Abstract
Background
The main objective of hospital hygiene and infection prevention is to protect patients from preventable nosocomial infections. It was recently stated that the proper goal should be for zero infection rates in sterile surgical procedures. In this article, we attempt to determine whether this demand is supported by the available literature.
Methods
We systematically searched the Medline and EMBASE databases for studies published in the last 10 years on the efficacy of infection control measures and carried out a meta-analysis according to the PRISMA tool. We used the following search terms: “aseptic surgery,” “intervention,” “surgical site infection,” “nosocomial infection,” “intervention,” and “prevention.”
Results
2277 articles were retrieved, of which 204 were acquired in full text and analyzed. The quantitative analysis included 7 prospective cohort studies on the reduction of nosocomial infection rates after aseptic surgery. The measures used included training sessions, antibiotic prophylaxis, and operative-site disinfection and cleaning techniques. These interventions succeeded in reducing postoperative wound infections (relative risk (RR] 0.99 [0.98; 1.00]). Subgroup analyses on antibiotic prophylaxis (RR 0.99 [0.98; 1.01]) and non-controlled trials (RR 0.97 [0.92; 1.02]) revealed small, insignificant effects.
Conclusion
A multimodal approach with the participation of specialists from various disciplines can further reduce the rate of postoperative infection. A reduction to zero is not realistic and is not supported by available evidence.
The most important goal of modern hospital hygiene and infection control is to give patients the best possible protection against avoidable hospital-acquired infections. The occurrence of these infections can be reduced by infection control measures (1– 3). Table 1 gives an overview of the incidence of postoperative wound infections after sterile surgical procedures. Values shown are mean rates of postoperative wound infection observed using various surveillance systems.
Table 1. Healthcare-associated infection rates for sterile operative procedures. Values are mean wound infection rates (%) per 100 procedures.
| Germany KISS Data 2010–2014 (26, 27) |
Europe ECDC Data 2010–2011 |
USA CDC Data for 2013 |
|
|---|---|---|---|
Orthopedic surgery
|
0.4 (0.0–1.0) 0.8 (0.3–1.6) |
0.7 1.0 |
0.6 0.7 |
Cardiac surgery
|
3.5 (2.6–4.2) | 3.5 | 0.6 |
Gynecology
|
0.4 (0.0–0.6) | 3.0 | |
Neurosurgery
|
0.1 (0.0–0.4) | ||
Visceral surgery
|
3.0 (1.7–4.6) | 0.6 |
The results of the SENIC study (Study on the Efficacy of Nosocomial Infection Control, aimed at the reduction of healthcare-associated infections [HAI]), have been available for many years (4). The study investigated the effect of infection control programs on the reduction of HAI over the course of 5 years in more than 300 US hospitals. It found that hospitals that employed hygiene personnel in a targeted way (one full-time person per 250 beds) and instituted suitable monitoring and feedback systems were able to reduce their HAI rates by 32%. In contrast, hospitals that did not implement this measure saw infection rates rise by 18% during the same period. On the basis of these findings, it was concluded that at least one-third of HAI can be avoided by maintaining standards of infection control measures (4).
However [so entspricht es dem deutschen Original], the potential for reducing HAI varies greatly, being strongly dependent on the hospital baseline situation. There is evidence, though, that significant reduction of HAI can be achieved by various interventions (5– 7). Postoperative wound infections are a problem in all surgical disciplines, although the infection risk is not the same for different kinds of surgical procedure. It depends on multiple factors such as local wound conditions, the patient’s immune status, and others. As an example, the differences between wound infection rates for cesarean sections (Table 1) in Germany and the European Union can be partly explained by the fact that in Germany even pregnant women with no risk factors may undergo cesarean sections.
Differences in wound infection rates are particularly due, however, to differences in the degree of contamination of the surgical site. Health authorities in the United States, for example, have set national reduction targets between 25% and 70% in their “Roadmap to Elimination” action plan (Table 2). Thus, the targets of the US action plan are seen not as zero values, but as percent reduction rates—an approach also followed by the German regional medical associations (Landesärztekammern) with their quality control data. These too monitor not outcome parameters alone, but also process parameters.
Table 2. United States national action plan for the prevention of health care–associated infections with targets up to the year 2020.
| Health care–associated infection/measure | Target reduction | Year | |
|---|---|---|---|
| 1 | Central venous catheter–related bloodstream infection | 50% | 2015 |
| 2 | Catheter-related urinary tract infection | 25% | 2007/2008 |
| 3 | Invasive MRSA infection | 75% | 2015 |
| 4 | Health care–associated MRSA infection | 50% | 2015 |
| 5 | Health care–associated Clostridium difficile diarrhea | 30% | 2015 |
| 6 | Clostridium difficile–related hospital admissions | 30% | 2015 |
| 7 | Postoperative wound infection | 30% | 2015 |
| 8 | Pre- or perioperative measures (e.g., timing of antibiotic prophylaxis, shaving) | To be implemented by 2020 | |
MRSA, methicillin-resistant Staphylococcus aureus
In Germany we are now seeing demands for zero infection rates in sterile operative procedures: “A national campaign for the creation of a culture of targeting zero health care-associated infections and zero tolerance for unsafe practices is long overdue” (8).
There is agreement that postoperative wound infections in general can and must be reduced. However, the reality is that an infection rate of zero in sterile operative procedures is not achievable. This article shows the extent to which the demand for “zero infections in sterile operations” is not evidence-based. It is based on a systematic literature review and meta-analysis of existing studies. The analysis is limited to the past 10 years, since the hypothesis that a zero infection rate is possible was first put forward in 2006.
Methods
The meta-analysis included interventional studies in which the aim was to reduce the number of postoperative wound infections after sterile operative procedures. Sterile procedures are defined as those in which the surgical site is free from infection and inflammation, and in which neither the respiratory nor the gastrointestinal nor the urinary tract are opened. This systematic review was carried out in accordance with the principles in the PRISMA Statement (Preferred Reporting Items for Systematic Reviews and Metaanalyses) (9).
Inclusion and exclusion criteria
The criteria for including studies were defined on the basis of PICO questions selected before the start of the study and laid down in the study protocol (PICO: Patient group = patients undergoing sterile procedures; Intervention = any infection control measure aimed at reducing postoperative wound infection, with the exception of care bundles for catheter infection management; Comparison/control intervention = compared to another preventive measure or no intervention; Outcome = incidence of postoperative wound infections after sterile procedures). We included only studies in adults (≥16 years) that investigated a direct relationship between an intervention and the occurrence of postoperative infections after sterile procedures. Intervention studies relating to other HAI, reviews, letters, editorials, and case reports were not included.
Study identification
Studies were identified through a computer-aided literature search of Medline and EMBASE, and in the references of the studies found by this search. Search terms on 31 December 2015 were: “aseptic surgery,” “intervention,” “surgical site infection rates,” and “interventions,” and “nosocomial infection” and “prevention.” Only studies with reduction of postoperative infections as an endpoint were included. Studies reporting other infections without interventions were not included. The search was restricted to studies published in German or English between June 2004 and December 2015, so as to reflect current literature. Studies that had not yet been published were not included in the meta-analysis. The first round of selection was by title. The abstracts of the selected studies were assessed by two authors independently for possible inclusion. Every article that met, or appeared to meet, the inclusion criteria was screened. Members of the assessment team were not permitted to assess studies to which they were themselves contributing authors. When there was a difference of opinion, the data selection was discussed, and if it was excluded, the reason for this was recorded.
Statistical analysis
To minimize the risk of systematic error (selection bias), the included studies were assessed according to detailed criteria developed by the Cochrane Effective Practice and Organisation of Care (EPOC) Group (10) and independently evaluated by two reviewers.
Discrepancies were resolved by discussion or, if necessary, by bringing in a third reviewer. Because of the presence of extra variability within studies, the DerSimonian and Laird random effects model was used. Analysis was carried out using the Mantel-Haenszel test. Begg’s funnel plot was used to evaluate publication bias. The overall heterogeneity of the studies was measured using the Q-statistic. Data were analyzed using STATA version 12.1 (Stata Corporation, College Station, USA).
Results
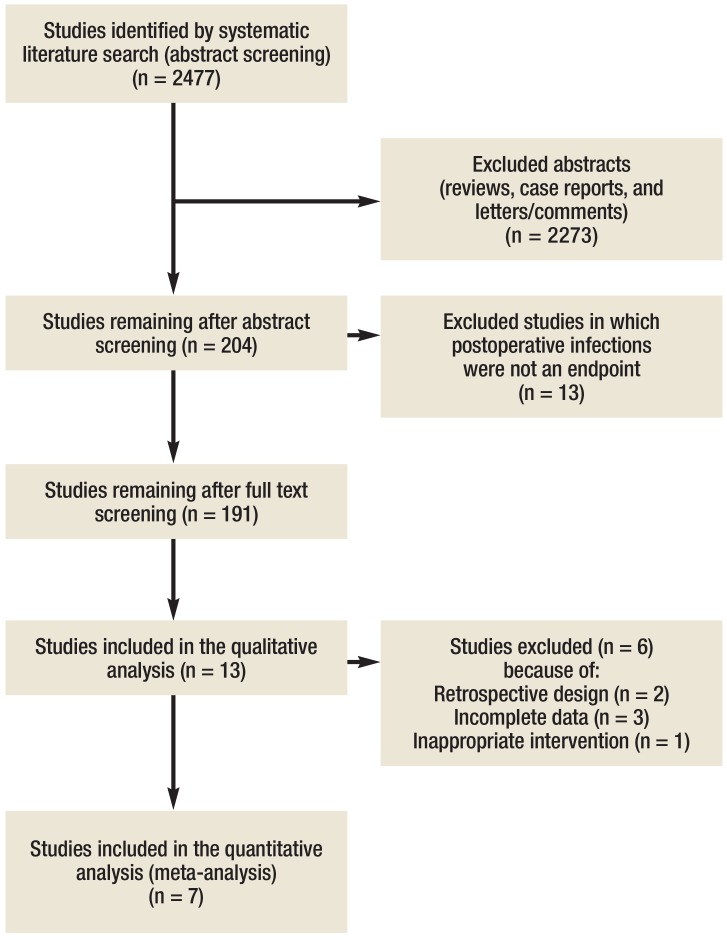
The literature search identified 2477 studies, 2273 of which were immediately excluded either because they were reviews, case reports, or letters/comments. Of the remaining 204 publications (8.2%), a further 191 were excluded because they were pediatric studies or single case reports, contained results in the form of point prevalences, or related to results that were unconnected with any intervention. The remaining 13 studies (with a total study population of 94 633 patients) were included in the analysis (eFigure 1).
eFigure 1.
Selection of studies for inclusion in the meta-analysis
Study characteristics
A full list of the studies included is given in Table 3. Five studies were carried out in Europe (14– 16, 19, 22), three in the USA and Canada (13, 17, 21), three each in South America and Central America (11, 18, 20), one in Asia (22), and one in Australia (12). Overall, there were three prospective cohort studies (before/after design) (11, 14, 18), eight other prospective studies (12, 15– 17, 19– 22), and two retrospective studies.
Table 3. Characteristics of the 11 studies included in the meta-analysis.
| Reference | N | Study design | Nature of surgery | Intervention | SSI (incidence) before intervention | SSI (incidence) after intervention |
|---|---|---|---|---|---|---|
| (16) | 1088 | Prospective cohort study |
Orthopedic procedure |
Setting up a team of hygiene specialists to promote adherence to surgical infection prophylaxis specified in standard operating procedure (SOP), with regular feedback | 3.3% | 2.0% |
| (17) | 811 | Prospective cohort study |
Abdominal surgery | Intervention 1: Daily requirement to justify need for an indwelling catheterIntervention 2: Intervention 1 plus sterile intraoperative catheter placement | 6.9% | Intervention 1: 2.7% Intervention 2: 0.8% |
| (13) | 1001 | Retrospective cohort study |
Coronary arterial bypass with sternotomy |
Implementation of intra- and postoperative prevention practices | 3.0% | 0%*1 |
| (19) | 1616 | Prospective cohort studies over two time periods |
Cesarean section | Before intervention: Prophylactic antibiotics only for elective sectionIntervention: Prophylactic antibiotics for all patients, supplemented by education of medical personnel in aseptic and scrub techniques | Elective C sections: 5.3% Wound infections: 4.5% |
Elective C sections: 0.9% Wound infections: 1.5% |
| (15) | 60 460 | Prospective cohort study |
Abdominal and chest surgery | Implementation of a surveillance program | 2.6% | 0.7%*2 |
| (21) | 192 | Prospective cohort study |
Abdominal hysterectomy | Education of personnel in antibiotic prophylaxis Change: From povidone-iodine solution to 4%chlorhexidine solution preoperatively |
10.7% | 1.2% |
| (11) | 3496 | Prospective cohort study (before/after study) |
All forms of surgery | Education of personnel in antibiotic prophylaxis for sterile surgery, followed by prospective observation | 3.2% | 1.9% |
| (14) | 3621 | Prospective cohort study (before/after study) |
Vascular, abdominal, gynecological, and orthopedic surgery | Optimization of SOPs for preoperative antibiotic prophylaxis | 5.3% | 4.5% |
| (18) | 12 299 | Prospective cohort study |
All forms of surgery | Single-dose antibiotic prophylaxis instead of 24-h regimen | 2.1% | 2.1% |
| (12) | 618 | Randomized controlled clinical trial |
Local excisions, mastectomy, and microdochectomy | Single dose of flucloxacillin vs. no antibiotic prophylaxis | 3.2% | 4.5% |
| (20) | 2338 | Prospective cohort study |
Surgery for breast cancer | Implementation of enhanced infection control measures | 33.3% | 18.9% |
| (23) | 341 | Retrospective cohort study |
Aseptic revision knee arthroplasty | Short antibiotic prophylaxis vs. extended antibiotic prophylaxis (5days postoperatively) | 6.9% | 2.2% |
| (22) | 2301 | Retrospective or prospective | General surgery | Implementation of hand hygiene | 5% | 6.5% |
*1All procedures were carried out by the same surgeons and higher-risk patients were excluded
*2Infection rate reduced by 29% at 2 years after implementation of the program. SSI, surgical site infection; SOP, standard operating procedure
Only one of these studies achieved a postoperative infection rate of 0% (13). This study retrospectively evaluated the bypass operations of a single surgeon with regard to deep sternal wound infections. None of the other studies achieved a zero infection rate.
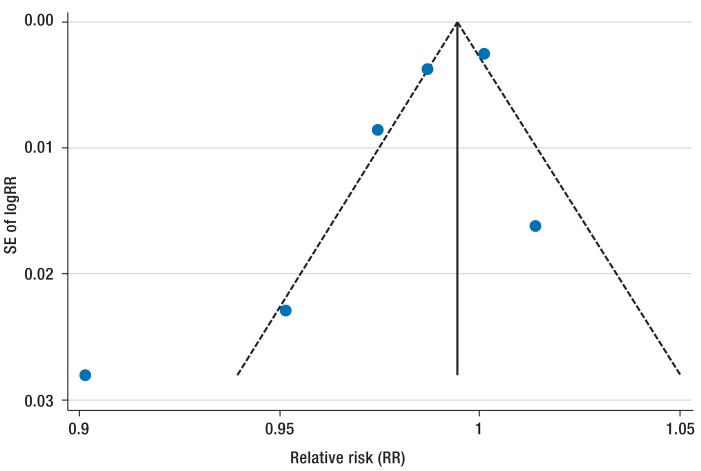
The interventions described included a wide variety of optimized perioperative antibiotic prophylaxis measures (8 studies) (11, 12, 14, 16, 18, 19, 21, 23), alterations to aseptic measures (5 studies) (13, 17, 20, 21, 22), education of personnel (3 studies) (11, 19, 21), and the introduction of surveillance programs and intensified aftercare programs. In these studies the baseline wound infection rates ranged from 2.1% for abdominal and thoracic procedures to up to 33.3% for breast procedures. The greatest reduction rates, of 83% (absolute risk reduction 4.25%) and 89% respectively, were shown by Nagle et al. (17) and Marchi et al. (15). The former study implemented a standard operating procedure (SOP) for patient care and for the use of urinary catheters during and after the operation. The rate of urinary tract infections was reduced from 6.9% to 0.8% (17). The latter study showed a significant reduction in postoperative wound infections in abdominal and chest procedures from 2.6% to 0.7% after introduction of a surveillance system (15). Two studies (12, 18), on the other hand, showed no reduction at all compared to the preintervention period. Underlying risk factors are shown in the eTable. Begg’s funnel plot analysis showed there was no publication bias (p = 0.11) (eFigure 2).
eTable. Risk factors and preventive measures in five studies from Table 3.
| Study (reference) | |||||
|---|---|---|---|---|---|
| Nagle D et al. (17) |
Lindsjö et al. (22) |
Marchi M et al. (15) |
Young H et al. (21) |
Vilar-Compte D et al. (20) |
|
| Obesity | + | + | |||
| Longer operative times | + | + | + | ||
| Diabetes mellitus | + | ||||
| Smoking | |||||
| Previous SSI | + | ||||
| Advanced age | + | ||||
| Hypertension | |||||
| American Society of Anesthesiologists score 3 or 4 | + | ||||
| Preoperative chemotherapy | + | ||||
| Increased intraoperative bleeding | + | ||||
| Postoperative drain | + | ||||
| Longer drainage time | + | + | |||
| Placement of a second drain | + | ||||
| Patients operated on by a junior doctor | + | ||||
SSI, surgical site infection
eFigure 2.
Funnel plot analyzing publication bias. SE, standard error
Effect of interventions
Thirteen studies (11– 23) investigated the influence of the implemented measures on postoperative infections. Seven studies were included in the meta-analysis (11, 12, 14, 18, 19, 21, 22). Five studies could not be included in the meta-analysis because of their retrospective design, or because their intervention focused on urinary catheters did not meet the inclusion criteria, or because of incomplete data (13, 16, 17, 20, 23).
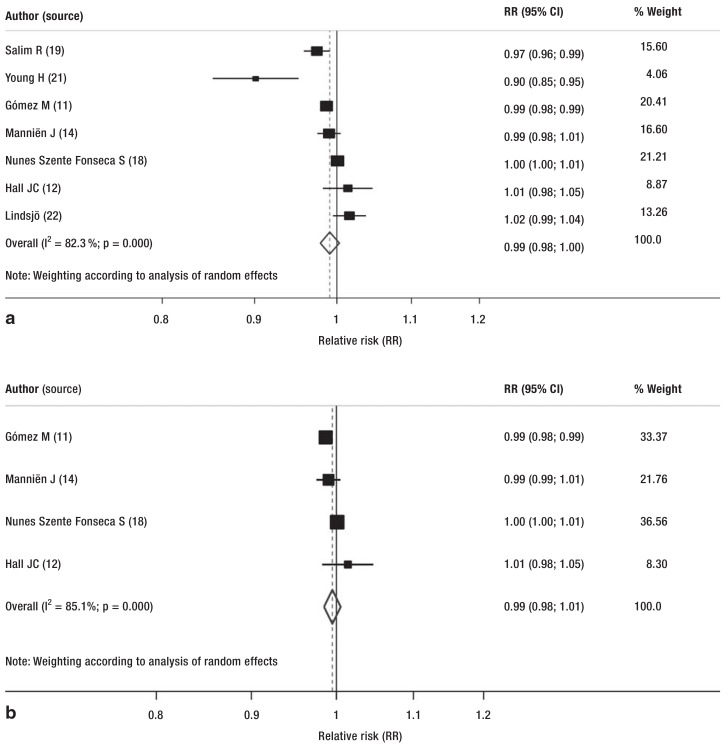
All implemented interventions emphasize the importance of increasing compliance with the recommended measures for best practice for surgery. These measures included education, antibiotic prophylaxis, and appropriate disinfection and cleaning of the operating area. These interventions reduced the rate of postoperative wound infections, although not significantly (pooled relative risk [RR] 0.99; 95% confidence interval [CI: 0.98; 1.00]; random effects model) (Figure, a).
Figure.
Subgroup analyses presented as forest plots:
a) Forest plot of all studies comparing postoperative infections before and after the intervention (relative risk [RR], unadjusted);
b) Forest plot of subgroup analysis of studies (before/after and RCT) in which antibiotic prophylaxis was the intervention
CI, confidence interval; RCT, randomized controlled trial
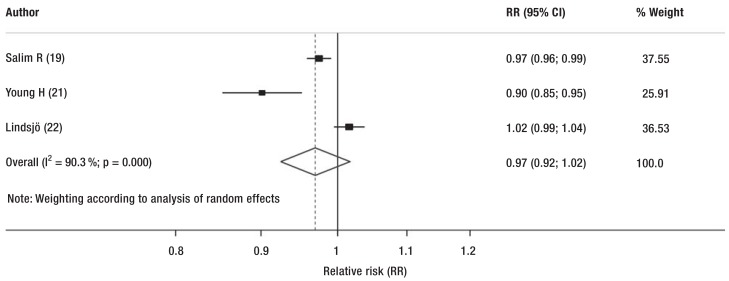
To reduce the effect of the heterogeneity of the studies included in the review, two analyses were carried out that focused on the implementation of a SOP for antibiotic administration. The meta-analyses showed a reduction, although nonsignificant, in randomized clinical trials and cohort studies (before/after design) (pooled RR 0.99; 95% CI: [0.98; 1.01]; χ2 73; random effects model) (Figure, b) and in prospective cohort studies (pooled RR 0.98; 95% CI: [0.92; 1.02]; random effects model) (eFigure 3).
eFigure 3.
Subgroup analysis presented as forest plot. Forest plot of subgroup analysis of studies without a control intervention.
CI, confidence interval
Discussion
Based on the analysis of current studies and the meta-analysis, it may be concluded that at present the evidence-based data are inadequate to support a concept of “zero infection” in the sense of prevention of all postoperative wound infection. None of the studies analyzed achieved this (Table 3).
One of the main reasons for the misinterpretation of the existing data has been that the data underlying the “zero target” are based on studies of prevention of the bloodstream infections associated with central venous catheters (CVC) (24). The studies cited by the German Society of Hospital Hygiene (Deutsche Gesellschaft für Krankenhaushygiene) showed a reduction in these infections of up to 68% (8). Compared with the SENIC study mentioned above, this represents a considerable step forward (4).
In the USA, the premise has long been defended that all CVC-related infections are avoidable. This has led to a situation where the two largest cost carriers in the US health system only partially reimburse costs incurred due to CVC-related bloodstream infections. Worth et al. analyzed the data available to them in 2012 and concluded that the goal of a complication rate of zero CVC-related bloodstream infections was only achievable in certain target groups (25). These target groups include intensive care patients with an indwelling catheter in place for less than 9 days. It should however be mentioned that these authors analyzed only studies published in English. Given in addition that negative results regarding reduced infection rates are often not published, this could in reality represent an even smaller evidence base regarding risk reduction. Up until 2012, none of the studies achieved reducing risk down to zero—even with quite extensive interventions.
Every surgical incision (even in sterile procedures) is potentially contaminated with bacteria. Most often these are bacteria from the patient’s own endogenous flora. Whether a clinical infection can be prevented depends partly on the local host defenses. For this reason, it should always be remembered that risk is individual to each patient. In seriously ill patients, risks for HAI exist on which prevention strategies can have little effect. These include complicated surgical procedures, especially in the abdomen, and natural bacterial colonization.
Nevertheless, it should be particularly emphasized that a multimodal approach involving a range of specialists (hygienists, infectiologists, microbiologists, surgeons, and pharmacists) can result in significant reduction of postoperative infections.
Regular audits and feedback for the personnel involved in care are essential. In addition, the BQS (Institute for Quality and Patient Safety, Institut für Qualität & Patientensicherheit GmbH) and the Office for Quality Assurance (Geschäftsstelle Qualitätssicherung) have for years kept records of wound infections following certain procedures (www.bqs-qualitätsreport.de). However, these data are usually collected retrospectively from hospitals where personnel are not used to recording infections according to standardized definitions, potentially leading to both underestimates and overestimates. The case is different for hospitals participating in KISS (Hospital Infection Surveillance System, Krankenhaus-Infektions-Surveillance-System), where as a rule infections are recorded by specialist hygienists with enough time at their disposal.
Whether there should be compulsory reporting of postoperative infections is hotly debated. No clear evidence exists that publishing HAI data would serve a useful purpose (26). The discussion is colored by political arguments, with the concomitant risk that the hope that the data would be useful is accorded more importance than any possible undesired effects. The risk that published data could be misinterpreted is one that must be taken seriously, however. Another danger is that improving the indicators could become the implicit goal without any increase in the quality of care (i.e., data hygiene rather than infection prevention).
In our opinion, appropriate use of valid surveillance data will allow the rate of postoperative wound infections to be reduced (27). However, it should not be forgotten that the law of diminishing returns, though mostly employed in business, applies in clinical medicine as well: that, as investment in any measure (in this case infection prevention) increases, the increased improvement tends to become ever smaller. Even in business, zero-error (totally error-free) production is regarded as improbable. This is referred to in the “Six Sigma” method, for example, in which the goal is not a zero-error process but an error level that is within six standard deviations of the mean.
We believe that the financial cost pressure on hospitals at present works against a significant reduction in the rate of HAI. More than this: a demand for “zero infections,” in combination with reduced reimbursement for infection-related complications, could even further increase the financial pressure. One expected direct consequence would be ever shorter hospital stays for patients and fewer personnel to care for them, which could have the opposite effect of leading to inadequate hygiene. We believe that other negative implications of a “zero infection” demand may also be anticipated.
Limitations
This meta-analysis has certain limitations. The great diversity of interventions in the studies we included did not allow adequate subgroup analysis to identify the source of heterogeneity. However, the aim of this article was to perform a systematic literature review and meta-analysis to assess the evidence on “zero infections” in real clinical life.
Conclusion
With sufficient availability of infection control personnel, it is possible to reduce health care–associated infections by around one-third. To achieve this, however, hospitals would have to make resources available. Reducing HAI to zero is not realistic, partly because of the existence of “high-performance medicine,” with invasive procedures frequently carried out even in patients with considerable risk factors, not least in order to achieve more convenient coding (diagnosis-related groups). These procedures increase the risk of HAI and also the selection of multiresistant pathogens.
The data collected, however, do show that a multimodal approach can lead to significant reduction of postoperative infections. As long as financial resources are not available to support structures that could prevent HAI in a targeted way, public health officials must face the fact that patients are exposed to a continuing risk of HAI. Fundamentally, it also appears debatable whether every demand really must be evidence-based. More to the point is the question whether the demand is one that can be met.
Key Messages.
Healthcare-associated infections are associated with increased mortality and morbidity.
Effective hospital infection control is needed to protect patients from infections.
However, a “zero infection” rate after sterile procedures is unrealistic.
Meta-analysis of the past 10 years shows no evidence to support “zero infections.”
Reduction of health care–associated infections is nevertheless possible using a multidisciplinary approach.
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Loveday HP, Wilson JA, Pratt RJ, et al. National evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect. 2014;86:1–70. doi: 10.1016/S0195-6701(13)60012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tacconelli E, Cataldo MA, Dancer SJ, et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014;20:1–55. doi: 10.1111/1469-0691.12427. [DOI] [PubMed] [Google Scholar]
- 3.Lee AS, Cooper BS, Malhotra-Kumar S, et al. MOSAR WP4 Study Group. Comparison of strategies to reduce meticillin-resistant Staphylococcus aureus rates in surgical patients: a controlled multicentre intervention trial. BMJ Open. 2013;19(3) doi: 10.1136/bmjopen-2013-003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes JM. Study on the efficacy of nosocomial infection control (SENIC Project): results and implications for the future. Chemotherapy. 1988;34:553–561. doi: 10.1159/000238624. [DOI] [PubMed] [Google Scholar]
- 5.Ng W, Brown A, Alexander D, et al. A multifaceted prevention program to reduce infection after cesarean section: Interventions assessed using an intensive postdischarge surveillance system. Am J Infect Control. 2015;43:805–809. doi: 10.1016/j.ajic.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Hickson E, Harris J, Brett D. A journey to zero: reduction of post-operative cesarean surgical site infections over a five-year period. Surg Infect. 2015;16:174–177. doi: 10.1089/sur.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mihaljevic AL, Müller TC, Kehl V, Friess H, Kleeff J. Wound edge protectors in open abdominal surgery to reduce surgical site infections: a systematic review and meta-analysis. PLoS One. 2015 10 doi: 10.1371/journal.pone.0121187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walger P, Popp W, Exner M. Stellungnahme der DGKH zu Prävalenz, Letalität und Präventionspotenzial nosokomialer Infektionen in Deutschland 2013. Hyg Med. 2013;38:329–338. [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group preferred reporting iItems for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cochrane Effective Practice and Organisation of Care Group. EPOC resources for review authors. http://epoc.cochrane.org/resources. (last accessed on 2 August 2015)
- 11.Gómez MI, Acosta-Gnass SI, Mosqueda-Barboza L, Basualdo JA. Reduction in surgical antibiotic prophylaxis expenditure and the rate of surgical site infection by means of a protocol that controls the use of prophylaxis. Infect Control Hosp Epidemiol. 2006;27:1358–1365. doi: 10.1086/509845. [DOI] [PubMed] [Google Scholar]
- 12.Hall JC, Willsher PC, Hall JL. Randomized clinical trial of single-dose antibiotic prophylaxis for non-reconstructive breast surgery. Br J Surg. 2006;93:1342–1346. doi: 10.1002/bjs.5505. [DOI] [PubMed] [Google Scholar]
- 13.Kieser TM, Rose S, Aluthman U, Montgomery M, Louie T, Belenkie I. Toward zero: Deep sternal wound infection after 1001 consecutive coronary artery bypass procedures using arterial grafts: Implications for diabetic patients. J Thorac Cardiovasc Surg. 2014;148:1887–1895. doi: 10.1016/j.jtcvs.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Manniën J, Van Kasteren MEE, Nagelkerke NJ, et al. Effect of optimized antibiotic prophylaxis on the incidence of surgical site infection. Infect Control Hosp Epidemiol. 2006;27:1340–1346. doi: 10.1086/509842. [DOI] [PubMed] [Google Scholar]
- 15.Marchi M, Pan A, Gagliotti C, et al. The Sorveglianza Nazionale Infezioni in Chirurgia (SNICh) Study Group. The Italian national surgical site infection surveillance programme and its positive impact, 2009 to 2011. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.21.20815. [DOI] [PubMed] [Google Scholar]
- 16.Molina-Cabrillana J, Chirino Cabrera A, Rodríguez-Alvarez JP, et al. Effect of surveillance on surgical site infection rate in knee and hiparthroplasty. Rev Clin Esp. 2007;207:388–393. doi: 10.1157/13108756. [DOI] [PubMed] [Google Scholar]
- 17.Nagle D, Curran T, Anez-Bustillo L, Poylin V. Reducing urinary tract infections in colon and rectal surgery. Dis Colon Rectum. 2014;57 doi: 10.1097/DCR.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 18.Nunes Szente Fonseca S, Melon Kunzle SR, Junqueira MJ, Teodoro Nascimento R, De Andrade JI, Levin AS. Implementing 1-dose antibiotic prophylaxis for prevention of surgical site infection. ARCH SURG. 2006 doi: 10.1001/archsurg.141.11.1109. [DOI] [PubMed] [Google Scholar]
- 19.Salim R, Braverman M, Berkovic I, Suliman A, Teitler N, Shalev E. Effect of interventions in reducing the rate of infection after cesarean delivery. Am J Infect Control. 2011;39:e73–e78. doi: 10.1016/j.ajic.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Vilar-Compte D, Rosales S, Hernandez-Mello N, Maafs E, Volkow P. Surveillance, control, and prevention of surgical site infections in breast cancer surgery: a 5-year experience. Am J Infect Control. 2009;37:674–679. doi: 10.1016/j.ajic.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Young H, Knepper B, Vigil C, Miller A, Carey JC, Price CS. Sustained reduction in surgical site infection after abdominal hysterectomy. Surg Infect. 2013;14:460–463. doi: 10.1089/sur.2012.113. [DOI] [PubMed] [Google Scholar]
- 22.Lindsjö C, Sharma M, Mahadik VK, Sharma S, Stålsby Lundborg C, Pathak A. Surgical site infections, occurrence, and risk factors, before and after an alcohol-based handrub intervention in a general surgical department in a rural hospital in Ujjain, India. Am J Infect Control. 2015;43:1184–1189. doi: 10.1016/j.ajic.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Claret G, Tornero E, Martinez-Pastor J-C, et al. A prolonged post-operative antibiotic regimen reduced the rate of prosthetic joint infection after aseptic revision knee arthroplasty. Surg Infect. 2015;16:775–780. doi: 10.1089/sur.2015.044. [DOI] [PubMed] [Google Scholar]
- 24.Carlet J, Fabry J, Amalberti R, Degos L. The „zero risk“ concept for hospital-acquired infections: a risky business! Clin Infect Dis. 2009;49:747–749. doi: 10.1086/604720. [DOI] [PubMed] [Google Scholar]
- 25.Worth LJ, McLaws ML. Is it possible to achieve a target of zero central line associated bloodstream infections? Curr Opin Infect Dis. 2012;25:650–657. doi: 10.1097/QCO.0b013e32835a0d1a. [DOI] [PubMed] [Google Scholar]
- 26.Haustein T, Gastmeier P, Holmes A, et al. Use of benchmarking and public reporting for infection control in four high-income countries. Lancet Infect Dis. 2011;11:471–481. doi: 10.1016/S1473-3099(10)70315-7. [DOI] [PubMed] [Google Scholar]
- 27.Gastmeier P, Schwab F, Sohr D, Behnke M, Geffers C. Reproducibility of the surveillance effect to decrease nosocomial infection rates. Infect Control Hosp Epidemiol. 2009;30:993–999. doi: 10.1086/605720. [DOI] [PubMed] [Google Scholar]
- 28.Gastmeier P, Behnke M, Breier AC, et al. Nosokomiale Infektionsraten: Messen und Vergleichen. Bundesgesundheitsbl. 2012;55:1363–1369. doi: 10.1007/s00103-012-1551-y. [DOI] [PubMed] [Google Scholar]
- 29.Geffers C, Gastmeier P. Nosocomial infections and multidrug-resistant organisms in Germany—epidemiological data from KISS (The Hospital Infection Surveillance System) Dtsch Arztebl Int. 2011;108:87–93. doi: 10.3238/arztebl.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]