Abstract
Purpose
The purpose of this research was to develop a 256-channel dense-array electroencephalography (dEEG) sensor net (the Ink-Net) using high-resistance polymer thick film (PTF) technology to improve safety and data quality during simultaneous dEEG/MRI.
Methods
Heating safety was assessed with temperature measurements in an anthropomorphic head phantom during a 30-minute, induced-heating scan at 7 T. MRI quality assessment used B1 field mapping and fMRI retinotopic scans in three humans at 3 T. Performance of the 256-channel PTF Ink-Net was compared to a 256-channel MR-conditional copper-wired EEG net and to scans with no sensor net. A visual evoked potential paradigm assessed EEG quality within and outside the 3T scanner.
Results
Phantom temperature measurements revealed non-significant heating (ISO 10974) in the presence of either EEG net. In human B1 field and fMRI scans, the Ink-Net showed greatly reduced cross-modal artifact and less signal degradation than the copper-wired net, and comparable quality to MRI without sensor net. Cross-modal ballistocardiogram artifact in the EEG was comparable for both nets.
Conclusion
High-resistance PTF technology can be effectively implemented in a 256-channel dEEG sensor net for MR-conditional use at 7 T, and with significantly improved structural and fMRI data quality as assessed at 3 T.
Keywords: fMRI/BOLD, eccentricity maps, anthropomorphic head phantom, ISO 10974, temperature measurements, B1 maps
Introduction
Researchers and clinicians increasingly employ multi-modal brain imaging to converge unique information afforded by each imaging technology. Foremost among these is the fusion of electroencephalography (EEG), a direct measure of electrical cortical activity with millisecond temporal resolution, and functional magnetic resonance imaging (fMRI), a measure of the hemodynamic response to cortical and subcortical activity with excellent spatial resolution (1,2). Simultaneous EEG/fMRI is leading to basic science advances in understanding neurovascular coupling and insights into acute and chronic brain disorders (3), but safety risks and cross-modal artifacts have so far limited widespread adoption (4).
Safety risks associated with torque and forces from static and spatial gradient fields on EEG instrumentation (5) are easily mitigated by using non-ferromagnetic materials (6), but without appropriate precautions being taken, large currents may be induced on the EEG leads (7) by applied radio frequency (RF) fields and there are potential increases of the RF power absorbed in the head leading to risks of tissue heating (8–13) and burns (14). Deposition of RF power in the head is affected by EEG lead orientation (15), RF frequency (15,16), number of EEG electrodes/leads (16), and EEG lead resistivity (17–19). EEG electrodes, leads, and conductive pastes can also create eddy currents, shielding, and other perturbations of the static and RF varying fields that degrade the quality of structural and functional MR images (20–25). EEG recording likewise suffers from cross-modal artifact (2,24,26–28), including 1) imaging artifact arising from gradient switching and RF pulses, 2) cryoballistic artifact arising from cryogenic cooling system vibrations, and 3) ballistocardiogram (BCG), or pulse, artifact arising from cardiac-related body and electrode movement. The imaging artifact can be effectively removed using phase-locked loop synchronization between the MRI and EEG systems to enable distortion-attenuated construction of a moving-average artifact template that can be used in subtraction (29), and the cryoballistic artifact is typically handled by temporarily turning off the cryogenic pump. The BCG artifact, however, continues to challenge (30). Both heating and MRI cross-modal artifacts scale with magnetic field strength and the number of EEG electrodes and leads, making simultaneous recordings even more challenging with high-field scanners and dense-array EEG (dEEG) systems, which are otherwise desirable for their increased accuracy in electrical source imaging (31,32).
The presence of large RF (and low frequency) currents induced in conventional EEG electrodes and wire leads can be reduced by the use of high-resistance materials, such as polymer thick film (PTF), allowing for safe and high-quality data up to 7 T (22,24,33). This was previously demonstrated in a 32-channel sparse-array InkCap prototype (24). The present research extends this early work by designing and manufacturing a 256-channel dEEG Ink-Net that demonstrates improved MR image quality over a commercial MR-conditional 256-channel dEEG system with wire leads. This ability to acquire 256-channel dEEG, for enhanced spatial sampling, head coverage, and source localization (34), along with only minimal MR image degradation, will allow for more accurate investigation of the relationship between brain activity and networks revealed by the fusion of these two modalities.
Methods
dEEG Sensor Nets
Cu-Net
The commercial 256-channel MR-conditional Geodesic Sensor Net, HC GSN 220 MR (Electrical Geodesics, Inc., Eugene, OR), was used for comparison with the Ink-Net in all tests. The GSN uses a geodesic elastomer tension structure to position 258 low-profile pedestals (256 standard electrodes, along with reference and common electrodes). An AgCl-coated pellet electrode is inserted into each pedestal and the cavity is filled with a sponge soaked in electrolyte solution (KCl). Leads are copper (Cu) wires (mean lead resistance = 1 Ω), with 10 kΩ resistors fitted at the interface to each electrode.
Ink-Net
The prototype 256-channel dEEG Ink-Net replaced the AgCl-coated pellet electrodes and highly conductive copper-wire leads of the Cu-Net with high-resistance PTF technology (mean lead resistance = 19 kΩ, SD = 4.75 kΩ). Conductive ink (Engineered Conductive Materials, LLC, Delaware, OH) electrode pads and leads were screen-printed (GM Nameplate, Inc., Seattle, WA) onto Melinex (DuPont Teijin Films U.S. Limited Partnership, Chester, VA) substrate (see Figure 1 for details). The Ink-Net pieces fit into the GSN elastomer structure, ensuring that the geometry of the two nets was otherwise identical. Ink leads were interfaced to traditional copper-wire leads 8–15 cm outside the head coil.
Figure 1.
A: Construction of the Ink-Net, a high-resistance PTF-based 256-channel dEEG sensor net: (1) Sample piece (from total of 32 pieces with 6–11 electrodes per piece) schematic showing (1) Melinex substrate (thickness = 130 µm), and printed, (2) custom-blend silver-carbon (Ag/C) ink leads (layer 1; width = 0.38 mm, thickness = 0.01 mm, length = 59–99 cm), (3) silver-chloride (AgCl) ink pad electrodes (layer 2; diameter = 5 mm, thickness = 0.01 mm), and (4) dielectric coating in green (layer 3) masked to leave electrodes exposed. Close-up of printed pieces inserted into EGI’s 256 Geodesic Sensor Net (HC GSN) pedestal & elastomer structure, showing exposed pad electrode at top, and with inserted sponge for electrolyte solution. B: Diagram of phantom temperature measurement set up. A thin rod (3-mm diameter) was used to create channels into the phantom to target measurement locations. Temperature probes were then inserted into the channels to lie 5-mm below the phantom surface under the selected electrode locations (and at head center). To make reliable contact between the temperature probes and surrounding phantom tissue, thereby avoiding measurement underestimation, channel cavities around the probes were filled by syringe using thermally conductive grease (Super Thermal Grease II, MG Chemicals, Surrey, B.C., Canada). C: Temperature increase (°C) during a high-SAR turbo-spin echo sequence at 7 T to induce heating in an anthropomorphic head phantom wearing: No-Net (left), Cu-Net (middle), and Ink-Net (right). D: Phantom with No-Net (left), Cu-Net (middle), and Ink-Net (right) with seven temperature probes (orange-encased optical fibers) inserted from base of neck to indicated positions, along with homologous positions AF8 and T8 on right side, and head center. Thermally conductive grease, used to ensure good probe contact with phantom tissue, can be seen at each location.
Heating Safety Assessment: Phantom Testing at 7 T
Temperature measurements were conducted using a homogeneous agar-based conductive head phantom (18), with conductivity (0.5 S/m) as per ASTM 2182 (35) extended to 300 MHz (36) and thermal conductivity (0.5 W m−1 K−1) similar to cerebral cortex (37), and seven optical temperature probes (OSENSA Innovations Corp., Coquitlam, BC, Canada). The KCl solution used as electrolyte in the sponge electrodes had conductivity (1.9 S/m at 127 MHz) similar to human tissue, as measured with an Agilent 85070E Dielectric Probe Kit (Agilent Technologies, Santa Clara, CA) connected to an E5061B Agilent network analyzer. Measurements were conducted across three test sessions: 1) phantom only (No-Net), 2) phantom with Ink-Net, and 3) phantom with Cu-Net. Following previous studies (12,18,24), five probes were inserted (Figure 1), one at the center of the phantom and four below 10-10 International System locations Cz, T7, T8, and the nasion. The remaining two probes were located under electrodes closest to the rods of the 0° (AF8) and 90° (AF7) ports of the transmit birdcage coil, previously shown to exhibit peak heating (12). To minimize temperature and Specific Absorption Rate (SAR) underestimation, probes were placed at a depth of approximately 5 mm, perpendicular to the electrode sponge surface (38). A high SAR sequence provided a strict test of RF-heating risk. First, the phantom was registered as a 91-kg male to permit increased power output. Following a localizer scan, the phantom was left at rest for 10 minutes, after which a high-power Turbo Spin Echo (TSE) sequence (12 slices, 1 mm3 voxels, TR/TE = 2150/226 ms, FA = 140°) delivered 100% SAR for 30 minutes (SAR 10g average = 9.991 +/− 0.009 W/kg). Temperature change relative to the temperature recorded just prior to the start of the TSE scan was measured continuously (1 S/s sampling rate) at each probe across the 30-minute TSE scan. During scanning, the ventilation fan was turned off to avoid an external cooling confound that would underestimate any temperature increases. The heating sequence was rerun 10 minutes after the first heating, and Pearson correlations were computed to assess replicability and measurement reliability. All scans used a custom birdcage transmit head coil, with 32-channel receive coil, built for the 7T Siemens scanner (Erlangen, Germany). Temperature thresholds and thermally safe scan time exposures were based on ISO 10974 (39).
Data Quality Assessment: Human Testing at 3T
To compare performance of the Ink-Net with the Cu-Net, which is currently approved for use at 3 T only, human testing was conducted at 3 T rather than 7 T. The study was approved by the Institutional Review Boards at University of Oregon and Electrical Geodesics, Inc. Three healthy, male volunteers (mean age = 50 years, range = 40–60 years) participated after written informed consent. Scans were repeated in three separate one-hour sessions (order counterbalanced) while wearing: 1) No-Net, 2) Ink-Net, and 3) Cu-Net. Participants lay on the bed of a Siemens Allegra head-only 3T scanner (Erlangen, Germany) with head positioned at the center of a circularly polarized head coil (USA Instruments, Inc., Aurora, OH) fit with a mirror for viewing back-projected stimuli. For Ink-Net and Cu-Net sessions, nets were plugged into the GES 300 MR amplifier (Electrical Geodesics Inc., Eugene, OR) placed just outside the bore and connected to an EEG recording system in the MR control room.
Structural MRI assessment: B1 field mapping
The Double Angle Method (40) assessed the influence of the EEG nets on B1 field uniformity. Two gradient echo sequences (32 slices, 3 × 3 × 3.6 mm3 voxels, TR/TE = 8000/15 ms) were conducted at two flip angles: 60° and 120° (S1 and S2, respectively). The magnitude from each of these volumes was used to calculate the flip angle (FA) in the S1 volume (40) as shown:
Gradient echo data from each of the EEG conditions were registered to the No-Net data for each participant. A combined mask was then created for each participant taking into account any region without signal (due to anatomical reasons, such as low signal in the skull, and to slice prescription differences between conditions) in the original magnitude S2 volumes for all three conditions. This mask was applied to the FA estimates data to enable both qualitative and quantitative comparisons of the B1 field.
Functional MRI assessment: Retinotopic mapping
An echo-planar imaging (EPI) sequence (32 slices, 3 mm3 voxels, TR/TE = 2000/30 ms, 25% distance spacing), with Prospective Acquisition Correction (PACE) (41) applied to compensate for head movement, was conducted while participants observed expanding and contracting multicolored checkerboard rings (42) presented in two separate 5-minute blocks. Data were analyzed using FreeSurfer and FS-Fast 5.1.0 (43). First, each participant's T1-weighted gradient echo image (MP-RAGE) was used to construct cortical surfaces (grey-white matter boundary and pial surface for each hemisphere). After applying an additional motion correction to the EPI-PACE prospective series data to remove residual motion misalignment, EPI-PACE data were registered to the MP-RAGE. Next, EPI-PACE data were resampled onto the grey-white matter boundary and smoothed with a 5-mm FWHM 2D smoothing kernel. Finally, phase-encoded retinotopic eccentricity maps were generated by a GLM-based F-test using the real and imaginary components of the eccentricity's phase. The region of interest (ROI) used for data extraction was created with a combined anatomical and functional mask. The anatomical mask consisted of 15 regions (selected to encompass the entire occipital lobe) from the Desikan and Killiany atlas (44). Log P-values for each condition were examined within this anatomical ROI. Then, any vertex from the anatomical ROI with an absolute uncorrected log P-value greater than 2 (i.e., uncorrected p < 0.01) in any of the three conditions was included in the functional mask used for comparison across conditions.
EEG Assessment
To assess EEG quality and severity of the challenging BCG artifact independently from gradient and cryogen artifacts, EEG was acquired with the cryogenic pump off and no active MR scanning engaged while subjects viewed simultaneous checkerboard wedges presented to the upper and lower visual field. To compare BCG artifact magnitude to a previous study using a 32-electrode MR-conditional cap (30), 32 matching electrode locations were retained from the dense sensor array for analysis and then an average BCG was computed for each subject across 380 cardiac cycles (30), time-locked to the R-slope (45) of the QRS complex from a simultaneously recorded ECG signal. Lastly, the root mean square (RMS) amplitude was computed over all 32 channels for each time point of the cardiac cycle (Figure 3B), and the peak RMS value across the cycle served as an index of the BCG artifact magnitude. A visual evoked potential (VEP) time-locked to stimulus onset (N = 1150) was computed after BCG artifact cleaning using the optimal basis sets (OBS) algorithm (46) and qualitatively compared to the VEP from recordings outside the magnet.
Figure 3.
A: Ink-Net and Cu-Net recording extracts illustrating ballistocardiogram (BCG) artifact in EEG from representative electrodes (e187-193 located over right occipito-temporal regions) (top) and QRS cardiac complex in the time-synchronized ECG recordings (bottom). The red rectangle outlines one cardiac cycle, showing the QRS complex in the ECG and corresponding BCG artifact in the EEG recording. B: Root mean square (RMS) BCG artifact amplitude computed across a sub-sampled 32-electrode montage (30). Dashed lines indicate the standard deviation across the three participants. C: Visual Evoked Potential (VEP) waveform at electrode 45 (location of yellow dot in topographic map inset) computed from Ink-Net (left) and Cu-Net (right) recordings outside (top) and inside (bottom) 3 T scanner field after BCG artifact cleaning. LORETA distributed linear inverse source localization computed at time of the negative peak (visual N1) using GeoSource software (Electrical Geodesics, Inc., Eugene, OR) shows maximal activity in medial occipital cortex (indicated by crosshair) in all cases. Additional lower-amplitude source activity in 3T solutions is likely due to misallocated residual BCG artifact. Data were filtered at 0.1 – 30 Hz, average referenced, and baselined to a 100-ms pre-stimulus window.
Results
Phantom temperature measurements during the 30-minute maximum SAR scan to induce heating revealed (Figure 1B) that none of the three conditions produced temperature increases greater than 2 °C (Table 1), which is well within ISO 10974 (39). In all cases, the highest temperature increase was registered at one or both of the probes closest to 0° and 90° port rods (under electrodes AF7 and/or AF8). The maximum temperature increase after 30 minutes for the No-Net condition (AF7 = 1.8 °C) was slightly higher than the Ink-Net (AF8 = 1.5 °C) and Cu-Net (AF8 = 1.6 °C). Temperature increases at retest substantially replicated the results in the first recording, but with an expected smaller increase due to a warmer starting condition. Temperature increases after a more typical 15-minute interval did not exceed 1 °C for either net.
Table 1.
Temperature increase (°C) during a high-SAR turbo-spin echo (TSE) sequence at 7 T to induce heating in an anthropomorphic head phantom wearing no EEG net, Ink-Net, and Cu-Net. Heating test and retest results at seven different locations in a head phantom at 15 minutes and 30 minutes after start of TSE scan at 100% SAR. Bold font indicates location of largest increase per net condition.
| Location | First TSE Scan (°C) | Re-Test TSE Scan (°C) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 minutes | 30 minutes | 15 minutes1 | 30 minutes2 | |||||||||
| No-Net | Ink-Net | Cu-Net | No-Net | Ink-Net | Cu-Net | No-Net | Ink-Net | Cu-Net | No-Net | Ink-Net | Cu-Net | |
| Center | 0.87 | 0.76 | 0.59 | 1.58 | 1.36 | 1.05 | 0.76 | 0.71 | 0.56 | 1.41 | 1.28 | 1.01 |
| Nasion | 0.89 | 0.62 | 0.42 | 1.59 | 1.09 | 0.83 | 0.77 | 0.53 | 0.37 | 1.41 | 1.00 | 0.77 |
| Cz | 0.87 | 0.54 | 0.30 | 1.69 | 1.09 | 0.71 | 0.70 | 0.46 | 0.29 | 1.45 | 1.00 | 0.64 |
| T7 | 0.85 | 0.60 | 0.58 | 1.51 | 1.08 | 1.00 | 0.77 | 0.56 | 0.5 | 1.43 | 0.98 | 0.94 |
| T8 | 0.74 | 0.63 | 0.63 | 1.29 | 1.07 | 1.10 | 0.63 | 0.56 | 0.58 | 1.17 | 1.01 | 1.04 |
| AF7 | 1.02 | 0.74 | 0.74 | 1.82 | 1.26 | 1.27 | 0.86 | 0.62 | 0.68 | 1.55 | 1.16 | 1.19 |
| AF8 | 0.73 | 0.87 | 0.99 | 1.33 | 1.47 | 1.60 | 0.61 | 0.74 | 0.98 | 1.19 | 1.27 | 1.55 |
Test-retest Pearson correlation coefficients: rNo-Net = 0.950; rInk-Net = 0.960; rCu-Net = 0.999.
Test-retest Pearson correlation coefficients: rNo-Net = 0.960; rInk-Net = 0.972; rCu-Net = 0.999.
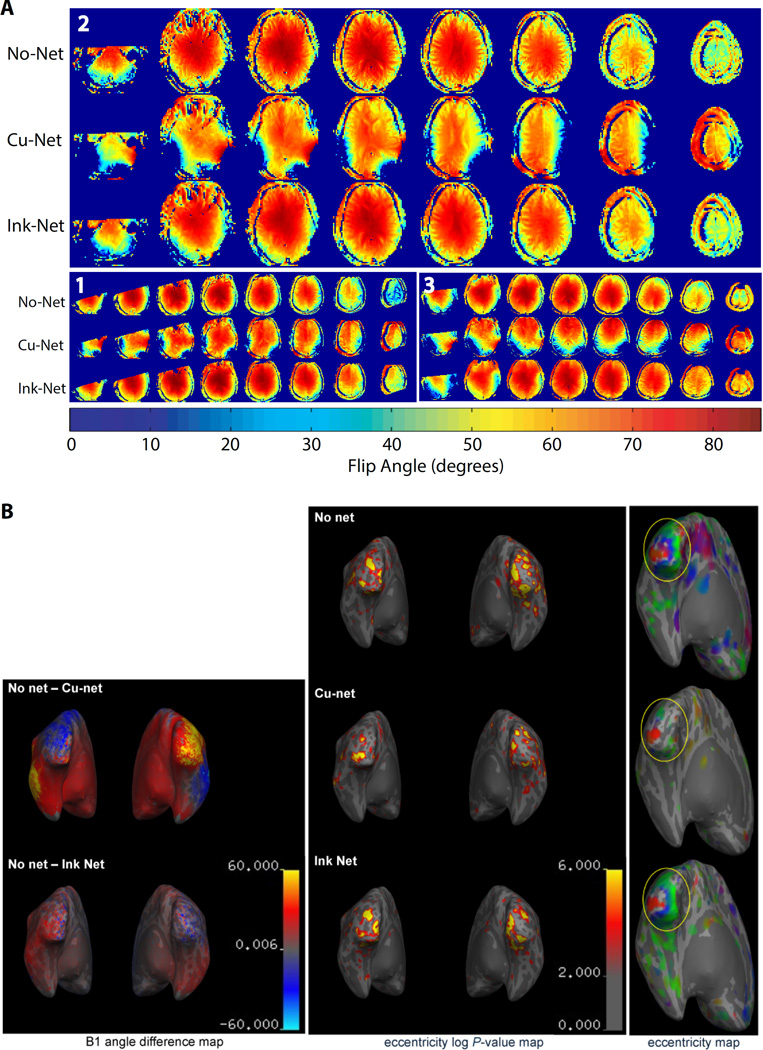
B1 flip angle standard deviations, averaged across the three participants, were 14.9° (No-Net), 13.8° (Ink-Net), and 21.6° (Cu-Net), indicating a decrease in B1 field homogeneity in the presence of the Cu-Net only (Table 2). The B1 field maps reveal where the Cu-Net consistently created large B1 artifacts. As seen in Figure 2A, the B1 field maps are largely consistent between the Ink-Net and No-Net conditions, but with the Cu-Net two large artifacts are evident: left inferior temporal lobe and right lateral occipital region. These Cu-Net artifacts are also evident when projected on inflated 3-D FA difference maps (Figure 2B, left).
Table 2.
Structural and functional magnetic resonance imaging assessment indices per condition for each participant.
| B1 Field Mapping | Retinotopic Mapping | ||
|---|---|---|---|
| Flip Angle SD | Log P-Values | No. Vertices, Log P > 2 | |
| Participant 1 | |||
| No net | 16.45° | 7.00 | 16,904 |
| Cu-Net | 20.27° | 3.35 | 12,134 |
| Ink-Net | 12.96° | 3.96 | 14,935 |
| Participant 2 | |||
| No net | 16.79° | 3.04 | 8,583 |
| Cu-Net | 22.22° | 1.91 | 5,233 |
| Ink-Net | 16.66° | 2.69 | 7,214 |
| Participant 3 | |||
| No net | 11.44° | 7.44 | 19,539 |
| Cu-Net | 22.34° | 3.21 | 12,465 |
| Ink-Net | 11.82° | 5.08 | 15,317 |
Figure 2.
A: Flip angle maps from representative participant 2 (and, as smaller insets, from participants 1 and 3) under each of three conditions show large signal loss artifacts from the presence of copper wires in the Cu-Net condition (middle rows). In contrast, the Ink-Net maps (lower rows) are comparable to No-Net (upper rows). Maps are in neurological convention. Target flip angle is 60 degrees. B: Structural and functional MRI results from representative participant 2: B1 angle difference maps (left). Retinotopic log-P values (center) and BOLD eccentricity maps (right). Both B1 and fMRI signals show larger difference relative to No-Net baseline when subject wears the Cu-Net as compared to the Ink-Net.
Mean log P-values of the fMRI eccentricity mapping (Table 2) revealed that the Ink-Net outperformed the Cu-Net, with log P-values 33% and 52% smaller, respectively, than No-Net. Furthermore, the number of vertices with log P > 2 was smallest for the Cu-Net, indicating the Cu-Net had a smaller significance volume. Figure 2B (center) shows that a larger portion of visual cortex displays significant activation in the Ink-Net condition than the Cu-Net. The Ink-Net map also has a smoother distribution, resulting in fewer patchy peaks.
BCG artifact (Figure 3A) peak RMS magnitude, averaged across the three participants, was moderately larger for the Ink-Net (mean = 112 µV, SD = 5 µV) than the Cu-Net (mean = 91 µV, SD = 24 µV). The VEP recorded outside the magnet was similar for both nets, with the characteristic visual P1, N1 and P2 clearly visible in single-subject average waveforms, and similar topographies and occipital source localization (Figure 3B, top). The VEP recorded within the 3T field was somewhat distorted by residual BCG artifact for both the Ink-Net and Cu-Net, but the main topographic features in the VEP and source localization were retained in both (Figure 3B, bottom).
Discussion and Conclusions
The prototype high-resistance 256-channel PTF-based dEEG sensor net (Ink-Net) and the commercial 256-channel copper-wired MR-conditional dEEG net (Cu-Net) both demonstrated excellent safety for MR-conditional use at 7 T. The Ink-Net, however, displayed greatly reduced cross-modal artifact in the MR images as compared to the copper-wired net. Cross-modal BCG artifact in the EEG recordings was comparable for both nets.
The 7T MRI high-power TSE scans to induce worst-case heating of a phantom wearing either the Cu-Net or Ink-Net showed superficial heating in the same range as the phantom with no net. However, this non-significant heating is lower than the three degrees or more of heating previously recorded both at 3 T and 7 T (12,18). These higher temperatures, however, were recorded inside the EEG paste or gel and were much closer to the metal electrodes for a worst-case measurement (38) and arguably too conservative for the case of an electrode embedded at the top of a 1-cm high pedestal. Furthermore, the sponges in the pedestal are generally filled with both electrolyte and air pockets, and we found that the temperature measurements in wet sponges were for the most part unreliable due to the presence of air pockets. When considering only temperatures measured in the actual phantom, adjacent to the surface to simulate scalp heating, our values are in agreement with previous recordings (18). In follow up, we also repeated the temperature measurement protocol using a lower-powered typical fMRI sequence (2D EPI sequence: 64 slices, 1.5 mm3 isotropic resolution, TR/TE = 3,160/21 ms, 12% distance spacing) for the Ink-Net condition (see supplementary information). As expected, heating was even less, showing less than 1 °C increase after 30 minutes for all locations in the phantom. Phantom estimation of heating in the human head is somewhat limited by the absence of perfusion and differences in electric field symmetry between the homogeneous phantom head and the heterogeneous human head (47) which has numerous tissue compartments with varying electrical properties (48). Furthermore, electrode position relative to MRI coils may vary somewhat across individuals and scanners (49). Despite these limitations, numerical simulations performed with electrically homogeneous and heterogeneous models have been shown to provide similar electric field estimations (50), suggesting the validity of experimental validations with a geometrically matched homogeneous phantom, commonly used for safety evaluation of MRI-conditional (18) devices.
Across all 3T human tests, the Ink-Net showed considerably reduced cross-modal structural and functional MRI artifact and less signal degradation than the Cu-Net. Structural MRI quality is highly sensitive to B1 field distortion. B1 field mapping (40) revealed severe FA map attenuations with the copper-wired net, replicating previous findings (51). In striking contrast, the FA map from scans with the Ink-Net was comparable to scans with no net at all. Follow-up analyses identified maximum FA peaks in the frontal areas for the copper-wired net, while the Ink-Net and No-Net both had the typical FA maximum peaks in the center of the head. Deformations of the B1 field due to wire leads have been observed at fields of 1.5 T through 7 T, and with electrode arrays as low as 32 channels (22), so the enhanced MR image quality due to high-resistance PTF leads is immediately relevant to structural clinical scans, and will permit future clinical assessment at higher field strengths and EEG channel counts without compromising important clinical MRI signal integrity.
Functional MRI retinotopic mapping is a highly sensitive paradigm selected here to rigorously test the robustness of the BOLD signal in the presence of EEG leads. Phase encoded retinotopic eccentricity maps while wearing the Ink-Net resulted in somewhat less robust maps than scans with no net, perhaps due to the greater distance of the occipital lobe from the head coil caused by the 1-cm height of the electrode pedestals in the EEG nets. Signal-to-noise ratio (SNR) of MRI data is greatly affected by distance of the coil from the scanned volume (in this case, occipital lobe) (52). Using the same 12-channel head coil as here, Wiggins and colleagues showed that SNR drops from ~53 % to ~42 % as one moves from the SNR peak in the occipital lobe to 1 cm anterior. Use of a blank net (i.e., pedestal structure without electrodes or leads) in future studies would help control for this potential confound. The Ink-Net did, however, perform considerably better than the Cu-Net, indicating that the Ink-Net caused less BOLD signal degradation than did traditional copper-wired EEG technology. These results differed from Luo et al. (51) who found comparable BOLD sensitivity with and without the presence of the 256-channel copper-wired HC GSN MR. The discrepancy is likely due to the following important differences. First, our voxel size was significantly smaller: ~27 mm3 versus ~46 mm3. Indeed, Luo et al. (51) predicted that voxel sizes smaller than 30 mm3 would show reduced BOLD due to shielding from the copper leads. Furthermore, the spiral in/out k-space sampling sequence used by Luo et al. (51) was designed to improve SNR and reduce dropout artifacts (53), while the sequence employed here was the more commonly used linear k-space sampling.
EEG quality recorded outside the scanner was similar for the Ink-Net and commercial Cu-Net. EEG quality and magnitude of cross-modal BCG artifact inside the 3T scanner (static field without active scanning) was also comparable for both nets. Indeed, lead resistance and other design and lead routing differences among the EEG nets used here and the EEG cap used by Mullinger et al. (30), appeared to have little impact on BCG artifact magnitude and quality of EEG signal. Future work should focus on improving BCG artifact separation and post-acquisition signal processing (54–56).
In summary, the present study showed that high-resistance, conductive inks printed on PTF substrate can be effectively applied to the construction of electrodes and leads for a dEEG system with up to 256 channels. The prototype 256-channel Ink-Net demonstrated safe use during scanning at 7T field strength, with comparable EEG quality and little to negligible MRI artifact or signal loss as tested in humans at 3 T, in contrast to considerable MRI degradation when using a 256-channel MR-conditional EEG net with conventional copper leads.
Supplementary Material
Acknowledgments
This research was supported by the grants R43NS071988 (NIH/NINDS), U01-NS075026 (NIH/NINDS), R21EB016449 (NIH/NIBIB), S10OD010759 (NIH/OD), P41RR14075 (NCRR), S10RR023043 (NCRR), S10RR023401 (NCRR), and by the MIND Institute. The authors acknowledge Leonardo Angelone, from the Food and Drug Administration, and Peter Serano for scientific discussion.
References
- 1.Herrmann CS, Debener S. Simultaneous recording of EEG and BOLD responses: a historical perspective. Int J Psychophysiol. 2008;67(3):161–168. doi: 10.1016/j.ijpsycho.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Mullinger K, Bowtell R. Combining EEG and fMRI. Methods Mol Biol. 2011;711:303–326. doi: 10.1007/978-1-61737-992-5_15. [DOI] [PubMed] [Google Scholar]
- 3.Laufs H. Functional imaging of seizures and epilepsy: evolution from zones to networks. Curr Opin Neurol. 2012;25(2):194–200. doi: 10.1097/WCO.0b013e3283515db9. [DOI] [PubMed] [Google Scholar]
- 4.Bonmassar G, Mullinger KJ. Specific Issues Related to EEG-fMRI at B0 > 3T. In: Mulert C, Lemieux L, editors. EEG-fMRI: Physiological Basis, Technique, and Applications. Berlin Heidelberg: Springer-Verlag; 2010. pp. 201–220. [Google Scholar]
- 5.Schenck J. Safety of strong, static magnetic fields. J Magn Reson Imaging. 2000;12(1):2–19. doi: 10.1002/1522-2586(200007)12:1<2::aid-jmri2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Purdon PL, Millan H, Fuller PL, Bonmassar G. An open-source hardware and software system for acquisition and real-time processing of electrophysiology during high field MRI. J Neurosci Methods. 2008;175(2):165–186. doi: 10.1016/j.jneumeth.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou CK, Bassen H, Osepchuk J, Balzano Q, Petersen R, Meltz M, Cleveland R, Lin JC, Heynick L. Radio frequency electromagnetic exposure: tutorial review on experimental dosimetry. Bioelectromagnetics. 1996;17(3):195–208. doi: 10.1002/(SICI)1521-186X(1996)17:3<195::AID-BEM5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim TS, Kangarlu A, Chakeress DW. Design and performance issues of RF coils utilized in ultra high field MRI: experimental and numerical evaluations. IEEE Trans Biomed Eng. 2005;52(7):1278–1284. doi: 10.1109/TBME.2005.847564. [DOI] [PubMed] [Google Scholar]
- 9.Collins CM, Liu W, Wang J, Gruetter R, Vaughan JT, Ugurbil K, Smith MB. Temperature and SAR calculations for a human head within volume and surface coils at 64 and 300 MHz. J Magn Reson Imaging. 2004;19(5):650–656. doi: 10.1002/jmri.20041. [DOI] [PubMed] [Google Scholar]
- 10.Finelli DA, Rezai AR, Ruggieri PM, Tkach JA, Nyenhuis JA, Hrdlicka G, Sharan A, Gonzalez-Martinez J, Stypulkowski PH, Shellock FG. MR imaging-related heating of deep brain stimulation electrodes: in vitro study. AJNR Am J Neuroradiol. 2002;23(10):1795–1802. [PMC free article] [PubMed] [Google Scholar]
- 11.Gajsek P, Walters TJ, Hurt WD, Ziriax JM, Nelson DA, Mason PA. Empirical validation of SAR values predicted by FDTD modeling. Bioelectromagnetics. 2002;23(1):37–48. doi: 10.1002/bem.96. [DOI] [PubMed] [Google Scholar]
- 12.Noth U, Laufs H, Stoermer R, Deichmann R. Simultaneous electroencephalography-functional MRI at 3 T: an analysis of safety risks imposed by performing anatomical reference scans with the EEG equipment in place. J Magn Reson Imaging. 2012;35(3):561–571. doi: 10.1002/jmri.22843. [DOI] [PubMed] [Google Scholar]
- 13.Shellock FG. Reference manual for magnetic resonance safety. Salt Lake City, Utah: Amirsys; 2003. p. 456. [Google Scholar]
- 14.Rezai AR, Phillips M, Baker KB, Sharan AD, Nyenhuis J, Tkach J, Henderson J, Shellock FG. Neurostimulation system used for deep brain stimulation (DBS): MR safety issues and implications of failing to follow safety recommendations. Invest Radiol. 2004;39(5):300–303. doi: 10.1097/01.rli.0000124940.02340.ab. [DOI] [PubMed] [Google Scholar]
- 15.Hamblin DL, Anderson V, McIntosh RL, McKenzie RJ, Wood AW, Iskra S, Croft RJ. EEG electrode caps can reduce SAR induced in the head by GSM900 mobile phones. IEEE Trans Biomed Eng. 2007;54(5):914–920. doi: 10.1109/TBME.2007.893486. [DOI] [PubMed] [Google Scholar]
- 16.Angelone L, Potthast A, Segonne F, Iwaki S, Belliveau J, Bonmassar G. Metallic Electrodes and leads in simultaneous EEG-MRI: Specific absorption rate (SAR) simulation studies. Bioelectromagnetics. 2004;25(4):285–295. doi: 10.1002/bem.10198. [DOI] [PubMed] [Google Scholar]
- 17.Guy A, Lin J, Chou CK. Electrophysiological Effects of Electromagnetic Fields on Animals. In: Michaelson S, Miller M, Magin R, Carstensen E, editors. Fundamental and Applied Aspects of Nonionizing Radiation. New York: Springer US; 1975. pp. 167–211. [Google Scholar]
- 18.Angelone LM, Vasios CE, Wiggins G, Purdon PL, Bonmassar G. On the effect of resistive EEG electrodes and leads during 7 T MRI: simulation and temperature measurement studies. Magn Reson Imaging. 2006;24(6):801–812. doi: 10.1016/j.mri.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Wakeman D, Keil B, McSwain B, Iacono M, Poulsen C, Bonmassar G. InkNet: Safe 256-Channel EEG at 7 T. Proceedings of the 21st Annual Meeting of ISMRM; Salt Lake City UT. #2841. [Google Scholar]
- 20.Bonmassar G, Hadjikhani N, Ives JR, Hinton D, Belliveau JW. Influence of EEG electrodes on the BOLD fMRI signal. Hum Brain Mapp. 2001;14(2):108–115. doi: 10.1002/hbm.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krakow K, Allen PJ, Lemieux L, Symms MR, Fish DR. Methodology: EEG-correlated fMRI. Adv Neurol. 1999;83:187–201. [PubMed] [Google Scholar]
- 22.Mullinger K, Debener S, Coxon R, Bowtell R. Effects of simultaneous EEG recording on MRI data quality at 1.5, 3 and 7 tesla. Int J Psychophysiol. 2008;67(3):178–188. doi: 10.1016/j.ijpsycho.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Scarff CJ, Reynolds A, Goodyear BG, Ponton CW, Dort JC, Eggermont JJ. Simultaneous 3-T fMRI and high-density recording of human auditory evoked potentials. NeuroImage. 2004;23(3):1129–1142. doi: 10.1016/j.neuroimage.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 24.Vasios CE, Angelone LM, Purdon PL, Ahveninen J, Belliveau JW, Bonmassar G. EEG/(f)MRI measurements at 7 Tesla using a new EEG cap ("InkCap") NeuroImage. 2006;33(4):1082–1092. doi: 10.1016/j.neuroimage.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 25.Klein C, Hanggi J, Luechinger R, Jancke L. MRI with and without a high-density EEG cap--what makes the difference? NeuroImage. 2015;106:189–197. doi: 10.1016/j.neuroimage.2014.11.053. [DOI] [PubMed] [Google Scholar]
- 26.Debener S, Mullinger KJ, Niazy RK, Bowtell RW. Properties of the ballistocardiogram artefact as revealed by EEG recordings at 1.5, 3 and 7 T static magnetic field strength. Int J Psychophysiol. 2008;67(3):189–199. doi: 10.1016/j.ijpsycho.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Grouiller F, Vercueil L, Krainik A, Segebarth C, Kahane P, David O. A comparative study of different artefact removal algorithms for EEG signals acquired during functional MRI. NeuroImage. 2007;38(1):124–137. doi: 10.1016/j.neuroimage.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 28.Mandelkow H, Halder P, Boesiger P, Brandeis D. Synchronization facilitates removal of MRI artefacts from concurrent EEG recordings and increases usable bandwidth. NeuroImage. 2006;32(3):1120–1126. doi: 10.1016/j.neuroimage.2006.04.231. [DOI] [PubMed] [Google Scholar]
- 29.Cohen MS. The Regents of the University of California, assignee. Method and apparatus for reducing contamination of an electrical signal. 7,286,871. United States patent. 2007 Oct 23;
- 30.Mullinger KJ, Havenhand J, Bowtell R. Identifying the sources of the pulse artefact in EEG recordings made inside an MR scanner. NeuroImage. 2013;71:75–83. doi: 10.1016/j.neuroimage.2012.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brodbeck V, Spinelli L, Lascano AM, Wissmeier M, Vargas MI, Vulliemoz S, Pollo C, Schaller K, Michel CM, Seeck M. Electroencephalographic source imaging: a prospective study of 152 operated epileptic patients. Brain. 2011;134(Pt 10):2887–2897. doi: 10.1093/brain/awr243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng R, Hu J, Pan L, Wu J, Lang L, Jiang S, Gu X, Guo J, Zhou L. Application of 256-channel dense array electroencephalographic source imaging in presurgical workup of temporal lobe epilepsy. Clin Neurophysiol. 2015 doi: 10.1016/j.clinph.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Serano P, Angelone LM, Katnani H, Eskandar E, Bonmassar G. A novel brain stimulation technology provides compatibility with MRI. Sci Rep. 2015;5:9805. doi: 10.1038/srep09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song J, Davey C, Poulsen C, Luu P, Turovets S, Anderson E, Li K, Tucker D. EEG source localization: Sensor density and head surface coverage. J Neurosci Methods. 2015;256:9–21. doi: 10.1016/j.jneumeth.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Standard test method for measurement of radio frequency induced heating on or near passive implants during magnetic resonance imaging. F2182-11a. West Conshohocken, PA: ASTM International; 2011. American Society for Testing and Materials. [Google Scholar]
- 36.Buchner R, Hefter GT, May PM. Dielectric Relaxation of Aqueous NaCl Solutions. The Journal of Physical Chemistry A. 1999;103(1):1–9. [Google Scholar]
- 37.Valvano JW, Cochran JR, Diller KR. Thermal conductivity and diffusivity of biomaterials measured with self-heated thermistors. Int J Thermophys. 1985;6(3):301–311. [Google Scholar]
- 38.Mattei E, Triventi M, Calcagnini G, Censi F, Kainz W, Bassen HI, Bartolini P. Temperature and SAR measurement errors in the evaluation of metallic linear structures heating during MRI using fluoroptic probes. Phys Med Biol. 2007;52(6):1633–1646. doi: 10.1088/0031-9155/52/6/006. [DOI] [PubMed] [Google Scholar]
- 39.International Standards Organization. Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device (ISO/TS 10974) 2012 [Google Scholar]
- 40.Insko E, Bolinger L. Mapping of the radiofrequency field. J of Magn Reson, Series A. 1993;103(1):82–85. [Google Scholar]
- 41.Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44(3):457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 42.Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268(5212):889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- 43.FsFast F. 2014 http://surfer.nmr.mgh.harvard.edu/fswiki/FsFast. [Google Scholar]
- 44.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Christov II. Real time electrocardiogram QRS detection using combined adaptive threshold. Biomedical engineering online. 2004;3(1):28. doi: 10.1186/1475-925X-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niazy RK, Beckmann CF, Iannetti GD, Brady JM, Smith SM. Removal of FMRI environment artifacts from EEG data using optimal basis sets. NeuroImage. 2005;28(3):720–737. doi: 10.1016/j.neuroimage.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 47.Carmichael DW, Thornton JS, Rodionov R, Thornton R, McEvoy A, Allen PJ, Lemieux L. Safety of localizing epilepsy monitoring intracranial electroencephalograph electrodes using MRI: radiofrequency-induced heating. J Magn Reson Imaging. 2008;28(5):1233–1244. doi: 10.1002/jmri.21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gabriel C, Gabriel S, Corthout E. The dielectric properties of biological tissues: I. Literature survey. Phys Med Biol. 1996;41(11):2231–2249. doi: 10.1088/0031-9155/41/11/001. [DOI] [PubMed] [Google Scholar]
- 49.Kainz W. MR heating tests of MR critical implants. J Magn Reson Imaging. 2007;26(3):450–451. doi: 10.1002/jmri.21020. [DOI] [PubMed] [Google Scholar]
- 50.Angelone LM, Ahveninen J, Belliveau JW, Bonmassar G. Analysis of the role of lead resistivity in specific absorption rate for deep brain stimulator leads at 3T MRI. IEEE Trans Med Imaging. 2010;29(4):1029–1038. doi: 10.1109/TMI.2010.2040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo Q, Glover GH. Influence of dense-array EEG cap on fMRI signal. Magn Reson Med. 2012;68(3):807–815. doi: 10.1002/mrm.23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiggins GC, Polimeni JR, Potthast A, Schmitt M, Alagappan V, Wald LL. 96-Channel receive-only head coil for 3 Tesla: design optimization and evaluation. Magn Reson Med. 2009;62(3):754–762. doi: 10.1002/mrm.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46(3):515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 54.Luo Q, Huang X, Glover GH. Ballistocardiogram artifact removal with a reference layer and standard EEG cap. J Neurosci Methods. 2014;233:137–149. doi: 10.1016/j.jneumeth.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krishnaswamy P, Bonmassar G, Poulsen C, Pierce ET, Purdon PL, Brown EN. Reference-Free Removal of EEG-fMRI Ballistocardiogram Artifacts with Harmonic Regression. NeuroImage. 2015 doi: 10.1016/j.neuroimage.2015.06.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krishnaswamy P, Bonmassar G, Purdon PL, Brown EN. Reference-free harmonic regression technique to remove EEG-fMRI ballistocardiogram artifacts. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:5426–5429. doi: 10.1109/EMBC.2013.6610776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.