event. In MCI patients, XR access may thus make it impossible to follow typical trauma procedures for imaging critically ill patients (i.e. triaged as red and yellow) with chest, cervical spine (C-spine) and pelvic radiographs in addition to other regions with penetrating injuries. Doing so would likely result in quick overcrowding of the radiology department and queues of critical patients waiting for XR imaging.36
In addition to operational issues of access, XR has limitations in detecting serious injuries. In a multicentre study of patients with serious blunt torso trauma, CT detected occult injuries not suspected by chest XR in 71% of patients, about 38% of which required further intervention.37 In another study, 69% of pneumothoraces were occult on XR.38 The American College of Radiology39 appropriateness criteria for C-spine trauma no longer indicate XR as a first line test because of its limited sensitivity and specificity. XR plays no role in patients with head or abdominal trauma because injuries to parenchymal organs such as the brain or liver cannot be diagnosed.
CT
CT is the imaging modality of choice in patients with major or multisystem trauma (polytrauma). Patients undergoing standardized whole-body CT have been found to have significantly better outcomes.40 CT can also detect indirect injuries that may not be suspected such as blunt traumatic injuries caused by the blast wave after explosions.9,41
The potential use of CT as a triage tool for MCI patients was first described in 2006, when the introduction of a dedicated CT whole-body protocol (head to pubic symphysis) during simulation increased throughput from 2.4 to 6.7 patients per hour.28 In two subsequent studies by the same team, CT examinations could be further increased up to 11 patients per hour.18,42 In both studies, the number of casualties arriving per hour was lower than the capacity for CT examinations. A recent report of the Boston Marathon bombing demonstrated reduced CT turnaround times accomplished by making additional scanners available.34 With appropriate availability, CT can consequently help to correct errors in prior triage and to prioritize need for further interventions and operations by reducing the overtriage rate.
One disadvantage of routine CT in MCI patients is the large number of images that are generated by whole-body CT examinations. To have all images viewable after a short period of time, dedicated three-dimensional (3D) viewing workstations with capacity to do secondary image reformations are very helpful, because the images do not have to be reformatted and processed by a technologist. This approach reduced technologist image processing time by 50% in a simulation study.42 In addition, reduction in the number of processed images speeds image transfer to picture archiving and communication system (PACS). Information technology issues related to network architecture and structure should be assessed in terms of reliability and speed when planning radiology department procedures for DMP activation conditions.
CT imaging protocols
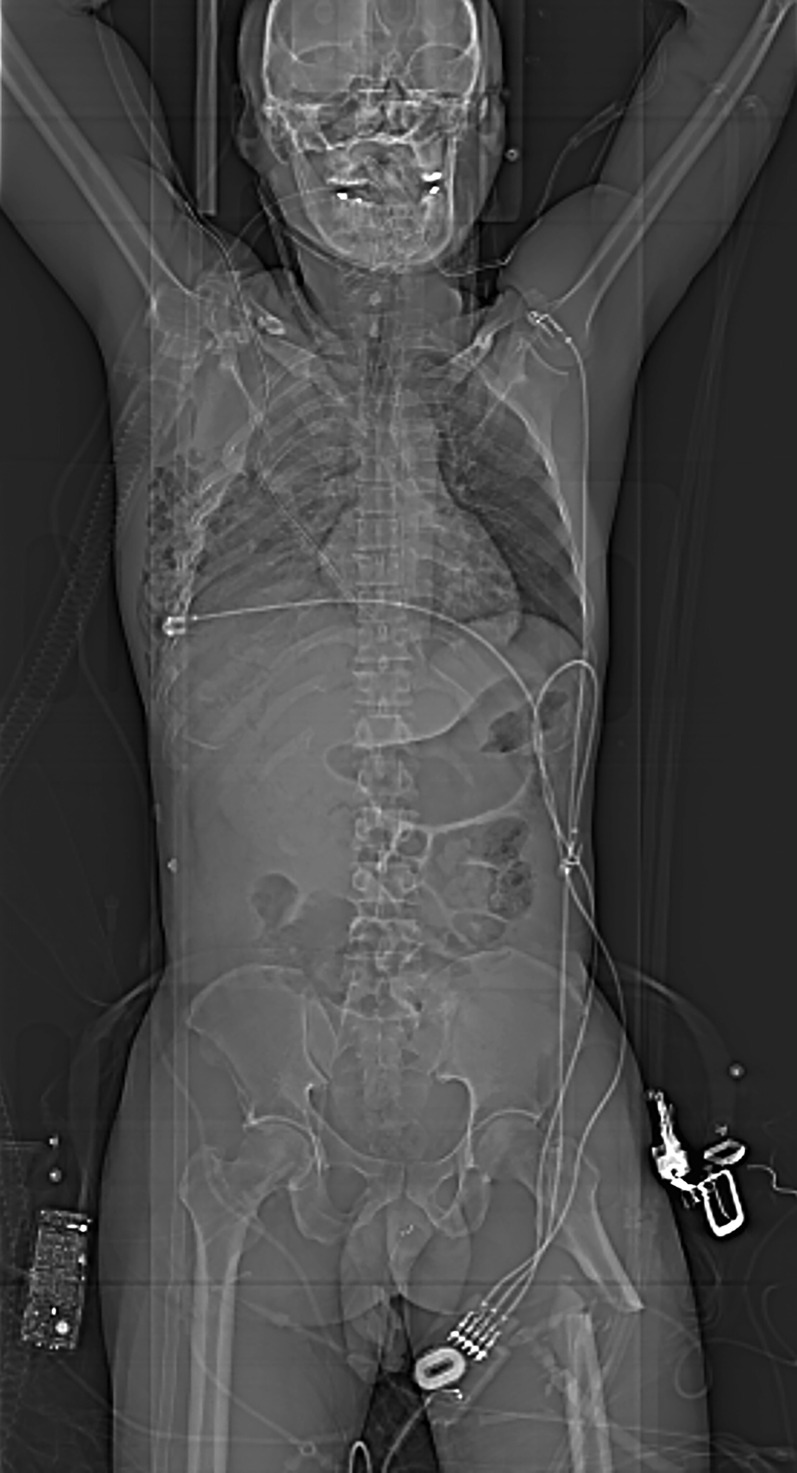
By screening with the planning CT projection radiograph images (called the scout, topogram, surview or planogram by different manufacturers), extremity fractures can be detected and the CT examination can be extended to cover injured body parts. There are some reports that standard reading of planning images reveals diagnoses of importance that would otherwise have been missed or not included in the CT scan volume.43–45 Whether the planning images are sufficient to eliminate XR examinations prior to CT is a matter of debate. However, in severely injured patients undergoing (near) total-body CT, a planning image may well suffice and conventional radiographs may then be reserved to regions not covered by the CT scan, such as the extremities, in order to save time (Figure 2).
Figure 2.
Planning projection radiograph obtained during a whole-body trauma CT, demonstrating serial rib fractures with pneumothorax and subcutaneuous emphysema on the right side, partial atelectasis of the right upper lung and a displaced proximal left femoral shaft fracture. The pelvis and spine show no obvious signs of injuries.
After planning images, a standardized CT protocol from head to pubic symphysis may be performed in every severely injured patient scheduled for CT. Attempts to limit CT coverage to certain body parts require time-consuming discussion. The more comprehensive standardized approach in the setting of MCI patients can reduce time to CT as well as the need for subsequent repeat visits to the CT suite. Lastly, in DMP activation, procedures should be exceptionally clear with minimal risk of confusion. The CT protocol should be optimized for time and robust image quality more than to high-end imaging. In older CT scanners, tube overheating is a serious problem.28 Reducing tube current can help to avoid this issue, though image quality will suffer. The head CT should be a non-enhanced scan, and helical scanning is preferred to a sequential (axial) scan mode. To save time, the torso CT (C-spine to pubic symphysis) may be performed in a single pass at an early venous phase without the use of bolus tracking or other features that may add preparation time. As an alternative, a fixed delay split-bolus technique combining arterial with portovenous phase could be used.46 To keep the image count low during the initial influx with patients still arriving, axial slices of 3- to 5-mm thickness should be first sent to PACS in a soft-tissue kernel. Secondary reconstructions can be performed later on specific request by the radiologist or to aid in final reporting after the influx abates. If there is a three-dimensional workstation linked to the CT machine, image review may be performed there from thin-section images because reformations can be performed rapidly.42
MR tomography, angiography and other modalities
There is no role for MR examinations during the surge period after a MCI when patients are overcrowding the admittance area. MR is time consuming, with limited capacities and available examination slots in most EDs, and patient safety cannot be ensured after any MCI with explosions and possible shrapnel injuries.
Interventional angiography can play a significant role in damage control of severe bleeding, but diagnostic angiography should typically be replaced by CT angiography when vascular imaging is needed. Although angiography suite staffing and availability are typically limited during the surge period, in appropriate circumstances, these interventions may be used to relieve pressure on OR capacity.
Image reading, reporting and communication of findings
Interpretation and reporting of the expected flood of images is a challenge for the involved radiologists. Reading high numbers of images under time pressure over a long period may result in diagnostic errors, and increasing radiologist staffing or dual reading is encouraged whenever possible. One advantage of CT is that images acquired during the CT triage examination can easily be stored in PACS for secondary review, and further image reconstructions and reformations may be performed after the surge of incoming patients has stopped. This may enable detection of subtle injuries not identified during the initial interpretation that may be important for the further treatment of patients.47
Examination reports need to be linked to the specific patients, which can be challenging in DMP-activated circumstances. A card with a unique identification code is often attached to patients triaged prior to arrival by an outside triage team, but this card may become detached or lost.18,34 One robust method to identify patients is to write the code on the patient's skin.3 There have been reports of internet-based or other electronic patient identification systems, but all require a working infrastructure including electricity, network connectivity or at least batteries. Depending on the mechanism of the MCI, these resources may be limited such that devices may not work properly.48,49
In a number of reports from simulated and real MCI events, hand-written preliminary reports have shown to be very useful because they are obtained quickly, can be copied and attached to the patient or the stretcher.3,18,34 In a study that simulated image reading with historic cases during a DMP activation drill, the number of hand-written reports produced per hour ranged from 7 to 8 per hour per reading team, which was lower than the theoretical maximum number of 9–10 per hour.18 Preliminary reports may be shortened by focusing on the most severe injuries that require rapid intervention, excluding other less important findings in the preliminary report. Pre-made forms can be used that allow the most important injury types to be identified by checking boxes, along with their locations. This preliminary paper report of major injuries can be rapidly produced, with a copy kept with the patient and treatment team, and a carbon copy kept in radiology for completion reporting at a later time.
Injuries that need immediate intervention must be communicated orally to the team leader. This is the fastest and most reliable way to communicate in a surge situation. When reports are handwritten, there is no possibility to look up findings in an electronic record, thus oral communication is essential.
SIMULATION STRATEGIES
Most critical care regions and individual hospitals within those regions organize MCI simulation trainings or drills to enhance their skills in dealing with these events. The prevalent use of imaging in the evaluation of trauma patients makes it imperative to consider its use in MCI patient evaluation. Radiology department logistics may create a bottleneck in patient throughput, and radiologists may need to fundamentally change their operations and communication strategies to adjust to a situation that is far more chaotic than day-to-day practice. It is thus paramount that the radiology department is involved in training drills for DMP activation, both in the preparation and in the execution of these simulations.
Preparation for and running of DMP activation drills involve substantial organizational effort as well as a considerable budget, therefore the training effect should be maximized. Department-specific learning objectives should be established, and observers not actively participating in the drill should take note of obstacles and opportunities for improvement. A thorough post-event evaluation of the drill is invaluable. All participants should give their comments on what went well and what should be improved. The results of the evaluation highlight learning points for the organization as a whole, which should be shared with the hospital staff and incorporated in the next simulation, which may be used to evaluate success of implementing the needed changes.
Executing MCI drills requires co-ordination, space, equipment and participation.50 It requires construction of a scenario (e.g. explosion, mass shooting, plane crash) and a decision about the timing relative to normal hospital activities. The number of casualties, the triage classes of the victims, types and severity of injuries, vital parameters (at presentation and during resuscitation), imaging study types and results, and the need for therapeutic interventions should be anticipated. The number of personnel to take part should be calculated and recruited. Using cases from a trauma patient registry database helps to select realistic scenarios. To adequately appreciate the role of imaging, the radiology personnel involved in devising the drill should prescribe imaging study durations that are as close to real life as possible. Examination time estimates should include entering examination orders, patient transportation to the radiology department, transfer on and off tables, as well as time for execution of the examination and image reconstruction. Varied choices of imaging modalities by the treatment teams should be anticipated, with results for the relevant imaging tests reported during the drill.
Some of the many possible questions to address in preparation include:
How are patients uniquely identified, and does radiology make use of the same pre-made registration numbers specific to DMP activation?
How do treatment teams order examinations?
Who is in charge of assigning priority for imaging?
Does a radiologist belong to a treatment team that goes with the T1 and T2 patients?
Will enough radiologists be available to assign a radiologist to each treatment team formed or to each modality in use?
Does the co-ordinating radiologist interpret studies or just provide logistic support and communication to colleagues in the DCC?
How are patients tracked within the radiology department and how is room availability tracked?
Who transports patients and where do they go after examination completion until a decision is made about disposition?
How are results conveyed, especially during times of potential electronic communication overload?
6Is there an oral provisional report of the preliminary interpretation or are there pre-made result forms, either paper or electronic?
Does the radiologist rendering the preliminary interpretation also make the final report, or do colleagues handle this, possibly in a more remote location?
If so, how do they become aware of the provisional results?
How many patients can undergo each examination type per hour and for how many hours?
Are imaging protocols amended to increase surge capacity?
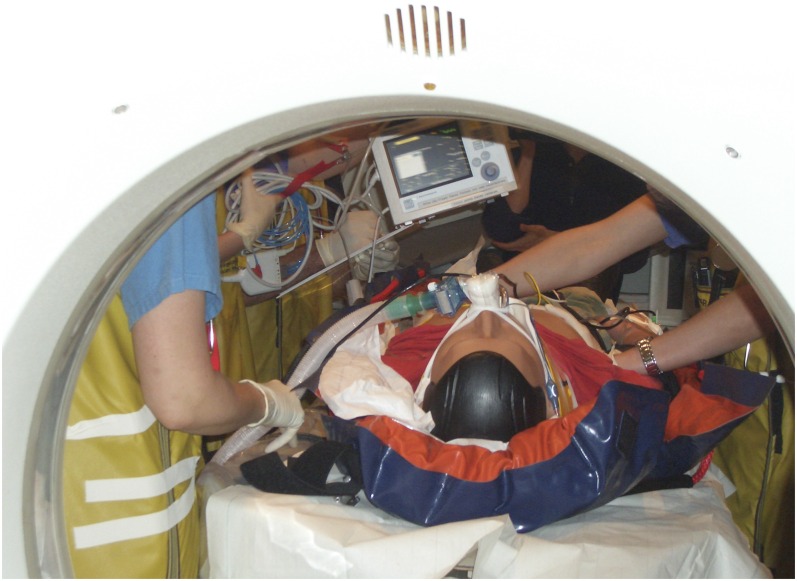
To optimize strategies after MCI events, simulations can be enacted in different ways, without solid proof in the literature of one ideal strategy.51 For simulation of various types, hospitals can use live moulaged actors (actors with mock injuries to very realistic extend and instructed scenario role play), computerized patients, high-fidelity simulators and virtual reality, amongst others.50,52–58 Live moulaged actors seemed to have the highest fidelity with students showing more accurate and more timely performance in one study of a small number of paediatric MCI victims but a large set of tested participants.50 In another recent study, virtual reality simulation proved to be valuable.52 All simulation techniques in these studies focused on clinical patient triage. To our knowledge, which method best simulates the role of radiology during DMP activation has not been specifically studied. Regardless of the method, in our experience, simulation drills bring many details to light about logistical challenges in the radiology departments during DMP activation and are very valuable to participate in (Figure 3).
Figure 3.
Simulation of performing a whole-body CT in a mass casualty incident patient by using a high-fidelity simulator. This will give insight in all components of throughput time (e.g. transfers to the room, transfers on and off the CT table, arrangements of equipment within the room).
SUMMARY
MCIs lead to stressful situations during activation of the hospital DMP, when resources become rapidly overwhelmed. To maximize capacity and patient throughput while minimizing error, delay, morbidity and mortality, all personnel have to know their role. These situations should be well thought out and trained for, since they occur infrequently and the goals of care are different from those of daily routine. Radiology departments and their staff are no exception, and a departmental DMP must be developed as an integral component of the hospital plan. Simulation drill training is a valuable way to test plan effectiveness and identify opportunities for improvement. Radiology leadership must engage fully in DMP activations, with thorough debriefing and evaluation following every event in order to face any future event in a better state of preparedness.
Contributor Information
Ferco H Berger, Email: fhberger@gmail.com.
Markus Körner, Email: markus@dr-koerner.net.
Mark P Bernstein, Email: markbernsteinmd@gmail.com.
Aaron D Sodickson, Email: ASODICKSON@bwh.harvard.edu.
Ludo F Beenen, Email: l.f.beenen@amc.uva.nl.
Patrick D McLaughlin, Email: mclaughlin.paddy@gmail.com.
Digna R Kool, Email: dignakool@gmail.com.
Ronald M Bilow, Email: Ronald.M.Bilow@uth.tmc.edu.
REFERENCES
- 1.Hirshberg A, Holcomb JB, Mattox KL. Hospital trauma care in multiple-casualty incidents: a critical view. Ann Emerg Med. 2001; 37: 647–52. doi: 10.1067/mem.2001.115650 [DOI] [PubMed] [Google Scholar]
- 2.Lee JS, Franc JM. Impact of a two-step emergency department triage model with START, then CTAS, on patient flow during a simulated mass-casualty incident. Prehosp Disaster Med 2015; 30: 390–6. doi: 10.1017/S1049023X15004835 [DOI] [PubMed] [Google Scholar]
- 3.VandenBerg SL, Davidson SB. Preparation for mass casualty incidents. Crit Care Nurs Clin North Am 2015; 27: 157–66. doi: 10.1016/j.cnc.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 4.Blom L, Black JJM. Major incidents. BMJ 2014; 348: g1144. doi: 10.1136/bmj.g1144 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Mass casualty management systems: strategies and guidelines for building health sector capacity. Geneva, Switzerland: WHO Press; 2007. Available from: http://www.int/hac/techguidance/MCM_guidelines_inside_final.pdf [Google Scholar]
- 6.Connor SB. When and why health care personnel respond to a disaster: the state of the science. Prehosp Disaster Med 2014; 29: 270–4. doi: 10.1017/S1049023X14000387 [DOI] [PubMed] [Google Scholar]
- 7.Culley J, McKnight S, Rivish VO, Moneda MD. Mass casualty information decision support. OJNI 2011; 15. [Google Scholar]
- 8.Pole T, Marcozzi D, Hunt RC. Interrupting my shift: disaster preparedness and response. Ann Emerg Med 2014; 63: 584–8. doi: 10.1016/j.annemergmed.2013.08.030 [DOI] [PubMed] [Google Scholar]
- 9.Raja AS, Propper BW, Vandenberg SL, Matchette MW, Rasmussen TE, Johannigman JA, et al. Imaging utilization during explosive multiple casualty incidents. J Trauma 2010; 68: 1421–4. doi: 10.1097/TA.0b013e3181cf7d32 [DOI] [PubMed] [Google Scholar]
- 10.Postma IL, Beenen LF, Bijlsma TS, Berger FH, Heetveld MJ, Bloemers FW, et al. Radiological work-up after mass casualty incidents: are ATLS guidelines applicable? Eur Radiol 2014; 24: 785–91. doi: 10.1007/s00330-013-3072-y [DOI] [PubMed] [Google Scholar]
- 11.Mohammed AB, Mann HA, Nawabi DH, Goodier DW, Ang SC. Impact of London's terrorist attacks on a major trauma center in London. Prehosp Disaster Med 2006; 21: 340–4. [DOI] [PubMed] [Google Scholar]
- 12.Boston Trauma Center Chiefs’ Collaborative. Boston marathon bombings: an after-action review. J Trauma Acute Care Surg 2014; 77: 501–3. doi: 10.1097/TA.0000000000000397 [DOI] [PubMed] [Google Scholar]
- 13.Powers R. Evidence-based ED disaster planning. J Emerg Nurs 2009; 35: 218–23. doi: 10.1016/j.jen.2008.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kearns RD, Cairns BA, Cairns CB. Surge capacity and capability. A review of the history and where the science is today regarding surge capacity during a mass casualty disaster. Front Public Health 2014; 2: 29. doi: 10.3389/fpubh.2014.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franc JM, Ingrassia PL, Verde M, Colombo D, Della Corte F. A simple graphical method for quantification of disaster management surge capacity using computer simulation and process-control tools. Prehosp Disaster Med 2015; 30: 9–15. doi: 10.1017/S1049023X1400123X [DOI] [PubMed] [Google Scholar]
- 16.Adini B, Aharonson-Daniel L, Israeli A. Load index model: an advanced tool to support decision making during mass-casualty incidents. J Trauma Acute Care Surg 2015; 78: 622–7. doi: 10.1097/TA.0000000000000535 [DOI] [PubMed] [Google Scholar]
- 17.Jenkins PC, Richardson CR, Norton EC, Cooke CR, Banerjee M, Nathens AB, et al. Trauma surge index: advancing the measurement of trauma surges and their influence on mortality. J Am Coll Surg 2015; 221: 729–38.e1. doi: 10.1016/j.jamcollsurg.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 18.Körner M, Krötz MM, Wirth S, Huber-Wagner S, Kanz KG, Boehm HF, et al. Evaluation of a CT triage protocol for mass casualty incidents: results from two large-scale exercises. Eur Radiol 2009; 19: 1867–74. doi: 10.1007/s00330-009-1361-2 [DOI] [PubMed] [Google Scholar]
- 19.Körner M, Geyer LL, Wirth S, Meisel CD, Reiser MF, Linsenmaier U. Analysis of responses of radiology personnel to a simulated mass casualty incident after the implementation of an automated alarm system in hospital emergency planning. Emerg Radiol 2011; 18: 119–26. doi: 10.1007/s10140-010-0922-7 [DOI] [PubMed] [Google Scholar]
- 20.Sierink JC, Saltzherr TP, Reitsma JB, Van Delden OM, Luitse JS, Goslings JC. Systematic review and meta-analysis of immediate total-body computed tomography compared with selective radiological imaging of injured patients. Br J Surg 2012; 99: 52–8. doi: 10.1002/bjs.7760 [DOI] [PubMed] [Google Scholar]
- 21.Cross KP, Petry MJ, Cicero MX. A better START for low-acuity victims: data-driven refinement of mass casualty triage. Prehosp Emerg Care 2015; 19: 272–8. doi: 10.3109/10903127.2014.942481 [DOI] [PubMed] [Google Scholar]
- 22.Lerner EB, McKee CH, Cady CE, Cone DC, Colella MR, Cooper A, et al. A consensus-based gold standard for the evaluation of mass casualty triage systems. Prehosp Emerg Care 2015; 19: 267–71. doi: 10.3109/10903127.2014.959222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahn CA, Schultz CH, Miller KT, Anderson CL. Does START triage work? An outcomes assessment after a disaster. YMEM 2009; 54: 424–30.e1. doi: 10.1016/j.annemergmed.2008.12.035 [DOI] [PubMed] [Google Scholar]
- 24.Culley JM, Svendsen E. A review of the literature on the validity of mass casualty triage systems with a focus on chemical exposures. Am J Disaster Med 2014; 9: 137–50. doi: 10.5055/ajdm.2014.0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zoraster RM, Chidester C, Koenig W. Field triage and patient maldistribution in a mass-casualty incident. Prehosp Disaster Med 2007; 22: 224–9. [DOI] [PubMed] [Google Scholar]
- 26.Jones N, White ML, Tofil N, Pickens M, Youngblood A, Zinkan L, et al. Randomized trial comparing two mass casualty triage systems (JumpSTART versus SALT) in a pediatric simulated mass casualty event. Prehosp Emerg Care 2014; 18: 417–23. doi: 10.3109/10903127.2014.882997 [DOI] [PubMed] [Google Scholar]
- 27.Frykberg ER. Medical management of disasters and mass casualties from terrorist bombings: how can we cope? J Trauma 2002; 53: 201–12. doi: 10.1097/00005373-200208000-00001 [DOI] [PubMed] [Google Scholar]
- 28.Körner M, Krötz M, Kanz KG, Pfeifer KJ, Reiser M, Linsenmaier U. Development of an accelerated MSCT protocol (Triage MSCT) for mass casualty incidents: comparison to MSCT for single-trauma patients. Emerg Radiol 2006; 12: 203–9. doi: 10.1007/s10140-006-0485-9 [DOI] [PubMed] [Google Scholar]
- 29.Peleg K, Rozenfeld M, Stein M. Poorer outcomes for mass casualty events victims: is it evidence based? J Trauma 2010; 69: 653–8. doi: 10.1097/TA.0b013e3181e7bbfc [DOI] [PubMed] [Google Scholar]
- 30.Wydo SM, Seamon MJ, Melanson SW, Thomas P, Bahner DP, Stawicki SP. Portable ultrasound in disaster triage: a focused review. Eur J Trauma Emerg Surg 2015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.West B, Cusser A, Etengoff S, Landsgaard H, LaBond V. The use of FAST scan by paramedics in mass-casualty incidents: a simulation study. Prehosp Disaster Med 2014; 29: 576–9. doi: 10.1017/S1049023X14001204 [DOI] [PubMed] [Google Scholar]
- 32.Körner M, Krötz MM, Degenhart C, Pfeifer KJ, Reiser MF, Linsenmaier U. Current role of emergency US in patients with major trauma. Radiographics 2008; 28: 225–42. doi: 10.1148/rg.281075047 [DOI] [PubMed] [Google Scholar]
- 33.Engel A, Soudack M, Ofer A, Nitecki SS, Ghersin E, Fischer D, et al. Coping with war mass casualties in a hospital under fire: the radiology experience. AJR Am J Roentgenol 2009; 193: 1212–21. doi: 10.2214/AJR.09.2375 [DOI] [PubMed] [Google Scholar]
- 34.Brunner J, Rocha TC, Chudgar AA, Goralnick E, Havens JM, Raja AS, et al. The boston marathon bombing: after-action review of the brigham and women’s hospital emergency radiology response. Radiology 2014; 273: 78–87. doi: 10.1148/radiol.14140253 [DOI] [PubMed] [Google Scholar]
- 35.Aylwin CJ, König TC, Brennan NW, Shirley PJ, Davies G, Walsh MS, et al. Reduction in critical mortality in urban mass casualty incidents: analysis of triage, surge, and resource use after the London bombings on July 7, 2005. The Lancet. 2006; 368: 2219–25. [DOI] [PubMed] [Google Scholar]
- 36.Goh SH. Bomb blast mass casualty incidents: initial triage and management of injuries. Singapore Med J 2009; 50: 101–6. [PubMed] [Google Scholar]
- 37.Langdorf MI, Medak AJ, Hendey GW, Nishijima DK, Mower WR, Raja AS, et al. Prevalence and clinical import of thoracic injury identified by chest computed tomography but not chest radiography in blunt trauma: multicenter prospective cohort study. Ann Emerg Med 2015; 66: 589–600. doi: 10.1016/j.annemergmed.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charbit J, Millet I, Maury C, Conte B, Roustan JP, Taourel P, et al. Prevalence of large and occult pneumothoraces in patients with severe blunt trauma upon hospital admission: experience of 526 cases in a French level 1 trauma center. Am J Emerg Med 2015; 33: 796–801. doi: 10.1016/j.ajem.2015.03.057 [DOI] [PubMed] [Google Scholar]
- 39.Radiology ACO. ACR Appropriateness Criteria®: suspected spine trauma. [Accessed 8 December 2015.] Available from: http://www.acr.org/∼/media/f579c123f999479c88390a3df976be77.pdf
- 40.Huber-Wagner S, Lefering R, Qvick LM, Körner M, Kay MV, Pfeifer KJ, et al. ; Working Group on Polytrauma of the German Trauma Society. Effect of whole-body CT during trauma resuscitation on survival: a retrospective, multicentre study. Lancet 2009; 373: 1455–61. doi: 10.1016/S0140-6736(09)60232-4 [DOI] [PubMed] [Google Scholar]
- 41.Hare SS, Goddard I, Ward P, Naraghi A, Dick EA. The radiological management of bomb blast injury. Clin Radiol 2007; 62: 1–9. doi: 10.1016/j.crad.2006.09.013 [DOI] [PubMed] [Google Scholar]
- 42.Körner M, Geyer LL, Wirth S, Reiser MF, Linsenmaier U. 64-MDCT in mass casualty incidents: volume image reading boosts radiological workflow. AJR Am J Roentgenol 2011; 197: W399–404. doi: 10.2214/AJR.10.5716 [DOI] [PubMed] [Google Scholar]
- 43.Daffner RH. Reviewing CT scout images: observations of an expert witness. AJR Am J Roentgenol 2015; 205: 589–91. doi: 10.2214/AJR.15.14405 [DOI] [PubMed] [Google Scholar]
- 44.Kung JW, Wu JS, Shetty SK, Khasgiwala VC, Appleton P, Hochman MG. Spectrum and detection of musculoskeletal findings on trauma-related CT torso examinations. Emerg Radiol 2014; 21: 359–65. doi: 10.1007/s10140-014-1201-9 [DOI] [PubMed] [Google Scholar]
- 45.Johnson PT, Scott WW, Gayler BW, Lewin JS, Fishman EK. The CT scout view: does it need to be routinely reviewed as part of the CT interpretation? AJR Am J Roentgenol 2014; 202: 1256–63. doi: 10.2214/AJR.13.10545 [DOI] [PubMed] [Google Scholar]
- 46.Beenen LF, Sierink JC, Kolkman S, Nio CY, Saltzherr TP, Dijkgraaf MG, et al. Split bolus technique in polytrauma: a prospective study on scan protocols for trauma analysis. Acta Radiol 2015; 56: 873–80. doi: 10.1177/0284185114539319 [DOI] [PubMed] [Google Scholar]
- 47.Geyer LL, Körner M, Linsenmaier U, Huber-Wagner S, Kanz KG, Reiser MF, et al. Incidence of delayed and missed diagnoses in whole-body multidetector CT in patients with multiple injuries after trauma. Acta Radiol 2013; 54: 592–8. doi: 10.1177/0284185113475443 [DOI] [PubMed] [Google Scholar]
- 48.Marres GM, Taal L, Bemelman M, Bouman J, Leenen LP. Online victim tracking and tracing system (ViTTS) for major incident casualties. Prehosp Disaster Med 2013; 28: 445–53. doi: 10.1017/S1049023X13003567 [DOI] [PubMed] [Google Scholar]
- 49.DeMers G, Kahn C, Johansson P, Buono C, Chipara O, Griswold W, et al. Secure scalable disaster electronic medical record and tracking system. Prehosp Disaster Med 2013; 28: 498–501. doi: 10.1017/S1049023X13008686 [DOI] [PubMed] [Google Scholar]
- 50.Claudius I, Kaji A, Santillanes G, Cicero M, Donofrio JJ, Gausche-Hill M, et al. Comparison of computerized patients versus live moulaged actors for a mass-casualty drill. Prehosp Disaster Med 2015; 30: 438–42. doi: 10.1017/S1049023X15004963 [DOI] [PubMed] [Google Scholar]
- 51.Hsu EB, Jenckes MW, Catlett CL, Robinson KA, Feuerstein C, Cosgrove SE, et al. Effectiveness of hospital staff mass-casualty incident training methods: a systematic literature review. Prehosp Disaster Med 2004; 19: 191–9. [DOI] [PubMed] [Google Scholar]
- 52.Luigi Ingrassia P, Ragazzoni L, Carenzo L, Colombo D, Ripoll Gallardo A, Corte Della F. Virtual reality and live simulation: a comparison between two simulation tools for assessing mass casualty triage skills. Eur J Emerg Med 2015; 22: 121–7. doi: 10.1097/MEJ.0000000000000132 [DOI] [PubMed] [Google Scholar]
- 53.Andreatta PB, Maslowski E, Petty S, Shim W, Marsh M, Hall T, et al. Virtual reality triage training provides a viable solution for disaster-preparedness. Acad Emerg Med 2010; 17: 870–6. doi: 10.1111/j.1553-2712.2010.00728.x [DOI] [PubMed] [Google Scholar]
- 54.Wallace D, Gillett B, Wright B, Stetz J, Arquilla B. Randomized controlled trial of high fidelity patient simulators compared to actor patients in a pandemic influenza drill scenario. Resuscitation 2010; 81: 872–6. doi: 10.1016/j.resuscitation.2010.02.026 [DOI] [PubMed] [Google Scholar]
- 55.Schulz CM, Skrzypczak M, Raith S, Hinzmann D, Krautheim V, Heuser F, et al. High-fidelity human patient simulators compared with human actors in an unannounced mass-casualty exercise. Prehosp Disaster Med 2014; 29: 176–82. doi: 10.1017/S1049023X14000223 [DOI] [PubMed] [Google Scholar]
- 56.Wilkerson W, Avstreih D, Gruppen L, Beier KP, Woolliscroft J. Using immersive simulation for training first responders for mass casualty incidents. Acad Emerg Med 2008; 15: 1152–9. doi: 10.1111/j.1553-2712.2008.00223.x [DOI] [PubMed] [Google Scholar]
- 57.Williams J, Nocera M, Casteel C. The effectiveness of disaster training for health care workers: a systematic review. Ann Emerg Med 2008; 52: 211–2. doi: 10.1016/j.annemergmed.2007.09.030 [DOI] [PubMed] [Google Scholar]
- 58.Ali J, Dunn J, Eason M, Drumm J. Comparing the standardized live trauma patient and the mechanical simulator models in the ATLS initial assessment station. J Surg Res 2010; 162: 7–10. doi: 10.1016/j.jss.2010.02.029 [DOI] [PubMed] [Google Scholar]