Abstract
Carboxylesterases (CE) are members of the esterase family of enzymes, and as their name suggests, they are responsible for the hydrolysis of carboxylesters into the corresponding alcohol and carboxylic acid. To date, no endogenous CE substrates have been identified and as such, these proteins are thought to act as a mechanism to detoxify ester-containing xenobiotics. As a consequence, they are expressed in tissues that might be exposed to such agents (lung and gut epithelia, liver, kidney, etc.). CEs demonstrate very broad substrate specificities and can hydrolyze compounds as diverse as cocaine, oseltamivir (Tamiflu), permethrin and irinotecan. In addition, these enzymes are irreversibly inhibited by organophosphates such as Sarin and Tabun. In this overview, we will compare and contrast the two human enzymes that have been characterized, and evaluate the biology of the interaction of these proteins with organophosphates (principally nerve agents).
Carboxylesterases
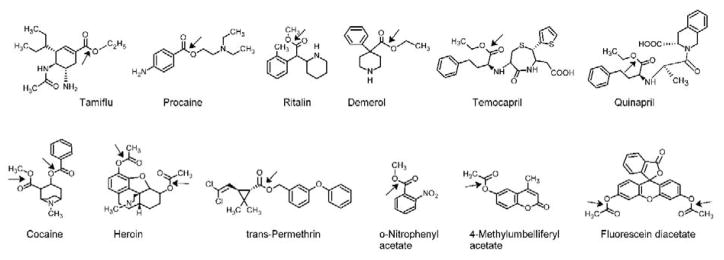
CEs are ubiquitous enzymes that have been identified in virtually all living organisms. The first report of an esterase was noted in 1906 [1], however after more than 100 years of biochemistry and molecular biology, no bona fide endogenous substrates have been identified. Consequently, it is believed that their sole function is protect cells from exogenous xenobiotics that contain the ester chemotype [2, 3]. Since the latter is present within hundreds of both naturally occurring and synthetic molecules, typically to improve the water solubility of the parent compound, the resulting hydrolysis effected by CEs is thought to be part of a detoxification process. As can be seen from Figure 1, the diversity in the chemical structures that can by hydrolyzed by these enzymes is considerable.
Figure 1. Carboxylesterase substrates.
The bond that is subject to hydrolysis is indicated by the arrow.
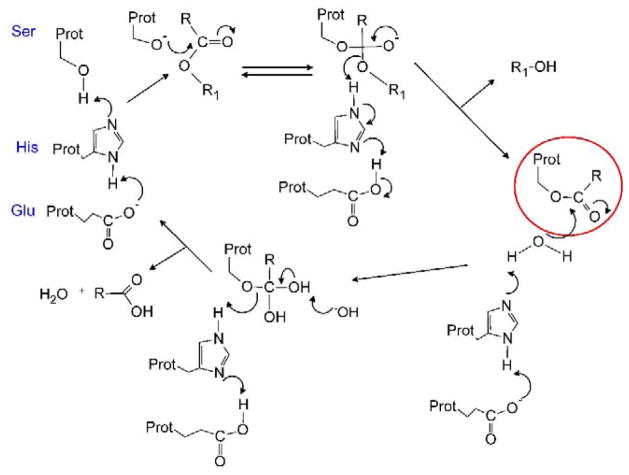
Hydrolysis of the ester bond is accomplished by the attack of a serine Oγ nucleophile towards the carbonyl carbon atom (Figure 2). The former is generated by a proton shuttle relay established by adjacent glutamic and histidine residues. The resulting tetrahedral intermediate that is formed, then collapses to release the alcohol, and to generate an esterified serine residue. A water molecule then acts as an intermediate nucleophile, essentially in exactly the same process, to remove the carboxylic acid. However for this to be achieved, the reactions needs to occur in a strictly controlled environment to prevent the serine nucleophile from reacting with free water molecules. This is accomplished by locating the catalytic amino acids at the base of a long, deep (~20Å) hydrophobic gorge (Figure 3), where access can be regulated [4–7]. Recent studies have confirmed that the flexibility of the loops at the entrance to the active site (and hence the size of the entrance to the catalytic gorge) can dramatically impact substrate hydrolysis [8], suggesting that specificity is determined, in part, by access of the ester to this domain.
Figure 2. The mechanism of substrate hydrolysis by CEs.
The essential catalytic amino acids are shown (Ser, His and Glu) and the mechanism depicts the hydrolysis of a generic ester (RCOOR1). It should be noted that an intermediate serine ester is generated (red circle) in this process, and that with molecules that contain poor leaving groups (e.g., organophosphates), the efficiency of the second hydrolysis step is markedly reduced leading to enzyme inhibition.
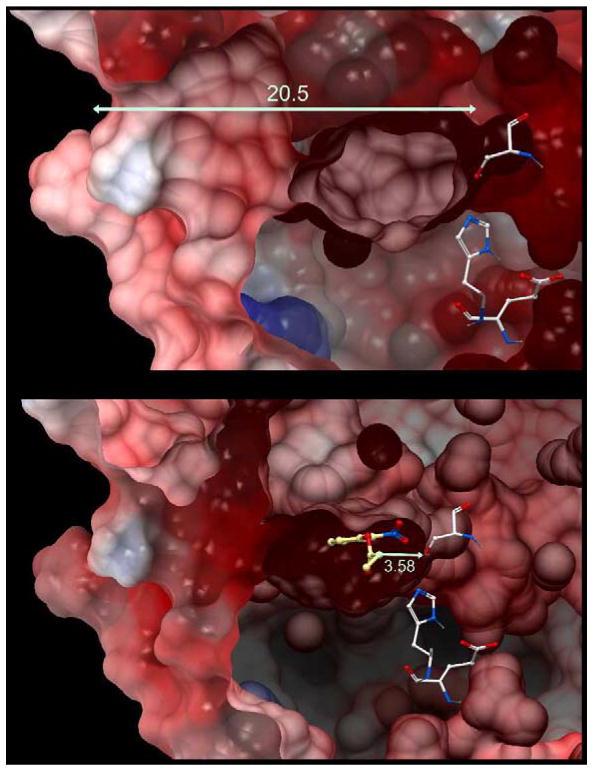
Figure 3. Structure of hCE1.
A – A cross section view of the protein. The entrance to the active site gorge is shown on the left hand side with the catalytic amino acids on the right. The distance from the active site serine to the entrance to the gorge in 20.5Å. The essential histidine and glutamic acid residues are also displayed.
B. The same image as A with o-nitrophenyl acetate docked into the active site. The distance from the serine Oγ atom to the carbonyl carbon atom is 3.58 Å.
CEs can be inhibited by organophosphates, where release of the adduct from the esterified serine does not occur [2, 9, 10]. This results in an inactivated, stalled enzyme. For example, agents such as bis-nitrophenyl phosphate and sarin lead to the phosphorylation of the Oγ atom, and since the resulting adducts are very poor leaving groups, the reactions essentially become irreversible, resulting in enzyme inactivation [11].
Carboxylesterase expression
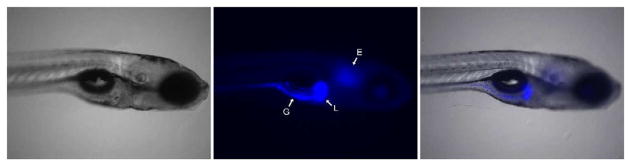
As indicated above, CEs are principally expressed in tissues that maintain a barrier function and are likely exposed to xenobiotics. This includes the epithelia of the lung and gut, liver, kidney and skin [12, 13]. An example of CE expression in vivo is demonstrated in Figure 4 in a zebrafish model. Here, larvae were exposed to 4-methylumbelifferyl acetate and CE-mediated hydrolysis of the substrate to the blue fluorescent product 4-methylumbelifferone was visualized in the live animal. This clearly depicts high functional levels of the enzyme(s) in the liver and gut, and lower levels in the ear. However, CE expression in blood varies significantly depending upon the species. For example, humans are essentially devoid of this activity, whereas small rodents have very high levels of circulating enzyme [14–17]. The reasons for this are not clear, but it appears as though through evolution, higher mammals (primates) have lost expression of these proteins in this fluid. Hence, the use of mice and rats as models to examine either, the hydrolysis of esterified substrates, and/or the disposition of metabolites, is problematic. To counteract this, plasma-esterase deficient mice have been generated and these animals have clearly demonstrated the impact of circulating CEs on the metabolism of esterified drugs [15, 16].
Figure 4. Expression of CEs in zebrafish.
The left panel shows a bright-field image of a zebrafish larvae at 5 days postfertilization; the middle panel indicates CE-mediated hydrolysis of 4-methylumbelifferyl acetate to the blue fluorescent product 4-methylumbelifferone; and the right panel shows an overlay of the images. L – liver; G – gut; E – ear.
The pattern of CE expression (i.e., in essentially any tissue that might contain a barrier to xenobiotic entry) indicates that essentially any drug, given either orally, via injection or dermally, would be subject to action by these enzymes. Therefore, any esterified molecule, assuming that it will fit within the active site of the protein, will be likely be hydrolyzed. This may be beneficial if the compound is a prodrug and the metabolite is produced in the tissue where enzyme expression occurs, however, more likely, the exact interplay of the different enzymes, their levels of expression, and their substrate specificities are not known. As a consequence, the actual amounts of drug and/or hydrolysis products present within target tissues is difficult to estimate. We would argue that the use of bioisosteres to replace the ester function in drug design will significantly reduce and/or eliminate the problems associated with compounds that contain this chemotype.
Human carboxylesterases
While 5 potential CE genes have been identified in the human genome, only two isoforms have been extensively studied with regard to their biochemical and cellular properties. Human liver CE (CES1, hCE1) demonstrates little variability in expression in liver microsomes, and primarily metabolizes small, planar substrates (e.g., Tamiflu, Ritalin, Plavix; [12]). It is a 60kDa protein that requires processing within the ER for enzymatic activity [18, 19]. Using molecular approaches, hCE1 has been engineered to be secreted and this has allowed large scale purification and the determination of the x-ray crystal structure of the protein (Figure 4; [4–7, 20]). These studies clearly identify a triad of catalytic amino acids (Ser221, Glu353, His464), a large hydrophobic catalytic gorge, and a potential ‘side door’ that may allow for exit of hydrolysis products from the enzyme (Figure 3). Interestingly, hCE1 is exceptionally stable and enzymatic activity can be maintained for samples stored at RT for many months. Therefore, studies have been proposed to use this enzyme as a detoxicant that could either be locally applied, or injected, to minimize agent toxicity [21].
The second isoform, human intestinal CE (CES2; hiCE), is expressed mainly in the epithelia of the gut, with highest levels in the duodenum [12]. Since this coincides with where the bile duct joins, it is thought that this may act as a defense against esterified agents that are secreted by the liver via the bile. Expression of the 60kDa hiCE enzyme in the liver is more variable than hCE1, and since the former enzyme demonstrates preferential activity toward larger, bulky substrates (including the anticancer agent CPT-11, and the narcotic cocaine), these differences in hiCE levels may account for the inter-individual variability of drug hydrolysis seen in vivo [12]. Due to the very poor in vitro stability of this enzyme, detailed structural analyses have not been successful. However it would seem likely that the architecture of the active site gorge and the catalytic amino acids would be very similar to that seen in hCE1.
Recently, pan CE, and selective hiCE, inhibitors have been identified [22, 23] and this has provided reagents to assess the level of xenobiotic hydrolysis in complex samples (e.g., liver extracts). While informative, results from these studies clearly indicate that other enzymes present within these samples (whether CEs, or other esterases, e.g., paraoxonases, cholinesterases) can also effect substrate hydrolysis [12]. These results argue that detailed biochemistry is required to completely document and characterize xenobiotic metabolism in complex samples.
Interaction of carboxylesterases by organophosphates
Several decades ago, it was realized that CEs could interact with organophosphates (OP) in a stoichiometric fashion [2, 9, 10]. This resulted in the inactivation of the enzyme, presumably by formation of an irreversible covalent bond with the catalytic serine (see Figure 2). Since OPs are ubiquitous agents used in both agriculture and chemical warfare, considerable interest has arisen in the use of CEs to mitigate toxicity from such compounds. Specifically, this has included the design of enzymes that might act in a catalytic manner resulting in the hydrolysis of OPs to non-toxic products, and the development of stabilized active enzymes that may be suitable for rapid detoxification of xenobiotics [24].
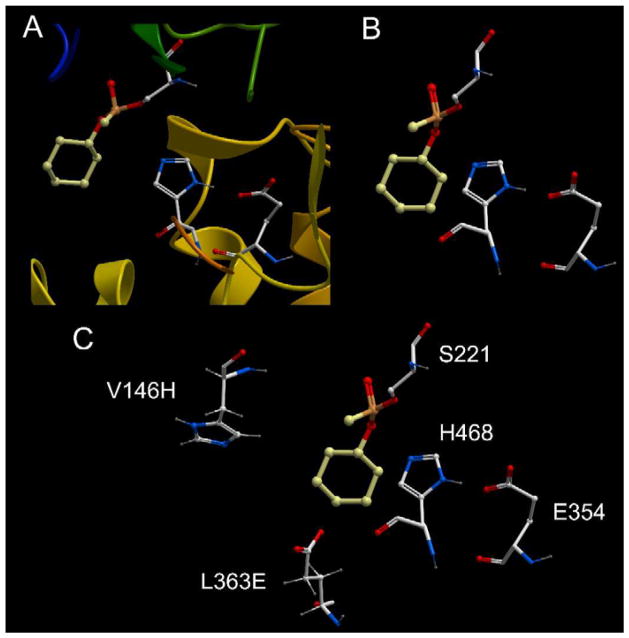
Recent structural studies with CEs have identified key residues within the active site that upon mutation can lead to a change in the enzymatic activity. For example, following determination of the crystal structures of hCE1 in complex with Soman, Tabun or cyclosarin [25, 26], it was observed that substitution of a pair of amino acids (V146H and L363E) might allow a water molecule to act as an intermediate nucleophile to remove the phosphorylated adducts on the serine Oγ atom. Mutagenesis, biochemistry and in vitro kinetic assays confirmed that this could be accomplished, with the greatest activity being observed with cyclosarin [24]. Figure 5 demonstrates the structure of inactivated hCE1 following incubation with the OP; a close up of the active site architecture and the catalytic amino acid residues (Ser221, His 468 and Glu354); and the juxtaposition of Val146 and Leu363, that when mutated to His and Glu, respectively, can effect water-mediated nucleophilic attack of the cyclohexyl phosphonyl group. These results suggest that CEs can tolerate significant changes in the geometry of the amino acids within the active site gorge, and that such changes may be directed towards specific substrates and/or covalent adducts. Hence the design of reagents that have specificity for different OPs may be practical, and potentially useful, in the in vivo detoxification of such agents.
Figure 5. The complex formed between the reaction of hCE1 and cyclosarin.
A – A close up view of the active site demonstrating the cyclohexyl phosphonyl adduct (yellow/orange) bound to the serine Oγ atom.
B – The same view as in A except that the ribbon structure has been removed.
C – Same view as in B except that the residues capable of directing a water molecule for attack of the phosphonyl adduct (V146H and L363E) are indicated.
Summary
CEs are ubiquitous enzymes that can hydrolyze very structurally diverse molecules. This include both naturally occurring and synthetic compounds, and this can result in either the activation or inactivation of the agent. Since no endogenous substrates have been identified, the hydrolysis of these molecules is thought to be a detoxification mechanism. However, while CEs may have some practical uses in the mitigation of OP toxicity, since the ester chemotype is present in numerous drugs, their impact on the disposition and distribution of both the parent compound and the derived metabolites, is a significant concern. Furthermore, since small animals appear to be poor models for assessing ester-mediated hydrolysis, it is likely that drug doses and schedules developed in these systems will be inappropriate for use in humans. We believe therefore, that in the early stages of development of novel clinical entities, ester groups should be replaced with suitable bioisosteres. The pharmacokinetics of such agents in preclinical models would likely be more representative of the exposure seen in humans thereby minimizing the time required for development. This will likely lead to more rapid translation into clinical trials with a reduction in costs for this process. Medicinal chemists and drug company executives should seriously consider these options prior to the initiation of drug development programs.
Supplementary Material
HIGHLIGHTS.
Carboxylesterase are general detoxifying enzymes that hydrolyze esterified molecules
They are expressed in tissues likely to be exposed to such agents
Humans and small mammals demonstrate different levels of carboxylesterase expression
Organophosphorus compounds are irreversibly inhibitors of these enzymes
Acknowledgments
This work was supported in part by NIH grants CA108775, AT007531, CA98468, DA018116, NS58089, NS082328, a NIH Cancer Center Core Grant CA21765, by St. Jude Children’s Research Hospital and by the American Lebanese Syrian Associated Charities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loevenhart AS. Further observations on the action of lipase. Am J Physiol. 1906;15:27. [Google Scholar]
- 2.Cashman J, Perroti B, Berkman C, Lin J. Pharmacokinetics and molecular detoxification. Environ Health Perspect. 1996;104:23–40. doi: 10.1289/ehp.96104s123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satoh T, Hosokawa M. The mammalian carboxylesterases: from molecules to function. Annu Rev Pharmacol Toxicol. 1998;38:257–288. doi: 10.1146/annurev.pharmtox.38.1.257. [DOI] [PubMed] [Google Scholar]
- 4.Bencharit S, Edwards CC, Morton CL, Howard-Williams EL, Kuhn P, Potter PM, Redinbo MR. Multisite promiscuity in the processing of endogenous substrates by human carboxylesterase 1. J Mol Biol. 2006;363:201–214. doi: 10.1016/j.jmb.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bencharit S, Morton CL, Howard-Williams EL, Danks MK, Potter PM, Redinbo MR. Structural insights into CPT-11 activation by mammalian carboxylesterases. Nat Struct Biol. 2002;9:337–342. doi: 10.1038/nsb790. [DOI] [PubMed] [Google Scholar]
- 6.Bencharit S, Morton CL, Hyatt JL, Kuhn P, Danks MK, Potter PM, Redinbo MR. Crystal structure of human carboxylesterase 1 complexed with the Alzheimer’s drug tacrine. From binding promiscuity to selective inhibition. Chem & Biol. 2003;10:341–349. doi: 10.1016/s1074-5521(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 7.Bencharit S, Morton CL, Xue Y, Potter PM, Redinbo MR. Structural basis of heroin and cocaine metabolism by a promiscuous human drug-processing enzyme. Nat Struct Biol. 2003;10:349–356. doi: 10.1038/nsb919. [DOI] [PubMed] [Google Scholar]
- 8.Wierdl M, Tsurkan L, Hyatt JL, Edwards CC, Hatfield MJ, Morton CL, Houghton PJ, Danks MK, Redinbo MR, Potter PM. An improved human carboxylesterase for enzyme/prodrug therapy with CPT-11. Cancer Gene Therapy. 2008;15:183–192. doi: 10.1038/sj.cgt.7701112. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton SE, Dudman AP, De Jersey J, Stoops JK, Zerner B. Organophosphate inhibitors: the reactions of bis(p-nitrophenyl) methyl phosphate with liver carboxylesterases and alpha-chymotrypsin. Biochim Biophys Acta. 1975;377:282–296. doi: 10.1016/0005-2744(75)90310-1. [DOI] [PubMed] [Google Scholar]
- 10.Cohen SD, Ehrich M. Cholinesterase and carboxylesterase inhibition by dichlorvos and interactions with malathion and triorthotolyl phosphate. Toxicol Appl Pharmacol. 1976;37:39–48. doi: 10.1016/s0041-008x(76)80006-3. [DOI] [PubMed] [Google Scholar]
- 11.Maxwell DM. The specificity of carboxylesterase protection against the toxicity of organophosphorus compounds. Toxicol Appl Pharmacol. 1992;114:306–312. doi: 10.1016/0041-008x(92)90082-4. [DOI] [PubMed] [Google Scholar]
- 12.Hatfield MJ, Tsurkan L, Garrett M, Shaver T, Edwards CC, Hyatt JL, Hicks LD, Potter PM. Organ-specific carboxylesterase profiling identifies the small intestine and kidney as major contributors of activation of the anticancer prodrug CPT-11. Biochem Pharmacol. 2011;81:24–31. doi: 10.1016/j.bcp.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams FM. Potential for metabolism locally in the skin of dermally absorbed compounds. Human Exp Toxicol. 2008;27:277–280. doi: 10.1177/0960327107085831. [DOI] [PubMed] [Google Scholar]
- 14.Li B, Sedlacek M, Manoharan I, Boopathy R, Duysen EG, Masson P, Lockridge O. Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem Pharmacol. 2005;70:1673–1684. doi: 10.1016/j.bcp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Morton CL, Iacono L, Hyatt JL, Taylor KR, Cheshire PJ, Houghton PJ, Danks MK, Stewart CF, Potter PM. Metabolism and antitumor activity of CPT-11 in plasma esterase-deficient mice. Cancer Chemother Pharmacol. 2005;56:629–636. doi: 10.1007/s00280-005-1027-y. [DOI] [PubMed] [Google Scholar]
- 16.Morton CL, Wierdl M, Oliver L, Ma M, Danks MK, Stewart CF, Eiseman JL, Potter PM. Activation of CPT-11 in mice: Identification and analysis of a highly effective plasma esterase. Cancer Res. 2000;60:4206–4210. [PubMed] [Google Scholar]
- 17.Guemei AA, Cottrell J, Band R, Hehman H, Prudhomme M, Pavlov MV, Grem JL, Ismail AS, Bowen D, Taylor RE, Takimoto CH. Human plasma carboxylesterase and butyrylcholinesterase enzyme activity: correlations with SN-38 pharmacokinetics during a prolonged infusion of irinotecan. Cancer Chemother Pharmacol. 2001;47:283–290. doi: 10.1007/s002800000258. [DOI] [PubMed] [Google Scholar]
- 18.Potter PM, Wolverton JS, Morton CL, Whipple DO, Danks MK. In situ subcellular localization of epitope tagged human and rabbit carboxylesterases. Cytometry. 1998;32:223–232. [PubMed] [Google Scholar]
- 19.Potter PM, Wolverton JS, Morton CL, Wierdl M, Danks MK. Cellular localization domains of a rabbit and a human carboxylesterase: Influence on irinotecan (CPT-11) metabolism by the rabbit enzyme. Cancer Res. 1998;58:3627–3632. [PubMed] [Google Scholar]
- 20.Fleming CD, Bencharit S, Edwards CC, Hyatt JL, Tsurkan L, Bai F, Fraga C, Morton CL, Howard-Williams EL, Potter PM, Redinbo MR. Structural insights into drug processing by human carboxylesterase 1: tamoxifen, mevastatin, and inhibition by benzil. J Mol Biol. 2005;352:165–177. doi: 10.1016/j.jmb.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Redinbo MR, Potter PM. Mammalian Carboxylesterases: From drug targets to protein therapeutics. Drug Discov Today. 2005;10:313–325. doi: 10.1016/S1359-6446(05)03383-0. [DOI] [PubMed] [Google Scholar]
- 22.Wadkins RM, Hyatt JL, Yoon KJ, Morton CL, Lee RE, Damodaran K, Beroza P, Danks MK, Potter PM. Identification of novel selective human intestinal carboxylesterase inhibitors for the amelioration of irinotecan-induced diarrhea: Synthesis, quantitative structure-activity relationship analysis, and biological activity. Mol Pharmacol. 2004;65:1336–1343. doi: 10.1124/mol.65.6.1336. [DOI] [PubMed] [Google Scholar]
- 23.Wadkins RM, Hyatt JL, Wei X, Yoon KJ, Wierdl M, Edwards CC, Morton CL, Obenauer JC, Damodaran K, Beroza P, Danks MK, Potter PM. Identification and characterization of novel benzil (diphenylethane-1,2-dione) analogues as inhibitors of mammalian carboxylesterases. J Med Chem. 2005;48:2905–2915. doi: 10.1021/jm049011j. [DOI] [PubMed] [Google Scholar]
- 24.Hemmert AC, Otto TC, Chica RA, Wierdl M, Edwards JS, Lewis SL, Edwards CC, Tsurkan L, Cadieux CL, Kasten SA, Cashman JR, Mayo SL, Potter PM, Cerasoli DM, Redinbo MR. Nerve agent hydrolysis activity designed into a human drug metabolism enzyme. PloS One. 2011;6:e17441. doi: 10.1371/journal.pone.0017441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleming CD, Edwards CC, Kirby SD, Maxwell DM, Potter PM, Cerasoli DM, Redinbo MR. Crystal structures of human carboxylesterase 1 in covalent complexes with the chemical warfare agents soman and tabun. Biochemistry. 2007;46:5063–5071. doi: 10.1021/bi700246n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemmert AC, Otto TC, Wierdl M, Edwards CC, Fleming CD, MacDonald M, Cashman JR, Potter PM, Cerasoli DM, Redinbo MR. Human carboxylesterase 1 stereoselectively binds the nerve agent cyclosarin and spontaneously hydrolyzes the nerve agent sarin. Mol Pharmacol. 2010;77:508–516. doi: 10.1124/mol.109.062356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.