Abstract
Objective:
The role of lung ultrasound (LUS) integrated with chest X-ray (CXR) for the first-line diagnosis of paediatric pneumonia; to define its role during the follow-up to exclude complications.
Methods:
We performed a retrospective review of a cohort including 84 consecutive children (age range: 3–16 years; mean age: 6 years; 44 males, 40 females) with clinical signs of cough and fever. All the patients underwent CXR at admission integrated with LUS. Those positive at LUS were followed up with LUS until the complete resolution of the disease.
Results:
CXR showed 47/84 pneumonic findings. LUS showed 60/84 pneumonic findings; 34/60 pneumonic findings had a typical pattern of lung consolidation; 26/60 pneumonic findings showed association of multiple B-lines, findings consistent with interstitial involvement, and small and hidden consolidations not achievable by CXR. One case was negative at LUS because of retroscapular location. 60 patients were followed up with LUS; 28/60 patients showed a complete regression of the disease; 23/60 patients had a significant decrease in size of consolidation; 9/60 patients showed disease stability or insignificant decrease in size, thus requiring adjunctive LUS examinations.
Conclusion:
LUS, integrated with CXR, revealed to be an accurate first-line technique to identify small pneumonic consolidations, especially for “CXR-occult” findings, and for early diagnosis of pleural effusion; furthermore, LUS follow-up allows complications to be verified and additional radiation exposures to be avoided.
Advances in knowledge:
The effective role of LUS in the diagnosis and follow-up of lung consolidations and pleural effusions in paediatric patients in an emergency setting.
INTRODUCTION
Community-acquired pneumonia (CAP) is one of the most common causes of illness in paediatric patients, causing more deaths than malaria and acquired immune deficiency syndrome.
According to the British Thoracic Society guidelines, it can be clinically defined as the presence of signs and symptoms of pneumonia, such as fever (>38.5° C), tachypnoea and respiratory distress in a healthy child, caused by an infection acquired outside the hospital.1
In most of the uncomplicated cases, chest X-ray (CXR) is not routinely recommended; however, it is considered as the standard of reference for its diagnosis in some selected patients and the first imaging step to perform in children. Nevertheless, CXR has some important concerns such as the exposure to ionizing radiation, which increases carcinogenic risk, lack of patient collaboration, low reproducibility and high interobserver and intra-observer variability owing to those consolidations affecting hidden areas such as the retrocardiac region and lung bases.2
In the recent years, lung ultrasound (LUS) has been demonstrated to be a feasible technique in the evaluation of pneumonia, pneumothorax, atelectasis, pleural effusion and alveolar-interstitial syndrome in adults;3–9 less is known about its role in paediatric patients.10 LUS is rapid, portable, repeatable and above all, it is a non-radiating technique, a feature allowing its application in children; in fact, the small body size of these patients, including a small thoracic width and lung mass, allows an easier detection of lung anomalies by LUS, because of a more rapid involvement of the pleura by the disease.11 The examination is performed at the patient's bedside and it can be repeated without the risk of ionizing radiation. Some authors demonstrated that the learning curve is faster with respect to other ultrasound examinations, and it requires less skill.12,13
The aim of this study was to evaluate the role of LUS in the diagnosis of pneumonia in paediatric patients in addition to CXR, and to study its feasibility in the follow-up in an emergency setting.
To our knowledge, this is the first study which focused on the role of LUS in the follow-up of CAP in an emergency department in a paediatric population.
METHODS AND MATERIALS
Between January 2013 and May 2015, we retrospectively evaluated 84 children admitted to the emergency department with the clinical suspect of pneumonia, presenting with fever (>38° C for more than 3 days) and cough. Age ranged from 3 to 16 years (mean age: 6 years; 44 M and 40 F). All patients underwent clinical examination, blood sample analysis, CXR and LUS at admission.
LUS was performed using a Siemens Acuson Sequoia 512 system (Siemens Medical Systems, Forchleim, Germany), equipped with a curved-array 4 MHz multifrequency probe and a linear probe (7.5–10 MHz). Both the anterior and posterior lungs were evaluated in both longitudinal and transversal sections in supine or lying position, scanning one hemithorax at a time: in particular, the anterior part, extending from the parasternal to the mid-axillary line, was evaluated by positioning the probe transversally to the chest, from the second to the fifth intercostal space, and longitudinally along the parasternal, mid-clavicular, anterior axillary and mid-axillary lines.
The posterior lung, extending from the mid-axillary line to the paravertebral line, was scanned by placing the probe transversally on the intercostal spaces below the scapular spine and longitudinally along the paravertebral and posterior axillary lines.
For each finding suggesting a consolidation, we evaluated: number, size, location, shape, echogenicity, echostructure and B-lines and its inner features such as the presence of air bronchogram, fluid bronchogram, vascularity and basal pleural effusion, defined as anechoic or hypoechoic fluid with or without debris. In addition, we also analysed pleural line abnormalities, such as the irregular appearance of the pleural line and subpleural lung consolidations, that is echo-poor or solid region with blurred margins with or without air bronchogram.
A normal pattern was defined as the presence of normal lung sliding with or without A-lines. The presence of focal and multiple B-lines or confluent B-lines suggested the presence of lung disease.14 The air bronchogram appears as multiple small air holes within the consolidation or as a tree-shaped echogenic structure (Figure 1). It can have an intrinsic movement consensual to breathing, helping to rule out an atelectasis.15 The fluid bronchogram appears as an echo-poor tubular structure, with hyperechoic walls, along the airways and, it can be differentiated from vessels using colour Doppler. It has been described in post-obstructive pneumonia.
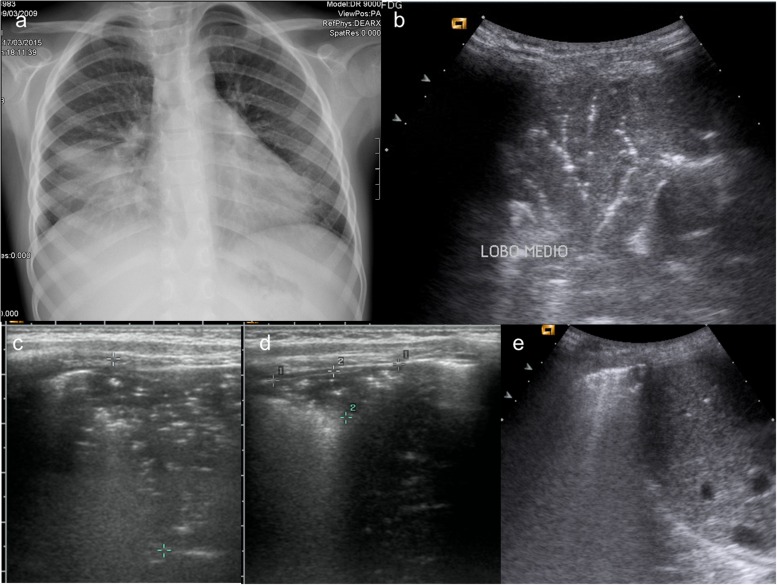
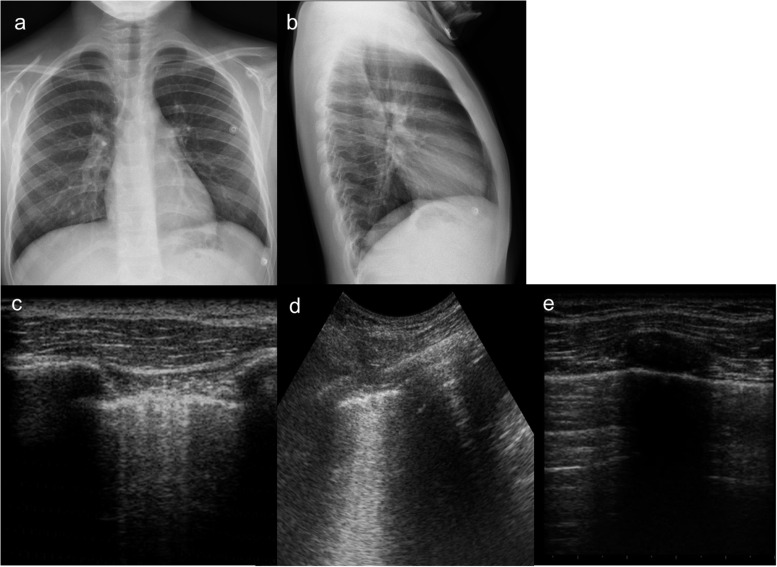
Figure 1.
A 6-year-old boy with cough and fever. (a) Chest X-ray (CXR) performed at admission in anterior–posterior projection shows an extensive medial lobe consolidation; (b) lung ultrasound (LUS) performed simultaneously with CXR shows a typical pneumonic consolidation with “arborescent bronchogram”; (c) in association, there was a wider consolidation with “parallel” bronchogram suggesting a prevalent atelectasis component, measures were taken to follow up it up; (d) follow-up at 7 days. LUS shows a significative reduction in size of lung consolidation, with persistent “parallel” bronchogram suggesting residual minimal atelectasis component; (e) follow-up at 14 days. LUS shows complete resolution of lung consolidation, with residual pleural line thickening.
The time-motion mode can be used to differentiate alveolar consolidation from pleural effusion, since the first one often contains water; the synusoid sign helps to confirm a low-viscosity pleural effusion.
CXR was performed with direct radiography system (Carestream DR 9000; Carestream Heath Inc., Rochester, NY) in posterior-anterior and lateral position (when possible).
When pleural effusion was identified only by ultrasound and not by CXR, it was chosen to perform just ultrasound until pleural effusion re-absorption.
LUS follow-up was performed only in positive patients at admission, after 5 and 14 days.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study (retrospective study), formal consent is not required. Informed consent was obtained from all individual participants included in the study.
RESULTS
In the 84 patients admitted to our emergency department for clinical signs of pneumonia, LUS showed findings consistent with pneumonia in 60/84 patients, while CXR was positive in 47/84 children. Only one patient with normal LUS showed a positive CXR, because of the retroscapular region of consolidation, hidden to the ultrasound beam. 13 patients with normal CXR had a positive LUS. In these 13 patients with discordant results, the clinical management was consistent with pneumonia. The cases not diagnosed by LUS or by CXR were discovered to be superior airway infections or interstitial pneumonia.
LUNG ULTRASOUND FINDINGS
At admission, LUS findings for pneumonia were:
34/60 (56%) children showed a typical pattern of lung consolidation. All the consolidations showed hypervascularity at colour-Doppler imaging (Figure 2).
26/60 (44%) patients showed association of multiple B-lines, expression of interstitial involvement and small subpleural consolidations consistent with small mucus plugs.
Air bronchogram was observed in 31/60 (70%) patients. Fluid bronchogram was observed in only 2 cases.
52/60 (86.6%) patients showed some pleural line abnormalities, such as thickening, irregularity, hypoechogenicity and a typical granular pattern.
18/60 (30%) children showed pleural effusion (of different entity), 5 of which with debris (Figure 3).
1 case positive at CXR was negative at LUS because of the retroscapular region, which is a difficult area to explore with ultrasound.
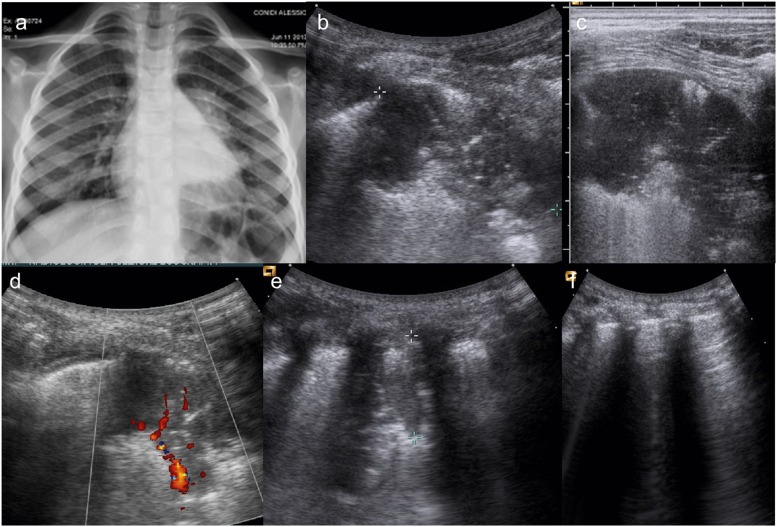
Figure 2.
An 8-year-old patient presenting with persistent cough and fever after therapy. (a) Chest X-ray shows a left parahilar consolidation; (b) lung ultrasound (LUS) performed simultaneously depicts well a well-defined wide pneumonic consolidation; (c) best detail of the well-defined wide pneumonic consolidation. (d) Colour-Doppler ultrasound shows its typical hypervascularity; (e) LUS follow-up (7 days after): wide reduction of pneumonic consolidation, but still present; (f) LUS follow-up (14 days after): pneumonic consolidation disappearance; regular “A-lines”.
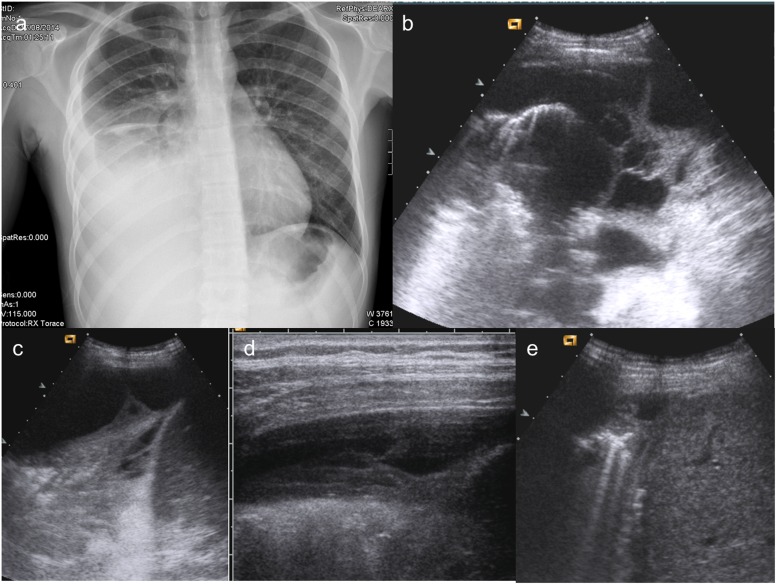
Figure 3.
A 15-year-old boy presenting at emergency department with dyspnoea and fever. (a, b) Chest X-ray shows rise of the right diaphragm, and the latero-lateral projection enhances the presence of posterior pleural effusion; (c) lung ultrasound (LUS) performed at the same time demonstrates a large amount of pleural effusion; (d) LUS, performed after tube insertion and drainage of partially corpusculated pleural effusion, shows a quite complete resolution of empyema; (e) follow-up at 7 days: LUS shows a complete resolution of empyema.
Consolidation sizes ranged from 1.5 to 7 cm, at first LUS evaluation.
The 60 patients with positive LUS findings were followed up with LUS for 14 days in order to monitor more accurately the evolution of the disease; 28/60 patients showed a complete regression of the disease; 23/60 children showed a significant decrease in size of lung consolidation; and 9/60 children showed disease stability or insignificant decrease in size of lung consolidation, requiring thus adjunctive LUS exams (at 21–28 days).
As for pleural effusion, in 13/18 children, there was complete fluid re-absorption, while in 5/18 children, there was a reduction in volume and an organization, because LUS showed a corpusculated component (Figure 4); 2/18 children needed chest-tube insertion for pleural effusion drainage.
Figure 4.
A 10-year-old patient presenting with dyspnoea, cough and fever after 5 days of pneumonia therapy. (a) Anterior–posterior Chest X-ray shows an inhomogeneous right basal opacification. (b, c) Lung ultrasound (LUS) performed simultaneously well depicts a multiloculated and corpusculated pleural effusion with a large posterobasal consolidation; (d) LUS at 7 days after pleural drainage shows a wide reduction in size of the multiloculated pleural effusion; (e) the follow-up at 14 days after pleural drainage demonstrates a complete re-absorption of the septated pleural effusion with some residual “B-lines”, expression of interstitial involvement.
All the cases were followed up until the complete resolution of the disease.
CHEST X-RAY FINDINGS
33/47 patients (70.2% of CXR-positive patients) had findings positive for lung consolidation; in all cases, there was air bronchogram. 6/33 of these patients showed pleural effusion.
17/47 children (36.2% of CXR-positive patients) showed an interstitial involvement; none of these showed pleural effusion.
In 13 patients with a negative CXR and positive LUS, the clinical presentation and management was typical for pneumonia.
DISCUSSION
Chest radiography is commonly performed to diagnose a lung consolidation in the presence of signs and symptoms suggesting pneumonia in adult patients, because of its rapidity, accessibility and convenience. However, previous studies reported some concerns about its application, owing to the high interobserver variability and the use of ionizing radiation, especially if performed on paediatric patients.16,17
In fact children are at least four times more sensitive than adults to ionizing radiation, because of a longer life expectancy and faster cell rate division.18,19
For a long time, ultrasound has been used to recognize just a pleural effusion because the normal aerated lung is not the ideal target for the ultrasound beam; it is in the recent years that evaluation of the lung has became possible owing to advances in technologies and the use of multifrequency probes. In fact, the presence of solid tissue or fluid can be easily detected by ultrasound, as many previous studies performed on adults and children reported;20–23 as Iuri et al20 demonstrated in their work, LUS applied on children showed a sensitivity of 91.67% in identifying parenchymal consolidation, recognizing the air bronchogram in 36.3% vs 29.1% of CXR. Another study by Copetti and Cattarossi21 reported a case series of 79 young patients with suspected pneumonia undergoing both CXR and LUS: the latter identified 60/79 lung consolidations whereas CXR identified 53 out of 60 patients with positive ultrasound findings. Reissig et al22 reported an excellent LUS sensitivity and specificity (94% and 98%, respectively) in CAP diagnosis in their large prospective study performed on adults.
The distinctive feature of this article is that the first diagnosis and follow-up are achieved in emergency setting; this highlights LUS feasibility and its high accuracy also in difficult conditions.
In our study, LUS recognized in total 47 lung consolidations, whereas CXR recognized 33 lung consolidations; this finding can be explained because of the location of some lesions (beyond the heart and mediastinum) and their small size, if we consider the small mucus plugs. Lung consolidations appeared as hypoechoic areas with blurred margins, and in all cases presenting as consolidation, air bronchogram was detected. This finding helped us to also recognize very small consolidations undetectable by CXR (Figure 5). In 13 cases, the lung disease appeared only as multiple B-lines, expression of non-specific interstitial involvement; most of those cases did not show pleural effusion (Figure 6). CXR recognized 17 interstitial diseases; this could be explained by the inherent high CXR interobserver variability.
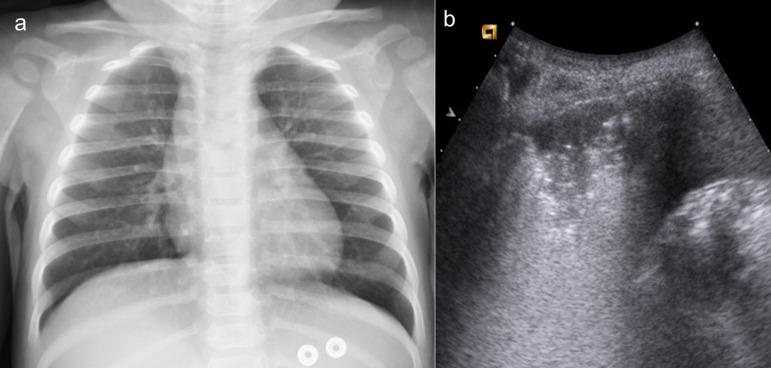
Figure 5.
A 14-month-old boy presenting with cough and fever. (a) Chest X-ray does not show any consolidation or evident interstitial disease; (b) lung ultrasound performed simultaneously depicts well a well-defined left posterobasal small consolidation.
Figure 6.
A 11-year-old boy presenting with mild dyspnoea, cough and fever. (a, b) Chest X-ray does not show any consolidation or interstitial disease. (c, d) Lung ultrasound performed simultaneously on lung bases depicts well multiple “B-lines”, ring-down, vertical artefacts, expression of interstitial involvement, so much different from the normal pattern of “A-lines”. (e) Artefacts parallel to pleural lines, which are present at upper lobes.
This result is in line with the literature data reported in a very recently published article;24 the authors, in fact, compared the accuracy of LUS in the detection of lung consolidation, taking CT scan as the gold standard: they demonstrated that in a subgroup of patients who underwent CXR also (in addition to CT and LUS), the sensitivity of LUS (81.4%) was significantly higher than that of CXR (64.3%), whereas specificity remained similar (94.2% vs 90%), showing that LUS can be a reliable alternative to CXR for the diagnosis of lung consolidation at the bedside of the patient.
A fluid bronchogram was identified in two cases because colour Doppler helped us to distinguish it from vessels; this result is in line with literature, since the rate of this finding ranges from 0% to 8.1%.25
LUS showed a higher sensitivity, with respect to CXR, in the identification of pleural effusion, because ultrasound can detect very small volume of fluid, allowing also a better characterization of its content, since it has been possible to recognize small debris within pleural effusision or a corpusculated component, as it can be observed in haematic collections (Figure 3). At LUS, 52/60 (86.6%) patients showed abnormality of the pleural line, presenting with a granular pattern; these are interesting data deserving further studies. There was only one case of false-negative LUS owing to the retroscapular localization of the lesion recognized by CXR.
Moreover, LUS helped to distinguish lung consolidation from atelectasis, as shown in Figure 1, since the latter shows the same echogenicity as the liver, and the air bronchogram is parallel and crowded; on the other hand, in pneumonia, the air bronchogram presents a branching pattern. In addition, the presence of dynamic air bronchograms helps to rule out atelectasis.
LUS performed during the follow-up allowed us to monitor the evolution of the disease in a better way than by CXR, especially when concerning small consolidations and the small amounts of pleural effusion not visible on the CXR.
We have to say that ultrasound has its own limitations, such as the inability to explore some regions (the retroscapular area); it is operator dependent and does not allow the evaluation of the real reduction in size of a finding because of superimposed artefacts. No specific LUS signs are detected in case of interstitial pneumonia; further studies are required to improve it. In addition, LUS requires time to explore the entire thorax; nevertheless, in the follow-up, LUS is really feasible and fast because the physician is aware of disease location once it has been identified, allowing to answer clinical questions such as an increase or reduction in the volume of pleural effusion or consolidation. It is very useful in young crying patients also because their movements do not cause any artefacts in CT or CXR.
Above all, the lack of ionizing radiation makes this technique really suitable and repeatable for these patients.26
The limitations of our study consist of the small sample size, retrospective approach and low concordance as for as consolidation dimensions measured by CXR and LUS; more studies with a larger sample size are required to overcome these limitations.
CONCLUSION
This study confirms the role of LUS in the diagnosis of pneumonia in paediatric patients as a significant tool in addition to CXR, and proves its feasibility in the follow-up in an emergency setting.
We can state that it is incorrect to perform CT just to evaluate or quantify a pleural effusion, considering the higher accuracy of LUS, especially in paediatric patients.
To our knowledge, this is the first study which focused on the role of LUS in the follow-up of CAP in an emergency department; further data are required to achieve a wider experience in this setting.
Contributor Information
Stefania Ianniello, Email: stefianni66@gmail.com.
Claudia Lucia Piccolo, Email: clapiccolo@libero.it.
Grazia L Buquicchio, Email: graziabuquicchio@gmail.com.
Margherita Trinci, Email: margherita.trinci@libero.it.
Vittorio Miele, Email: vmiele@sirm.org.
REFERENCES
- 1.British Thoracic Society of standards of care committee. BTS guidelines for the management of community acquired pneumonia in childhood. Thorax 2002; 57(Suppl. 1): 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies H, Wang E. Reliability of the chest radiograph in the diagnosis of lower respiratory infections in young children. Pediatr Infect Dis J 1996; 15: 600–4. doi: 10.1097/00006454-199607000-00008 [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstein DA, Lascols N, Prin S, Meziere G. The “lung pulse”: an early ultrasound sign of complete atelectasis. Intensive Care Med 2003; 29: 2187–92. doi: 10.1007/s00134-003-1930-9 [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein DA, Lascols N, Meziere G, Gepner A. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med 2004; 30: 276–81. doi: 10.1007/s00134-003-2075-6 [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest 1995; 108: 1345–8. doi: 10.1378/chest.108.5.1345 [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein DA, Meziere G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: the comet-tail artifact. Intens Care Med 1998; 24: 1331–4. doi: 10.1007/s001340050771 [DOI] [PubMed] [Google Scholar]
- 7.Lichtenstein DA, Meziere G, Biderman P, Gepner A, Barrè O. The comet-tail artifact, An ultrasound sign of alveolar-interstitial syndrome. Am J Reso Crit Care Med 1997; 156: 1640–6. doi: 10.1164/ajrccm.156.5.96-07096 [DOI] [PubMed] [Google Scholar]
- 8.Ianniello S, Di Giacomo V, Sessa B, Miele V. First-line sonographic diagnosis of pneumothorax in major trauma: accuracy of e-FAST and comparison with multidetector computed tomography. Radiol Med 2014; 119: 674–80. doi: 10.1007/s11547-014-0384-1 [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Zhang Z. Bedside ultrasonography for diagnosis of pneumothorax. Quant Imaging Med Surg 2015; 5: 618–23. doi: 10.3978/j.issn.2223-4292.2015.05.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urbankowska E, Krenke K, Drobczynski L, Korczynski P, Urbankowski T, Krawiec M, et al. Lung ultrasound in the diagnosis and monitoring of community acquired pneumonia in children. Respir Med 2015; 109: 1207–12. doi: 10.1016/j.rmed.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 11.Ho MC, Ker CR, Hsu JH, Wu JR, Dai ZK, Chen IC. Usefulness of lung ultrasound in the diagnosis of community-acquired pneumonia in children. Pediatr Neonatal 2012; 56: 40–5. [DOI] [PubMed] [Google Scholar]
- 12.Bedetti G, Gargani L, Corbisiero A, Frassi F, Poggianti E, Mottola G. Evaluation of ultrasound lung comets by hand-held echocardiography. Cardiovasc Ultrasound 2006; 4: 34. doi: 10.1186/1476-7120-4-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.See KC, Ong V, Wong SH, Leanda R, Santos J, Taculod J, et al. Lung ultrasound training: curriculum implementation and learning trajectory among respiratory therapists. Intensive Care Med 2015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Blaivas M. Lung ultrasound in evaluation of pneumonia. J Ultrasound Med 2012; 31: 823–6. [DOI] [PubMed] [Google Scholar]
- 15.Caiulo VA, Gargani L, Caiulo S, Fisicaro A, Moramarco F, Latini G, et al. Lung ultrasound characteristics of community acquired pneumonia in hospitalized children. Pediatr Pulmonol 2013; 48: 280–7. doi: 10.1002/ppul.22585 [DOI] [PubMed] [Google Scholar]
- 16.Frush DP. Radiation, thoracic imaging, and children: radiation safety. Radiol Clin North Am 2011; 49: 1053–69. doi: 10.1016/j.rcl.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 17.Ye X, Xiao H, Chen B, Zhang S. Accuracy of lung ultrasonography versus chest radiography for the diagnosis of adult community-acquired pneumonia: review of the literature and meta-analysis. PLoS One 2015; 10: e0130066. doi: 10.1371/journal.pone.0130066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strauss K, Kaste S. The ALARA (as low as reasonably achievable) concept in pediatric interventional and fluoroscopic imaging: striving to keep radiation doses as low as possible during fluoroscopy of pediatric patients- a white paper executive summary. Pediatr Radiol 2006; 36(Suppl. 2): 110–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas KE, Parnell-Parmley JE, Haidar S, Moineddin R, Charkot E, BenDavid G, et al. Assessment of radiation dose awareness among pediatricians. Pediatr Radiol 2006; 36: 823–32. doi: 10.1007/s00247-006-0170-x [DOI] [PubMed] [Google Scholar]
- 20.Iuri D, De Candia A, Bazzocchi M. Evaluation of the lung in children with suspected pneumonia: usefulness of ultrasonography. Radiol Med 2009; 114: 321–30. doi: 10.1007/s11547-008-0336-8 [DOI] [PubMed] [Google Scholar]
- 21.Copetti R, Cattarossi L. Ultrasound diagnosis of pneumonia in children. Radiol Med 2008; 113: 190–8. doi: 10.1007/s11547-008-0247-8 [DOI] [PubMed] [Google Scholar]
- 22.Reissig A, Copetti R, Mathis G, Mempel C, Schuler A, Zechner P, et al. Lung ultrasound in the diagnosis and follow-up of community acquired pneumonia: a prospective multicentre diagnostic accuracy study. Chest 2012; 142: 965–72. doi: 10.1378/chest.12-0364 [DOI] [PubMed] [Google Scholar]
- 23.Pagano A, Numis FG, Visone G, Pirozzi C, Massarone M, Olibet M, et al. Lung ultrasound for diagnosis of pneumonia in emergency department. Intern Emerg Med 2015; 10: 851–4. doi: 10.1007/s11739-015-1297-2 [DOI] [PubMed] [Google Scholar]
- 24.Nazerian P, Volpicelli G, Vanni S, Gigli C, Betti L, Bartolucci M, et al. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am J Emerg Med 2015; 33: 620–5. doi: 10.1016/j.ajem.2015.01.035 [DOI] [PubMed] [Google Scholar]
- 25.Reissig A, Kroegel C. Sonographic diagnosis and follow-up of pneumonia: a prospective study. Respiration 2007; 74: 537–47. doi: 10.1159/000100427 [DOI] [PubMed] [Google Scholar]
- 26.Chen SW, Zhang MY, Liu J. Application of lung ultrasonography in the diagnosis of childhood lung diseases. Chin Med J 2015; 128: 2672–8. doi: 10.4103/0366-6999.166035 [DOI] [PMC free article] [PubMed] [Google Scholar]