Abstract
The respiratory system may be involved in all systemic vasculitides, although with a variable frequency. The aim of our review is to describe radiographic and high-resolution CT (HRCT) findings of pulmonary vasculitides and to correlate radiological findings with pathological results. Lung disease is a common feature of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitides, including granulomatosis with polyangiitis (Wegener's), eosinophilic granulomatosis with polyangiitis (Churg–Strauss) and microscopic polyangiitis. Pulmonary involvement is less frequent in immune-complex-mediated small-vessel vasculitides, such as Behçet's disease and Goodpasture's syndrome. Pulmonary involvement associated to large-vessel (gigantocellular arteritis and Takayasu's disease) or medium-vessel (nodose polyarteritis and Kawasaki's disease) vasculitides is extremely rare. The present review describes the main clinical and radiological features of pulmonary vasculitides with major purpose to correlate HRCT findings (solitary or multiple nodules, cavitary lesions, micronodules with centrilobular or peribronchial distribution, airspace consolidations, “crazy paving”, tracheobronchial involvement, interstitial disease) with pathological results paying particular attention to the description of acute life-threatening manifestations. A thorough medical history, careful clinical examination and the knowledge of radiological patterns are mandatory for a correct and early diagnosis.
INTRODUCTION
The systemic vasculitides are a heterogeneous group of disorders characterized by an inflammatory destructive process affecting blood vessels.1 The lung is frequently involved with various clinical presentations. Pulmonary vasculitides may be primary and, in most cases, idiopathic disorder or secondary to other conditions such as infectious diseases, connective tissue diseases, malignancies and hypersensitivity disorders.2 Primary pulmonary vasculitides are rare disorders, with an incidence of 20–100 cases per million and a prevalence of 150–450 per million.2–5
The most widely used approach to classifying primary vasculitides is based on the size of the affected vessels (large, medium, small). Primary vasculitides most commonly associated with thoracic involvement are small-vessel antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitides represented by granulomatosis with polyangiitis (Wegener's) (GPA), eosinophilic granulomatosis with polyangiitis (EGPA) (Churg–Strauss) and microscopic polyangiitis.
Pulmonary involvement is less frequently associated to small-vessel immune-complex-mediated vasculitides as Goodpasture's syndrome and Behçet's disease. Pulmonary involvement related to large-vessel vasculitides (Takayasu arteritis, giant-cell arteritis) and medium-sized-vessel vasculitides (polyarteritis nodosa and Kawasaki disease) is rare.2
Radiological manifestations of primary pulmonary vasculitides are extremely variable and can be characterized by several findings including small-vessel wall thickening, nodular lesions, cavitary lesions, micronodules with centrilobular and peribronchial distribution, “ground-glass” opacities and/or consolidations, “crazy-paving” pattern, tracheobronchial stenoses and aneurysmatic dilatation of pulmonary arteries.
The knowledge of these signs and a careful correlation of specific clinical, laboratory, radiological and pathological features are mandatory for early diagnosis of vasculitides also in biopsy-proven cases.
Chest radiography is often non-specific and does not show the right pattern and extent of thoracic involvement. High-resolution CT (HRCT) is the most effective method to evaluate the characteristics, distribution and evolution of lung disorders.
As mentioned above, the primitive pulmonary vasculitides are rare disorders and represent one of the most complex diagnosis in medicine characterized by non-specific symptoms and signs common to other diseases such as infectious, connective tissue and neoplastic disease; in addition, the diagnosis of vasculitis is based on the identification of specific patterns of clinical, radiological, laboratory and histopathological abnormalities. The association of serological testing for ANCA with other immunopathological markers such as immunoglobulin A, serum cryoglobulins and anti-glomerular basement membrane is equally important for the diagnosis.
CLASSIFICATION OF THE VASCULITIDES
The size of the predominantly involved vessels strongly influences the clinical and radiological features of the different forms of vasculitides and is therefore one major criterion for the classification of vasculitides. Primary vasculitides are classified into large-vessel vasculitides, medium-sized-vessel vasculitides and small-vessel vasculitides. Large-vessel vasculitides affect the aorta and its largest branches; the medium-sized-vessel vasculitides affect the main visceral arteries and their branches; and the small-vessel vasculitides affect the capillaries, venules and arterioles. Note how all three categories affect arteries, but only small-vessel vasculitis affects also capillaries and venules. The 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides elaborated a new classification system6 (Table 1).
Table 1.
Classification of vasculitides
| Large-vessel vasculitis |
| Giant-cell arteritis |
| Takayasu arteritis |
| Medium-sized-vessel vasculitis |
| Polyarteritis nodosa |
| Kawasaki disease |
| Small-vessel vasculitis |
| Antineutrophil cytoplasmic antibody-associated vasculitis |
| Microscopic polyangiitis |
| Granulomatosis with polyangiitis (Wegener's) |
| Eosinophilic granulomatosis with polyangiitis (Churg–Strauss) |
| Immune complex SVV |
| Antiglomerular basement membrane disease |
| Cryoglobulinaemic vasculitis |
| Immunoglobulin A vasculitis (Henoch–Schönlein) |
| Hypocomplementemic urticarial vasculitis (anti-C1q vasculitis) |
| Variable-vessel vasculitis |
| Behcet's disease |
| Cogan's syndrome |
| Single-organ vasculitis |
| Cutaneous leukocytoclastic angiitis |
| Cutaneous arteritis |
| Primary central nervous system vasculitis |
| Isolated aortitis |
| Others |
| Vasculitis associated with systemic disease |
| Lupus vasculitis |
| Rheumatoid vasculitis |
| Sarcoid vasculitis |
| Others |
| Vasculitis associated with probable aetiology |
| Hepatitis C virus–associated cryoglobulinaemic vasculitis |
| Hepatitis B virus–associated vasculitis |
| Syphilis-associated aortitis |
| Drug-associated immune complex vasculitis |
| Drug-associated ANCA-associated vasculitis |
| Cancer-associated vasculitis |
| Others |
ANCA, anti-neutrophil cytoplasmic autoantibody; SVV, small vessel vasculitides.
In this review, we describe the radiological, pathological and clinical features of primary vasculitides that most frequently involve the lung, represented by primary small-vessel ANCA-associated vasculitides [EGPA (Churg–Strauss), granulomatosis with polyangiitis (Wegener's) and microscopic polyangiitis] and primary immune-complex-mediated vasculitides (Behcet's disease and Goodpasture's syndrome).
We also discuss the radiological findings and the underlying causes of diffuse alveolar haemorrhage (DAH) which is a clinical syndrome defined by the presence of haemoptysis, diffuse alveolar infiltrates and a drop in haematocrit level. It is usually related to a primary pulmonary small-vessel vasculitides, but it can also be associated with other diseases such as idiopathic alveolar haemorrhage, collagen vascular diseases, drug reactions and anticoagulation disorders. Granulomatosis with polyangiitis (Wegener's) and microscopic polyangiitis are the most common causes of DAH, representing 45% of the cases.5
PRIMARY SMALL-VESSEL VASCULITIDES
Small-vessel vasculitides are defined as inflammatory diseases that affect vessels smaller than arteries, such as arterioles, venules and capillaries; however, small-vessel vasculitides may also affect arteries, although, and in some cases, they may be also associated with larger calibre vessels involvement.
The diagnosis of small-vessel vasculitides needs pathological manifestations of vasculitic involvement of capillaries and venules, such as glomerulonephritis, purpura or pulmonary capillaritis. Primary, idiopathic, ANCA-associated small-vessel vasculitides are characterized by a more frequent lung involvement; ANCAs are antibodies directed against the intracellular antigens of neutrophils and monocytes.
The most common primary systemic small-vessel vasculitides in adults are ANCA-associated small-vessel vasculitides which include three major categories: EGPA (Churg–Strauss), granulomatosis with polyangiitis (Wegener's) and microscopic polyangiitis.
EGPA (Churg–Strauss) and granulomatosis with polyangiitis (Wegener's) appear with as necrotizing granulomatous inflammation, whereas microscopic polyangiitis appears with as necrotizing inflammation without granulomatosis.6
These three nosological entities are joined by particular clinical features, histopathological involvement of small vessels, similar response to immunosuppressive treatment and positivity for ANCA. ANCA positivity is common but not always present in these vasculitides, thus ANCA negativity does not completely rule out these vasculitides (negative-predictive value of 90%).6
In addition to these three diseases, there are other two forms of small-vessel vasculitides not associated with ANCA, but related to immune complexes deposition, such as Behçet's disease and Goodpasture's syndrome—the latter characterized by the presence of antibodies that selectively target antigens of the glomerular and alveolar basement membrane; it is a cause of pulmonary–renal syndrome.
Small-vessel vasculitides can affect people of all ages but are most common in adults 50–69 years of age, and they affect males slightly more frequently than they affect females.6
GRANULOMATOSIS WITH POLYANGIITIS (WEGENER'S)
The aetiopathogenesis of granulomatosis with polyangiitis is unknown and probably multifactorial; ANCAs certainly have a key role in vascular inflammation pathogenesis.
The prevalence is about 1.5–3 cases per 100,000 inhabitants; adults between 30 and 50 years of age are mainly affected, without sex predilection. The advanced age of onset and the early renal involvement are negative prognostic factors.
The clinical disease's onset is generally acute and characterized by dyspnoea, cough and haemoptysis due to lower respiratory tract involvement. The upper airway involvement is frequent (50–70% of patients); the kidneys are involved in 75–85% of patients; the nervous system is involved in 20–35%; the eye is involved in 10–15%; the skin is involved in 10–15%, and the muscles and joints are involved in 30%.2,7
The typical histopathological lesions of granulomatosis with polyangiitis are necrosis, vasculitides (which generally affects arterioles, venules and capillaries and is usually focal and eccentric to the lumen) and granulomatous inflammation (inflammatory infiltrate is composed by neutrophils, lymphocytes, plasma cells, macrophages, eosinophils and giant cells). The presence of a rim of ground-glass opacity in the pulmonary parenchyma surrounding lung nodules is frequent and is expression of alveolar haemorrhage that sometimes can spread and represent the predominant HRCT abnormality.
An association with immunological diseases such as calcinosis, Raynaud phenomenon, oesophageal dysmotility, sclerodactyly and telangiectasia (CREST) syndrome and Hashimoto's thyroiditis is described.
Radiological findings
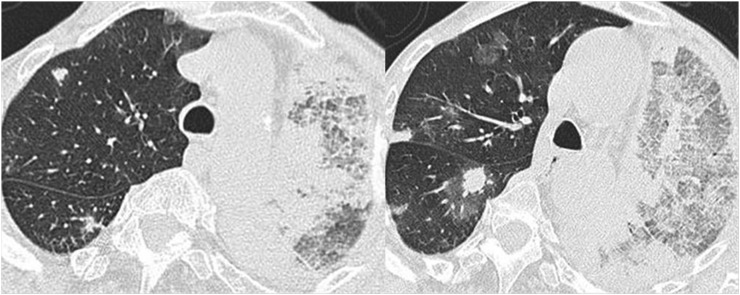
The most common HRCT abnormalities are lung nodules, usually multiple and bilateral which tend to increase with disease progression. It can range from few millimeters to >10 cm in diameter and become cavitated. Cavities are usually thick walled and characterized by an irregular inner margin (Figure 1) and absent calcification. The nodular lesions are often related to the vessels, and they tend to involve mainly the subpleural regions but have no predilection for the upper or lower lung zones. The presence of a rim of ground-glass opacity around the nodules (halo sign) is frequent and is the expression of alveolar haemorrhage (Figure 2); the presence of an air bronchogram within pulmonary nodules is equally typical (Figure 3). After therapy, the lesions usually show a slow resolution characterized by a marked wall thinning (Figure 4). In cases of DAH, HRCT features consist of bilateral ground-glass opacities and consolidations usually prominent in the perihilar areas with a relative sparing of the subpleural pulmonary parenchyma (Figure 5), pulmonary apices and costophrenic angles. The crazy paving pattern characterized by a smooth and regular interlobular septal thickening associated with ground-glass opacity may be seen some days later in an acute episode of haemorrhage (Figure 6).
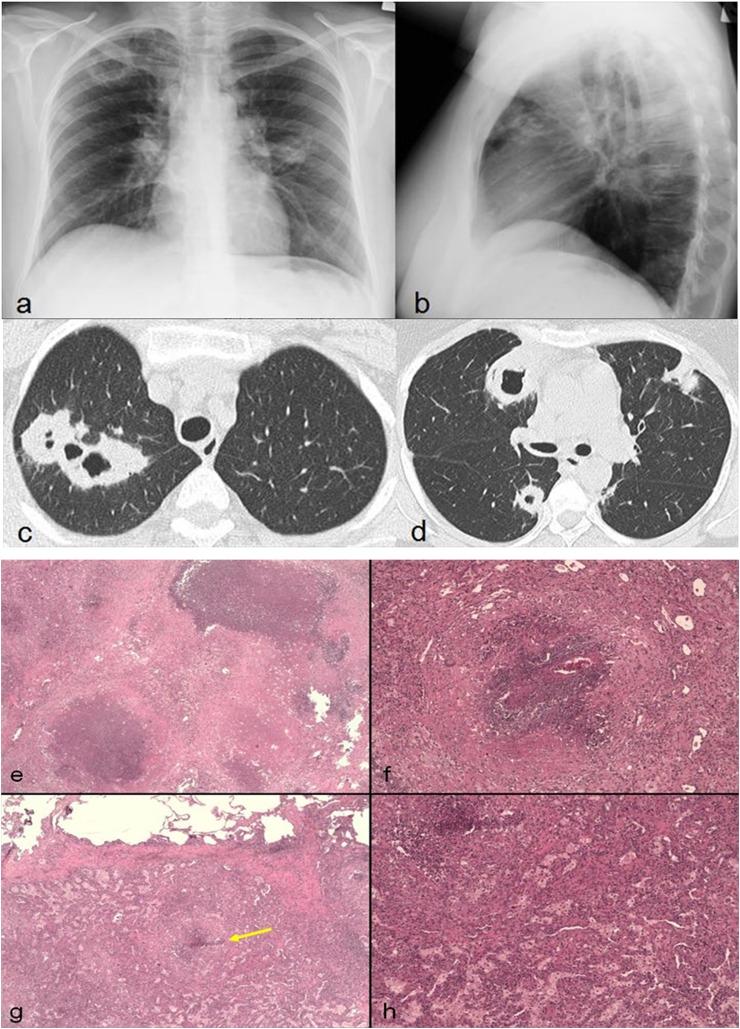
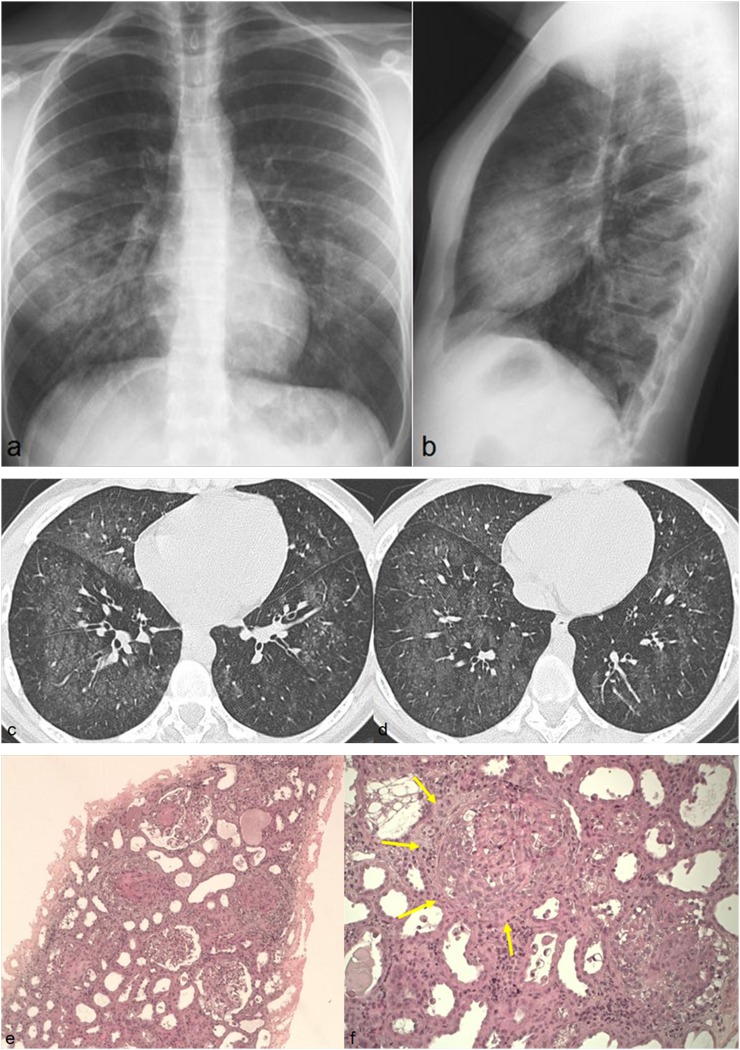
Figure 1.
A 39-year-old female with haemoptysis. Chest radiographs show bilateral round opacities, one of which located in the right upper lobe appears cavitated (a, b). Axial high-resolution CT images show multiple bilateral cavitary lesions with irregular and thickened walls (c, d). Notice that some lesions are related to pulmonary vessels. Open biopsy of a pulmonary nodule shows necrotic nodules in fibrotic organization (e), fibrinoid necrosis of pulmonary vessel wall with necrotizing granulomas (f) and necrotizing granuloma (arrow) near normally aerated alveolar structures with associated histiocytic infiltration (g). A detail of massive histiocytic infiltration of the pulmonary parenchyma (h).
Figure 2.
A 42-year-old female with biopsy-proved granulomatosis with polyangiitis (Wegener's). Axial high-resolution CT images show multiple bilateral pulmonary nodules surrounded by a rim of ground-glass opacity (halo sign).
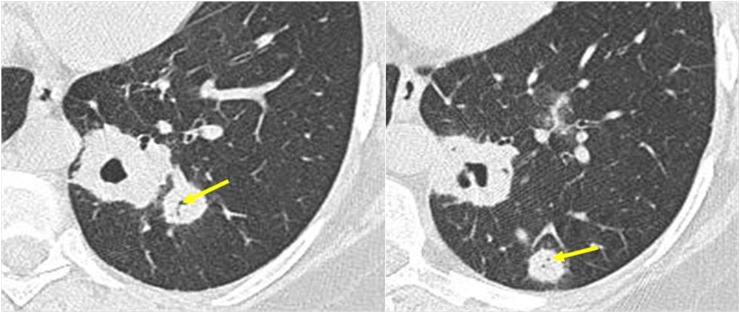
Figure 3.
Parenchymal nodules in a patient with granulomatosis with polyangiitis (Wegener's). Note the presence of the patent's bronchi inside the nodules (arrows).
Figure 4.
The same patient of Figure 1 with granulomatosis with polyangiitis (Wegener's) and multiple nodular cavitary lesions in both lungs (a, b). CT images, obtained after treatment, show a favourable response characterized by marked wall thinning of residual lesions (c, d).
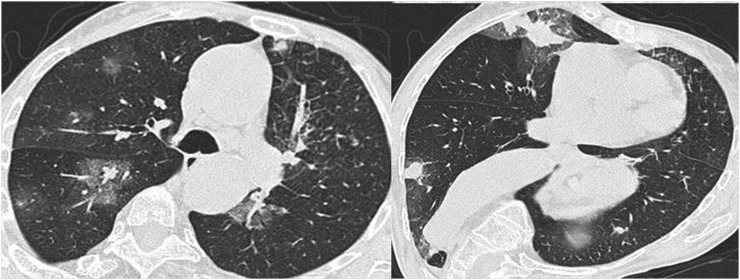
Figure 5.
A 42-year-old female with histologically proven granulomatosis with polyangiitis (Wegener's) obtained from renal biopsy. Axial high-resolution CT images show bilateral ground-glass opacities with a predominantly perihilar distribution and a relative sparing of the subpleural parenchyma.
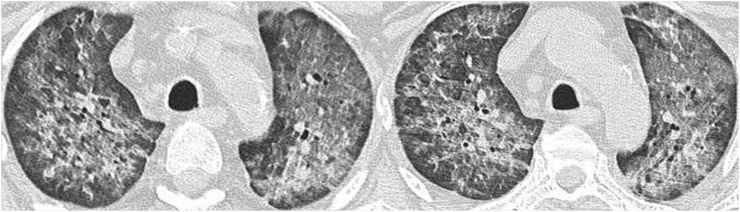
Figure 6.
A 50-year-old female with granulomatosis with polyangiitis (Wegener's). Axial high-resolution CT images show reticular pattern on a background of ground-glass attenuation, representing haemorrhagic alveolitis.
Diagnosis
In presence of an appropriate clinical context and compatible radiological findings, the ANCA positivity should be considered highly suggestive of Wegener. The evidence of necrotizing granulomatous vasculitides in the affected sites (lung, kidney, skin etc.) allows the histological confirmation of the disease.
Surgical open-lung biopsy is the gold standard technique for the definitive diagnosis while transbronchial biopsy often does not provide diagnostic material.
EOSINOPHILIC GRANULOMATOSIS WITH POLYANGIITIS (CHURG–STRAUSS)
Also known as allergic granulomatous angiitis, eosinophilic granulomatosis with polyangiitis (Churg–Strauss) is characterized by a clinical triad: asthma, hypereosinophilia and necrotizing systemic vasculitis.8 The diagnosis is based on the presence of four or more of the following six findings: asthma, >10% eosinophilia in a differential white blood cell count, mononeuropathy or polyneuropathy due to a systemic vasculitis, paranasal sinus abnormalities, migratory or transient pulmonary opacities and histological evidence of extravascular eosinophils in a biopsy specimen.9 Late age of onset (mean age of 32 years) of asthma allows us to distinguish EGPA (Churg–Strauss) asthma from typical asthma in the general population.10 The lung is the most commonly involved organ, followed by the skin. DAH (haemorrhagic alveolitis) and glomerulonephritis are more or less common than in the other forms of vasculitides.
The heart is an important target organ in EGPA (Churg–Strauss), and the main causes of morbidity and mortality are represented by coronary arteritis.11
Histological findings include granulomatous necrotizing vasculitis of the small arteries and eosinophil-rich inflammatory infiltrate.12
Clinically, EGPA (Churg–Strauss) shows three distinct phases: (a) the prodromal period, which may persist for several years, consisting of asthma and allergic rhinitis; (b) a phase of marked peripheral blood eosinophilia and eosinophil tissue infiltrates; (c) and a life-threatening vasculitic phase.13
The first two prevasculitic phases are characterized by marked tissue eosinophilia, which manifests in the lung as eosinophilic pneumonia.14
Radiological findings
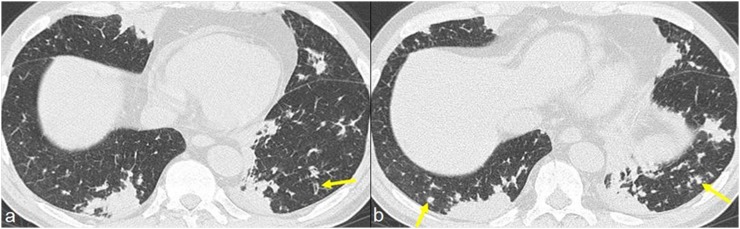
The most frequently observed radiological sign in patients with EGPA (Churg–Strauss) consists of transient and often migrant opacities with bilateral and non-segmental distribution, mostly peripheral, without predilection for any craniocaudal lung zone.14 This aspect is similar to eosinophilic pneumonia and organizing pneumonia.15,16
The most common abnormality at HRCT, seen in up to 90% of patients, is bilateral areas of ground-glass opacity or consolidation with a bilateral symmetric distribution and a peripheral predominance. Other relatively common findings are the presence of airway involvement consisting of bronchial dilatation, bronchial wall thickening and small peribronchial and centrilobular nodules (Figures 7 and 8) related to eosinophilic infiltration of the bronchial wall and asthma.14
Figure 7.
A 50-year-old male with histologically proven eosinophilic granulomatosis with polyangiitis (Churg–Strauss). Axial high-resolution CT images show bilateral areas of consolidation with a predominantly peripheral distribution. Note the association with dilated bronchi (arrow in a) and micronodules with centrilobular distribution (arrows in b).
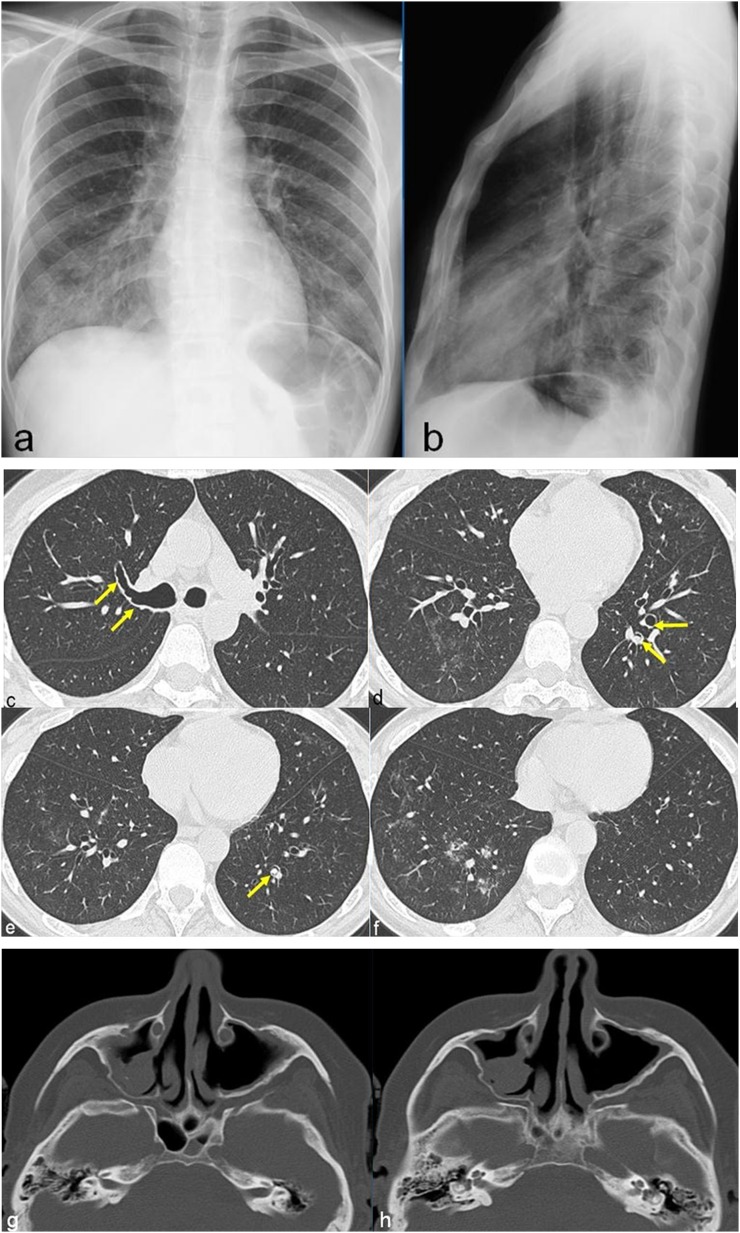
Figure 8.
A 50-year-old female with allergic asthma, rhinitis and peripheral neuropathy. Chest radiographs show hazy areas of increased opacity prominent in the middle and right lower lobes (a, b). CT images, obtained after 10 days from chest radiographs, show diffuse and irregular bronchial wall thickening (arrows in c) associated with the presence of nodules with endo- (arrows in d, arrow in e) and peribronchial (f) distribution. CT of the facial bones shows bilateral meatal antrostomy of maxillary sinuses with signs of chronic sinusopathy (g, h).
Regular and smooth interlobular septal thickening, which may reflect the presence of oedema secondary to cardiac involvement or eosinophilic septal infiltration, can be seen in 50% of patients (Figure 9).
Figure 9.
A 35-year-old female with previously diagnosed eosinophilic granulomatosis with polyangiitis (Churg–Strauss) presenting with acute dyspnoea and haemoptysis. Chest radiographs show reticular interstitial pattern with gravitational distribution and bilateral pleural effusion (a, b). CT images show peribroncovascular interstitial thickening and smooth interlobular septal thickening with gravitational distribution; bilateral pleural effusion is also present (c, d). CT signs are suggestive of interstitial pulmonary oedema.
Diagnosis
In asthmatic patient, the presence of bilateral parenchymal areas of consolidation with prevalent peripheral distribution can suggest a diagnosis of chronic eosinophilic pneumonia, EGPA (Churg–Strauss) or organizing pneumonia. The presence of blood eosinophilia is typical of the first two diseases but is not frequent in organizing pneumonia. The presence of systemic manifestations related to EGPA (Churg–Strauss), including rash, peripheral neuropathy, sinusopathy and the presence of perinuclear ANCA in serum, allow to make the diagnosis even without biopsy. In case of ANCA negativity (30–40%), the biopsy confirmation is recommended. Biopsy confirmation is recommended when easily accessible lesional tissue, e.g. skin, is available.
MICROSCOPIC POLYANGIITIS
Microscopic polyangiitis is a non-granulomatous necrotizing systemic vasculitis with the involvement of small vessels. It is the most common cause of pulmonary–renal syndrome, a syndrome characterized by the coexistence of alveolar haemorrhage, due to alveolar capillaritis, and necrotizing glomerulonephritis.17
More than 80% of patients have a rapidly progressive glomerulonephritis at presentation, associated to diffuse pulmonary haemorrhage in 30–50% of the cases.7,18,19 Thoracic symptoms include dyspnoea, haemoptysis and progressive anaemia; other common manifestations include skin lesions, peripheral neuritis and ocular symptoms such as other small-vessel vasculitides.
It is considered to be the small-vessel variant of polyarteritis nodosa which affects only medium-sized muscular arteries and does not involve the lungs.
Over the differences in the involved vessels' size, other differences from polyarteritis include the following: lung involvement is unusual in polyarteritis nodosa; microscopic polyangiitis is almost never associated with a previous hepatitis B virus and hepatitis C virus infections; the rapidly progressive glomerulonephritis does not occur in polyarteritis nodosa.
Radiological findings
The radiological lung features consist of diffuse bilateral airspace opacities related to DAH or haemorrhagic diffuse alveolitis.
The HRCT pattern is characterized by ground-glass opacities more or less associated with areas of consolidation (related to the presence of completely blood-filled alveoli) usually bilateral, diffused or patchy (Figure 10).
Figure 10.
A 63-year-old female with cough, haemoptysis and progressive anaemization. CT images show areas of consolidation and ground-glass opacity associated with interlobular septal thickening (a, b). CT images through the upper abdomen show extensive perirenal and subcapsular haematomas with fluid–fluid levels. Haemorrhagic imbibition of the peri- and pararenal fat is clearly seen (c, d). Histological diagnosis of necrotizing non-granulomatous vasculitis obtained from renal biopsy. Diseased artery characterized by duplication of the elastic lamina due to necrotizing vasculitis (arrows in e). Area of glomerular infarction (arrow in f) and glomerular sclerosis (arrowhead in f).
The clinical evolution of haemorrhagic alveolitis is often dramatic and can be fatal if not promptly treated.
In favourable cases, the parenchymal opacities usually resolve within 1–2 weeks, slower than acute pulmonary oedema that is the most important radiological differential diagnosis.
The differential diagnosis with diffuse alveolar involvement secondary to infective diseases is more difficult; in these cases, clinical signs are mandatory for diagnosis.
After the acute phase, a reticular pattern could be present. It can persist when recurrent haemorrhage occurs and may progress to pulmonary fibrosis.
Diagnosis
The diagnosis of microscopic polyangiitis should be suspected in patients with clinical and radiological findings of diffuse pulmonary haemorrhage and ANCA-positive rapidly progressive glomerulonephritis. Clinically, the main differential diagnosis is with Goodpasture's syndrome, granulomatosis with polyangiitis and systemic lupus erythematosus characterized by pulmonary and renal manifestations. In these cases, serum tests are fundamental for the correct diagnosis characterized by the presence of antiglomerular basement membrane antibodies; renal biopsy is generally performed to differentiate microscopic polyangiitis (non-granulomatous necrotizing systemic vasculitis) from granulomatosis with polyangiitis (granulomatous necrotizing vasculitis) and systemic lupus erythematosus (immune complex deposition vasculitis).20
DIFFUSE ALVEOLAR HAEMORRHAGE
DAH can be defined by the presence of haemoptysis, diffuse alveolar infiltrate and a drop in haematocrit. It is a clinical syndrome usually related to all primary or secondary small-vessel vasculitides with capillaritis as granulomatosis with polyangiitis (Wegener's), EGPA (Churg–Strauss), microscopic polyangiitis, Henoch–Schönlein purpura, Behçet's disease, connettivitis (especially systemic lupus erythematosus), antiphospholipid antibody syndrome and Goodpasture's syndrome.2,18,21
These conditions must be differentiated from other diseases responsible of diffuse pulmonary haemorrhage such as drug-inducted pulmonary damage or inhalation of toxic substances.
The radiological findings are common among all these forms, and it is already described in microscopic polyangiitis.
BEHÇET'S DISEASE
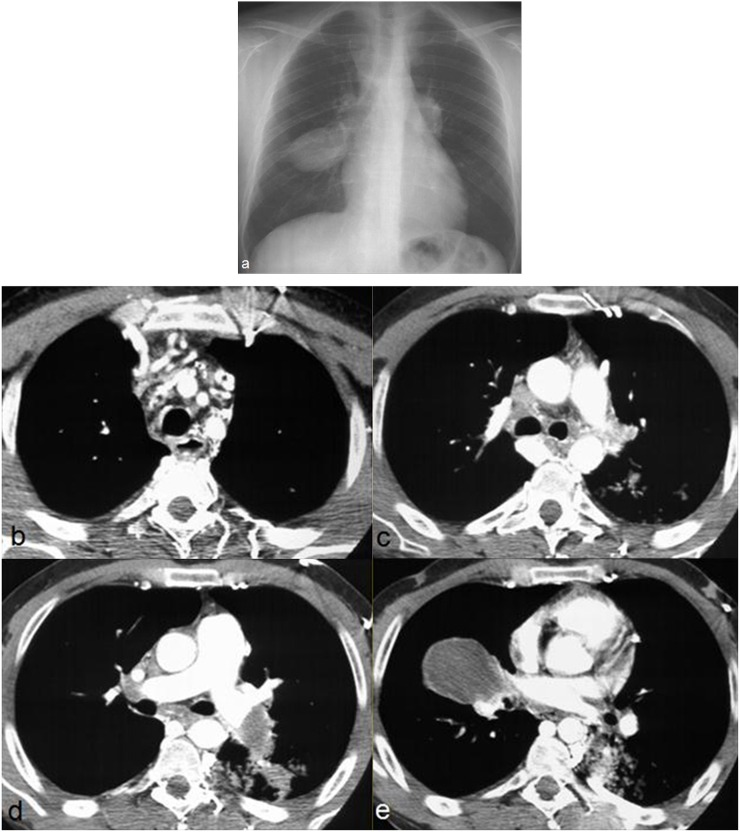
Behçet' s disease is a rare chronic multisystemic vasculitis that usually appears during the second and third decades of life and is characterized by ocular anomalies (uveitis), ulcers affecting the mouth and genitals and additional clinical manifestations in multiple organ systems. Pulmonary involvement is present in 10% of cases. The vasculitis can involve large, medium and small vessels of both the arterial and venous circulation.22 Large-vessel involvement is characterized by arterial or venous occlusion and the presence of aneurysms. The pulmonary arteries are the second most common site of arterial involvement, preceded by the aorta. Behçet's disease is the most common cause of pulmonary artery aneurysms (Figure 11) caused by inflammation of the vasa vasorum of the tunica media with destruction of the elastic fibres and dilatation of the vessel lumen.23,24 Haemoptysis is the most common presenting symptom and is one of the leading causes of death.23 Pulmonary aneurysms in Behçet's disease are fusiform to saccular, generally multiple and bilateral, frequently partially or totally thrombosed. The aneurysms frequently involve main or lower lobe pulmonary arteries.24,25 Thrombosis of the superior vena cava and of other mediastinal veins is often present (Figure 11). In untreated patients, mortality rate is higher, whereas they are much lower in patients receiving immunosuppressant treatment.18,25
Figure 11.
Behçet disease in a 29-year-old male with cough and haemoptysis. Chest radiograph shows aneurysmatic dilatation of pulmonary arteries (a). Axial post-contrast CT images through the upper chest show thrombosis of the superior vena cava and of brachiocephalic veins with collateral venous vessels in the mediastinum and the posterior chest wall (b, c). CT images at the level of the pulmonary arteries reveal enlarged and completely thrombosed right middle lobar artery and left descending artery (d, e).
The most common parenchymal lesions are ill-defined areas of increased opacity, which represent focal vasculitis and thrombosis of pulmonary vessels resulting in infarction, haemorrhage and focal atelectasis.23,25
GOODPASTURE'S SYNDROME
Goodpasture's syndrome is an extremely rare small-vessel vasculitis (one case per million per year) characterized by the presence of antibodies that selectively targets antigens of the glomerular and alveolar basement membrane; it is a cause of pulmonary–renal syndrome. The radiographic and CT lung features are consistent with DAH (Figure 12).
Figure 12.
A 22-year-old female with haemoptysis. Chest radiographs show bilateral alveolar opacities with relative sparing of the apical regions (a, b). Axial CT images show diffuse areas of ground-glass opacities involving both lungs (c, d). Histological diagnosis of Goodpasture's syndrome obtained from renal biopsy which shows a rapidly progressive glomerulonephritis (e) and the presence of glomerular crescent (arrows in f) caused by proliferation of parietal epithelial cell.
CONCLUSION
The diagnosis of vasculitis is often delayed because several other diseases have similar clinical manifestations.
The knowledge of the main radiographic and HRTC findings, in association with clinical, laboratoristic and serum data, often enable a non-invasive diagnosis of pulmonary vasculitis.
The presence of specific radiological findings related to each disorder is essential for a correct differential diagnosis, even in biopsied cases.
Contributor Information
Beatrice Feragalli, Email: beatriceferagalli@hotmail.com.
Cesare Mantini, Email: cesare.mantini@gmail.com.
Marco Sperandeo, Email: sperandeom@libero.it.
Michele Galluzzo, Email: mgalluzzo@scamilloforlanini.rm.it.
Giovanni Belcaro, Email: giovanni.belcaro@unich.it.
Armando Tartaro, Email: a.tartaro@radiol.unich.it.
Antonio R Cotroneo, Email: ar.cotroneo@rad.unich.it.
REFERENCES
- 1.Heeringa P, Schreiber A, Falk RJ, Jennette JC. Pathogenesis of pulmonary vasculitis. Semin Respir Crit Care Med 2004; 25: 465–74. doi: 10.1055/s-2004-836140 [DOI] [PubMed] [Google Scholar]
- 2.Brown KK. Pulmonary vasculitis. Proc Am Thorac Soc 2006; 3: 48–57. doi: 10.1513/pats.200511-120JH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.González-Gay MA, García-Porrúa C. Systemic vasculitis in adults in northwestern Spain, 1988–1997. Clinical and epidemiologic aspects. Medicine (Baltimore) 1999; 78: 292–308. doi: 10.1097/00005792-199909000-00002 [DOI] [PubMed] [Google Scholar]
- 4.Watts RA, Lane SE, Bentham G, Scott DG. Epidemiology of systemic vasculitis: a ten-year study in the United Kingdom. Arthritis Rheum 2000; 43: 414–19. doi: [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Colby TV, Lombard C, Carpenter HA. Clinicopathologic study of 34 cases of diffuse pulmonary hemorrhage with lung biopsy confirmation. Am J Surg Pathol 1990; 14: 1112–25. doi: 10.1097/00000478-199012000-00003 [DOI] [PubMed] [Google Scholar]
- 6.Jennette JC. Overview of the 2012 Revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol 2013; 17: 603–6. doi: 10.1007/s10157-013-0869-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frankel SK, Cosgrove GP, Fischer A, Meehan RT, Brown KK. Update in the diagnosis and management of pulmonary vasculitis. Chest 2006; 129: 452–65. doi: 10.1378/chest.129.2.452 [DOI] [PubMed] [Google Scholar]
- 8.Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med 1997; 337: 1512–23. doi: 10.1056/NEJM199711203372106 [DOI] [PubMed] [Google Scholar]
- 9.Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP. The American College of Rheumatology 1990 criteria for the classification of Churg–Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 1990; 33: 1094–100. doi: 10.1002/art.1780330806 [DOI] [PubMed] [Google Scholar]
- 10.Choi YH, Im JG, Han BK, Kim JH, Lee KY, Myoung NH. Thoracic manifestation of Churg–Strauss syndrome: radiologic and clinical findings. Chest 2000; 117: 117–24. doi: 10.1378/chest.117.1.117 [DOI] [PubMed] [Google Scholar]
- 11.Solans R, Bosch JA, Pérez-Bocanegra C, Selva A, Huguet P, Alijotas J. Churg–Strauss syndrome: outcome and long-term follow-up of 32 patients. Rheumatology (Oxford) 2001; 40: 763–71. doi: 10.1093/rheumatology/40.7.763 [DOI] [PubMed] [Google Scholar]
- 12.Katzenstein AL. Diagnostic features and differential diagnosis of Churg–Strauss syndrome in the lung: a review. Am J Clin Pathol 2000; 114: 767–72. [DOI] [PubMed] [Google Scholar]
- 13.Kim YK, Lee KS, Chung MP, Han J, Chong S, Chung MJ. Pulmonary involvement in Churg–Strauss syndrome: an analysis of CT, clinical, and pathologic findings. Eur Radiol 2007; 17: 3157–65. doi: 10.1007/s00330-007-0700-4 [DOI] [PubMed] [Google Scholar]
- 14.Silva CI, Müller NL, Fujimoto K, Johkoh T, Ajzen SA, Churg A. Churg–Strauss syndrome: high resolution CT and pathologic findings. J Thorac Imaging 2005; 20: 74–80. doi: 10.1097/01.rti.0000155268.00125.de [DOI] [PubMed] [Google Scholar]
- 15.Worthy SA, Müller NL, Hansell DM, Flower CD. Churg–Strauss syndrome: the spectrum of pulmonary CT findings in 17 patients. AJR Am J Roentgenol 1998; 170: 297–300. doi: 10.2214/ajr.170.2.9456932 [DOI] [PubMed] [Google Scholar]
- 16.Müller NL, ed. Imaging of the chest. Philadelphia, PA: Saunders-Elsevier; 2008. [Google Scholar]
- 17.Bosch X, Guilabert A, Font J. Antineutrophil cytoplasmic antibodies. Lancet 2006; 368: 404–18. doi: 10.1016/S0140-6736(06)69114-9 [DOI] [PubMed] [Google Scholar]
- 18.Marten K, Schnyder P, Schirg E, Prokop M, Rummeny EJ, Engelke C. Pattern-based differential diagnosis in pulmonary vasculitis using volumetric CT. AJR Am J Roentgenol 2005; 184: 720–33. doi: 10.2214/ajr.184.3.01840720 [DOI] [PubMed] [Google Scholar]
- 19.Sorensen SF, Slot O, Tvede N, Petersen J. Prospective study of vasculitis patients collected in a five year period: evaluation of the Chapel Hill nomenclature. Ann Rheum Dis 2000; 59: 478–82. doi: 10.1136/ard.59.6.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller NL. Microscopic polyangiitis. In: Müller NL, ed. Imaging of the chest. Philadelphia, PA: Saunders-Elsevier; 2008. pp. 834–7. [Google Scholar]
- 21.Green RJ, Ruoss SJ, Kraft SA, Duncan SR, Berry GJ, Raffin TA. Pulmonary capillaritis and alveolar hemorrhage: update on diagnosis and management. Chest 1996; 110: 1305–16. doi: 10.1378/chest.110.5.1305 [DOI] [PubMed] [Google Scholar]
- 22.Erkan F, Gül A, Tasali E. Pulmonary manifestations of Behçet's disease. Thorax 2001; 56: 572–8. doi: 10.1136/thorax.56.7.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiller N, Lieberman S, Chajek-Shaul T, Bar-Ziv J, Shaham D. Thoracic manifestations of Behçet disease at CT. RadioGraphics 2004; 24: 801–8. doi: 10.1148/rg.243035091 [DOI] [PubMed] [Google Scholar]
- 24.Chae EJ, Do KH, Seo JB, Park SH, Kang JW, Jang YM. Radiologic and clinical findings of Behçet disease: comprehensive review of multisystemic involvement. RadioGraphics 2008; 28: e31. doi: 10.1148/rg.e31 [DOI] [PubMed] [Google Scholar]
- 25.Tunaci M, Ozkorkmaz B, Tunaci A, Gül A, Engin G, Acunaş B. CT findings of pulmonary artery aneurysms during treatment for Behçet's disease. AJR Am J Roentgenol 1999; 172: 729–33. doi: 10.2214/ajr.172.3.10063870 [DOI] [PubMed] [Google Scholar]