Abstract
Extensive evidence has demonstrated that psychological stress has detrimental effects on psychological health, cognitive function, and ultimately well-being. While stressful events are a significant cause of psychopathology, most individuals exposed to adversity maintain normal psychological functioning. The mechanisms underlying such resilience are poorly understood, and there is an urgent need to identify and target these mechanisms to promote resilience under stressful events. Botanicals have been used throughout history to treat various medical conditions; however, the development of botanical compounds into potential preventative and therapeutic agents in studies promoting brain health is hindered by the fact that most orally consumed botanicals are extensively metabolized during absorption and/or by post-absorptive xenobiotic metabolism. Therefore, the primary objective of this review article is to provide recommendations for developing natural compounds as novel therapeutic strategies to promote resilience in susceptible subjects. The development of botanical polyphenols to ultimately attenuate mood disorders and cognitive impairment will rely on understanding (1) the absorption and bioavailability of botanical polyphenols with emphasis on flavan-3-ols, (2) the characterization of tissue specific accumulation of biologically available polyphenols and their mechanisms of action in the brain, and eventually (3) the characterization of biologically available polyphenol metabolites in mechanisms associated with the promotion of resilience against mood disorders and cognitive impairment in response to stress. We also summarize exciting new lines of investigation about the role of botanicals such as polyphenols in the promotion of cognitive and psychological resilience. This information will provide a strategical framework for the future development of botanicals as therapeutic agents to promote resilience, ultimately preventing and/or therapeutically treating cognitive impairment and psychological dysfunction.
Keywords: Polyphenols, Botanicals, Resilience, Stress-Induced Depression, Sleep Deprivation-Induced Cognitive Impairment, Microbiome
Introduction
Over the past decade, there has been increasing attention paid to the phenomenon of resilience: the ability to maintain normal psychological and physical functioning and avoid serious mental illness when exposed to stress and trauma, even at extraordinary levels (Aburn et al., 2016; Kimhi, 2016). Although resilience has been identified across the spectrum of psychiatric disorders, we focus here on resilience as it relates to stress-induced depression and sleep deprivation (SD)-induced cognitive impairment. In this context, resilience refers to the capacity of an individual to cope with negative psychological and biological consequences of extreme stress that would otherwise compromise their psychological or physical well-being (Kimhi, 2016; Kreutzer et al., 2016; Russo et al., 2012). Recent reports indicate that resilience in humans is an active, adaptive process and not simply the absence of pathological responses that occur in more susceptible individuals (Russo et al., 2012). Anxiety and stress-related disorders are widespread psychological conditions with broad health implications, including negative impacts on cardiovascular and metabolic functions, as well as on mental health, leading to depression and memory dysfunction (Farzaei et al., 2016). The concept of human resilience is difficult to operationalize as it encapsulates many divergent behavioral phenotypes (Russo et al., 2012). Indeed, the study of human resilience is still a mostly phenomenological assessment of biological factors in resilient individuals that are associated with more successful coping responses (Kimhi, 2016; Russo et al., 2012). While stressful events are a significant cause of psychopathology, most individuals exposed to adversity maintain normal psychological functioning. The mechanisms underlying such resilience are poorly understood, and there is an urgent need to identify and target these mechanisms to promote resilience under stressful events. Certain botanicals, used throughout history to treat various medical conditions, provide a unique opportunity to target these mechanisms and ultimately promote neuroresilience in response to stress. The development of botanical polyphenols to ultimately attenuate mood disorders and cognitive impairment will rely on understanding (1) the absorption and bioavailability of botanical polyphenols with emphasis on flavan-3-ols, (2) the characterization of tissue specific accumulation of biologically available polyphenols and their mechanisms of action in the brain, and eventually (3) the characterization of biologically available polyphenol metabolites in mechanisms associated with the promotion of resilience against mood disorders and cognitive impairment in response to stress. We also summarize exciting new lines of investigation about the role of botanicals such as polyphenols in the promotion of cognitive and psychological resilience. This information will provide a strategical framework for the future development of botanicals as therapeutic agents to promote resilience, ultimately preventing and/or therapeutically treating cognitive impairment and psychological dysfunction.
A Historical Perspective of Botanical Drug Discovery in Medicine
Throughout history, the development of major drug blockbusters by the pharmaceutical industry has capitalized on the mechanistic investigation of natural compounds followed by subsequent clinical, pharmacological and chemical studies. Since the 1940s, 131 (74.8%) out of 175 small molecule anticancer drugs are natural product-based/inspired, with 85 (48.6%) being either natural products or derived from natural products (Newman and Cragg, 2012). Among the 20 approved small molecule New Chemical Entities in 2010, half of them are natural products (Newman and Cragg, 2012). The history of natural product discovery is full of remarkable stories of how the discovery of a natural product profoundly impacted advances in biology and therapy. An archetypical example is the discovery of the anti-inflammatory agent, acetylsalicyclic acid, commonly known as aspirin, derived from the natural product Filipendula ulmaria (Schmidt et al., 2008). Similarly, the discovery of the natural product Papaver somniferum (opium poppy) resulted in the isolation of several alkaloids including morphine (Schmidt et al., 2008). Another typical example is Digitalis (foxglove), an herbaceous genus, which in the last three centuries was shown to produce a bioactive metabolite digitoxin, currently used to treat congestive heart failure (Schmidt et al., 2008). Most recently the Chinese traditional medicine Artemisinin, isolated from the plant sweet wormwood, is now part of standard anti-malarial regimens; this discovery supported a Nobel Prize in Physiology or Medicine in 2015 (Kiani et al., 2016). Undoubtedly the discovery of new natural products promises significant advances in many fields of medicine, especially neurology and psychiatry. Unfortunately, the much needed research ranging from rigorous bioavailability studies to characterization of the bioactive metabolites to pharmacokinetic and pharmacodynamic studies are still for the most part unmet in the field of brain research. Remarkable progress has been made in the last few years in relation to the characterization of certain bioavailable bioactive polyphenolic metabolites that are capable of accumulating in the brain, which promises a great deal of opportunities for future development in the promotion of healthy cognitive function (Chen et al., 2015; Ferruzzi et al., 2009; Gasperotti et al., 2015; Ho et al., 2013; Margalef et al., 2016; Wang et al., 2015; Wang et al., 2012b). Thanks to the support of federal funding, in particular from the National Center for Complementary and Integrative Health (NCCIH), the characterization of bioavailable bioactive polyphenolic metabolites will undoubtedly allow unparalleled new breakthroughs in the field of brain health rivaling the impact of acetylsalicylic acid (aspirin) on anti-inflammatory research.
Dietary Polyphenols in the Promotion of Cognitive and Psychological Resilience
Polyphenols are a class of phenolic compounds characterized by two or more benzene rings that each has at least one hydroxyl group (OH) attached, and are found abundantly in fruits, such as berries and grapes, vegetables, tea, and other plant sources. Polyphenols have been found to possess a variety of health benefits, including cancer prevention (Chen and Chen, 2013), heart disease risk reduction (Jiang et al., 2010), and protection against neurodegenerative disorders (Pasinetti, 2012). In preclinical studies, we found certain bioavailable, bioactive and brain-penetrating polyphenols, particularly those among the flavonoid subclass found in commercially available Concord grape juice (CGJ) and grape-seed polyphenol extract (GSPE), effectively promote neuronal plasticity mechanisms that play a major role in learning and memory functions (Ho et al., 2013; Wang et al., 2012b). Consistent with these observations, Krikorian et al. demonstrated that 16-weeks of dietary supplementation with CGJ (15 – 21 oz. per day) significantly improved cognitive function of older adult subjects with mild cognitive impairment (Krikorian et al., 2010). Increasing evidence indicates that dietary polyphenols may help promote cognitive and/or psychological resilience. Primary polyphenolic constituents include flavonoids, which can further be subdivided into flavonols and flavanols, phenolic acids, and stilbenes. Previous bio-guided fractionation and bioavailability studies from our research group revealed that GSPE is rich in bioavailable flavanols (flavan-3-ols), including anthocyanidins such as catechin and epicatechin, and microbiome-derived phenolic acids; we also found that CGJ is rich in bioavailable flavonols, such as quercetin (Fig. 1; Ferruzzi et al., 2009; Ho et al., 2013; Wang et al., 2015). Studies from our lab and others also suggest that flavanol metabolites may benefit cognition, in part, by improving synaptic plasticity. Our observations provide the impetus to further develop select brain bioavailable, bioactive catechin, epicatechin and other brain-targeted proanthocyanidin metabolites to promote cognitive function (Kimhi, 2016; Wang et al., 2008; Wang et al., 2012b).
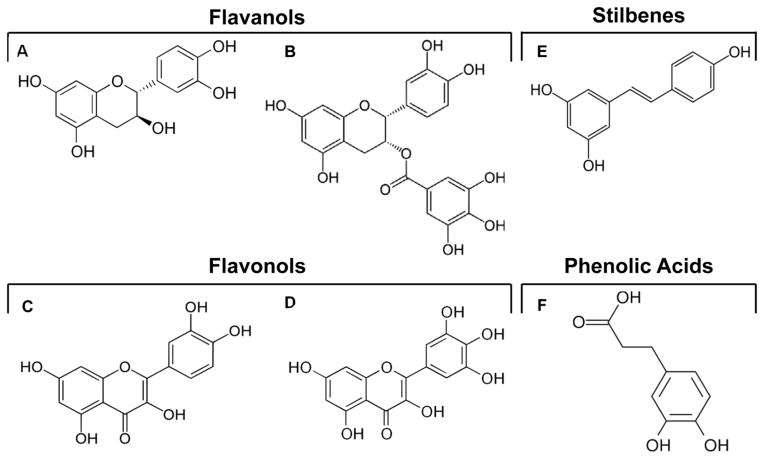
Fig. 1. Representative structures of polyphenols from grape and grape-derived products.
Representatives of flavanols: A) catechin and B) epicatechin gallate. Representatives of flavonols: C) quercetin and D) myricetin. Representative of stilbenes: E) RSV. Representative of phenolic acids: F) dihydrocaffeic acid.
Implications of Bioavailability in the Characterization of Bioactive Polyphenol Metabolites
In order to develop polyphenols for the promotion of cognitive and psychological resilience, it is essential to explore the pharmacokinetics of specific polyphenolic preparations and the mechanistic basis of their bioactivities in the brain. Since orally consumed polyphenols almost exclusively accumulate in the brain in metabolite form, these studies are extremely important for the development of novel therapeutic drugs to deliver specific brain available, bioactive polyphenol metabolites to recapitulate the biological activities of the original botanical polyphenolic compounds. Previous studies from us and others were designed to identify specific metabolites that may benefit brain function, for example, synaptic plasticity. We previously demonstrated that oral administration of GSPE from the monomeric form of proanthocyanidins is able to improve cognitive function, and only metabolites derived from a monomeric form of GSPE were able to selectively reach and accumulate in the brain at a concentration of ~400 nM (Wang et al., 2012b). For example, we reported that a biosynthetic epicatechin metabolite, 3′-O-methyl-epicatechin-5-O-β-glucuronide (3′-O-Me-EC-Gluc), one of the proanthocyanidin metabolites identified in the brain following oral GSPE treatment, promotes basal synaptic transmission and long term potentiation at physiologically relevant concentrations in hippocampal slices through mechanisms associated with cAMP response element binding protein (CREB) signaling (Fig. 2, adapted from (Spencer, 2009)). These studies suggest that certain forms of proanthocyanidin metabolites may target the brain and benefit cognitive function through modulation of synaptic plasticity. These studies provide the impetus to develop the polyphenol, 3′-O-Me-EC-Gluc metabolite, among others, for further studies of learning and memory function (Wang et al., 2012b), and demonstrate the importance of exploring the pharmacokinetics of specific polyphenolic preparations. Our studies have also demonstrated the essential approach of identifying select polyphenol-derived metabolites for targeting specific brain functions, which is consistent with previous evidence that catechin and epicatechin glucuronide metabolites may reach the brain and exert bioactivity (Abd El Mohsen et al., 2002; Abd El-Mohsen et al., 2006; Milbury and Kalt, 2010). Collectively, these studies strongly support the concept that characterization of select metabolites is a fundamental part of investigating dietary botanicals through bioavailability studies which can be eventually developed to selectively target specific brain functions.
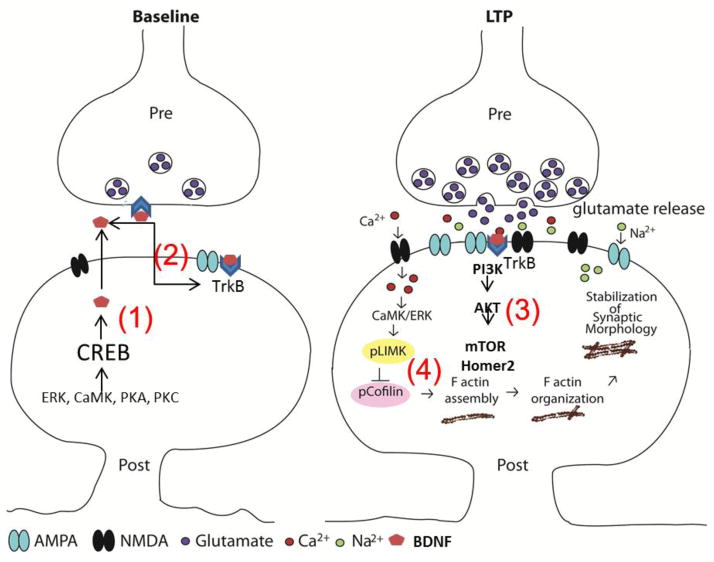
Fig. 2. Brain-bioavailable polyphenol metabolites promote neuroplasticity mechanisms.
Polyphenols modulate processes leading to synapse growth and increased receptor density. (1) Polyphenol metabolites activate the CREB pathway, thus increasing the expression and release of brain-derived neurotrophic factor from the synapse. (2) Elevated brain-derived neurotrophic factor expression binds to pre- and postsynaptic tropomyosin receptor kinase B receptors. (3) This triggers glutamate release, PI3K/mTOR signaling, and Arc IEG synthesis. (4) Sustained activation of mTOR leads to enhanced translational efficiency, while IEGs may influence expression of scaffolding proteins, increase receptor density and ultimately synaptic efficacy. Adapted from (Spencer, 2009).
Microbiome Associated Metabolism of Polyphenols into Phenolic Metabolites
Development of polyphenols for brain health is complicated by the fact that most orally consumed polyphenols are extensively metabolized by gastrointestinal (GI) epithelial cells during absorption and/or by post-absorptive xenobiotic metabolism. The majority of dietary polyphenols consumed are not absorbed by the upper intestinal track, and are further broken down by the gut microbiota in the colon into low molecular weight phenolic compounds, such as phenolic acids, that can be more efficiently absorbed by GI epithelial cells (Fig. 3; Calani et al., 2012; Monagas et al., 2010; Ozdal et al., 2016). In vitro studies have demonstrated that isolated human fecal microbiota are capable of metabolizing polyphenol components from botanical supplements into smaller phenolic metabolites, particularly phenolic acids, including 3-hydroxyphenolic acetic acid, 3,4-dihydroxyphenolactic acid, 3-(3′-hydroxyphenyl)propionic acid (3-HPP), and 3-(3′,4′-dihydroxyphenyl)propionic acid (Aura et al., 2008). The formation of phenolic compounds from GSPE was anticipated based on previous observations from in vitro colonic microflora metabolism of flavonoids, including proanthocyanidins, and urinary output of phenolic acid following oral administration of flavanoids (Aura et al., 2002; Prasain et al., 2009; Rechner et al., 2004; Ward et al., 2004).
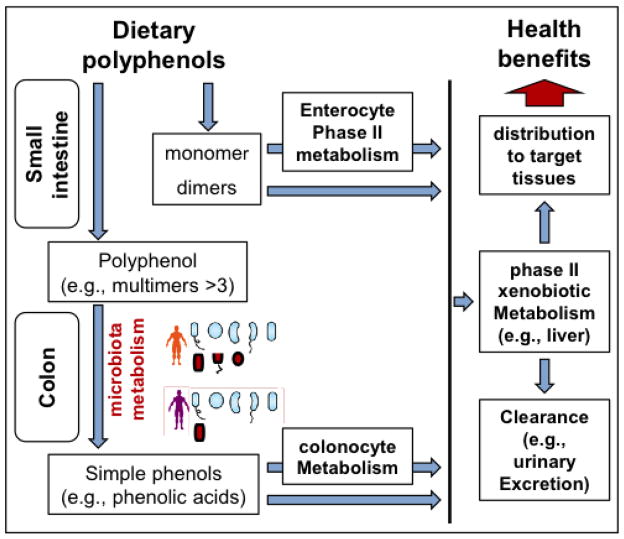
Fig. 3. Interpersonal differences in gut microbiota composition affect health benefits of polyphenol-rich botanicals.
After oral consumption, monomeric (and to a lesser extent, dimeric) polyphenols are absorbed by the small intestine, with or without enterocyte modification by phase II metabolism (e.g., glucuridation). Most dietary polyphenols, particularly multimeric ones, are not absorbed by the small intestine and are passed to the colon where microbial metabolism converts them into simple phenols, such as phenolic acids. Some of these phenolic acids are absorbed, with or without colonocyte modification by phase II metabolism. Absorbed polyphenol metabolites and phenolic acids may undergo additional phase II metabolism, primarily by the liver, before they are delivered to target tissues, such as blood cells and the brain. The schematic depicts interpersonal microbiota diversity with “red” bacterial strains representing those required to generate select bioactive phenolic acids capable of modulating the cellular/molecular mechanisms underlying psychological and/or cognitive resilience.
Published evidence has also demonstrated that the gut microbial community (i.e., gut microbiota) plays a critical role in modulating bioconversion and bioavailability of bioactive phenolic metabolites, particularly phenolic acids, from orally consumed polyphenol-rich botanical supplements (Bolca et al., 2013; Chen et al., 2012; Selma et al., 2009; Wang et al., 2012a). For example, evidence from our group revealed oral administration of certain botanical supplements leads to accumulation of multiple phenolic acids, such as 3,4-dihydroxydrocinnamic acid, 3-(3′-hydroxyphenyl) propionic acid and homovanillic acid in circulating blood (Wang et al., 2015). We observed an approximately 10-fold reduction in the content of 3,4-dihydroxydrocinnamic acid and approximately 50% reduction in content of 3-(3′-hydroxyphenyl) propionic acid and homovanillic acid in the plasma of germ-free gnotobiotic mice compared to conventionalized gnotobiotic mice (with normal microbiota harvested from wild-type mice) following oral administration of the botanical polyphenols (Pasinetti, unpublished observation). These studies from our group and others strongly support the pivotal contribution of the GI microbiota in modulating bioconversion and bioavailability of bioactive phenolic acids from the botanical polyphenols that are relevant for health benefits, including the preservation of cognitive and psychological resilience (Bolca et al., 2013; Chen et al., 2012; Selma et al., 2009; Wang et al., 2012a).
Based on these key issues in the characterization of bioactive polyphenol metabolites, we have been conducting studies on the role of polyphenols in the promotion of cognitive and psychological resilience; next we summarize exciting new lines of investigation that were made possible through the consideration of these factors.
Towards the Promotion of Resilience Against Stress-Induced Depression
An exciting line of investigation using bioactive bioavailable polyphenolic metabolites is the promotion of resilience against stress-induced depression and anxiety. Depression and anxiety are estimated to affect upwards of 50 million people in the US alone. There is no effective strategy of prevention, and it is estimated that currently available antidepressants produce remission in less than 50% of patients (Rush et al., 2009), which highlights the need for new, more selective therapeutics targeting underlying disease mechanisms. A number of recent human studies indicate that psychosocial stressors increase peripheral cytokine production and may be an important factor in the development of depression and anxiety (Dowlati et al., 2010; Maes et al., 1992; Maes et al., 1997; Maes et al., 2011). Subsets of patients with major depressive disorder (MDD) have higher levels of multiple inflammatory markers, including the cytokine Interleukin 6 (IL-6), the most consistently looked for cytokine (Maes et al., 1992; Maes et al., 1997; Miller et al., 2009). Although biological criteria are not used to diagnose depression, it is noteworthy that the newest diagnostic criteria for this disorder identifies inflammation as a possible cause (Association AP, 2013). Moreover, a recent meta-analysis indicates that IL-6 is the most consistently elevated pro-inflammatory cytokine in the blood of patients with MDD (Dowlati et al., 2010) and levels correlate with symptom severity in patients that do not respond to antidepressant treatment (Lanquillon et al., 2000). It is estimated that approximately 30–60% of patients with depression do not respond to approved antidepressant treatments (Krishnan and Nestler, 2008), which may reflect a heterogeneity in the mechanisms of depression that cannot be ubiquitously treated with standard antidepressants, including mechanisms such as systemic inflammation.
Thus, a promising approach to psychiatric drug development centers on screening compounds that promote resilience through anti-inflammatory properties. We have found that certain grape-derived polyphenol-rich botanicals have strong anti-inflammatory properties and can therefore promote psychological resilience and brain health through mediating IL-6 (Pasinetti, in preparation). In addition, we have strong evidence that by reducing peripheral inflammation, such as IL-6 release from leukocytes, we can promote psychological resilience and brain health (Pasinetti, in preparation). Thus, it is very likely that select bioavailable and bioactive phenolic metabolites reduce peripheral inflammation within leukocytes to reduce IL-6 production. In addition to reducing leukocyte-derived IL-6, select phenolic metabolites may also help promote psychological resilience by modulating expression of synaptic genes, and therefore, promote brain health and prevent maladaptive synaptic plasticity induced by environmental stressors, such as repeated social defeat stress (RSDS). Based on evidence that dietary polyphenols are primarily biologically available in vivo as phenolic metabolites we schematically summarize in Fig. 4 how dietary supplementation with specific bioactive polyphenol-rich preparations (e.g., CGJ/GSPE/resveratrol (RSV)) may mechanistically promote preservation of psychological resilience.
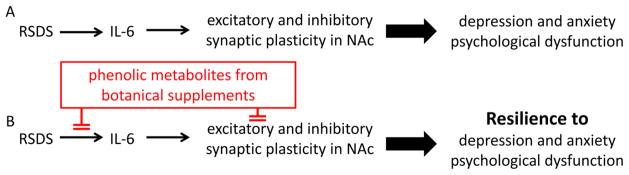
Fig. 4. Resilience to depression and anxiety.
Phenolic metabolites from dietary botanical supplements (i) reduce RSDS-induced inflammation and (ii) directly attenuate synaptic plasticity changes, which will lead to resilience to psychological dysfunction.
Towards the Promotion of Resilience Against Sleep Deprivation-Induced Cognitive Impairment
Another exciting line of investigation using bioactive bioavailable polyphenolic metabolites is the promotion of resilience against SD-induced cognitive impairment. Chronic sleep loss is a common problem in our society. An estimated 50–70 million US adults have sleep or wakefulness disorder (Colten et al., 2006). Insufficient sleep is co-morbid with chronic problems such as heart disease, kidney disease, high blood pressure, diabetes, obesity, and mental illness (Ford and Kamerow, 1989; Gillin, 1998; Hirotsu et al., 2010; Knutson and Van, 2008; Najafian et al., 2013; Palagini et al., 2013; Vijayan, 2012). Sleep loss can also contribute to irritability, aggression, inattentiveness, and diminished psychomotor vigilance (Kamphuis et al., 2012; Rajaratnam, 2001; Van Dongen et al., 2003). The negative impact of sleep loss on physical and mental health places a strain on our healthcare system (Kapur et al., 2002) and a large financial burden on our economy (Goel et al., 2009). Unfortunately, many people are unable to obtain sufficient sleep on a daily basis. Therefore, it is important to explore the molecular and cellular impacts of sleep loss in an effort to identify novel therapeutic approaches to counteract these effects.
Natural dietary supplements have been used to treat stress and insomnia since Hippocrates, and often have only mild side effects that are easily managed (Kinrys et al., 2009; Wollen, 2010). Select bioactive bioavailable polyphenolic metabolites derived from flavan-3-ols have extensive pharmacological action, including a pivotal role in mechanisms related to learning and memory consolidation. We recently demonstrated that oral administration with the botanical supplement mixture of GSPE, CGJ and RSV attenuates SD-mediated memory impairments assessed by contextual fear conditioning assay, and the improvement of memory consolidation processes coincided with the induction of the CREB transcription factor activity and induce the expression of immediate early genes (IEGs) in primary cortico-hippocampal neurons (Fig. 5). This evidence was exciting especially in view of the fact that these changes were associated with an increased expression of proteins involved in the consolidation of short into long term memory in the brain. Thus, this evidence demonstrates that certain dietary polyphenols may beneficially promote resilience towards the preservation of cognitive function in models of SD and supports the better characterization and development of bioavailable bioactive polyphenolic metabolites from complex botanicals for clinical applications.
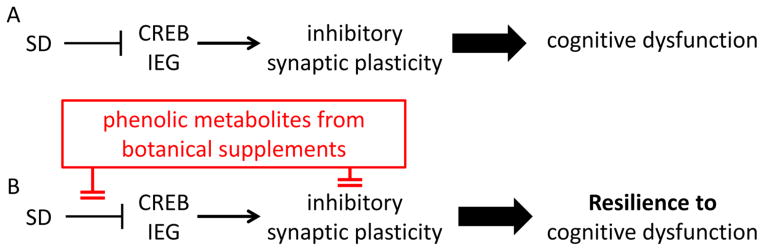
Fig. 5. Resilience to sleep deprivation-induced cognitive dysfunction.
Phenolic metabolites (a) promote CREB signaling and (b) modulate the expression of IEGs that are necessary for memory consolidation, which will lead to resilience to SD-mediated cognitive dysfunction.
Conclusion
While there has been significant effort within the pharmaceutical and medical industries to develop treatments for mood disorders, such as anxiety and depression, and SD-induced cognitive dysfunction, currently available treatments often have extreme side effects and adverse drug reactions, as well as inconvenient drug-drug and food-drug interactions. It is therefore necessary to challenge the prevailing and failing approach of the pharmaceutical industry by demonstrating the safe and efficacious role of novel botanical supplements (Qureshi and Al-Bedah, 2013). Moreover, characterizing GI microbial strains critical for the generation of specific bioactive phenolic metabolites that contribute to cognitive wellness and psychological resilience is highly innovative and will provide insight into broader issues related to the eventual goal of translation.
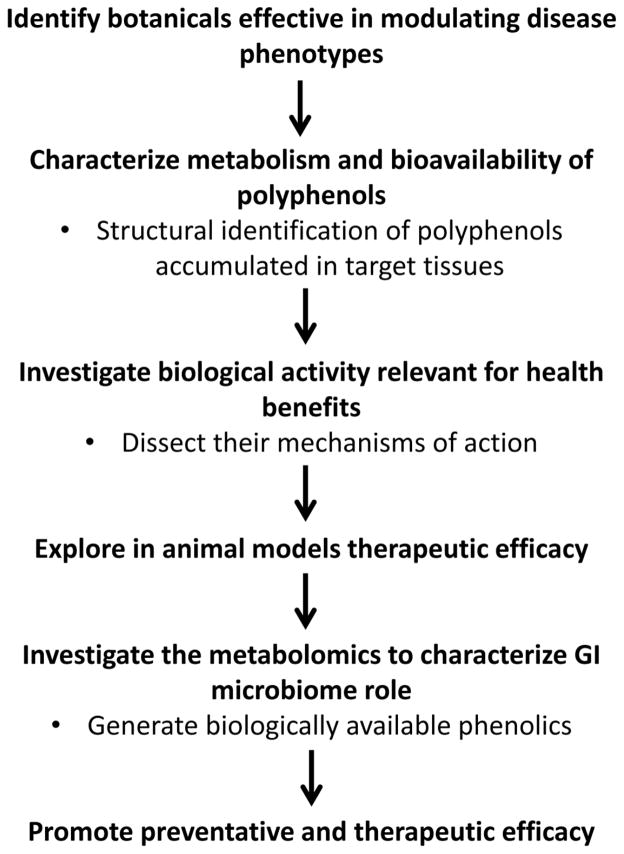
As schematized in Fig. 6, successful translational studies of polyphenols will require coordinated research efforts bridging together innovative technologies with studies (1) identifying botanical preparations effective in modulating specific conditions and/or phenotypes, (2) characterizing dose-response tolerability and safety of specific bioactive botanical preparations in preclinical models, (3) characterizing metabolism and bioavailability of polyphenols leading to the detection and structural identification of polyphenol metabolites accumulated in target tissue(s), (4) investigating in vitro and in vivo the identified bioavailable forms for bioactivity relevant for specific health benefits under investigation and dissecting their mechanisms of action, (5) exploring in vivo in animal model systems the efficacy of the bioactive polyphenol metabolites to influence the desired phenotype, and finally (6) further investigating the role of the GI microbiome in the generation of select biologically available phenolic forms from orally consumed botanicals to promote therapeutic efficacy of the bioactive botanical preparation, which will eventually lead to better understanding the role of novel probiotics involved in phenolic acid generation. These considerations are critical for the development of botanical polyphenols into “natural drugs” for the promotion of resilience against stress-induced depression and cognitive impairment, among other conditions.
Fig. 6.
Schematic of recommendations for the development of botanical polyphenols into “natural drugs”
Acknowledgments
This publication was supported by Grant Number P50 AT008661-01, titled “Dietary Botanicals in the Preservation of Cognitive and Psychological Resilience,” from the National Center for Complementary and Integrative Health (NCCIH) and the Office of Dietary Supplements (ODS). In addition, Dr. Pasinetti holds a Career Scientist Award in the Research and Development unit and is the Director of the Basic and Biomedical Research and Training Program, GRECC, James J. Peters Veterans Affairs Medical Center. We acknowledge that the contents of this manuscript do not represent the views of the NCCIH, ODS, National Institutes of Health, U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Reference List
- Abd El Mohsen MM, Kuhnle G, Rechner AR, Schroeter H, Rose S, Jenner P, Rice-Evans CA. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic Biol Med. 2002;33:1693–1702. doi: 10.1016/s0891-5849(02)01137-1. [DOI] [PubMed] [Google Scholar]
- Abd El-Mohsen M, Bayele H, Kuhnle G, Gibson G, Debnam E, Kaila SS, Rice-Evans C, Spencer JP. Distribution of [3H]trans-resveratrol in rat tissues following oral administration. Br J Nutr. 2006;96:62–70. doi: 10.1079/bjn20061810. [DOI] [PubMed] [Google Scholar]
- Aburn G, Gott M, Hoare K. What is resilience? An Integrative Review of the empirical literature. J Adv Nurs. 2016;72:980–1000. doi: 10.1111/jan.12888. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and statistical manual of mental disorders. Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- Aura AM, Mattila I, Seppanen-Laakso T, Miettinen J, Oksman-Caldentey KM, Oresic M. Microbial metabolism of catechin stereoisomers by human faecal microbiota: Comparison of targeted analysis and a non-targeted metabolomics method. Phytochemistry Letters. 2008;1:18–22. [Google Scholar]
- Aura AM, O’Leary KA, Williamson G, Ojala M, Bailey M, Puupponen-Pimia R, Nuutila AM, Oksman-Caldentey KM, Poutanen K. Quercetin derivatives are deconjugated and converted to hydroxyphenylacetic acids but not methylated by human fecal flora in vitro. J Agric Food Chem. 2002;50:1725–1730. doi: 10.1021/jf0108056. [DOI] [PubMed] [Google Scholar]
- Bolca S, Van de WT, Possemiers S. Gut metabotypes govern health effects of dietary polyphenols. Curr Opin Biotechnol. 2013;24:220–225. doi: 10.1016/j.copbio.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Calani L, Dall’Asta M, Derlindati E, Scazzina F, Bruni R, Del RD. Colonic metabolism of polyphenols from coffee, green tea, and hazelnut skins. J Clin Gastroenterol. 2012;46(Suppl):S95–S99. doi: 10.1097/MCG.0b013e318264e82b. [DOI] [PubMed] [Google Scholar]
- Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013;138:2099–2107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Hayek S, Rivera GJ, Gillitt ND, Ibrahim SA, Jobin C, Sang S. The microbiota is essential for the generation of black tea theaflavins-derived metabolites. PLoS One. 2012;7:e51001. doi: 10.1371/journal.pone.0051001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TY, Kritchevsky J, Hargett K, Feller K, Klobusnik R, Song BJ, Cooper B, Jouni Z, Ferruzzi MG, Janle EM. Plasma bioavailability and regional brain distribution of polyphenols from apple/grape seed and bilberry extracts in a young swine model. Mol Nutr Food Res. 2015;59:2432–2447. doi: 10.1002/mnfr.201500224. [DOI] [PubMed] [Google Scholar]
- Colten HR, Altevogt BM Institute of Medicine (and Committee on Sleep Medicine and Research) Sleep disorders and sleep deprivation an unmet public health problem. Washington, DC: Institute of Medicine; 2006. [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Farzaei MH, Bahramsoltani R, Rahimi R, Abbasabadi F, Abdollahi M. A Systematic Review of Plant-Derived Natural Compounds for Anxiety Disorders. Curr Top Med Chem. 2016;16:1924–1942. doi: 10.2174/1568026616666160204121039. [DOI] [PubMed] [Google Scholar]
- Ferruzzi MG, Lobo JK, Janle EM, Cooper B, Simon JE, Wu QL, Welch C, Ho L, Weaver C, Pasinetti GM. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: implications for treatment in Alzheimer’s disease. J Alzheimers Dis. 2009;18:113–124. doi: 10.3233/JAD-2009-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Gasperotti M, Passamonti S, Tramer F, Masuero D, Guella G, Mattivi F, Vrhovsek U. Fate of microbial metabolites of dietary polyphenols in rats: is the brain their target destination? ACS. Chem Neurosci. 2015;6:1341–1352. doi: 10.1021/acschemneuro.5b00051. [DOI] [PubMed] [Google Scholar]
- Gillin JC. Are sleep disturbances risk factors for anxiety, depressive and addictive disorders? Acta Psychiatr Scand Suppl. 1998;393:39–43. doi: 10.1111/j.1600-0447.1998.tb05965.x. [DOI] [PubMed] [Google Scholar]
- Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–339. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu C, Tufik S, Bergamaschi CT, Tenorio NM, Araujo P, Andersen ML. Sleep pattern in an experimental model of chronic kidney disease. Am J Physiol Renal Physiol. 2010;299:F1379–F1388. doi: 10.1152/ajprenal.00118.2010. [DOI] [PubMed] [Google Scholar]
- Ho L, Ferruzzi MG, Janle EM, Wang J, Gong B, Chen TY, Lobo J, Cooper B, Wu QL, Talcott ST, Percival SS, Simon JE, Pasinetti GM. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013;27:769–781. doi: 10.1096/fj.12-212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Chang CW, Dusting GJ. Cytoprotection by natural and synthetic polyphenols in the heart: novel mechanisms and perspectives. Curr Pharm Des. 2010;16:4103–4112. doi: 10.2174/138161210794519174. [DOI] [PubMed] [Google Scholar]
- Kamphuis J, Meerlo P, Koolhaas JM, Lancel M. Poor sleep as a potential causal factor in aggression and violence. Sleep Med. 2012;13:327–334. doi: 10.1016/j.sleep.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Kapur VK, Redline S, Nieto FJ, Young TB, Newman AB, Henderson JA. The relationship between chronically disrupted sleep and healthcare use. Sleep. 2002;25:289–296. [PubMed] [Google Scholar]
- Kiani BH, Suberu J, Mirza B. Cellular engineering of Artemisia annua and Artemisia dubia with the rol ABC genes for enhanced production of potent anti-malarial drug artemisinin. Malar J. 2016;15:252. doi: 10.1186/s12936-016-1312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhi S. Levels of resilience: Associations among individual, community, and national resilience. J Health Psychol. 2016;21:164–170. doi: 10.1177/1359105314524009. [DOI] [PubMed] [Google Scholar]
- Kinrys G, Coleman E, Rothstein E. Natural remedies for anxiety disorders: potential use and clinical applications. Depress Anxiety. 2009;26:259–265. doi: 10.1002/da.20460. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Van CE. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer JS, Marwitz JH, Sima AP, Bergquist TF, Johnson-Greene D, Felix ER, Whiteneck GG, Dreer LE. Resilience Following Traumatic Brain Injury: A Traumatic Brain Injury Model Systems Study. Arch Phys Med Rehabil. 2016;97:708–713. doi: 10.1016/j.apmr.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Krikorian R, Nash TA, Shidler MD, Shukitt-Hale B, Joseph JA. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br J Nutr. 2010;103:730–734. doi: 10.1017/S0007114509992364. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, ing-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, De JR, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Obuchowiczwa E, Goehler L, Brzeszcz J. Depression’s multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuro Endocrinol Lett. 2011;32:7–24. [PubMed] [Google Scholar]
- Maes M, Van der PM, Stevens WJ, Peeters D, DeClerck LS, Bridts CH, Schotte C, Cosyns P. Leukocytosis, monocytosis and neutrophilia: hallmarks of severe depression. J Psychiatr Res. 1992;26:125–134. doi: 10.1016/0022-3956(92)90004-8. [DOI] [PubMed] [Google Scholar]
- Margalef M, Pons Z, Iglesias-Carres L, Arola L, Muguerza B, rola-Arnal A. Gender related similarities and differences in the body distribution of grape seed flavanols in rats. Mol Nutr Food Res. 2016 doi: 10.1002/mnfr.201500717. [DOI] [PubMed] [Google Scholar]
- Milbury PE, Kalt W. Xenobiotic metabolism and berry flavonoid transport across the blood-brain barrier. J Agric Food Chem. 2010;58:3950–3956. doi: 10.1021/jf903529m. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monagas M, Urpi-Sarda M, Sanchez-Patan F, Llorach R, Garrido I, Gomez-Cordoves C, ndres-Lacueva C, Bartolome B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010;1:233–253. doi: 10.1039/c0fo00132e. [DOI] [PubMed] [Google Scholar]
- Najafian J, Mohamadifard N, Siadat ZD, Sadri G, Rahmati MR. Association between sleep duration and diabetes mellitus: Isfahan Healthy Heart Program. Niger J Clin Pract. 2013;16:59–62. doi: 10.4103/1119-3077.106756. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdal T, Sela DA, Xiao J, Boyacioglu D, Chen F, Capanoglu E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients. 2016;8 doi: 10.3390/nu8020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palagini L, Bruno RM, Gemignani A, Baglioni C, Ghiadoni L, Riemann D. Sleep loss and hypertension: a systematic review. Curr Pharm Des. 2013;19:2409–2419. doi: 10.2174/1381612811319130009. [DOI] [PubMed] [Google Scholar]
- Pasinetti GM. Novel role of red wine-derived polyphenols in the prevention of Alzheimer’s disease dementia and brain pathology: experimental approaches and clinical implications. Planta Med. 2012;78:1614–1619. doi: 10.1055/s-0032-1315377. [DOI] [PubMed] [Google Scholar]
- Prasain JK, Peng N, Dai Y, Moore R, Arabshahi A, Wilson L, Barnes S, Michael WJ, Kim H, Watts RL. Liquid chromatography tandem mass spectrometry identification of proanthocyanidins in rat plasma after oral administration of grape seed extract. Phytomedicine. 2009;16:233–243. doi: 10.1016/j.phymed.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi NA, Al-Bedah AM. Mood disorders and complementary and alternative medicine: a literature review. Neuropsychiatr Dis Treat. 2013;9:639–658. doi: 10.2147/NDT.S43419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaratnam SM. Legal issues in accidents caused by sleepiness. J Hum Ergol (Tokyo) 2001;30:107–111. [PubMed] [Google Scholar]
- Rechner AR, Smith MA, Kuhnle G, Gibson GR, Debnam ES, Srai SK, Moore KP, Rice-Evans CA. Colonic metabolism of dietary polyphenols: influence of structure on microbial fermentation products. Free Radic Biol Med. 2004;36:212–225. doi: 10.1016/j.freeradbiomed.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Warden D, Wisniewski SR, Fava M, Trivedi MH, Gaynes BN, Nierenberg AA. STAR*D: revising conventional wisdom. CNS Drugs. 2009;23:627–647. doi: 10.2165/00023210-200923080-00001. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, Ribnicky DM, Poulev A, Logendra S, Cefalu WT, Raskin I. A natural history of botanical therapeutics. Metabolism. 2008;57:S3–S9. doi: 10.1016/j.metabol.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selma MV, Espin JC, Tomas-Barberan FA. Interaction between phenolics and gut microbiota: role in human health. J Agric Food Chem. 2009;57:6485–6501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- Spencer JP. The impact of flavonoids on memory: physiological and molecular considerations. Chem Soc Rev. 2009;38:1152–1161. doi: 10.1039/b800422f. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med. 2012;6:557–566. doi: 10.1586/ers.12.44. [DOI] [PubMed] [Google Scholar]
- Wang D, Ho L, Faith J, Ono K, Janle EM, Lachcik PJ, Cooper BR, Jannasch AH, D’Arcy BR, Williams BA, Ferruzzi MG, Levine S, Zhao W, Dubner L, Pasinetti GM. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease beta-amyloid oligomerization. Mol Nutr Food Res. 2015;59:1025–1040. doi: 10.1002/mnfr.201400544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Xia M, Yan X, Li D, Wang L, Xu Y, Jin T, Ling W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res. 2012a;111:967–981. doi: 10.1161/CIRCRESAHA.112.266502. [DOI] [PubMed] [Google Scholar]
- Wang J, Ferruzzi MG, Ho L, Blount J, Janle EM, Gong B, Pan Y, Gowda GA, Raftery D, rrieta-Cruz I, Sharma V, Cooper B, Lobo J, Simon JE, Zhang C, Cheng A, Qian X, Ono K, Teplow DB, Pavlides C, Dixon RA, Pasinetti GM. Brain-targeted proanthocyanidin metabolites for Alzheimer’s disease treatment. J Neurosci. 2012b;32:5144–5150. doi: 10.1523/JNEUROSCI.6437-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ho L, Zhao W, Ono K, Rosensweig C, Chen L, Humala N, Teplow DB, Pasinetti GM. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J Neurosci. 2008;28:6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NC, Croft KD, Puddey IB, Hodgson JM. Supplementation with grape seed polyphenols results in increased urinary excretion of 3-hydroxyphenylpropionic Acid, an important metabolite of proanthocyanidins in humans. J Agric Food Chem. 2004;52:5545–5549. doi: 10.1021/jf049404r. [DOI] [PubMed] [Google Scholar]
- Wollen KA. Alzheimer’s disease: the pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Altern Med Rev. 2010;15:223–244. [PubMed] [Google Scholar]