Abstract
Maintaining the central nervous system microenvironment after injury, infection, and inflammatory and degenerative diseases is contingent upon adequate control of glial homeostatic functions. Disease is caused by microbial, environmental and endogenous factors that compromise ongoing nervous system function. The final result is neuronal dysfunction, dropout and nerve connection loss, and these underlie the pathobiology of Alzheimer's and Parkinson's disease, amyotrophic lateral sclerosis, stroke, and bacterial, parasitic and viral infections. However, what promotes disease are homeostatic changes in the brain's microenvironment affected by innate glial immune pro-inflammatory and adaptive immune responses. These events disturb the brain's metabolic activities and communication abilities. How the process affects the brain's regulatory functions that can be harnessed for therapeutic gain is the subject at hand. Specific examples are provided that serve to attenuate inflammation and improve disease outcomes specifically for HIV-associated neurocognitive disorders.
Keywords: astrocytes, microglia, neuroinflammation, human immunodeficiency virus-associated neurocognitive disorders, neurodegenerative disorders, Alzheimer's disease, Parkinson's disease, glioblastoma multiforme
Introduction
For infectious, degenerative and inflammatory disorders of the central nervous system (CNS), microglial responses directly participate in disease. This occurs as a consequence of the cells’ reaction to infectious, immune and degenerative processes (Perry et al. 2010; Streit et al. 1988). First, viral infections, for example, can lead to a parainfectious encephalomyelitis. This is commonly referred to as an acute disseminated encephalomyelitis (ADEM) where disease is driven pathobiologically by antigen-antibody attack. Autoimmunity occurs as a consequence of generalized neuroinflammation in the brain and spinal cord with profound myelin damage and white matter destruction. ADEM is commonly triggered after infection with a spectrum of viruses that include, but are not limited to influenza, measles, mumps, rubella, varicella zoster, Zika, Epstein Bar, cytomegalo-, entero- and herpes simplex viruses. ADEM can also be driven by vaccinations (Hemachudha et al. 1987; Ozawa et al. 2000). In both instances, brain injury is commonly self-limited, and permanent neurological sequel can occur but not commonly. Second, direct viral invasion of the CNS induces disease as a consequence of productive infection in neurons or accessory glial or endothelial cells. The process is active within the nervous system parenchyma. Such invasion is dependent on the pathogen, the immune status of the host and related genetics, and host immune responses. Such pathogenic events are commonly associated with significant morbidity and mortality. The former is seen with altered levels of consciousness and focal neurological symptoms commonly include seizures (Whitley 1990). A third cause of encephalitis is linked to inflammatory responses that occur as a consequence of viral infection of accessory cells and notably, monocytes, macrophages and microglia (He et al. 1997; Koenig et al. 1986). This directly involves neuronal destructive activities induced by viral and cellular neurotoxic proteins (Sørensen et al. 2008). A singular example of accessory cell infection-associated encephalitis is human immunodeficiency virus type one (HIV-1) infection. Apropos of HIV, a vigorous innate immune response occurs as a direct consequence of brain mononuclear phagocytes (MP; monocyte, perivascular macrophage and microglial) infection that drives pro-inflammatory and virotoxin-associated neurotoxic responses (Koenig et al. 1986; Persidsky and Gendelman 2003). This is perpetuated by autocrine and paracrine amplification of cytokine activities and by ongoing infection in the setting of a failure of immune responses or in patients where antiretroviral therapy has not been initiated (Schifitto et al. 2007). Neuronal loss in HIV-1 encephalitis is strongly associated with synaptic and dendritic damage (Masliah et al. 1996). Overt synaptic loss is in turn linked to severe cognitive impairment and brain viral load. Interestingly, levels of virus can vary dependent on brain region and underlies that it is the inducer of immune secretory factors, the perpetrators of disease (Kumar et al. 2007). Indeed, the development of dementia commonly but not always is associated with virus (Gray 1999). Interestingly, compensatory immune responses can restrict virus and attenuate disease; also, the advent of combination antiretroviral therapy has limited viral infection in the brain and facilitated host antiretroviral activities. Nonetheless, given the limited penetrance of antiretroviral drugs across blood-brain barrier (BBB) and comorbid conditions such as aging, drugs abuse and coinfection of hepatitis viruses in the HIV-infected population (Devlin et al. 2011), persistent neuroinflammation and viral reservoirs hiding in the brain remain to prevent the CNS function from restoring (Alexaki et al. 2008; Thompson et al. 2011).
For the degenerative Alzheimer's and Parkinson's diseases (AD and PD), the accumulation of misfolded, oxidized and aggregated proteins affect neuroinflammation and rapidly lead to neuronal dysfunction (Lashley et al. 2015; Polymeropoulos et al. 1997; Spillantini et al. 1997). Control of disease can also be associated with inflammation and immunity but how this occurs and can be harnessed for therapeutic gain is less clear (McGeer and McGeer 2004; Salminen et al. 2009). In general terms, attenuation of inflammation either by drugs or by relevant adaptive immune responses can facilitate disease outcomes, but the corollary provides opposite endpoints. Altogether, with the importance of neuroinflammation as a cause for a range of disorders of varying etiology, a primary therapeutic goal is the control of brain's microenvironment regardless of the disease. Obviously, the removal of the root cause such as the infectious agent or the misfolded proteins remains primary. This can be realized by therapeutics and vaccination strategies (Benner et al. 2004; Buchbinder et al. 2008; Doody et al. 2014; Masliah et al. 2005; Morgan et al. 2000; Schneeberger et al. 2015). However, what must follow is the restoration of the brain's innate immune function in sustaining its metabolic function and homeostasis. This is what underlies restorative neuronal vitality (Benarroch 2005; Ransohoff and Brown 2012).
Here, we discuss the means to achieve the goals of controlling the brain's microenvironment. This will be highlighted through others and our own developments for therapeutics that are aimed towards restoring neural homeostasis. Underpinning this goal is a singular limitation that rests in drug penetrance across the BBB and as such access of therapeutic agents to damaged brain areas (Pardridge 2005). To this end, questions still exist on how BBB disruption occurs and can be reversed, how altering vascular channels can occur, and how chemical modification of drugs can be better developed to maximize therapeutic delivery (Baseri et al. 2012; Gabathuler 2010; Meairs and Alonso 2007; Wolak and Thorne 2013). Although each approach has its limitations and drawbacks, several chemical modification of drugs to increase lipophilicity to move through lipophilic BBB, disguising them with lipophilic molecules to make pro-drugs, attaching targeting molecules to get the drugs to pass through the BBB, and packaging them into nanoparticles or exosomes were investigated (Dash et al. 2011; El-Andaloussi et al. 2012; Hall et al. 2016; Pathan et al. 2009). Yet another means rests in the delivery system where we can harness MP as carriers of antiviral drug nanoparticles as well as disease fighting proteins and nucleic acids (Mallapragada et al. 2015). The delivery of such agents across the BBB remains a singular goal (Brynskikh et al. 2010; Dou et al. 2009; Nowacek and Gendelman 2009; Zhao et al. 2011). Overall, we discuss the feasibility of modulating the CNS microenvironment for therapeutic benefit. We also reviewed the recent advances in delivery of therapeutic agents to the brain with a particular emphasis on the means to restore the brain's microenvironment during disease using a spectrum of adjunctive therapies that include those that attenuate inflammation and deliver growth factors to the sites of injury (Biju et al. 2010; Eggert et al. 2010; Marker et al. 2013; Zhao et al. 2014).
Attenuating Neuroinflammation
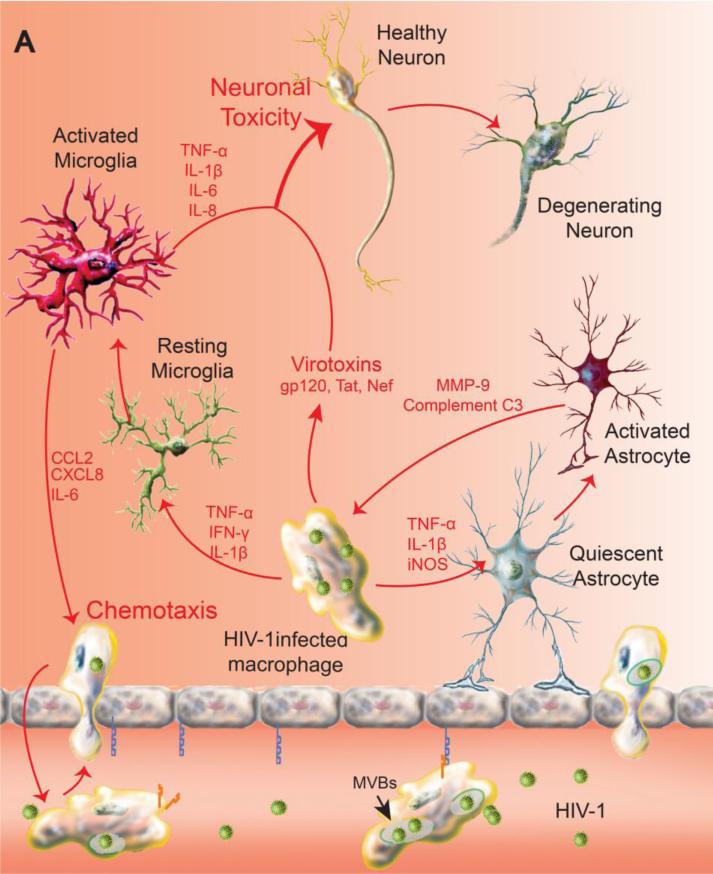
Neurodegenerative conditions like AD, PD and HAND (HIV-associated neurocognitive disorders) are accompanied by over active inflammatory responses that herald onset and tempo of disease progression (Combs et al. 2000; Harezlak et al. 2011; McGeer and McGeer 2004). The etiologies of each of these disorders reside in distinct agents that include aggregation of misfolded protein, immune attack and viral infection that lead to a commonly deleterious consequence that interrupts the homeostasis of the local microenvironment. Microglia, a sensor and scavenger for pathological events in the CNS, become rapidly activated in response to environmental, infectious and immunotoxin signals. These include in the case of neurodegenerative diseases with misfolded proteins. Microglia subsequently undergo a series of morphological, molecular and secretory changes that affect the brain's microenvironment (Aguzzi et al. 2013; Cameron and Landreth 2010; Kim and Joh 2006). Additionally, the interplay between microglia and astrocytes further polarizes the former towards a neurotoxic phenotype (Shih et al. 2006; Verderio and Matteoli 2001). Such changes in phenotype can affect the brain's microenvironment by producing a pro-inflammatory milieu that speeds neuronal injury and ongoing disease pathogenesis (Heneka et al. 2014). Although the CNS was historically considered to be immune-privileged, activation and transformation of resident and circulating immune cells in pathogenesis of neurodegenerative diseases indicate that brain hemostasis and immune system status are tightly coupled. Evidence also suggests that there are lymphoid controls operative in the brain (Wilson et al. 2010). Nonetheless, MP activation and subsequent induction of chemokines like CCL2, CXCL8 and pro-inflammatory mediators such as inducible nitric oxide synthase (iNOS), tumor necrosis factor–α (TNF-α) and interleukin-6 (IL-6) collectively serve to speed the infiltration of circulating immune cells into the CNS (Czlonkowska et al. 1999; Rivest 2009) (Figure 1A). The migration of effector memory T cells from the periphery generates leukocyte-microglial crosstalk that exacerbates neuroinflammation and restricts neuronal survival (Mallapragada et al. 2015). Another important player of immune origin contributing to the pro-inflammatory signals is mast cells, which are found in most tissues; they transverse compromised BBB to become CNS residents. Mast cell–glia communication also opens new perspectives for the development of therapies targeting neuroinflammation by differentially modulating activation of non-neuronal cells that normally control neuronal sensitization (Skaper et al. 2014). Immunopharmacologics are being developed as neuroprotective strategies for disease either to block the attraction of the circulating immune cells, T cells, macrophages and mast cells to the CNS by restoring the BBB or to induce anti-inflammatory responses in the CNS (Wilson et al. 2010). Disease-modifying treatments can control inflammation and restore neural function (Figure 1B, C). Such adjunctive therapies take into account that modulating both innate and adaptive immune responses are necessary for success in ameliorating an inflammatory CNS microenvironment and as such restore neuronal function during disease (Popovich and Longbrake 2008).
Figure 1.
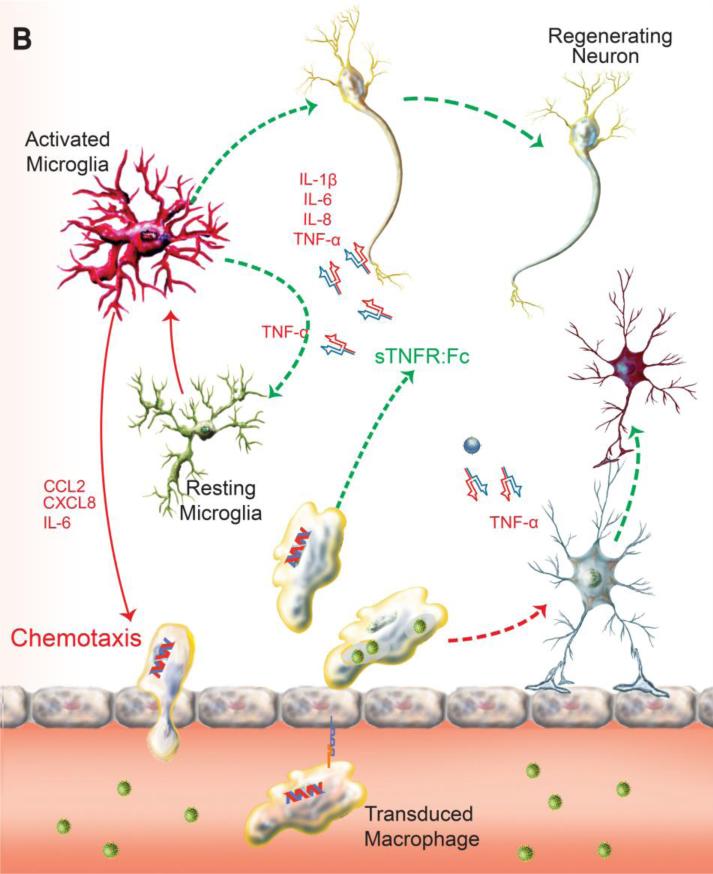
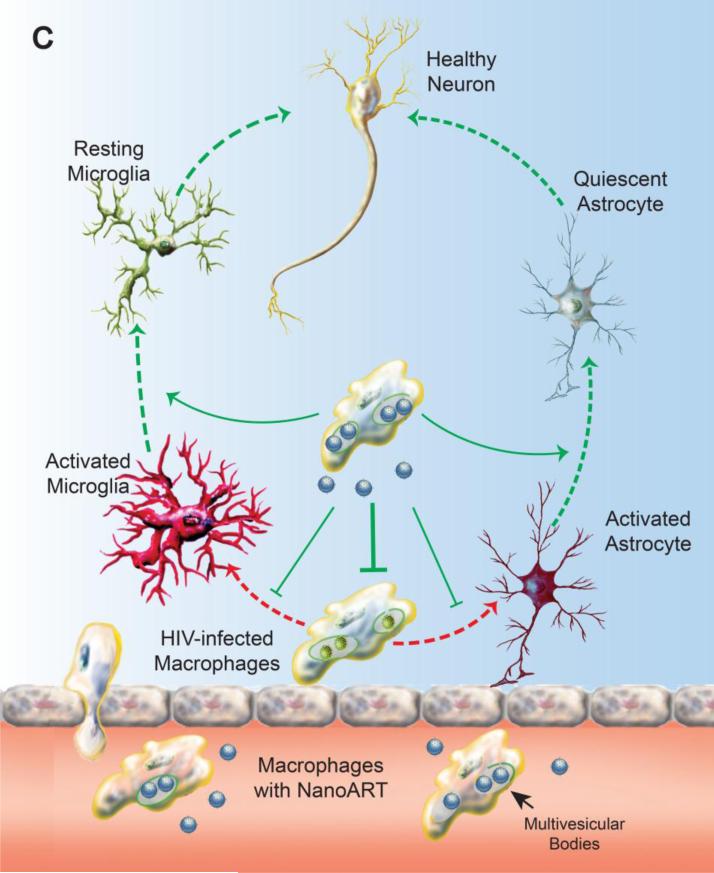
Combination of adjunctive and antiretroviral treatments for HAND. A vigorous innate immune response occurs as a direct consequence of brain mononuclear phagocytes (perivascular macrophages, microglia) infection that drives a pro-inflammatory and virotoxin-associated neurotoxic response. This is perpetuated by an autonomous amplification of cytokine activities like TNF-α and by a persistent chemotaxis of HIV-1 infected macrophages from periphery in the setting of a failure of immune responses or an absence of antiretroviral therapy. Meanwhile HIV-1 infected macrophages interrupt astroglial function and integrity by pro-inflammatory mediators, compromising the metabolic function and homeostasis of the CNS. Neuronal loss in HIV-1 encephalitis is associated with synaptic and dendritic damage. Overt synaptic loss is in turn linked to cognitive impairment induced by the brain's viral load (A). The chemokine gradient in diseased brain drives transduced macrophage constitutively expressing sTNFR:Fc to the inflammatory site. Local release of sTNFR:Fc reverses the pro-inflammatory microenvironment and blocks a cycle of autocrine and paracrine cytokines produced by sequestering the major pro-inflammatory mediator TNF-α (B). Delivery of nanoART using macrophage as carriage serves to control HIV-1 replication in the brain by bringing antiretroviral drugs to local sites of viral growth. The long-acting nanoformulated-antiretroviral medicines are in the same subcellular viral compartment within multivesicular bodies (MVBs). This serves to reduce viral loads and numbers of peripheral HIV-1 infected CD4+ T-cells. Drug levels are sustained in reservoirs of viral infection (C). Upon the suppression of persistent inflammation and clearance of viral brain reservoirs, the function and homeostasis of microglia, astrocyte and most neurons are maintained.
The pragmatic question that is continuous over many years is why have so many anti-inflammatory therapies failed to improve disease outcomes for neurodegenerative disorders? (Nowacek et al. 2009). This is believed to be due to the overall pro-inflammatory microenvironment that remained uncontrolled or even deteriorated as diseases progress, which counteract the benefit from the blockage of over-activated excitatory synapses. In this regard, comprehensive management of neuroinflammation needs be taken at a more fundamental level. MP, microglia and macrophages, activated by HIV-1 facilitate neuroinflammation by upregulating pro-inflammatory cytokines and monocyte-chemotaxis signals, which in turn further recruit circulating MP to the diseased brain (Williams et al. 2012). For example, for HAND, attempts were made to use memantine, an uncompetitive antagonist of the N-methyl-D-aspartate (NMDA) receptor, as an adjunctive agent to antiretroviral therapies aimed at reducing excitatory neurotoxicity, failed to improve cognitive function (Schifitto et al. 2007). This was seen in a phase II double-blinded, randomized, placebo- controlled trial, despite positive preclinical investigations (Anderson et al. 2004).
Another example is with a mixed lineage kinase-3 (MLK3) inhibitor. MLK3, a widely expressed kinase in neurons (Maroney et al. 2001), astrocytes (Falsig et al. 2004), microglia (Hidding et al. 2002) and myeloid lineage cells (Handley et al. 2007), regulate the c-Jun N-terminal Kinase (JNK) MAPK and p38 signaling pathways, which is tightly related to monocyte chemotaxis via CCL2 and CXCL8 and to cytokine production by microglia and brain macrophages (Ahmed et al. 2009; Young and Arndt 2009; Zheng et al. 2008). It has been demonstrated that HIV Tat and gp120 protein can induce autophosphorylation at the activation loop of MLK3 in primary rat neurons and human monocytes, which can be abolished by CEP-1347, a large molecular inhibitor of MLK3 (Sui et al. 2006). Further in vivo studies also indicated the neuroprotective effect of CEP-1347 with apparent reduction in microgliosis, neuronal loss and preserved dendritic integrity in an HIV encephalitis (HIVE) model (Eggert et al. 2010). The neuroprotective attribute of CEP-1347 has also been shown in studies of other neurodegenerative disorders such as Huntington's disease (Apostol et al. 2008), AD (Bozyczko-Coyne et al. 2001) and PD (Lotharius et al. 2005; Mathiasen et al. 2004; Saporito et al. 1999; Saporito et al. 2002). The promising results obtained in animal models of neurodegenerative disorders stretched to CEP-1347 clinical trials in PD patients. However, a phase II clinical trial concluded CEP-1347 as ineffective treatment in early PD (Investigators 2007). Symptoms of PD start when about 70–80% of striatal dopamine and about half of nigral dopamine neurons have been lost (Bernheimer et al. 1973; McGeer et al. 1988). Neuronal loss occurs generally over the course of a preclinical period of several years (Dunnett and Bjorklund 1999). Therefore, the irreversible degeneration may have started before CEP-1347 could be given in this set of patients; moreover, single neuroprotective agent may not be sufficient to reverse the disease progression. Implementation of strategies for primary prevention will require the identification of markers that would permit early diagnosis prior to the onset of symptoms. Another concern resides in the limited distribution of CEP-1347 to the CNS due to its naturally polar structure and large molecular weight (Kaneko et al. 1997). Indeed, a new MLK-3 inhibitor, URMC-099, with a favorable chemical profile and CNS penetrant property was designed. This compound demonstrated a significant amount of the neuroprotection imparted by inhibiting MLK3 in immune cells and microglia (Marker et al. 2013).
The effectiveness of CEP1347 and URMC-099 in the control of neuroinflammation and protecting neurons from injury is significant with the latter showing dramatic effects in the treatment of experimental HAND models (Eggert et al. 2010; Marker et al. 2013). The known etiology and definite onset point of HAND possibly render this an advantage. Nevertheless, the power of these in vivo studies was partially limited until very recently when URMC-099 was shown to have both neuroprotective and unique antiretroviral effects (Zhang et al. 2016). Indeed, during studies to extend the half-life of crystalline nanoformulated antiretroviral therapy (nanoART), URMC-099, which was originally developed as an adjunctive neuroprotective agent, was demonstrated to broaden antiviral responses and improve ART biodistribution. Co-administration of long-acting ritonavir-boosted atazanavir (nanoATV/r) nanoformulations with URMC-099 not only reduced viral load and the numbers of HIV-1 infected CD4+ T-cells in lymphoid tissues but sustained drug levels in reservoirs of viral infection. These results were realized more than either drug alone when administered in infected humanized NOD/SCID/IL2Rγc−/− mice and paralleled the formation of drug depots within the reticuloendothelial system. The autophagosome appears to be that depot and to lead to the sequestration of nanoATV/r in Rab-associated recycling and late endosomes (Zhang et al. 2016). These results provide an example for how unique immune modulatory reactions can also improve antiretroviral responses. As such the drug combinations are being developed beyond protease inhibitors to involve other drug classes that include integrase inhibitors to facilitate cell-based viral clearance.
Cell-based Gene and Immune Modulatory Therapies
Well known is the fact that MP can cause BBB breakdown following brain inflammation, migrating toward the inflammation site via processes known as diapedesis and chemotaxis (Woodward and Troedsson 2015). Relying on the natural inflammatory responses, macrophages have been widely applied for the active delivery of therapeutic agents, including nucleic acids, peptides, drugs and enzymes, to the brain. Indeed, delivery of nanoformulated antiretroviral drugs (nanoART) using macrophages as carriers has significantly increased drug concentration and reduced HIV-1 replication in the diseased compartments of HIVE rodents (Dou et al. 2009). Limited effect on the ongoing neuroinflammation in this study, however, reinforced the importance of combining immune-modulatory therapy to restore the brain's innate immune and metabolic homeostasis.
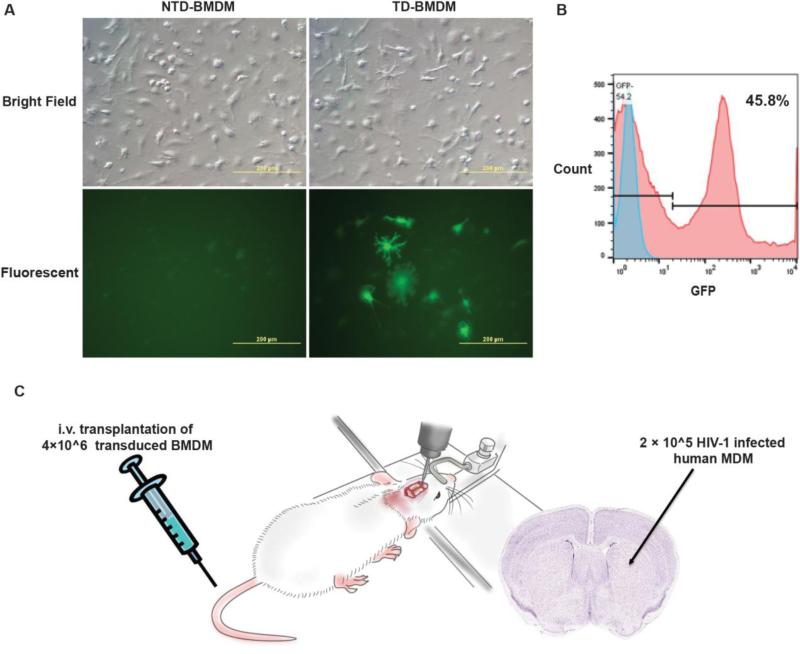
Gene therapy is the therapeutic approach of precise manipulation of the host genome, ultimately the function of targeted cells. Recent studies and clinical trials have employed a variety of vehicles to deliver protective genes to the CNS (Dey et al. 2010; Duebgen et al. 2014; Munoz et al. 2013; Zappia et al. 2005) (NCT01446614, NCT01883661). With the known effectiveness of soluble TNF-receptor (sTNFR) in the treatment of peripheral autoimmune and inflammatory conditions including rheumatoid arthritis (Moreland et al. 1999), we reasoned that a parallel approach could be applied for HIV infections of the nervous system where a pro-inflammatory environment seen within the brain incites neuronal-drop out and related cognitive dysfunction. We showed that the bicistronic plasmid construct, sTNFR-Fc-eGFP, expressing the sTNFR-Fc fusion protein and the green fluorescent protein (GFP) was suitable for stable expression of sTNFR-Fc in human macrophages and neuronal cells as a potential therapy for neuroAIDS (Cao et al. 2011). To this end, we transduced bone marrow-derived macrophages (BMDM) with the defective lentiviral vector encoding sTNFR:Fc-eGFP (MOI =15). In BMDM sTNFR-GFP (below this used as shorter abbreviation of transcript) was expressed with efficiency greater than 45% of transduced cells (Figure 2A). The transduction efficiency of the injected BMDM was confirmed by flow cytometry (ten days post transduction), which demonstrated on average more than 45% of BMDM were GFP+ (Figure 2B). Two hours prior to adoptive transfer of the recombinant sTNFR-GFP transduced BMDM, the immune deficient NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were injected intracranially (i.c.) in the caudate putamen with 2 × 105 HIV-1ADA infected monocyte-derived macrophages (MDM) as described previously (Dou et al. 2009). This procedure induced encephalitis referred to as HIVE, which paralleled what is operative, in infected patients (Figure 2C).
Figure 2.
Adoptive transplantation of sTNFR-GFP transduced BMDM into HIVE mice. BMDM transduced with the sTNFR-GFP at day 7 in vitro were shown positive for enhanced GFP expression and started to exhibit macrophage-like branching appearance. One of three representative experiments is shown (A). Transduction efficiency measured by flow cytometry ten days post transduction demonstrated more than 45% transduced BMDM were GFP+, one of three representative experiments is shown (B). Four-week-old female NSG mice were stereotactically injected intracranially into the caudate putamen with HIV-1ADA–infected MDM (2 × 105 cells in 5μl) after 1 d of viral infection and referred to as HIVE mice (C). These mice were subsequently transplanted with 4×106 transduced (TD, n=12) or non-transduced (NTD, n=10) BMDMs in 200 μl PBS through tail vein. HIVE controls (HIV-Ctl) were injected with same volume of PBS (n=8); SHAM controls were only intracranially injected with 5 μl PBS (n=6). The mouse sketch was provided by Encapsula NanoSciences with copyright permission.
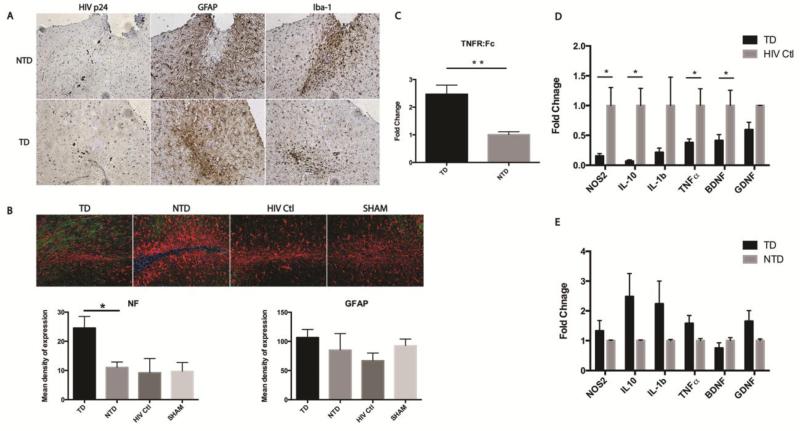
Adoptive transfer of sTNFR transduced BMDM showed no change in numbers of HIV-1p24 positive infected cells or the intensity of astrocyte responses, but a modest reduction in microglial staining was evident (Figure 3A). In this survey histopathological analyses of brain sections were acquired at sites of HIV-infection-mediated injury. Here, Iba-1 staining showed that adoptive transfer of sTNFR-GFP transduced BMDM attenuated microgliosis in brain areas surrounding the injection sites of HIV-1 infected MDM, when compared to adoptive transfer of control BMDM. In Figure 3B, neurofilament staining was evaluated in and around the injection site and quantitated by Nuance multispectral imaging system. Transduced HIV-1 infected mice have significantly higher NF content staining than control groups; GFAP intensities were not significantly different amongst the groups (Figure 3B, lower panel). Parallel analyses of synaptic markers that include microtubule associated protein 2 (MAP-2) and synaptophysin (SYN) failed to demonstrate changes as a result of therapy and when quantitated by similar multispectral imaging markers (data not shown).
Figure 3.
Delivery of sTNFR by transduced BMDM demonstrated an anti-inflammatory effect in an HIVE model. Immunohistochemical staining of HIV p24 protein identified HIV-1 infected human MDM at injection site. Evaluation of astrogliosis and microgliosis by GFAP and Iba-1 staining revealed adoptive transplantation of sTNFr:Fc-GFP transduced BMDM attenuated the microgliosis surrounding injection site compared to transplantation of NTD cells. But no apparent reduction of astrogliosis was observed (A). GFAP/Neurofilament staining in the injection site were analyzed by Nuance multispectral imaging system (20X objective). The representative images are shown. Transduced group have significantly higher content of NF (green) staining in comparison with other groups. However, the difference in the intensity of GFAP was not statistically significant among groups (B). The expression of genes encoding TNFR:Fc, NOS2, IL1-β, TNF-α, NOS2, BDNF, and GDNF in brain subregions containing injection site were measured by real-time RT-PCR using SYBR green system and specific primers. n=5 in TD group, n=6 in NTD group, n=3 in HIV Ctl group. The expression of TNFR:Fc is more than two-fold higher in TD than in NTD brain (C). The expression of inflammatory cytokines including NOS2, IL-10, IL-1β, TNF-α and neurotrophic factors like BDNF and GDNF, is lower in TD than they are in HIV-Ctl brain (D). No significant differences in expression in TD group when compared to NTD group were found (E). For multispectral image quantifications, images were converted to 12 bit grayscale and quantified using ImagePro plus software. There are 3 animals in each group, and 4 sections of selected regions from each animal were analyzed and quantified. Each bar represented as mean ± SEM, P values were obtained using one way ANOVA test, * p < 0.05. Antibodies used include mouse anti-Neurofilament (1:200, Clone 2F11, Dako, CA), polyclonal rabbit anti-GFAP (1:1000, Dako, CA), and rabbit anti- Iba1 (1:500, Wako, VA). For real-time RT-PCR, Ct values of each target gene were normalized to values for GAPDH in each sample. To calculate relative amounts mRNA, the average Ct values were subtracted from GAPDH values for each target gene to provide changes in Ct value. Fold Change in expression was calculated as log2 relative units. Multiple t tests using Holm-Sidak method were applied for statistical analysis. Each bar represent mean ± SEM with *p < 0.05, **p < 0.01
We next evaluated by real-time polymerase chain reaction (RT-PCR) the gene expression levels of sTNFR-Fc in the brain samples HIVE animals injected with transduced BMDM; pro-inflammatory cytokine and neurotrophic factor (GDNF and BDNF) expression levels were measured in brains of animals that received sTNFR-Fc or control vector containing BMDM. Notably, expression of sTNFR-Fc was detected in the brains of animals that received transduced BMDM (Figure 3C). No statistically significant differences in NOS2, IL-10, IL-1b, TNF-α, BDNF and GDNF were noted between animals treated with transduced (TD) and non-transduced (NTD) BMDM (Figure 3E). However, in these experiments measurement of pro- and anti- inflammatory and neurotrophic factors were also compared in brain tissues of HIVE controls (HIV-Ctl) with animals adoptively transferred with TD BMDM. Here, decreased expression of NOS2, IL-10, IL-1b and TNF-α shown in the TD animals compared to HIV-Ctl group (Figure 3D). Adoptive transfer of macrophages in an HIVE experimental model might have by itself negative effects associated with additional influx of macrophages (with control vectors or non-transduced). Delivery of sTNFR by transduced BMDM at the initiation of inflammation showed positive anti-inflammatory effect. The reduction in microgliosis by sTNFR-GFP transduced BMDM and the induction of anti-inflammatory milieu might be contributing to the neuroprotective response with increased NF immunostaining around the injected area (Figure 3B).
A similar approach has been applied in the PD study to deliver neurotropic and protective factors to attenuate neuroinflammatory processes linked to neuronal death (Zhao et al. 2014). Glial cell line–derived neurotrophic factor (GDNF) is the most potent survival factor for the nigrostriatal dopaminergic neurons that degenerate in PD (Bjorklund et al. 1997; Gash et al. 1996; Kordower et al. 2000; Pascual et al. 2008; Ramaswamy et al. 2009). Current GDNF-based therapies have been seriously hindered by the difficulty in delivering these therapies to the brain. Moreover, the progressive nature of PD requires continuous and sustained delivery of GDNF over months or years in order to maintain dopamine neuron survival and function. Conjugation of GDNF with other molecules enabling BBB penetration may partially overcome these difficulties and increase the feasibility of this approach for long-term sustained delivery of GDNF remains uncertain. Advancement in the development of cell-based gene therapy that can be applied systemically and achieve sustained release of moderate amounts of GDNF opened a new window (Figure 4). Adoptive transfer of genetically modified macrophage expressing GDNF dramatically ameliorated neuroinflammation and reduced degeneration of the tyrosine hydroxylase positive (TH+) neurons of the substantia nigra and TH+ terminals in the striatum of the 1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine (MPTP) and the 6-hydroxydopamine (6-OHDA) PD mice models (Biju et al. 2010; Zhao et al. 2014). Genetically modified macrophages acting as carriages for GDNF were recruited into the substantia nigra of MPTP-treated mice, both the number of dopaminergic neurons and the level of dopamine were rescued in the striatum of MPTP-treated GDNF mice (Biju et al. 2010). In another study, exosomes released from GDNF-transfected macrophages resulted in the drastic increase of neuronal maturation with a pronounced outgrowth of axons and dendrites in PC12 neurons in vitro (Zhao et al. 2014). This attributes to the potential mechanism of efficient interactions between exosomal carriers and plasma membranes of dopaminergic neurons that facilitate GDNF binding to their receptors, resulting in the profound therapeutic effect. Nevertheless, due to the limited understanding, the mechanism responsible for the therapeutic effects of cell-based GDNF delivery remains inconclusive. Recently the hypothesis that growth factors could effectively protect from endoplasmic reticulum stress, augment autophagy and brake or significantly reduce speed of misfolded proteins accumulation in PD, HD, ALS and AD was reviewed (Cai et al. 2016; Garcia-Huerta et al. 2016). A comprehensive examination and detailed understanding of the mechanisms would be of great value in designing strategies for cell-mediated drug delivery.
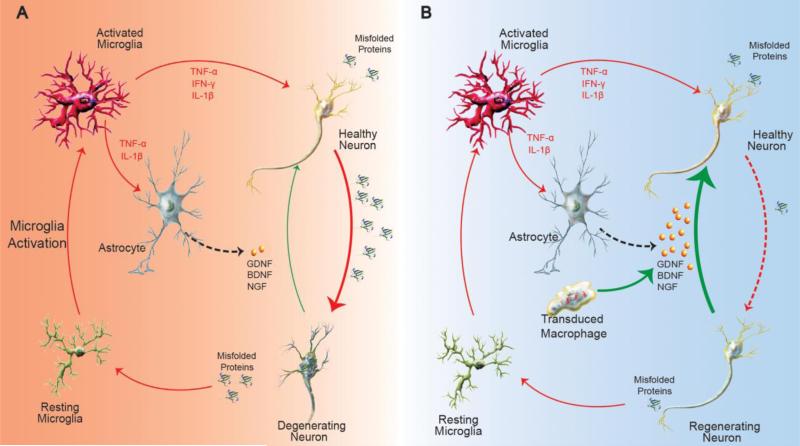
Figure 4.
Common mechanism of neurodegenerative pathology mediated by microglia and astrocyte activation, and reversal of neuronal dropout by introducing neurotrophic factors by transduced macrophages. Activated microglia undergo a series of morphological, molecular and secretory changes in the presence of abnormal proteins generated by neurons (a-synuclein, amyloid etc.) that lead to a pro-inflammatory microenvironment in the CNS, and, as results progressively increasing build-up of misfolded proteins. These include the induction pro-inflammatory mediators such as IFN-γ, NF-α and IL-1β speeding the neuronal dysfunction and degeneration. The interplay between microglia and astrocytes further impairs the astrocytes capability of maintaining homeostasis and nourishing neurons with neurotrophic factors like GDNF, BDNF, and NGF (A). Adoptive transfer of genetically modified macrophage expressing neurotrophic factors dramatically ameliorated neuroinflammation and reduced degeneration of neurons in rodent models of AD and PD. Uptake of exosomes containing neurotrophic factors released from transduced macrophages resulted in a reduction of misfolded protein production via autophagy and should significantly delay their accumulation (B). The efficient interactions between exosomal carriers and plasma membranes of neurons that facilitate growth factor binding to their receptors may contribute to therapeutic end points.
As inflammation has been implicated as a critical mechanism in AD pathogenesis, suppression of microglial activation and subsequent neuroinflammation was investigated employing several anti-inflammatory drugs in trial, including natural nonsteroidal anti-inflammatory drugs (NSAIDs), polyphenols and new drugs synthesized based on multi-target directed ligand (MTDL) design (Shi et al. 2013). TNF-alpha inhibitor administered perispinally in patients with mild-to-severe AD showed promise as potential treatment approach (Tobinick et al. 2006). Adjunctive therapies targeting multiple factors in AD processes might be the best potential treatment strategy as in any other neurodegenerative disease.
Conclusion
In summary, this review underscores the current approaches of modulating the altered microenvironment under various pathological conditions. With a primary goal of eradicating root causes of diseases, development of adjunctive therapies will not only enhance the efficacy of primary therapy but will contribute pivotally in restoring homeostasis and function of the CNS. For instance, transduced BMDM produced high levels of sTNFR in vitro. Adoptive transfer of the transduced BMDM to a mouse model of HIVE demonstrated a trend for anti-inflammatory effects at disease site. Stable expression of immune modulatory peptides like sTNFR could readily work in conjunction with nanoART in dampening local inflammation. This suggested the future direction of using cell-based delivery of nanoART and neuroprotective peptide/proteins in achieving pathological and functional recovery simultaneously in HAND patients (Figure 1B and C).
Parallel systems were devised for treatment of age-linked neurodegenerative disorders. This includes AD and PD. The developed delivery systems are honed to overcome the BBB and deliver drugs to brain lesions. To rescue and recover the degenerating neurons, cell-based gene and peptide delivery could ensure a sustained supply of neurotrophic factors in the local microenvironment.
Acknowledgements
This work was supported, in part, by the University of Nebraska Foundation, which includes but is not limited to individual donations from Carol Swarts, M.D., and Frances and Louie Blumkin, and the National Institutes of Health grants P01 DA028555, R01 NS36126, P01 NS31492, 2R01 NS034239, P01 MH64570, P01 NS43985, P30 MH062261 and R01 AG043540 (HEG); R24 OD 018546-01 (LP); and R01 MH079717 (YL). We thank the UNMC - Flow Cytometry Core facility for its help in data acquisition and analysis. We appreciate the Encapsula NanoSciences for providing the mouse artwork.
Footnotes
Conflicts of Interest: The authors declare they have no conflicts of interest.
References
- Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed RAM, Murao K, Imachi H, Yoshida K, Dobashi H, Hosomi N, Ishida T. c-Jun N-terminal kinases inhibitor suppresses the TNF-α induced MCP-1 expression in human umbilical vein endothelial cells. Endocrine. 2009;35:184–188. doi: 10.1007/s12020-008-9136-0. [DOI] [PubMed] [Google Scholar]
- Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Current HIV research. 2008;6:388. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ER, Gendelman HE, Xiong H. Memantine protects hippocampal neuronal function in murine human immunodeficiency virus type 1 encephalitis. The Journal of neuroscience. 2004;24:7194–7198. doi: 10.1523/JNEUROSCI.1933-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol BL, et al. CEP-1347 reduces mutant huntingtin-associated neurotoxicity and restores BDNF levels in R6/2 mice. Molecular and Cellular Neuroscience. 2008;39:8–20. doi: 10.1016/j.mcn.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Baseri B, et al. Activation of signaling pathways following localized delivery of systemically administered neurotrophic factors across the blood–brain barrier using focused ultrasound and microbubbles. Physics in medicine and biology. 2012;57:N65. doi: 10.1088/0031-9155/57/7/N65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Mayo Clinic Proceedings. Vol. 10. Elsevier; 2005. Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. pp. 1326–1338. [DOI] [PubMed] [Google Scholar]
- Benner EJ, et al. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9435–9440. doi: 10.1073/pnas.0400569101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations Journal of the neurological sciences. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Biju K, et al. Macrophage-mediated GDNF delivery protects against dopaminergic neurodegeneration: a therapeutic strategy for Parkinson's disease. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18:1536–1544. doi: 10.1038/mt.2010.107. doi:10.1038/mt.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund A, Rosenblad C, Winkler C, Kirik D. Studies on neuroprotective and regenerative effects of GDNF in a partial lesion model of Parkinson's disease. Neurobiol Dis. 1997;4:186–200. doi: 10.1006/nbdi.1997.0151. doi:10.1006/nbdi.1997.0151. [DOI] [PubMed] [Google Scholar]
- Bozyczko-Coyne D, O'Kane TM, Wu ZL, Dobrzanski P, Murthy S, Vaught JL, Scott RW. CEP-1347/KT-7515, an inhibitor of SAPK/JNK pathway activation, promotes survival and blocks multiple events associated with A β-induced cortical neuron apoptosis. Journal of neurochemistry. 2001;77:849–863. doi: 10.1046/j.1471-4159.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- Brynskikh AM, et al. Macrophage delivery of therapeutic nanozymes in a murine model of Parkinson's disease. Nanomedicine : nanotechnology, biology, and medicine. 2010;5:379–396. doi: 10.2217/nnm.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchbinder SP, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. The Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Arikkath J, Yang L, Guo ML, Periyasamy P, Buch S. Interplay of endoplasmic reticulum stress and autophagy in neurodegenerative disorders. Autophagy. 2016;12:225–244. doi: 10.1080/15548627.2015.1121360. doi:10.1080/15548627.2015.1121360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron B, Landreth GE. Inflammation, microglia, and Alzheimer's disease. Neurobiology of disease. 2010;37:503–509. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Wu C, Yang Y, Sniderhan LF, Maggirwar SB, Dewhurst S, Lu Y. Lentiviral vector-mediated stable expression of sTNFR-Fc in human macrophage and neuronal cells as a potential therapy for neuroAIDS. J Neuroinflammation. 2011;8:48. doi: 10.1186/1742-2094-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer's disease: inhibition of β-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARγ agonists. The Journal of neuroscience. 2000;20:558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czlonkowska A, Czlonkowski A, Kohutnicka M, Kurkowska-Jastrzebska I, Wronska A. MHC class II positive microglia and lymphocytic infiltration are present in the substantia nigra and striatum in mouse model of Parkinson's disease. Acta Neurobiologiae Experimentalis. 1999;59:1–8. doi: 10.55782/ane-1999-1289. [DOI] [PubMed] [Google Scholar]
- Dash PK, et al. Loss of neuronal integrity during progressive HIV-1 infection of humanized mice. The Journal of Neuroscience. 2011;31:3148–3157. doi: 10.1523/JNEUROSCI.5473-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin KN, et al. Neurocognitive effects of HIV, hepatitis C, and substance use history. Journal of the International Neuropsychological Society. 2011;18:68. doi: 10.1017/S1355617711001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey ND, et al. Genetically engineered mesenchymal stem cells reduce behavioral deficits in the YAC 128 mouse model of Huntington's disease. Behavioural brain research. 2010;214:193–200. doi: 10.1016/j.bbr.2010.05.023. doi:10.1016/j.bbr.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Doody RS, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. New England Journal of Medicine. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- Dou H, et al. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. Journal of immunology (Baltimore, Md : 1950) 2009;183:661–669. doi: 10.4049/jimmunol.0900274. doi:10.4049/jimmunol.0900274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duebgen M, Martinez-Quintanilla J, Tamura K, Hingtgen S, Redjal N, Wakimoto H, Shah K. Stem cells loaded with multimechanistic oncolytic herpes simplex virus variants for brain tumor therapy. Journal of the National Cancer Institute. 2014;106 doi: 10.1093/jnci/dju090. dju090 doi:10.1093/jnci/dju090. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Bjorklund A. Prospects for new restorative and neuroprotective treatments in Parkinson's disease. Nature. 1999;399:A32–39. doi: 10.1038/399a032. [DOI] [PubMed] [Google Scholar]
- Eggert D, et al. Neuroprotective activities of CEP-1347 in models of neuroAIDS. Journal of immunology (Baltimore, Md : 1950) 2010;184:746–756. doi: 10.4049/jimmunol.0902962. doi:10.4049/jimmunol.0902962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Andaloussi S, et al. Exosome-mediated delivery of siRNA in vitro and in vivo. Nature protocols. 2012;7:2112–2126. doi: 10.1038/nprot.2012.131. doi:10.1038/nprot.2012.131. [DOI] [PubMed] [Google Scholar]
- Falsig J, Pörzgen P, Lotharius J, Leist M. Specific modulation of astrocyte inflammation by inhibition of mixed lineage kinases with CEP-1347. The Journal of Immunology. 2004;173:2762–2770. doi: 10.4049/jimmunol.173.4.2762. [DOI] [PubMed] [Google Scholar]
- Gabathuler R. Approaches to transport therapeutic drugs across the blood–brain barrier to treat brain diseases. Neurobiology of disease. 2010;37:48–57. doi: 10.1016/j.nbd.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Garcia-Huerta P, Troncoso-Escudero P, Jerez C, Hetz C, Vidal RL. The intersection between growth factors, autophagy and ER stress: A new target to treat neurodegenerative diseases? Brain Res. 2016 doi: 10.1016/j.brainres.2016.02.052. doi:10.1016/j.brainres.2016.02.052. [DOI] [PubMed] [Google Scholar]
- Gash DM, et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. doi:10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- Gray F. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathology and applied neurobiology. 1999;25:123–133. doi: 10.1046/j.1365-2990.1999.00167.x. [DOI] [PubMed] [Google Scholar]
- Hall J, Prabhakar S, Balaj L, Lai CP, Cerione RA, Breakefield XO. Delivery of Therapeutic Proteins via Extracellular Vesicles: Review and Potential Treatments for Parkinson's Disease, Glioma, and Schwannoma. Cellular and molecular neurobiology. 2016 doi: 10.1007/s10571-015-0309-0. doi:10.1007/s10571-015-0309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley ME, Rasaiyaah J, Barnett J, Thakker M, Pollara G, Katz DR, Chain BM. Expression and function of mixed lineage kinases in dendritic cells. International immunology. 2007;19:923–933. doi: 10.1093/intimm/dxm050. [DOI] [PubMed] [Google Scholar]
- Harezlak J, et al. Persistence of HIV– Associated Cognitive Impairment, Inflammation and Neuronal Injury in era of Highly. Active Antiretroviral Treatment AIDS (London, England) 2011;25:625. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, et al. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. 1997. [DOI] [PubMed]
- Hemachudha T, Griffin DE, Giffels JJ, Johnson RT, Moser AB, Phanuphak P. Myelin basic protein as an encephalitogen in encephalomyelitis and polyneuritis following rabies vaccination. The New England journal of medicine. 1987;316:369–374. doi: 10.1056/NEJM198702123160703. doi:10.1056/nejm198702123160703. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nature Reviews Immunology. 2014;14:463–477. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- Hidding U, et al. The c-Jun N-terminal kinases in cerebral microglia: immunological functions in the brain. Biochemical pharmacology. 2002;64:781–788. doi: 10.1016/s0006-2952(02)01139-5. [DOI] [PubMed] [Google Scholar]
- Investigators PSGP Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early. Parkinson disease Neurology. 2007;69:1480–1490. doi: 10.1212/01.wnl.0000277648.63931.c0. [DOI] [PubMed] [Google Scholar]
- Kaneko M, et al. Neurotrophic 3, 9-bis [(alkylthio) methyl]-and-bis (alkoxymethyl)-K-252a derivatives. Journal of medicinal chemistry. 1997;40:1863–1869. doi: 10.1021/jm970031d. [DOI] [PubMed] [Google Scholar]
- Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson's disease. Experimental and Molecular Medicine. 2006;38:333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- Koenig S, et al. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Kordower JH, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Borodowsky I, Fernandez B, Gonzalez L, Kumar M. Human immunodeficiency virus type 1 RNA Levels in different regions of human brain: quantification using real-time reverse transcriptase-polymerase chain reaction. Journal of neurovirology. 2007;13:210–224. doi: 10.1080/13550280701327038. doi:10.1080/13550280701327038. [DOI] [PubMed] [Google Scholar]
- Lashley T, Rohrer JD, Mead S, Revesz T. Review: An update on clinical, genetic and pathological aspects of frontotemporal lobar degenerations. Neuropathology and applied neurobiology. 2015;41:858–881. doi: 10.1111/nan.12250. [DOI] [PubMed] [Google Scholar]
- Lotharius J, Falsig J, van Beek J, Payne S, Dringen R, Brundin P, Leist M. Progressive degeneration of human mesencephalic neuron-derived cells triggered by dopamine-dependent oxidative stress is dependent on the mixed-lineage kinase pathway. The Journal of neuroscience. 2005;25:6329–6342. doi: 10.1523/JNEUROSCI.1746-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapragada SK, et al. Enabling nanomaterial, nanofabrication and cellular technologies for nanoneuromedicines. Nanomedicine : nanotechnology, biology, and medicine. 2015;11:715–729. doi: 10.1016/j.nano.2014.12.013. doi:10.1016/j.nano.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker DF, et al. The new small-molecule mixed-lineage kinase 3 inhibitor URMC-099 is neuroprotective and anti-inflammatory in models of human immunodeficiency virus-associated neurocognitive disorders. The Journal of Neuroscience. 2013;33:9998–10010. doi: 10.1523/JNEUROSCI.0598-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroney AC, et al. Cep-1347 (KT7515), a semisynthetic inhibitor of the mixed lineage kinase family. Journal of Biological Chemistry. 2001;276:25302–25308. doi: 10.1074/jbc.M011601200. [DOI] [PubMed] [Google Scholar]
- Masliah E, Ge N, Mucke L. Pathogenesis of HIV-1 associated neurodegeneration. Critical reviews in neurobiology. 1996;10:57–67. doi: 10.1615/critrevneurobiol.v10.i1.30. [DOI] [PubMed] [Google Scholar]
- Masliah E, et al. Effects of α-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Mathiasen JR, et al. Inhibition of mixed lineage kinase 3 attenuates MPP+-induced neurotoxicity in SH-SY5Y cells. Brain research. 2004;1003:86–97. doi: 10.1016/j.brainres.2003.11.073. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Akiyama H, McGeer EG. Rate of cell death in parkinsonism indicates active neuropathological process. Annals of neurology. 1988;24:574–576. doi: 10.1002/ana.410240415. doi:10.1002/ana.410240415. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson's disease. Parkinsonism & related disorders. 2004;10:S3–S7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Meairs S, Alonso A. Ultrasound, microbubbles and the blood–brain barrier. Progress in biophysics and molecular biology. 2007;93:354–362. doi: 10.1016/j.pbiomolbio.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Moreland LW, et al. Etanercept therapy in rheumatoid arthritis: a randomized, controlled trial. Annals of internal medicine. 1999;130:478–486. doi: 10.7326/0003-4819-130-6-199903160-00004. [DOI] [PubMed] [Google Scholar]
- Morgan D, et al. Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of Functional Anti-miR-9 by Mesenchymal Stem Cell-derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity Molecular therapy. Nucleic acids. 2013;2:e126. doi: 10.1038/mtna.2013.60. doi:10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacek A, Gendelman HE. NanoART, neuroAIDS and CNS drug delivery. Nanomedicine : nanotechnology, biology, and medicine. 2009;4:557–574. doi: 10.2217/nnm.09.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacek A, Kosloski LM, Gendelman HE. Neurodegenerative disorders and nanoformulated drug development. Nanomedicine (London, England) 2009;4:541–555. doi: 10.2217/nnm.09.37. doi:10.2217/nnm.09.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa H, Noma S, Yoshida Y, Sekine H, Hashimoto T. Acute disseminated encephalomyelitis associated with poliomyelitis vaccine. Pediatric neurology. 2000;23:177–179. doi: 10.1016/s0887-8994(00)00167-3. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nature neuroscience. 2008;11:755–761. doi: 10.1038/nn.2136. doi:10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Pathan SA, et al. CNS drug delivery systems: novel approaches. Recent patents on drug delivery & formulation. 2009;3:71–89. doi: 10.2174/187221109787158355. [DOI] [PubMed] [Google Scholar]
- Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nature Reviews Neurology. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Gendelman HE. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. Journal of leukocyte biology. 2003;74:691–701. doi: 10.1189/jlb.0503205. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nature reviews Neuroscience. 2008;9:481–493. doi: 10.1038/nrn2398. doi:10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Soderstrom KE, Kordower JH. Trophic factors therapy in Parkinson's disease. Progress in brain research. 2009;175:201–216. doi: 10.1016/S0079-6123(09)17514-3. doi:10.1016/s0079-6123(09)17514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. The Journal of clinical investigation. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nature reviews Immunology. 2009;9:429–439. doi: 10.1038/nri2565. doi:10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in Alzheimer's disease: amyloid-β oligomers trigger innate immunity defence via pattern recognition receptors. Progress in neurobiology. 2009;87:181–194. doi: 10.1016/j.pneurobio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Saporito MS, Brown EM, Miller MS, Carswell S. CEP-1347/KT-7515, an inhibitor of c-jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons in vivo. Journal of Pharmacology and Experimental Therapeutics. 1999;288:421–427. [PubMed] [Google Scholar]
- Saporito MS, Hudkins RL, Maroney AC. 2 Discovery of Cep-1347/Kt-7515, an Inhibitor of the Jnk/Sapk Pathway for the Treatment of Neurodegenerative Diseases. Progress in medicinal chemistry. 2002;40:23–62. doi: 10.1016/s0079-6468(08)70081-x. [DOI] [PubMed] [Google Scholar]
- Schifitto G, et al. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. Aids. 2007;21:1877–1886. doi: 10.1097/QAD.0b013e32813384e8. [DOI] [PubMed] [Google Scholar]
- Schneeberger A, Tierney L, Mandler M. Active immunization therapies for Parkinson's disease and multiple system atrophy Movement Disorders. 2015. [DOI] [PubMed]
- Shi S, Wang Z, Qiao Z. The multifunctional anti-inflammatory drugs used in the therapy of Alzheimer's disease. Current medicinal chemistry. 2013;20:2583–2588. doi: 10.2174/0929867311320200006. [DOI] [PubMed] [Google Scholar]
- Shih AY, et al. Policing the police: astrocytes modulate microglial activation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:3887–3888. doi: 10.1523/JNEUROSCI.0936-06.2006. doi:10.1523/jneurosci.0936-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD, Facci L, Giusti P. Mast cells, glia and neuroinflammation: partners in crime? Immunology. 2014;141:314–327. doi: 10.1111/imm.12170. doi:10.1111/imm.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen LN, Reinert LS, Malmgaard L, Bartholdy C, Thomsen AR, Paludan SR. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. The Journal of Immunology. 2008;181:8604–8612. doi: 10.4049/jimmunol.181.12.8604. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM-Y, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Graeber MB, Kreutzberg GW. Functional plasticity of microglia: a review. Glia. 1988;1:301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Sui Z, et al. Inhibition of mixed lineage kinase 3 prevents HIV-1 Tat-mediated neurotoxicity and monocyte activation. The Journal of Immunology. 2006;177:702–711. doi: 10.4049/jimmunol.177.1.702. [DOI] [PubMed] [Google Scholar]
- Thompson KA, Cherry CL, Bell JE, McLean CA. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. The American journal of pathology. 2011;179:1623–1629. doi: 10.1016/j.ajpath.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobinick E, Gross H, Weinberger A, Cohen H. TNF-alpha modulation for treatment of Alzheimer's disease: a 6-month pilot study. MedGenMed : Medscape general medicine. 2006;8:25. [PMC free article] [PubMed] [Google Scholar]
- Verderio C, Matteoli M. ATP mediates calcium signaling between astrocytes and microglial cells: modulation by IFN-gamma. Journal of immunology (Baltimore, Md : 1950) 2001;166:6383–6391. doi: 10.4049/jimmunol.166.10.6383. [DOI] [PubMed] [Google Scholar]
- Whitley RJ. Viral encephalitis New England. Journal of Medicine. 1990;323:242–250. doi: 10.1056/NEJM199007263230406. [DOI] [PubMed] [Google Scholar]
- Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. Journal of leukocyte biology. 2012;91:401–415. doi: 10.1189/jlb.0811394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest. 2010;120:1368–1379. doi: 10.1172/JCI41911. doi:10.1172/jci41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolak DJ, Thorne RG. Diffusion of macromolecules in the brain: implications for drug delivery. Molecular pharmaceutics. 2013;10:1492–1504. doi: 10.1021/mp300495e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward E, Troedsson M. Inflammatory mechanisms of endometritis. Equine veterinary journal. 2015;47:384–389. doi: 10.1111/evj.12403. [DOI] [PubMed] [Google Scholar]
- Young SK, Arndt PG. c-Jun NH2-terminal kinase regulates lipopolysaccharide-induced pulmonary mononuclear cell recruitment via CCL2. Experimental lung research. 2009;35:682–700. doi: 10.3109/01902140902853168. [DOI] [PubMed] [Google Scholar]
- Zappia E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. doi:10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- Zhang G, et al. The mixed lineage kinase-3 inhibitor URMC-099 improves therapeutic outcomes for long-acting antiretroviral therapy. Nanomedicine: Nanotechnology, Biology and Medicine. 2016;12:109–122. doi: 10.1016/j.nano.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Haney MJ, Gupta R, Bohnsack JP, He Z, Kabanov AV, Batrakova EV. GDNF-transfected macrophages produce potent neuroprotective effects in Parkinson's disease mouse model. PLoS One. 2014;9:e106867. doi: 10.1371/journal.pone.0106867. doi:10.1371/journal.pone.0106867. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhao Y, et al. Active targeted macrophage-mediated delivery of catalase to affected brain regions in models of Parkinson's disease. Journal of nanomedicine & nanotechnology. 2011 doi: 10.4172/2157-7439.S4-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JC, et al. HIV-1-infected and/or immune-activated macrophages regulate astrocyte CXCL8 production through IL-1β and TNF-α: Involvement of mitogen-activated protein kinases and protein kinase. R Journal of neuroimmunology. 2008;200:100–110. doi: 10.1016/j.jneuroim.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]