Abstract
Men have lower rates of HIV testing and higher rates of AIDS-related mortality compared to women in sub-Saharan Africa. To assess whether there is an opportunity to increase men’s uptake of testing by correcting misperceptions about testing norms, we compare men’s perceptions of their closest friend’s HIV testing behaviors with the friend’s actual testing self-report using a unique dataset of men sampled within their social networks (n = 59) in Dar es Salaam, Tanzania. We examine the accuracy and bias of perceptions among men who have tested for HIV (n = 391) and compare them to the perceptions among men who never tested (n = 432). We found that testers and non-testers did not differ in the accuracy of their perceptions, though non-testers were strongly biased towards assuming that their closest friends had not tested. Our results lend support to social norms approaches designed to correct the biased misperceptions of non-testers to promote men’s HIV testing.
Keywords: HIV testing, Misperceptions, Men, Tanzania
Introduction
Men experience significantly higher rates of AIDS-related mortality compared to women in sub-Saharan Africa [1]. This is thought to be a combined result of men’s low rates of HIV testing [2], delayed treatment initiation [3], as well as lower levels of treatment adherence [4]. HIV testing serves as a gateway to the treatment and care continuum for individuals who test positive as well as an entryway to prevention interventions including pre-exposure prophylaxis for those who test negative. Men’s low rates of HIV testing are frequently attributed to their adherence to traditional norms of masculinity, which value self-reliance and consequently discourage health-seeking behaviors like HIV testing [1, 5]. Additionally, men’s low rates of HIV testing may be an unintended consequence of national testing programs that prioritize women and the prevention of maternal to child transmission of HIV due to women’s heightened susceptibility and vulnerability to HIV infection [5]. Given men’s increased risk of AIDS-related mortality and the important role of HIV testing, several calls to increase HIV testing among men in the region have been made [6, 7].
One promising approach to increasing men’s HIV testing may be leveraging peer norms around testing. Peers have been found to play an important role in shaping health behaviors including decisions to test for HIV, as reported in a review of qualitative studies examining the uptake of HIV testing in sub-Saharan Africa [8]. HIV testing behaviors were also found to cluster within men’s social networks in Tanzania, suggesting peer networks may be influencing the adoption of HIV testing [9]. Peers likely influence HIV testing through social norms, which provide important information on perceived or actual prevalence (descriptive norms) and appropriateness (injunctive norms) of behaviors [10]. Importantly, perceptions of social norms may be inaccurate and systematically biased. Individuals may overestimate or under-estimate the true prevalence or appropriateness of actual peer behaviors [11]. Moreover, scholars have found that in many cases, individuals have a tendency to underestimate health behaviors (e.g. exercising) among their peers while overestimating risky behaviors (e.g. engaging in substance use) [12], a phenomenon known as pluralistic ignorance [13, 14]. In other cases, individuals have been found to report peer behaviors that are very similar to their own behaviors. This false consensus effect [15] results in individuals who engage in a behavior assuming that their friends also engage in the behavior. Misperceptions of actual peer behaviors are important because individual’s behaviors are thought to conform to their own perceptions. Therefore, comparing perceptions with actual peer behaviors is a way to identify misperceptions, which can subsequently be intervened upon. Interventions using a “social norms approach” [16] can be implemented to rectify these misperceptions with the aim of reducing risky behaviors or increasing health-promoting behaviors by revealing actual healthier norms. These approaches are not designed to broadly transform or shift entrenched societal norms, as is done in other public health approaches that often strive for structural changes [17]. Rather, a social norms approach focuses precisely on correcting identified discrepancies between perceived and actual behaviors to drive behavior change. One social norms approach provides tailored feedback to individuals who incorrectly perceive their peers’ behaviors. This feedback includes information about their peers’ actual behaviors to correct the individual’s erroneous perceptions. Such normative feedback interventions may be easier to implement than structural approaches and have been effective in reducing risky behaviors like alcohol misuse [18].
Despite the potential utility of correcting misperceptions of HIV testing norms to increase HIV testing among men, no studies have evaluated the accuracy and/or bias of perceived HIV testing norms by comparing them to actual HIV testing behaviors reported by peers. The purpose of this paper, therefore, is to evaluate whether there is an opportunity to promote men’s HIV testing by correcting misperceptions using a social norms approach. We do this by comparing men’s perceptions of their closest friend’s HIV testing behavior with the friend’s actual testing self report using a unique dataset of urban men (n = 823) sampled within their social networks, locally referred to as “camps,” in Dar es Salaam, Tanzania. We specifically examine and compare the accuracy and bias of perceptions for men who have previously tested for HIV (n = 391) with those who have not tested (n = 432) to assess the effects of individual HIV testing status on perceptions of peer HIV testing behavior.
Methods
Setting
This study took place in Dar es Salaam, the commercial capital and largest city in Tanzania. We conducted our research within four wards (analogous to US census tracts) of Kinondoni District, the most populated and impoverished of the three districts in Dar es Salaam. HIV prevalence in Dar es Salaam is 6.9 % which is higher than the national average of 5 % [19]. The HIV prevalence among men in Dar es Salaam is 5.3 % [19]. HIV testing in Tanzania is free of charge and is provided by the government, private sector and other organizations through health facilities, stand-alone facilities as well as outreach services. In the study area, free testing is available in multiple venues and has also been offered through the Tanzania AIDS Prevention Program (TAPP) using mobile caravans since 2008. It is reported that 46.7 % of men ages 15–49 in Dar es Salaam have ever tested for HIV [19].
Study
Data for this study come from the baseline assessment of an on-going cluster-randomized HIV and gender-based violence prevention trial with urban social networks of mostly young men in Dar es Salaam, Tanzania that are locally referred to as “camps”. Camps are stable social networks with an elected leadership structure that are formed by members to socialize and support one another. Previous research with these camps suggests that most camp members are men who are not in school and who are unemployed or only occasionally employed [20]. Men sometimes reported joining camps closest to where they lived and often talked about relying on one other to cope with financial stress and family emergencies [21]. Additionally, camp members reported engaging in activities such as playing sports or participating in camp-led business enterprises [21]. Camps have been described in more detail in other publications [21–23]. The on-going trial is evaluating the effectiveness of a camp-randomized microfinance and health leadership intervention on sexually transmitted infections, intimate partner violence and HIV risk behaviors [24].
Prior to the start of this trial, we used community informants to enumerate all camp-based social networks in operation within the study area (n = 294). Of these, 172 camps were eligible and we randomly selected 60 for inclusion in our trial. Next, we conducted a census of the selected camp networks by collecting camp rosters and then contacted each camp member to confirm his or her eligibility. To be eligible, individuals had to be over 15 years old, have been a camp member for more than 3 months, intend to live in Dar es Salaam for the next 30 months, and be willing to provide contact information for a friend or family member for follow-up purposes. Of the 1836 potentially eligible participants, we collected baseline behavioral data from a sample of n = 1491 (1249 men and 242 women) (response rate = 81.2 %). Informed consent was obtained from all individual participants included in the study. The study procedures and instruments were approved by the University of North Carolina at Chapel Hill Institutional Review Board as well as the Muhimbili University of Health and Allied Sciences (MUHAS) Senate Research and Publications Committee. Data were collected between October 8, 2013 and March 23, 2014.
Behavioral and Social Network Assessment
Trained interviewers, fluent in English and Swahili, conducted the baseline behavioral assessment using tablets programmed with a custom-designed CAPI (computer-assisted personal interviewing) instrument. All study instruments were developed in English, translated to Swahili, and then checked for accuracy. Further revisions were made until the Swahili translation matched the English version of the questionnaire. Questionnaires were piloted before the baseline assessment. A social network assessment, assessing social ties among camp members, was built into the assessment tool and displayed the camp roster associated with each participant. Participants were asked whether they knew each camp member. Next, from a list of all known individuals, participants were asked to state whether each of these known members was a friend, acquaintance, or somebody they didn’t get along with. From the list of friends (or acquaintances if no friends were identified), each participant was asked to identify his closest friend within the camp. Participants were then asked a series of follow-up questions about this individual, including their perceptions about whether the friend had ever tested for HIV.
Sample
Given our interest in comparing perceived versus actual HIV testing norms among men, the sample for this analysis was restricted to men with complete data on their perceptions of their closest friend’s HIV testing behavior, the closet friend’s self-reported HIV testing behavior, as well as the participant’s own HIV testing status. Specifically, 10.41 % of the 1249 men interviewed (n = 130) selected a closest friend who did not participate in the baseline assessment him/herself. These men were omitted from the analysis because we were not able to compare the respondent’s perceptions of the friend’s HIV testing behaviors to the friend’s actual self-reported testing behaviors. An additional 23.62 % of the sample of men (n = 295) refused/declined to say whether they perceived their closest friend to have ever tested for HIV or not. These men were also omitted because we did not have data on perceived norms to make an assessment of their accuracy or bias. One additional participant who declined to say whether he had ever tested for HIV was also excluded since our primary interest was comparing accuracy and bias of perceptions among prior testers versus non-testers. Thus, the final analytic sample is n = 823 men (65.9 % of the men enrolled at baseline).
Measures
Individual HIV Testing Behavior
Each participant was asked “Have you ever had a test for HIV/AIDS?” Response options included yes, no, don’t know, and declined to answer.
Perceived HIV Testing Behavior
After identifying their closest friend within the camp network, participants were asked a series of questions about this individual. Perceived HIV testing was assessed using the following question “Do you think [Name of closest friend] ever had an HIV test?”
Friend-Reported HIV Testing
Since all eligible and willing camp members were interviewed during our baseline assessment, those participating camp members who were identified by others as closest friends also completed baseline assessments. During the baseline assessment, they were asked to self-report their HIV testing behavior with the question “Have you ever had a test for HIV/AIDS?”
Data Analysis
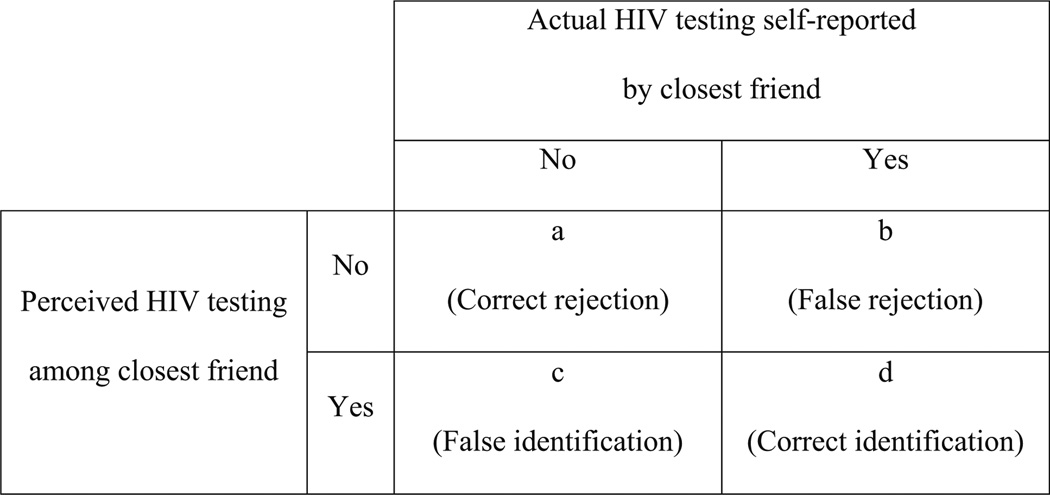
We used descriptive statistics to examine the proportion of men and their closest friends who had ever tested for HIV. We then compared perceived HIV testing behavior of the closest friend to actual self-reported HIV testing behavior of this friend and determined the number of men who correctly rejected (i.e. the perception that the friend had never tested was concordant with the friend’s actual behavior), falsely rejected (i.e. the perception that the friend had not tested when the friend reported having tested), falsely identified (i.e. the perception that the friend had tested when the friend reported not having tested), and correctly identified HIV testing in their closest friends (i.e. the perception that the friend had tested was concordant with the friend’s actual behavior). These four categories are illustrated in Fig. 1. We used these values to compute the rate of correctly identifying HIV testing by computing proportion of correct identifications among all cases where the friend had actually tested. The complement of this rate was the false rejection rate, or the proportion of false rejections among all cases where the friend had actually tested. We also computed the false identification rate, based on the proportion of false identifications among all cases where the friend had not actually tested. Finally, we computed the correct rejection rate as the complement to the false identification rate. This rate represented the proportion of correct rejections among all cases where the friend had not actually tested. Next, we used these rates to calculate nonparametric measures of accuracy [25] and bias [26] of the perceptions using a signal detection analysis approach. We performed these calculations separately for HIV testers and non-testers. Accuracy values range from 0 to 1 with 0 being completely inaccurate and 1 being completely accurate. Bias values range from −1 to 1 with −1 being a complete liberal bias where participants perceive HIV testing regardless of its presence and 1 being a complete conservative bias against perceiving HIV testing even when it is present. This approach was previously used to assess the accuracy and bias of perceptions of friend’s substance use behaviors [27]. Finally, we used multinomial logistic regression models using SAS PROC SURVEY LOGISTIC with a glogit link to examine whether individual testing status (i.e. non-testers vs. testers) was significantly associated with accuracy or bias of perceptions while accounting for the nested structure of our data (men nested within camp networks). The outcome variables for the multinomial models were the log-odds of the perception being a correct rejection (cell a in Fig. 1), a false rejection (cell b), or a false identification (cell c) versus a correct identification (cell d). The same modeling strategy was used in a previous study of accuracy and bias [27]. We began with an unadjusted model and then controlled for age, education, SES (assessed using principal components analysis (PCA) of responses to a wealth index assessing ownership of 10 different household assets [28] that was then categorized into terciles based on the entire sample of men and women in our baseline dataset), marital history, and number of children. We conducted our analyses using SAS software Version 9.4 [29].
Fig. 1.
Accuracy and bias of perceptions of closest friend’s HIV testing behavior
Results
The characteristics of the men included in our sample are presented in Table 1. The mean age of the sample was 26.0 years (range 15.0–59.0, data not shown). The majority (57.8 %) of men had a primary school education or less, 10.7 % finished some secondary school, and 31.1 % completed secondary school or more. Men were fairly equally distributed in terms of SES, with 31.8 % falling in the low SES category, 36.0 % falling in the middle SES category, and 32.1 % falling in the high SES category. Most men were unmarried (77.8 %) and did not have any children (67.1 %). The majority of men (80.2 %) had at least 1 sexual partner within the last year, with 62.3 % reporting having 1 partner, 9.0 % reporting 2 partners, and 8.9 % reporting 3 or more sexual partners. HIV testing was reported by almost half of the participants (47.5 %). The closest friends were overwhelmingly male (98.4 %) and had an average age of 26.4 years (range 15–59, data not shown). HIV tested was reported by 49.0 % of the closest friends. The majority of participants (64.9 %) perceived their closest friend as not having ever tested for HIV.
Table 1.
Characteristics and HIV testing behaviors of men (n = 823) and their closest friends
| Variable | % | n |
|---|---|---|
| Age | ||
| 15–19 | 17.9 | 147 |
| 20–24 | 30.6 | 252 |
| 25–29 | 26.1 | 215 |
| 30+ | 25.4 | 209 |
| Education | ||
| Primary school or less | 57.8 | 476 |
| Some secondary school | 10.7 | 88 |
| Secondary school completed or greater | 31.1 | 256 |
| SES | ||
| Low | 31.8 | 262 |
| Medium | 36.0 | 296 |
| High | 32.1 | 264 |
| Married | ||
| No | 77.8 | 640 |
| Yes | 22.0 | 181 |
| Number of past-year sexual partners | ||
| 0 | 19.8 | 163 |
| 1 | 62.3 | 513 |
| 2 | 9.0 | 74 |
| 3+ | 8.9 | 73 |
| Number of children | ||
| 0 | 67.1 | 552 |
| 1 | 20.1 | 165 |
| 2+ | 12.9 | 106 |
| Tested for HIV | ||
| No | 52.5 | 432 |
| Yes | 47.5 | 391 |
| Close friend’s age | ||
| 15–19 | 13.4 | 110 |
| 20–24 | 32.0 | 263 |
| 25–29 | 27.0 | 222 |
| 30+ | 27.7 | 228 |
| Close friend’s gender | ||
| Male | 98.4 | 810 |
| Female | 1.6 | 13 |
| Actual close friend’s HIV testing | ||
| No | 51.0 | 420 |
| Yes | 49.0 | 403 |
| Perceptions of close friend’s HIV testing | ||
| No | 64.9 | 534 |
| Yes | 35.1 | 289 |
We present the rate of correct identifications, false rejections, false identifications, and correct rejections for non-testers and testers in Table 2. When non-testers (n = 432) had a closest friend who had actually tested for HIV (n = 190), only about a quarter of participants accurately perceived HIV testing in their friend (correct identification rate = .26). The vast majority of non-testers falsely rejected this behavior in their closest friend when it actually occurred (false rejection rate = .74). Testers (n = 391), on the other hand, had a correct identification rate of .54 and a false rejection rate of .46 when their closest friend had actually tested (n = 213). When non-testers had a closest friend who had not tested for HIV (n = 242), they were more likely to correctly perceive the behavior (correct rejection rate = .80) rather than misperceive their closest friend to have tested (false identification rate = .20). Testers who had friends who had not tested (n = 178) had a false identification rate of .43 and a correct rejection rate of .57. The accuracy of perceptions for testers and non-testers was similar (.58 for non-testers and .60 for testers). However, non-testers were strongly biased to not perceiving HIV testing behavior in their closest friends (.84) while testers had only a slight bias (.06).
Table 2.
Rate of correct identifications, false rejections, false identifications, and correct rejections as well as estimates of accuracy and bias of perceptions among non-testers (n = 432) and testers (n = 391)
| Non-testers | Prior testers | |
|---|---|---|
| Rate (n) | ||
| Correct identification | 0.26 (49) | 0.54 (116) |
| False rejection | 0.74 (141) | 0.46 (97) |
| False identification | 0.2 (48) | 0.43 (76) |
| Correct rejection | 0.8 (194) | 0.57 (102) |
| Accuracy | 0.58 | 0.60 |
| Bias | 0.84 | 0.06 |
We present the odds ratio (OR) estimates, the 95 % Wald confidence intervals, and the corresponding p values of the Wald Chi square statistics for the accuracy and bias of perception for non-testers versus testers in Table 3. In both unadjusted and adjusted models, individual testing status was a significant predictor of the odds of a correct rejection versus correct identification (a vs. d in Fig. 1) as well as the odds of a false rejection versus correct identification (b vs. d). In the unadjusted model, non-testers had 4.50 times greater odds of correctly rejecting versus correctly identifying HIV testing (95 % CI 2.48–8.18) and 3.44 times greater odds of falsely rejecting vs. correctly identifying HIV testing in their close friends (95 % CI 2.09–5.66) compared to testers. Non-testers did not differ compared to testers in their odds of falsely identifying testing versus correctly identifying HIV testing in their close friends. When controlling for age, education, SES, marital history, and number of children, the OR estimates are slightly attenuated, but remain highly significant. Specifically, even when controlling for demographic characteristics, non-testers had 4.28 times greater odds of correctly rejecting versus correctly identifying HIV testing (95 % CI 2.34–7.83) than testers and 3.27 times greater odds of falsely rejecting versus correctly identifying HIV testing in their close friends (95 % CI 2.00–5.33) compared to men who had previously tested. In the adjusted model, non-testers still did not differ in their odds of falsely identifying versus correctly identifying HIV testing in their close friends compared to testers.
Table 3.
Multinomial logistic regression models of accuracy and bias of HIV testing perceptions for non-testers (n = 432) versus testers (n = 391)
| Effect | Contrasta | Unadjusted model |
Adjusted modelb |
||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95 % confidence limit | p value | Odds ratio | 95 % confidence limit | p value | ||
| HIV testing (no vs. yes) | a versus d | 4.50 | (2.48, 8.18) | <.001 | 4.28 | (2.34, 7.83) | <.001 |
| b versus d | 3.44 | (2.09, 5.66) | <.001 | 3.27 | (2.00, 5.33) | <.001 | |
| c versus d | 1.50 | (0.80, 2.79) | 0.21 | 1.39 | (0.75, 2.58) | 0.30 | |
Contrasts: a versus d = correct rejection versus correct identification; b versus d false rejection versus correct identification; c versus d = false identification versus correct identification
Adjusted models control for age, education, SES, marital history, and number of children
Discussion
This is the first study, to our knowledge, that has compared perceptions with actual reports of friend’s HIV testing behaviors. We found that while HIV testing was reported by almost half of the men in this study (47.5 %) and by approximately half of their closest friends (49.0 %), the majority of participants (64.9 %) thought that their closest friend had never tested for HIV. Testers and non-testers did not differ in the accuracy of their perceptions, meaning both groups had similar rates of correctly matching their perceptions to their friend’s self-reported behavior, but they did differ substantially with regard to the nature of their misperceptions. While testers had only a slightly higher rate of perceiving that the friend had not tested when their friends had actually tested compared to their rate of perceiving that the friend had tested when their closest friends had not tested, non-testers were strongly biased in their misperceptions. Almost 75 % of non-testers falsely rejected testing in their closest friends when those friends had actually tested. Even after controlling for their demographic characteristics, compared to testers, non-testers had over three times greater odds of falsely rejecting versus correctly identifying HIV testing and over four times greater odds of correctly rejecting versus correctly identifying testing in their close friends.
Since this is the first study to assess the accuracy and bias of perceived HIV testing norms, it is not possible to compare our findings to results obtained in other settings. However, we can relate our results to studies of other behaviors. Our findings are consistent with pluralistic ignorance [13, 14], suggesting that individuals tend to underestimate protective or healthy behaviors and overestimate risky behaviors in their peers [12]. For example, one recent study that looked at perceptions of both risky and protective sexual behaviors within online social networks and found that participants underestimated protective/ healthy behaviors like talking about and using condoms and overestimated risky behaviors like having multiple sexual partners [11]. Other studies have repeatedly shown that college students generally overestimate risky drinking norms on their campuses [30–32]. In our study, men underestimated the level of HIV testing among their closest friends. The degree to which non-testers did so was far greater than testers, but all men were biased towards assuming their closest friend had not tested. These results suggest HIV testing is not perceived to be a normative behavior even among men who have tested, but is particularly misperceived by non-testers. This provides an important opportunity to increase testing by correcting misperceived norms, particularly among non-testers.
Most other studies that have examined the accuracy and bias of perceived norms have focused on assessing perceptions of risky behaviors. Many of these studies found that people tend to misperceive peer behavior in a direction that is consistent with their own behavior. This tendency has been called the false consensus effect [15] or normative fallacy and is thought to occur when individuals project their own behaviors on to their peers [33]. For example, one study that used social network data and compared perceptions of peer substance use to actual self-reports of peer substance use found that non-users were biased in their misperceptions and often assumed that their friends were also non-users while substance users had a liberal bias and assumed that their friends were also using substances [27]. Support of the false consensus effect has also been found in studies of adolescent perceptions of deviant and health risk behaviors [34]. In contrast to the false consensus effect, we did not find evidence suggesting men who had tested for HIV were biased to misperceiving their friends as also having tested. In fact, testers in our study had a slight bias towards falsely assuming their friends had not tested for HIV. There was no difference in odds of falsely detecting versus correctly identifying HIV testing among their close friends for testers compared to non-testers in our study. This may be because our study was examining perceptions of a healthy and protective behavior while much of the literature on the false consensus effect has been done with perceptions of risky behaviors.
Innovative strategies are needed to increase men’s rates of HIV testing in sub-Saharan Africa. Men in the region experience significantly higher rates of AIDS-related mortality compared to women [1] and their low rates of testing are thought to contribute to their poor outcomes. A recent systematic review of strategies to increase men’s HIV testing in sub-Saharan Africa found that few interventions target men specifically [35]. Our findings suggest that a “social norms approach” [16] may be effective in increasing men’s testing for HIV in this setting. Interventions incorporating a social norms approach are designed to correct misperceptions with the aim of reducing risky behaviors or increasing health-promoting behaviors by revealing actual healthier norms [16]. This approach is most commonly implemented either through social marketing campaigns or through individual normative feedback interventions [18]. Social marketing campaigns may be implemented to provide information about actual norms through electronic and/or print media or even through discussions and workshops within social networks. Individual normative feedback, on the other hand, is a personalized approach to correcting misperceptions by providing participants with feedback on the actual norms of their peers [16]. This feedback may be provided through a personalized mailed leaflet, a web/computer interface, or as part of a motivational interviewing intervention [18]. A social norms approach was first recommended after a study found that students overestimated the degree to which their peers found excessive drinking to be acceptable [36]. A recent review of interventions designed to reduce alcohol consumption among college students who engaged in heavy drinking found that interventions with a social norms approach were effective in changing normative perceptions and in reducing harmful alcohol misuse [37]. Our results suggest we have an opportunity to increase men’s uptake of HIV testing by correcting misperceptions with a similar approach.
Decreasing men’s misperceptions of HIV testing norms may also be accomplished by promoting communication about HIV testing within networks or by encouraging friends to test together. Calls to encourage communication within social networks to increase men’s HIV testing have been made with regard to African American men who have sex with men [38]. We echo these calls to increase communication about HIV testing within social networks to increase men’s testing for HIV in sub-Saharan Africa. The fact that men in our study underestimated the level of HIV testing among their closest friends may be due to HIV-related stigma that may limit communication about testing. Other studies in sub-Saharan Africa have documented an inverse relationship between HIV stigma and HIV testing behaviors, particularly among men [39, 40]. Correcting misperceptions of HIV testing behaviors of closet friends may serve to promote testing among men by decreasing the stigma associated with testing in this population.
Strengths and Limitations
Our study is the first to evaluate the accuracy and bias of perceived HIV testing behaviors of men’s closest friends. A key strength of our study is our network sampling approach that allowed us to collect data on individuals sampled within their social networks, assess their closest friendship within the network, collect data on perceptions of this friend’s behavior, and then compare those perceptions with the friend’s own self-report of that behavior. Another strength of this study is the focus on a health-promoting behavior. Much of the literature comparing perceptions to actual norms has focused on perceptions of risky behaviors like alcohol and substance use. In theory, social norms approaches may be implemented to either reduce risky behavior or increasing health-promoting behaviors [16]. In practice, this approach has almost exclusively focused on reducing risky behavior [18]. This may be because the misperceptions of norms for health-promoting behaviors have been under-studied.
Our findings should be considered in light of their limitations. First, our data come from men who are members of camp-based social networks in Dar es Salaam, and as such, may not be generalizable to other networks of men in sub-Saharan African settings. Second, we only assessed the perceptions of HIV testing for men’s closest friends and thus were not able to examine the accuracy or bias of perceptions for men’s larger peer groups. However, close friendships, relationships that are characterized by trust, may be particularly important because people may be more influenced by the individuals that they care more about and value above others. Finally, our analytic sample was restricted to men with complete data on their perceptions and actual friend’s self-reported HIV testing behavior as well as their own HIV testing status. As a result, we excluded 34.1 % of the larger study’s sample of men. The majority of these men were omitted because they refused or declined to say whether they perceived their close friend to have ever tested for HIV or not. Men may have refused to answer this question because they truly did not know whether their friend had ever tested or because they were not comfortable speculating or sharing their thoughts about their friend’s behavior. All but one of these men were comfortable disclosing their own testing history with the interviewer. The fact that a significant proportion of the men enrolled in our trial either did not know or were not comfortable speculating as to whether their closest friend had ever tested for HIV underscores the likelihood that this population of men is likely not communicating about HIV testing with even their closest friends.
Conclusion
To our knowledge, this is the first study that has examined the accuracy and bias of perceived HIV testing norms among men in sub-Saharan Africa. We found that men who have not tested for HIV were strongly biased in their misperceptions of their closest friend’s testing behavior and tended to assume these friends had not tested. In summary, HIV testing is not perceived as a normative behavior within these urban social networks, especially among men who have not yet tested. Our results suggest we have an opportunity to increase men’s uptake of HIV testing and lend support to social norms approaches designed to correct the biased misperceptions of non-testers by revealing the true extent to which close friends are actually testing for HIV.
Acknowledgments
We wish to acknowledge the work and dedication of our study interviewers as well as our research team in Dar es Salaam, including Mrema Noel Kilonzo, Deus Kajuna, Brenda Mkony, Joyce Kondela and Gema Lambert. We also thank the anonymous reviewers for their comments on prior drafts. Finally, we would like to thank the participants of our study for their contributions.
Funding Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Numbers R01MH098690 and F31MH103062. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with Ethical Standards
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Dovel K, Yeatman S, Watkins S, Poulin M. Men’s heightened risk of AIDS-related death: the legacy of gendered HIV testing and treatment strategies. AIDS. 2015;29(10):1123–1125. doi: 10.1097/QAD.0000000000000655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatesh KK, Madiba P, De Bruyn G, Lurie MN, Coates TJ, Gray GE. Who gets tested for HIV in a South African urban township? Implications for test and treat and gender-based prevention interventions. J Acquired Immune Defic Syndr. 2011;56(2):151–165. doi: 10.1097/QAI.0b013e318202c82c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muula AS, Ngulube TJ, Siziya S, Makupe CM, Umar E, Prozesky HW, et al. Gender distribution of adult patients on highly active antiretroviral therapy (HAART) in Southern Africa: a systematic review. BMC Public Health. 2007;7:63. doi: 10.1186/1471-2458-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor-Smith K, Tweya H, Harries A, Schoutene E, Jahn A. Gender differences in retention and survival on antiretroviral therapy of HIV-1 infected adults in Malawi. Malawi Med J. 2010;22(2):49–56. doi: 10.4314/mmj.v22i2.58794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins JA, Hoffman S, Dworkin SL. Rethinking gender, heterosexual men, and women’s vulnerability to HIV/AIDS. Am J Public Health. 2010;100(3):435–445. doi: 10.2105/AJPH.2009.159723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills EJ, Ford N, Mugyenyi P. Expanding HIV care in Africa: making men matter. Lancet. 2009;374(9686):275–276. doi: 10.1016/S0140-6736(09)61348-9. [DOI] [PubMed] [Google Scholar]
- 7.Mills EJ, Beyrer C, Birungi J, Dybul MR. Engaging men in prevention and care for HIV/AIDS in Africa. PLoS Med. 2012;9(2):e1001167. doi: 10.1371/journal.pmed.1001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musheke M, Ntalasha H, Gari S, McKenzie O, Bond V, Martin-Hilber A, et al. A systematic review of qualitative findings on factors enabling and deterring uptake of HIV testing in Sub-Sa-haran Africa. BMC Public Health. 2013;13:220. doi: 10.1186/1471-2458-13-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulawa M, Yamanis TJ, Hill LM, Balvanz P, Kajula LJ, Maman S. Evidence of social network influence on multiple HIV risk behaviors and normative beliefs among young Tanzanian men. Soc Sci Med. 2016;153:35–43. doi: 10.1016/j.socscimed.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cialdini RB, Reno RR, Kallgren CA. A focus theory of normative conduct: recycling the concept of norms to reduce littering in public places. J Pers Soc Psychol. 1990;58(6):1015–1026. [Google Scholar]
- 11.Black SR, Schmiege S, Bull S. Actual versus perceived peer sexual risk behavior in online youth social networks. Transl Behav Med. 2013;3(3):312–319. doi: 10.1007/s13142-013-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkowitz AD. Applications of social norms theory to other health and social justice issues. In: Perkins WH, editor. The social norms approach to preventing school and college age substance abuse: a handbook for educators, counselors, and clinicians. San Francisco: Jossey-Bass; 2003. [Google Scholar]
- 13.Prentice DA, Miller DT. Pluralistic ignorance and the perpetuation of social norms by unwitting actors. In: Mark PZ, editor. Advances in experimental social psychology. Vol. 28. Cambridge: Academic Press; 1996. pp. 161–209. [Google Scholar]
- 14.Toch H, Klofas J. Pluralistic ignorance, revisited. In: Stephenson GM, Davis JH, editors. Progress in applied social psychology. Vol. 2. New York: Wiley; 1984. [Google Scholar]
- 15.Ross L, Greene D, House P. The false consensus effect: an egocentric bias in social perception and attribution processes. J Exp Soc Psychol. 1977;13(3):279–301. [Google Scholar]
- 16.Berkowitz AD. An overview of the social norms approach. In: Lederman LC, Stewart CP, editors. Challenging the culture of college drinking: a socially situated health communication campaign. Cresskill: Hampton Press; 2005. pp. 193–214. [Google Scholar]
- 17.Gupta GR. Gender, sexuality, and HIV/AIDS: the what, the why, and the how. Can HIV AIDS Policy Law Rev. 2000;5(4):86–93. [PubMed] [Google Scholar]
- 18.Moreira MT, Smith LA, Foxcroft D. Social norms interventions to reduce alcohol misuse in university or college students. Cochrane Database Syst Rev. 2009;3:CD006748. doi: 10.1002/14651858.CD006748.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Tanzania Commission for AIDS (TACAIDS) ZACZ, National Bureau of Statistics (NBS), Office of the Chief Government Statistician (OCGS), and ICF International. Tanzania HIV/AIDS and malaria indicator survey 2011-12. Dar es Salaam: TACAIDS, ZAC, NBS, OCGS, and ICF International. 2013 [Google Scholar]
- 20.Yamanis TJ, Doherty IA, Weir SS, Bowling JM, Kajula LJ, Mbwambo JK, et al. From coitus to concurrency: sexual partnership characteristics and risk behaviors of 15–19 year old men recruited from urban venues in Tanzania. AIDS Behav. 2012;17(7):2405–2415. doi: 10.1007/s10461-012-0312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamanis TJ, Maman S, Mbwambo JK, Earp JA, Kajula LJ. Social venues that protect against and promote HIV risk for young men in Dar es Salaam, Tanzania. Soc Sci Med. 2010;71(9):1601–1609. doi: 10.1016/j.socscimed.2010.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamanis TJ, Fisher JC, Moody JW, Kajula LJ. Young men’s social network characteristics and associations with sexual partnership concurrency in Tanzania. AIDS Behav. 2015 doi: 10.1007/s10461-015-1152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maman S, Kajula L, Balvanz P, Kilonzo M, Mulawa M, Yamanis T. Leveraging strong social ties among young men in Dar es Salaam: a pilot intervention of microfinance and peer leadership for HIV and gender-based violence prevention. Glob Public Health. 2015;20:1–14. doi: 10.1080/17441692.2015.1094105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kajula L, Balvanz P, Kilonzo MN, Mwikoko G, Yamanis T, Mulawa M, et al. Vijana Vijiweni II: a cluster-randomized trial to evaluate the efficacy of a microfinance and peer health leadership intervention for HIV and intimate partner violence prevention among social networks of young men in Dar es Salaam. BMC Public Health. 2016;16(1):1–12. doi: 10.1186/s12889-016-2774-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grier JB. Nonparametric indexes for sensitivity and bias: computing formulas. Psychol Bull. 1971;75(6):424–429. doi: 10.1037/h0031246. [DOI] [PubMed] [Google Scholar]
- 26.Donaldson W. Measuring recognition memory. J Exp Psychol Gen. 1992;121(3):275–277. doi: 10.1037//0096-3445.121.3.275. [DOI] [PubMed] [Google Scholar]
- 27.Henry DB, Kobus K, Schoeny ME. Accuracy and bias in adolescents’ perceptions of friends’ substance use. Psychol Addict Behav. 2011;25(1):80–89. doi: 10.1037/a0021874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography. 2001;38(1):115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 29.SAS Institute Inc. SAS® 9.4 Cary. 2011 [Google Scholar]
- 30.Perkins HW. Misperceptions of peer drinking norms in Canada: another look at the “reign of error” and its consequences among college students. Addict Behav. 2007;32(11):2645–2656. doi: 10.1016/j.addbeh.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 31.McAlaney J, McMahon J. Normative beliefs, misperceptions, and heavy episodic drinking in a british student sample. J Stud Alcohol Drugs. 2007;68(3):385–392. doi: 10.15288/jsad.2007.68.385. [DOI] [PubMed] [Google Scholar]
- 32.Perkins HW, Haines MP, Rice R. Misperceiving the college drinking norm and related problems: a nationwide study of exposure to prevention information, perceived norms and student alcohol misuse. J Stud Alcohol. 2005;66(4):470–478. doi: 10.15288/jsa.2005.66.470. [DOI] [PubMed] [Google Scholar]
- 33.Kandel DB. The parental and peer contexts of adolescent deviance: an algebra of interpersonal influences. J Drug Issues. 1996;26(2):289. [Google Scholar]
- 34.Prinstein MJ, Wang SS. False consensus and adolescent peer contagion: examining discrepancies between perceptions and actual reported levels of friends’ deviant and health risk behaviors. J Abnorm Child Psychol. 2005;33(3):293–306. doi: 10.1007/s10802-005-3566-4. [DOI] [PubMed] [Google Scholar]
- 35.Hensen B, Taoka S, Lewis JJ, Weiss HA, Hargreaves J. Systematic review of strategies to increase men’s HIV-testing in sub-Saharan Africa. AIDS. 2014;28(14):2133–2145. doi: 10.1097/QAD.0000000000000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perkins HW, Berkowitz AD. Perceiving the community norms of alcohol use among students: some research implications for campus alcohol education programming. Int J Addict. 1986;9–10:961–976. doi: 10.3109/10826088609077249. [DOI] [PubMed] [Google Scholar]
- 37.Miller MB, Leffingwell T, Claborn K, Meier E, Walters S, Neighbors C. Personalized feedback interventions for college alcohol misuse: an update of Walters & Neighbors (2005) Psy-chol Addict Behav. 2013;27(4):909–920. doi: 10.1037/a0031174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tobin KE, Yang C, Sun C, Spikes P, Latkin CA. Discrepancies between HIV prevention communication attitudes and actual conversations about HIV testing within social and sexual networks of African American men who have sex with men. Sex Transm Dis. 2014;41(4):221–226. doi: 10.1097/OLQ.0000000000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Babalola S. Readiness for HIV testing among young people in northern Nigeria: the roles of social norm and perceived stigma. AIDS Behav. 2007;11(5):759–769. doi: 10.1007/s10461-006-9189-0. [DOI] [PubMed] [Google Scholar]
- 40.Mburu G, Ram M, Siu G, Bitira D, Skovdal M, Holland P. Intersectionality of HIV stigma and masculinity in eastern Uganda: implications for involving men in HIV programmes. BMC Public Health. 2014;14:1061. doi: 10.1186/1471-2458-14-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]