Summary
Vitamin D influences allergen-induced pathways in the innate and adaptive immune system, and its potential immunomodulatory role in allergic skin disorders has been explored. This comprehensive review article provides an overview of the role of vitamin D in three common dermatologic conditions: atopic dermatitis (AD), chronic urticaria, and allergic contact dermatitis (ACD). Whereas the literature regarding vitamin D and AD has resulted in mixed findings, several studies have described an inverse relationship between vitamin D levels and AD severity, and improvement in AD with vitamin D supplementation. Similarly, several studies report an inverse relationship between vitamin D levels and severity of chronic urticaria. Although current research in humans remains limited, an increased likelihood of ACD has been demonstrated in vitamin D-deficient mice. Additional well-designed clinical trials will be necessary to determine whether vitamin D supplementation should be recommended for prevention or adjuvant treatment of these common dermatologic conditions.
Keywords: Allergy, Atopic Dermatitis, Allergic Contact Dermatitis, Chronic Urticaria, Immunomodulation, Vitamin D
1. Introduction
Vitamin D, which plays a critical role in calcium and phosphate balance, is acquired from two major sources. Whereas ultraviolet radiation from sun exposure gives rise to the production of cholecalciferol (D3), ergocalciferol (D2) and D3 can be obtained from our diet or in supplemental vitamins. Vitamin D2 and D3 are then hydroxylated to 25-hydroxyvitamin D (25[OH]D) in the liver. Clinically, 25[OH]D is measured in the serum. 25[OH]D is then further hydroxylated to the active form 1,25-dihydroxyvitamin D (1,25[OH]2D) in the kidney.
Vitamin D deficiency, generally defined as 25[OH]D levels less than 20 ng/mL, is a common public health issue, although the optimal amount of vitamin D remains controversial [1]. The ability to obtain vitamin D from sun exposure is diminished in the elderly, those with darker skin types, and those living at high latitudes [2]. Because vitamin D is a fat-soluble vitamin, malabsorption can also contribute to vitamin D deficiency. Not only can vitamin D deficiency contribute to rickets or osteomalacia, but it also has been connected to cardiovascular disease, diabetes mellitus, multiple sclerosis, infectious diseases, and cancer [3].
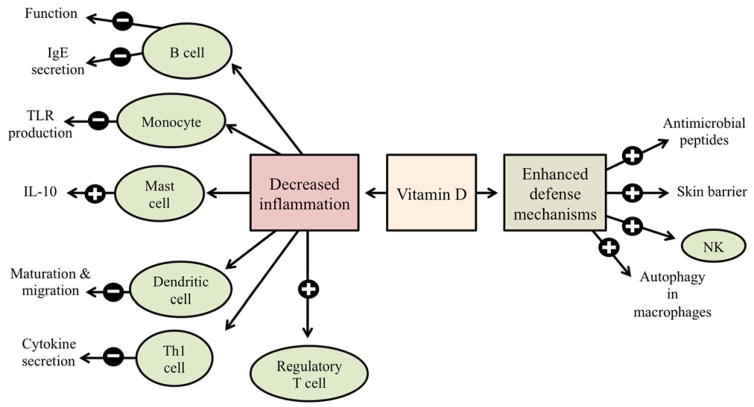
As shown in Figure 1, vitamin D enhances defense mechanisms against microbes and inhibits inflammation in the innate and adaptive immune system [4]. The vitamin D receptor (VDR) finds expression in several inflammatory cells, including T cells, B cells, neutrophils, macrophages, and dendritic cells [5]. Vitamin D enhances expression of antimicrobial peptides (including cathelicidin and β-defensins), enhances skin barrier function, induces autophagy in macrophages, and induces natural killer cells via increased cathelicidin [1, 6, 7]. Vitamin D is also involved in decreasing excessive inflammation by suppressing Toll-like receptor production by monocytes, enhancing mast cell production of interleukin-10 (IL-10, an anti-inflammatory cytokine), inhibiting dendritic cell activation by lipopolysaccharide (LPS), decreasing cytokine secretion from Th1 cells, inducing regulatory T cells, and inhibiting B lymphocyte function and immunoglobulin E (IgE) secretion [1]. Because atopic dermatitis (AD), chronic urticaria, and allergic contact dermatitis (ACD) all involve immune dysregulation, the role of vitamin D has been explored in these three common allergic skin disorders.
Fig. 1.
Potential role of vitamin D on various immune cells to decrease inflammation and enhancing defense mechanisms. The
 and
and
 signs represent stimulatory or inhibitory effect, respectively, of vitamin D on the release of cytokines or the function, as mentioned in the diagram.
signs represent stimulatory or inhibitory effect, respectively, of vitamin D on the release of cytokines or the function, as mentioned in the diagram.
2. Atopic Dermatitis
2.1 Background
AD, also known as atopic eczema, is a chronic and relapsing skin condition that affects more than 20% of children in most developed countries, and continues to increase in prevalence in low-income countries [8]. Whereas AD is common in children and adolescents, the prevalence in adults ranges from 1% to 3% [9]. AD involves a pruritic and erythematous rash that can occur anywhere, but commonly affects the face and extensor surfaces of infants, and the flexural areas in children. Those with AD can also develop other atopic disorders, such as food allergies, allergic rhinitis, and asthma [10].
The pathogenesis of AD is complex and multifactorial, involving abnormalities in cells of the immune system and the skin barrier. An imbalance between Th1 and Th2 cells exists. Whereas Th2 cells and associated interleukins, including IL-4, IL-5, and IL-13, play a role in the acute phase of AD, Th1 cells and associated cytokines, including interferon gamma (IFN-γ), granulocyte macrophage colony-stimulating factor (GM-CSF), IL-5 and IL-12, are predominant in the chronic phase [11]. AD also occurs because of deficiencies in the epidermal skin barrier. Patients with AD are more likely to acquire staphylococcal or viral infections of the skin due to three major factors: a compromised physical barrier of the epidermis, defects in pattern recognition receptors, and diminished production of antimicrobial peptides (AMPs) during inflammation [12].
2.2 Vitamin D levels and the severity of atopic dermatitis
(a) Inverse relationship between high vitamin D levels and atopic dermatitis
Despite conflicting results (Table 1), the majority of studies suggest that vitamin D is protective against AD. In one study, obese individuals with vitamin D deficiency, (serum 25[OH]D level < 25 nmol/L), were 5 times more likely to report AD than counterparts who were vitamin D replete (serum 25[OH]D level ≥ 75 nmol/L) [13]. Similarly, a cross-sectional study involving 15,212 adults in Korea found that mean serum 25[OH]D levels were significantly lower in AD participants compared to those without AD [14]. In this study, odds ratios of AD were significantly higher in those with inadequate or deficient vitamin D levels.
Table 1.
Serum 25-hydroxyvitamin D (25[OH]D) and atopic dermatitis (AD)
| Inverse relationship between high 25[OH]D levels and AD | Direct relationship between high 25[OH]D levels and AD | No significant relationship between 25[OH]D levels and AD |
|---|---|---|
AD severity has also been studied in regards to vitamin D. For example, an index for determining the severity of atopic dermatitis called SCORing Atopic Dermatitis (SCORAD) has been utilized in several studies. Peroni et al. [15] found that serum levels of 25[OH]D were significantly higher in children with mild AD compared to those with moderate or severe disease, based on SCORAD. Moreover, the number of children with specific IgE (sIgE) to Staphylococcus aureus enterotoxins and to Malassezia furfur was positively correlated with the severity of AD and the degree of vitamin D deficiency.
Similarly, a Korean study utilizing SCORAD demonstrated that mean serum levels of 25[OH]D were significantly lower in 91 children with AD compared to 32 healthy controls [16]. Furthermore, children with moderate and severe AD had significantly less serum 25[OH]D level than children with mild AD. Levels of serum IL-31, which is related to pruritus in AD, were not related to AD group, SCORAD index, or 25[OH]D levels.
In 226 infants with AD or food allergy, low levels of 25[OH]D were associated with a higher SCORAD index as well as an increased risk of food allergen sensitization [17]. Another study involving 498 children with AD in China also showed that compared to 328 healthy controls, levels of 25[OH]D were significantly lower in children with AD, and vitamin D deficiency was associated with elevated levels of IgE [18]. Serum levels of 25[OH]D correlated inversely with both the short-term severity of AD measured by SCORAD, and the long-term severity measured by the Nottingham Eczema Severity Score (NESS). SCORAD was also used in a study in Korea that compared the relationship between serum 25[OH]D level and severity of AD in children and adults [19]. Whereas serum 25[OH]D levels were lower in children with AD than healthy children, the same relationship was not found in adults. An important distinction with this study was that serum 25[OH]D levels were not significantly correlated with AD severity based on SCORAD scores or serum cathelicidin (LL-37) production.
Akan and colleagues [20] looked at the role of allergic sensitization in the relationship between vitamin D levels and AD severity in a study involving 73 patients with AD. Allergic sensitization was determined by epidermal prick tests as well as serum specific IgE, serum total IgE, and peripheral blood eosinophil count and percentage. In the 33 AD patients with allergic sensitization, there was a significant negative correlation between SCORAD values and serum vitamin D levels. However, no correlation was reported for those without allergic sensitization. Similarly, Lee et al. [21] found no significant correlation between vitamin D levels and AD severity (based on SCORAD) among 157 AD patients, but they did find that in 36 AD patients with food sensitization there was an inverse relationship between high vitamin D levels and AD severity.
The skin of patients with AD frequently becomes colonized with Staphylococcus aureus. Because AD can be worsened by an overlying bacterial infection, an observational, cross-sectional study investigated the relationship between levels of vitamin D and S. aureus virulence factors [22]. Several virulence factors, including tsst-1, eta, cna, aur, and sec, were associated with lower 25[OH]D levels, indicating that vitamin D deficiency can be associated with more virulent overlying S. aureus infections in patients with AD.
(b) Direct relationship between high vitamin D levels and atopic dermatitis
On the other hand, one cross-sectional study in Germany has suggested the opposite from those detailed above. Serum 25[OH]D levels were significantly higher in children and adolescents with AD compared to controls, although causality cannot be inferred [23]. Similarly, in a cross-sectional analysis involving 2,815 10-year-old children from Germany, there was a slightly increased prevalence of AD in children with high serum 25[OH]D levels [24].
(c) No relationship between high vitamin D levels and atopic dermatitis
Several studies have found no significant relationship between vitamin D levels and AD. For example, Chiu et al. [25] found that the serum level of 25[OH]D was not significantly correlated with the severity of AD based on the SCORAD index in their cross-sectional study of 94 children with AD. Limitations to this study include the inability to control for factors like sunlight exposure, intake of vitamin D, and prior treatment of AD. Similarly, Samochocki et al. [26] found no significant difference in serum 25[OH]D levels in 95 patients with AD compared to 58 controls. However, these authors describe an increased frequency of cutaneous bacterial infections in AD patients with low serum 25[OH]D levels. Lastly, a recent prospective study found no statistically significant associations between levels of 25[OH]D and prevalence or incidence of atopy, AD, asthma, wheezing, or lung function [27].
2.3 Vitamin D supplementation and atopic dermatitis
(a) Vitamin D supplementation is beneficial in atopic dermatitis
It has been suggested that vitamin D supplementation (via ultraviolet light exposure or oral supplements) is beneficial for AD. For example, Norwegian children with AD who lived in a sunny subtropical climate in Gran Canary for 4 weeks had significantly improved SCORAD indices and improved values for the Children’s Dermatology Life Quality Index (CDLQI) [28]. These beneficial effects persisted for 3 months upon return to Norway, but the role of sun-derived vitamin D is not specifically mentioned.
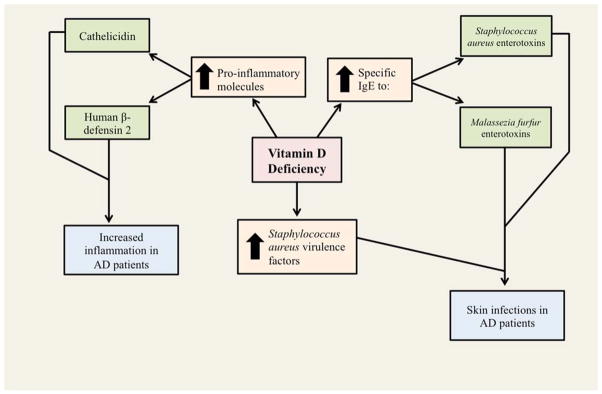
The ability of narrowband ultraviolet B (NB-UVB) treatment to augment systemic vitamin D status in AD patients (and also psoriasis patients) has been investigated [29]. In this study, 16 out of 18 psoriasis patients (89%), 17 out of 18 AD patients (94%), and 8 out of 15 healthy controls (53%) had insufficient levels of vitamin D (<30 ng/mL) prior to 15 NB-UVB treatments, administered three times per week. After these treatments, there was a significant increase in 25[OH]D levels, Psoriasis Area and Severity Indices, and SCORAD indices. Furthermore, although the pro-inflammatory molecules cathelicidin and human beta-defensin 2 (HBD-2) were both elevated prior to NB-UVB treatments, after treatments there were increased levels of cathelicidin and decreased levels of HBD-2. These changes in cathelicidin and HBD-2 may help explain the mechanism behind vitamin D in AD, and deserve our attention in future studies. Several mechanisms of action in the effect of vitamin D in atopic dermatitis have been proposed (Figure 2).
Fig. 2.
Mechanisms explaining the role of Vitamin D deficiency in atopic dermatitis.
The role of oral vitamin D supplementation has also begun to find expression in scientific literature. Oral supplementation would bypass the risks of potentially harmful ultraviolet (UV) radiation, including skin cancer. For example, one case report describes an adolescent girl with severe vitamin D deficiency, causing hypocalcemic rickets [30]. She also had a history of severe AD since childhood. She was treated with cholecalciferol (Vitamin D3) with an initial dose of 150,000 IU followed by 10,000 IU per week for 6 months, and her AD significantly improved.
Although the sample size of 11 patients is low, one study found that in patients with AD, supplementation with 1,000 IU of ergocalciferol daily for one month significantly improved the Investigator’s Global Assessment (IGA) score compared to AD patients who received a placebo [31]. However, the change in the Eczema Area and Severity Index (EASI) was not statistically significant. In a randomized, double blind, placebo-controlled study in Iran, 30 children and adolescents with AD treated with 1,600 IU of cholecalciferol for 60 days had significant improvement in SCORAD and TIS (Three Item Severity Score) values compared to 30 controls [32]. Similarly, in a population of 104 Mongolian children with winter-related AD, there were significant improvements in EASI and the IGA score after one month of vitamin D supplementation (1,000 IU daily) compared to controls [33]. Furthermore, a recent study showed that after three months of supplementation with vitamin D (1,000 IU daily), children with AD had a significant reduction in both the SCORAD index and various cytokines, including IL-2, IL-4, IL-6, and IFN-γ [34]. Therefore, vitamin D supplementation helped to normalize the serum pattern of Th1 and Th2 cytokines in AD. Samochocki et al. [26] also found that in patients with AD and serum 25[OH]D levels less than 15 ng/mL, supplementation with 2,000 IU of vitamin D daily for three months resulted in a decreased SCORAD index and objective SCORAD.
Lastly, Javanbakht et al. [35] performed a randomized double-blind trial involving 45 AD patients that investigated the role of both vitamin D and vitamin E treatment in AD for 60 days. The dose for vitamin D supplementation was 1,600 IU, and the dose for vitamin E supplementation was 600 IU of synthetic all-rac-α-tocopherol. Compared to the placebo group, there was a significant decrease in SCORAD in patients receiving vitamin D supplementation and a vitamin E placebo (by 34.8%), as well as patients receiving vitamin E supplementation and a vitamin D placebo (by 35.7%). In patients receiving both vitamin D and vitamin E supplementation, the decrease in SCORAD was greater (64.3%). This indicates that both of these vitamins could play a role in improving AD.
(b) Vitamin D supplementation is not beneficial in atopic dermatitis
In a prospective birth cohort study performed in Sweden, larger amounts of dietary vitamin D3 intake between 5 and 10 months of age was positively correlated with the development of AD at age 6 [36]. As opposed to the findings described in the previous section, atopic manifestations (AD, allergic rhinitis, and asthma) were more prevalent in children who had a higher intake of vitamin D3 during infancy in this study.
The pathway through which vitamin D could potentially be involved in the onset of AD and other atopic diseases has been proposed. It has been shown that active ligands of the vitamin D receptor induce expression of thymic stromal lymphopoietin (TSLP), a cytokine that contributes to the initiation of a phenotype that resembles AD in mice [37].
2.4 Vitamin D levels during pregnancy and atopic dermatitis
Vitamin D is believed to cross the placenta, and cord blood is another source of vitamin D for a developing fetus [38]. Consequently, vitamin D levels in mothers are correlated with vitamin D levels in their offspring, and the association between vitamin D levels in pregnant women and the incidence of AD in newborns has been investigated. The results thus far have been conflicting (Table 2).
Table 2.
Maternal serum vitamin D and atopic dermatitis (AD) in infants
| Increased maternal vitamin D is associated with AD in infants | Decreased maternal vitamin D is associated with AD in infants | There is no relationship between maternal vitamin D and AD in infants |
|---|---|---|
(a) Increased maternal vitamin D is associated with atopic dermatitis in infants
First, studies suggesting that increased maternal vitamin D could contribute to infantile and childhood AD will be reviewed. In one study, levels of 25[OH]D in the umbilical cord blood were inversely associated with transient early wheezing and AD by age 5 [39]. Furthermore, the risk of AD on examination at 9 months was increased in children whose mothers had a serum 25[OH]D concentration during pregnancy of greater than 75 nmol/L compared to children of mothers with less than 30 nmol/L of serum 25[OH]D level [40].
A study in Japan found that higher maternal intake of vitamin D in pregnancy may increase the risk of infantile AD, although higher maternal intake of dairy products during pregnancy was significantly associated with reduced risk of infantile AD [41]. In this study, 1,354 mother-child pairs from southern Japan were surveyed on their dietary history. Objective information about maternal vitamin D status was not included, and neither was data on vitamin D supplementation during pregnancy.
(b) Decreased maternal vitamin D is associated with atopic dermatitis in infants
Despite studies showing an increased risk of infantile AD with increasing maternal vitamin D levels, there have also been studies showing the opposite. For instance, Chi et al [42] looked at mother infant pairs with family history of asthma from areas in Baltimore, Boston, New York and St. Louis where at least 20% of the population was below the federal poverty level. Serum cord blood levels of vitamin D were assessed as a marker for maternal vitamin D status during pregnancy. Higher vitamin D levels were associated with lower number of regulatory T cells, which could affect immune function later in life, although this study did not specifically mention AD.
Several studies have found relationships between low maternal vitamin D and the development of AD in offspring. For example, an Australian study looked at cord blood levels of 25[OH]D and the risk of childhood AD in a cohort of 231 children [43]. They found a significantly decreased risk of AD in infants with increasing levels of cord blood 25[OH]D, but there were no associations with cord blood levels of 25[OH]D and allergic sensitization, IgE-mediated food allergy, or AD severity based on SCORAD. Jones et al [44] found that infants with higher 25[OH]D levels at birth had lower production of IL-5 and IL-13, which could suggest a protective effect against developing atopic disease.
High prenatal levels of vitamin D have been associated with decreased odds of the development of AD [45], and an additional study in Taiwanese children found that low maternal vitamin D is associated with the development of AD in offspring [46].
(c) There is no relationship between maternal vitamin D and atopic dermatitis in infants
Several studies have found no relationship between AD in infants and maternal vitamin D levels. For instance, Weisse et al [47] found no increased risk of atopic disease with increasing maternal vitamin D levels, although they did find a positive correlation between maternal and cord blood vitamin D levels and the development of food allergy. Pike et al [48] looked at a population of mother-infant pairs from the Southampton Women’s Study. In this study, maternal vitamin D levels were sampled at 34 weeks gestation and the frequencies of vitamin D consumption were recorded at 11 weeks gestation and 34 weeks gestation. Maternal 25[OH]D status at 34 weeks gestation was not associated with atopy at 1, 3, or 6 years of age for the offspring. Wills et al [49] also found no difference in maternal vitamin D levels during pregnancy and the development of AD in their children. In this prospective population-based study in the United Kingdom, they looked at maternal vitamin D levels at any time during pregnancy and assessed atopy when children were 7 years of age. There was no association between maternal vitamin D and the development of AD. Lastly, Chawes et al [50] conducted a prospective study and found that in a cohort of 257 children, deficient levels of 25[OH]D were associated with a 2.7 fold increased risk of troublesome lung symptoms, including recurrent coughing, wheezing, or dyspnea. However, there was no association with AD, respiratory infections, asthma, lung function, allergic sensitization, or rhinitis.
There has been one randomized controlled trial to assess the effect of maternal vitamin D levels of the development of AD in the offspring [51]. In this study, participants were randomized to three groups: no treatment, 800 IU of ergocalciferol daily until delivery or a single dose of 200,000 IU cholecalciferol. This study found no difference between groups in the prevalence of AD.
2.5 Summary of vitamin D and atopic dermatitis
Some studies have found that vitamin D is protective against AD and that vitamin D supplementation can be helpful in AD patients. Because there are limited double-blind placebo control studies, at this time there is insufficient evidence to support the role of vitamin D supplementation in AD patients, especially in children with AD. Results regarding maternal vitamin D status and AD in infants have been inconclusive.
3. Chronic Urticaria
3.1 Background
Urticaria is characterized by the development of wheals (often associated with burning or itching sensation), angioedema, or both [52]. Urticaria, commonly referred to as hives, can be idiopathic or mediated by type I hypersensitivity. Histamine released from mast cells contributes to the clinical symptoms. In addition to mast cells, chronic urticaria involves basophils, dendritic cells, monocytes, and pro-inflammatory cytokines and chemokines [53].
By definition, chronic urticaria consists of almost daily repeated episodes of urticaria that lasts for 6 weeks or longer [54]. The prevalence of chronic urticaria is estimated to be between 0.5–5% of the population, and the incidence is 1.4% per year [55]. Recent research has shown 30–50% of patients with chronic spontaneous urticaria produce an immunoglobulin IgG type auto-antibody against either the high affinity receptor FcεRIα or IgE [56]. The autologous serum skin test (ASST) has been used to test for chronic autoimmune urticaria by detecting auto-antibodies against FcεRIα [53]. The chronic urticaria index is a blood test that is commonly used to determine whether there is an autoimmune basis for urticaria in a particular patient.
3.2 Vitamin D levels in urticaria
Numerous studies have shown that that serum 25[OH]D levels are significantly lower in patients with chronic spontaneous urticaria than controls. The major differences between these studies will be reviewed here.
Whereas Thorp et al. [57] did not report a correlation between vitamin D levels and duration or severity of chronic urticaria, Woo et al. [53] and Chandrashekar et al. [58] found a negative correlation between the severity of urticaria and serum 25[OH]D levels. Furthermore, whereas Thorp et al. [57] and Grzanka et al. [59] found that ASST was not correlated with serum 25[OH]D levels, Chandrashekar et al. [58] and Woo et al. [53] found that in ASST-positive patients, serum 25[OH]D levels were significantly lower than the ASST-negative group. Although more research is needed in this area, these findings suggest that vitamin D deficiency may be a supplementary marker for autoimmune urticaria [53].
Of note, Woo et al. [53] were the only authors to retrospectively compare 4 different groups: a chronic urticaria group of 72 patients, an acute urticaria group of 26 patients, an AD group of 26 patients, and 72 healthy controls. They found that in comparison to the other groups, serum 25[OH]D levels were significantly reduced in the chronic urticaria group. Critically low vitamin D levels (<10 ng/ml) were more prevalent in the chronic urticaria group. Furthermore, these authors suggest that vitamin D deficiency may increase the probability that acute urticaria will progress into chronic urticaria. Among 10 acute urticaria patients with critically low vitamin D levels (<10 ng/mL), 5 cases progressed to the chronic form.
IgE is typically increased in urticaria patients, and the relationship between serum vitamin D and levels of IgE has been investigated. One study found that in vitro IgE production by stimulated B cells is markedly decreased after the administration of vitamin D [60]. It is possible that vitamin D could play a role in urticaria via immunomodulation of IgE-mediated pathways. However, because both immune and non-immune mechanisms give rise to the activation of mast cells in urticaria, the precise mechanistic role of vitamin D in urticaria remains unknown.
3.3 Vitamin D supplementation and chronic urticaria
The role of vitamin D supplementation in chronic urticaria has also been explored. For example, a case report of a 58-year old man with a 10 year history of chronic urticaria was reported to have a serum 25[OH]D level of 4.7 ng/ml, indicating severe vitamin D deficiency [61]. Upon supplementation, he had complete resolution of his chronic urticaria with a vitamin D dose of 2,000 IU daily. Similarly, a retrospective case-series showed that of the 28 urticaria, and angioedema patients with vitamin D levels less than 32 ng/mL, 61% of the patients had a positive response to vitamin D supplementation demonstrated by complete clearance of their symptoms [62]. In a study by Boonpiyathad et al. [63], chronic spontaneous urticaria patients with serum levels of 25[OH]D less than 30 ng/ml were treated with vitamin D2 supplementation. After 6 weeks, these patients showed significant improvement in their Urticaria Activity Score and their Dermatology Life Quality Index scores compared to controls.
A prospective study found that a high dose vitamin D3 supplementation was safe and beneficial in patients with chronic urticaria [64]. The study randomized 42 subjects with chronic urticaria into high (4,000 IU/d) or low (600 IU/d) vitamin D3 supplementation for 12 weeks in addition to a triple drug therapy consisting of cetirizine, ranitidine, and montelukast. Although the triple-drug therapy decreased total Urticaria Symptom Severity (USS) scores by 33% in the first week, subjects receiving the high dose vitamin D3 had a further decreases by 40% by week 12. The patients on high dose vitamin D3 had fewer days with urticaria as well as decreased body distribution of urticaria.
Lastly, a recent prospective case-control study comparing vitamin D levels in 58 chronic spontaneous urticaria (CSU) patients and 45 controls found that vitamin D levels were significantly lower in CSU patients [65]. Patients with 25[OH]D levels less than 30 ng/mL were treated with 300,000 IU/month of vitamin D3 supplementation. After 12 weeks, CSU patients showed significant improvements in urticaria activity score (UAS4) and Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL).
3.4 Summary of vitamin D and chronic urticaria
Patients with chronic urticaria have been shown to have lower serum vitamin D levels, and vitamin D supplementation has been beneficial for several chronic urticaria patients. Due to the limited number of studies highlighting the benefits of vitamin D in chronic urticaria, there is currently insufficient evidence to support vitamin D supplementation in these patients.
4. Allergic Contact Dermatitis
4.1 Background
Contact dermatitis is an inflammation of the skin upon exposure to a particular substance. Allergic contact dermatitis (ACD) and irritant contact dermatitis (ICD) are the two major subtypes. The focus of this paper is on ACD, which is characterized by redness, and papules with our without vesicles, followed by scaling and dryness [66, 67].
As opposed to AD and chronic urticaria, ACD involves a Type IV cell mediated hypersensitivity (also known as delayed type hypersensitivity) with Th1 lymphocytes [68]. Small molecular weight haptens or haptens conjugated to proteins induce keratinocytes to release pro-inflammatory cytokines [69]. The activated keratinocytes migrate to regional draining lymph nodes where native Th0 cells are activated. These cells mature into hapten-specific T Regulatory cells and Th1 cells, which remain behind at the site. Subsequent exposure to the same substance results in a shortened latent period [69].
Currently, a topical corticosteroid is the first-line treatment for ACD. For more extensive and severe cases, systemic corticosteroids are often used. Other modes of therapy for ACD are calcineurin inhibitors, UV light treatment and immunomodulating agents [69].
4.2 Vitamin D and allergic contact dermatitis in mice
One animal study has investigated the role of vitamin D in ACD. Male mice with normal levels of vitamin D were shown to be more responsive at repressing ACD compared to mice that were lacking vitamin D, although this relationship was not found in female mice [70]. The mechanism behind the different responses in male and female mice remains unknown. This animal study indicates that vitamin D could potentially be beneficial in ACD in humans. However, there is limited research on vitamin D supplementation for humans with ACD, highlighting the need for future investigation in this area.
4.3 Summary of vitamin D and allergic contact dermatitis
Only one mouse study has found that mice with higher serum vitamin D levels could be more likely to repress ACD. To date, there are no studies that suggest that vitamin D may play a role in ACD in humans. Therefore, vitamin D supplementation in ACD is not recommended and warrants clinical studies.
5. Expert Commentary and Five-Year View
5.1 Atopic Dermatitis
Since antimicrobial peptide (AMP) is decreased in AD, resulting in compromised innate immunity, a recent study questioned whether supplementation with vitamin D would increase AMP production and consequently improve the ability of people with AD to prevent bacterial and viral infections of the skin [71]. After 21 days of receiving 4000 IU of cholecalciferol daily, measurements of AMPs such as cathelicidin, human beta-defensin 3 (HBD-3), and IL-13 were unchanged in patients with AD along with the EASI remaining unchanged over the 21-day period. Therefore, more research is needed regarding the role of vitamin D in preventing skin infections in AD via alterations in AMPs.
One potential reason for the conflicting results about the role of vitamin D in AD involves variations in the vitamin D receptor (VDR) gene. Four different VDR single nucleotide polymorphisms (SNPs) were shown to have increased expression in patients with AD, compared to healthy controls [72]. Moreover, the VDR haplotype GCT was correlated with AD severity. Due to altered expression of VDR in GCT carriers, epidermal barrier function and local skin immunity could be jeopardized, leading to a higher likelihood of skin infections and subsequent AD exacerbations. Additional environmental and genetic factors clearly also play a part in AD severity, but a patient’s particular VDR genotype is a notable cofactor.
Genotypes were also investigated in a recent study by Hallau et al. [73]. They particularly looked at genes encoding for the vitamin D synthesizing enzyme Cyp27b1 and the vitamin D inactivating enzyme Cyp24a1. In 281 patients with AD, the Cyp24a1 rs2248359 SNP C allele was over-represented, in comparison to 278 healthy controls. Halotypes of genes for Cyp27b1 and Cyp24a1 were also associated with AD. Therefore, patients with AD and certain genotypes may have decreased VDR activity, which could contribute to the pathogenesis of AD. This further suggests that future studies investigating the relationship between vitamin D and AD should take individual genotypes into consideration.
There are several other important factors that deserve future attention. First, since the dose of vitamin supplementation in the current literature ranges anywhere from 1,000 IU to 4,000 IU daily, with variable duration of treatment, more standardization in this area would be helpful. Because most supplementation studies last for one to three months, more long-term data will also be necessary. Furthermore, instead of utilizing a standardized scoring system, current studies utilize variable tools to quantify AD severity, including SCORAD, EASI, and several other severity measures. Standardization in this area would also be helpful. In essence, future research involving large prospective studies would help elucidate whether supplemental vitamin D should be considered as an adjuvant treatment for AD, particularly in the winter months and in vitamin D deficient patients.
5.2 Chronic Urticaria
Whereas the role of vitamin D in AD has produced conflicting results in the literature, studies on chronic urticaria are more unanimous in their findings, indicating that it may be beneficial to assess vitamin D status in these patients. However, it is important to note that overall there have been significantly less studies involving vitamin D and chronic urticaria than AD. Prospective clinical studies with large sample sizes will be necessary before any definitive conclusions about the relationship between vitamin D and chronic urticaria can be made. Further studies should also elucidate the most beneficial dose of vitamin D supplementation for chronic urticaria. Lastly, continued research about the mechanism of action of vitamin D in chronic urticaria would be useful.
5.3 Allergic Contact Dermatitis
Low-dose UV radiation (UVR) is a possible therapy for ACD that needs further clinical investigations as UVR contributes to vitamin D stores. As previously mentioned, studies involving vitamin D levels in human with ACD would help clarify its role in this particular allergic skin condition.
6. Key Issues.
Although the precise mechanism of vitamin D in inflammatory skin conditions like AD, chronic urticaria, and ACD remains unknown, it may be related to its modulatory function on various immune cells and the barrier function of the skin.
Vitamin D enhances defense mechanisms within the skin barrier and generally decreases inflammation in the innate and adaptive immune system.
The relationship between vitamin D levels and AD has resulted in mixed findings, with the majority of studies favoring an inverse relationship between elevated serum vitamin D levels and AD severity.
Genetic differences in the vitamin D receptor and/or vitamin D synthesizing and inactivating enzymes may help explain the conflicting results in studies on the role of vitamin D in AD.
An inverse relationship between elevated serum vitamin D levels and chronic urticaria severity has been shown in several studies, but there are significantly less studies involving chronic urticaria than AD.
Whereas an animal study demonstrated an inverse relationship between vitamin D levels and ACD severity, human studies in this area are non-existent at this time.
Future large-scale prospective should assess the role of vitamin D as a possible supplemental therapy for allergic skin conditions.
Serum levels of vitamin D should be further investigated as a potential marker for disease progression and severity in these three conditions.
Footnotes
Declaration Interests
This work was supported by research grants R01 HL116042, R01 HL112597, and R01 HL120659 to DK Agrawal from the Office of the Dietary Supplement, NIH Director’s Office and the National Heart, Lung and Blood Institute, NIH, USA. The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1*.Muehleisen B, Gallo RL. Vitamin D in allergic disease: Shedding light on a complex problem. J Allergy Clin Immunol. 2013;131(2):324–9. doi: 10.1016/j.jaci.2012.12.1562. This elucidates the role of vitamin D in the immune system. [DOI] [PubMed] [Google Scholar]
- 2.Poduje S, Sjerobabski-Masnec I, Ozanić-Bulić S. Vitamin D—the true and the false about vitamin D. Coll Antropol. 2008;32(Suppl 2):159–62. [PubMed] [Google Scholar]
- 3.Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2(1):76–89. doi: 10.1016/S2213-8587(13)70165-7. [DOI] [PubMed] [Google Scholar]
- 4.Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Rheum Dis Clin North Am. 2012;38(1):125–39. doi: 10.1016/j.rdc.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Baeke F, Takiishi T, Korf H, Gysemans C, et al. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10(4):482–96. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 7.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179(4):2060–3. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 8.Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy. 2014;69(1):3–16. doi: 10.1111/all.12270. [DOI] [PubMed] [Google Scholar]
- 9.Scaria S, James E, Dharmaratnam AD. Epidemiology and treatment pattern of atopic dermatitis in patients attending a tertiary care teaching hospital. Int J Res Pharm Sci. 2011;2(11):38–44. [Google Scholar]
- 10.Hoffjan S, Stemmler S. Unravelling the complex genetic background of atopic dermatitis: from genetic association results towards novel therapeutic strategies. Arch Dermatol Res. 2015;307(8):659–70. doi: 10.1007/s00403-015-1550-6. [DOI] [PubMed] [Google Scholar]
- 11.Bieber T. Atopic dermatitis. New Engl J Med. 2008;358(14):1483–94. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 12.Hata TR, Gallo RL. Antimicrobial peptides, skin infections and atopic dermatitis. Semin Cutan Med Surg. 2008;27(2):144–50. doi: 10.1016/j.sder.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oren E, Bamerji A, Camargo CA. Vitamin D and atopic disorders in an obese population screened for vitamin D deficiency. J Allergy Clin Immunol. 2008;121(2):533–4. doi: 10.1016/j.jaci.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Cheng HM, Kim S, Park GH, et al. Low vitamin D levels are associated with atopic dermatitis, but not allergic rhinitis, asthma, or IgE sensitization, in the adult Korean population. J Allergy Clin Immunol. 2014;133(4):1048–55. doi: 10.1016/j.jaci.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 15.Peroni DG, Piacentini GL, Cametti E, et al. Correlation between serum 25-hydroxyvitamin D levels and severity of atopic dermatitis in children. Br J Dermatol. 2011;164(5):1078–82. doi: 10.1111/j.1365-2133.2010.10147.x. [DOI] [PubMed] [Google Scholar]
- 16.Cheon BR, Shin JE, Kim YJ. Relationship between serum 25-hydroxyvitamin D and interleukin-31 levels, and the severity of atopic dermatitis in children. Korean J Pediatr. 2015;58(3):96–101. doi: 10.3345/kjp.2015.58.3.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baek JH, Shin YH, Chung IH, et al. The link between serum vitamin D level, sensitization to food allergens, and the severity of atopic dermatitis in infancy. J Pediatr. 2014;165(4):849–54. doi: 10.1016/j.jpeds.2014.06.058. [DOI] [PubMed] [Google Scholar]
- 18.Wang SS, Hon KL, Kong AP, et al. Vitamin D deficiency is associated with diagnosis and severity of childhood atopic dermatitis. Pediatr Allergy Immunol. 2014;25(1):30–5. doi: 10.1111/pai.12167. [DOI] [PubMed] [Google Scholar]
- 19.Han TY, Kong TS, Kim MH, et al. Vitamin D status and its association with the SCORAD score and serum LL-37 level in Korean adults and children with atopic dermatitis. Ann Dermatol. 2015;27(1):10–14. doi: 10.5021/ad.2015.27.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akan A, Azkur D, Ginis T, et al. Vitamin D level in children is correlated with severity of atopic dermatitis but only in patients with allergic sensitizations. Pediatr Dermatol. 2013;30(3):359–63. doi: 10.1111/pde.12058. [DOI] [PubMed] [Google Scholar]
- 21.Lee SA, Hong S, Kim HJ, et al. Correlation between serum vitamin D level and the severity of atopic dermatitis associated with food sensitization. Allergy Asthma Immunol Res. 2013;5(4):207–10. doi: 10.4168/aair.2013.5.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilaberte Y, Sanmartín R, Aspiroz C, et al. Correlation between serum 25-hydroxyvitamin D and virulence genes of Staphylococcus aureus isolates colonizing children with atopic dermatitis. Pediatr Dermatol. 2015;34(4):506–13. doi: 10.1111/pde.12436. [DOI] [PubMed] [Google Scholar]
- 23.Heimbeck I, Wjst M, Apfelbacher CJ. Low vitamin D serum level is inversely associated with eczema in children and adolescents in Germany. Allergy. 2013;68(7):906–10. doi: 10.1111/all.12167. [DOI] [PubMed] [Google Scholar]
- 24.Wawro N, Heinrich J, Thiering E, et al. Serum 25(OH)D concentrations and atopic diseases at age 10: results from the GINIplus and LISAplus birth cohort studies. BMC Pediatr. 2014;14:286. doi: 10.1186/s12887-014-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu YE, Havens PL, Siegel DH, et al. Serum 25-hydroxyvitamin D concentration does not correlate with atopic dermatitis severity. J Am Acad Dermatol. 2013;69(1):40–6. doi: 10.1016/j.jaad.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samochocki Z1, Bogaczewicz J, Jeziorkowska R, et al. Vitamin D effects in atopic dermatitis. J Am Acad Dermatol. 2013;69(2):238–44. doi: 10.1016/j.jaad.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Thuesen BH, Heede NG, Tang L. No association between vitamin D and atopy, asthma, lung function or atopic dermatitis: a prospective study in adults. Allergy. 2015;70(11):1501–4. doi: 10.1111/all.12704. [DOI] [PubMed] [Google Scholar]
- 28.Byremo G, Rød G, Carlsen KH. Effect of climatic change in children with atopic eczema. Allergy. 2006;61(12):1403–10. doi: 10.1111/j.1398-9995.2006.01209.x. [DOI] [PubMed] [Google Scholar]
- 29*.Vähävihu K, Ala-Houhala M, Peric M, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol. 2010;163(2):321–8. doi: 10.1111/j.1365-2133.2010.09767.x. This elucidates the role of NB-UVB in increasing serum 25[OH]D levels, increasing cathelicidin, and decreasing HBD-2 in AD patients. [DOI] [PubMed] [Google Scholar]
- 30.Borzutzky A, Grob F, Camargo CA, Martinez-Aguayo A. Vitamin D deficiency rickets in an adolescent with severe atopic dermatitis. Pediatrics. 2014;133(2):e451–4. doi: 10.1542/peds.2013-1114. [DOI] [PubMed] [Google Scholar]
- 31.Sidbury R, Sullivan AF, Thadhani Rl, Camargo CA., Jr Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: a pilot study. Br J Dermatol. 2008;159(1):245–7. doi: 10.1111/j.1365-2133.2008.08601.x. [DOI] [PubMed] [Google Scholar]
- 32.Amestejani M, Salehi BS, Vasigh M, et al. Vitamin D supplementation in the treatment of atopic dermatitis: A clinical trial study. J Drugs Dermatol. 2012;11(3):327–30. [PubMed] [Google Scholar]
- 33.Camargo CA, Ganmaa D, Sidbury R, et al. Randomized trial of vitamin D supplementation for winter-related atopic dermatitis in children. J Allergy Clin Immunol. 2014;134(4):831–5. doi: 10.1016/j.jaci.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Di Filippo P, Scaparrotta A, Rapino D, et al. Vitamin D supplementation modulates the immune system and improves atopic dermatitis in children. Int Arch Allergy Immunol. 2015;166(2):91–6. doi: 10.1159/000371350. [DOI] [PubMed] [Google Scholar]
- 35*.Javanbakht MH, Keshavarz SA, Djalali M, et al. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J Dermatolog Treat. 2011;22(3):144–50. doi: 10.3109/09546630903578566. This randomized double-blind trial suggests that both vitamins D and E could be beneficial in treating AD. [DOI] [PubMed] [Google Scholar]
- 36.Bäck O, Blomquist H, Hernell O, Stenberg B. Does vitamin D intake during infancy promote the development of atopic allergy? Acata Derm Venereol. 2009;89(1):28–32. doi: 10.2340/00015555-0541. [DOI] [PubMed] [Google Scholar]
- 37.Li M, Hener P, Zhang Z, et al. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci USA. 2006;103:11736–41. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salle BL, Delvin EE, Lapillonne A, et al. Perinatal metabolism of vitamin D. Am J Clin Nutr. 2000;71(5 Suppl):1317S–24S. doi: 10.1093/ajcn/71.5.1317s. [DOI] [PubMed] [Google Scholar]
- 39.Baïz N, Dargent-Molina P, Wark JD, et al. Cord serum 25-hydroxyvitamin D and risk of early childhood transient wheezing and atopic dermatitis. J Allergy Clin Immunol. 2014;133(1):147–53. doi: 10.1016/j.jaci.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Gale CR, Robinson SM, Harvey NC, et al. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62(1):68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyake Y, Tanaka K, Okubo H, et al. Maternal consumption of dairy products, calcium and vitamin D during pregnancy and infantile allergic disorders. Ann Allergy Asthma Immunol. 2014;113:82–7. doi: 10.1016/j.anai.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 42.Chi A, Wildfire J, McLoughlin R. Umbilical cord plasma 25-hydroxyvitamin D concentration and immune function at birth: the Urban Environment and Childhood Asthma study. Clin Exp Allergy. 2011;41(6):842–50. doi: 10.1111/j.1365-2222.2011.03712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones AP, Palmer D, Zhang G, et al. Cord blood 25-hydroxyvitamin D3 and allergic disease during infancy. Pediatrics. 2012;130(5):e1128–35. doi: 10.1542/peds.2012-1172. [DOI] [PubMed] [Google Scholar]
- 44.Jones AP, D’Vaz N, Meldrum S, et al. 25-hydroxyvitamin D3 status is associated with developing adaptive and innate immune responses in the first 6 months of life. Clin Exp Allergy. 2015;45(1):220–31. doi: 10.1111/cea.12449. [DOI] [PubMed] [Google Scholar]
- 45.Wegienka G, Havstad S, Zoratti EM, et al. Association between vitamin D levels and allergy related outcomes vary by race and other factors. J Allergy Clin Immunol. 2015;136(5):1309–1314. e4. doi: 10.1016/j.jaci.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiu CY, Huang SY, Peng EC, et al. Maternal vitamin D levels are inversely related to allergic sensitization and atopic diseases in early childhood. Pediatr Allergy Immunol. 2015;26(4):337–43. doi: 10.1111/pai.12384. [DOI] [PubMed] [Google Scholar]
- 47.Weisse K, Winkler S, Hirche F, et al. Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy. 2013;68:220–8. doi: 10.1111/all.12081. [DOI] [PubMed] [Google Scholar]
- 48.Pike KC, Inskip MH, Robinson S, et al. Maternal late-pregnancy serum 25-hydroxyvitamin D in relation to childhood wheeze and atopic outcomes. Thorax. 2012;67:950–6. doi: 10.1136/thoraxjnl-2012-201888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wills AK, Shaheen SO, Granell R, et al. Maternal 25-hydroxyvitamin D and its association with childhood atopic outcomes and lung function. Clin Exp Allergy. 2013;43(10):1180–8. doi: 10.1111/cea.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chawes BL, Bønnelykke K, Jensen PF, et al. Cord blood 25(OH)-vitamin D deficiency and childhood asthma, allergy and eczema: the COPSAC2000 Birth Cohort Study. PLoS One. 2014;9(6):e99856. doi: 10.1371/journal.pone.0099856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldring ST, Griffiths CJ, Martineau AR, et al. Prenatal vitamin D supplementation and child respiratory health: A randomised controlled trial. PLoS One. 2013;8(6):e66627. doi: 10.1371/journal.pone.0066627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA2LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69:868–87. doi: 10.1111/all.12313. [DOI] [PubMed] [Google Scholar]
- 53*.Woo YR, Jung KE, Koo DW, Lee JS. Vitamin D as a marker for disease severity in chronic urticaria and its possible role in pathogenesis. Ann Dermatol. 2015;27(4):423–30. doi: 10.5021/ad.2015.27.4.423. This study retrospectively compared serum vitamin D levels in patients with chronic urticaria, patients with AD, and healthy controls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Powell RJ, Du Toit GL, Siddique N, et al. British Society for Allergy and Clinical Immunology (BSACI). BSACI guidelines for the management of chronic urticaria and angio-oedema. Clin Exp Allergy. 2007;37:631–50. doi: 10.1111/j.1365-2222.2007.02678.x. [DOI] [PubMed] [Google Scholar]
- 55.Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133(5):1270–7. doi: 10.1016/j.jaci.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 56.Ferrante G, Scavone V, Muscia MC, et al. The care pathway for children with urticaria, angioedema, mastocytosis. World Allergy Organization J. 2015;8(5):1–10. doi: 10.1186/s40413-014-0052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thorp WA, Goldner W, Meza J, Poole JA. Reduced vitamin D levels in adult subjects with chronic urticaria. J Allergy Clin Immunol. 2010;126(2):413. doi: 10.1016/j.jaci.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 58.Chandrashekar L, Rajappa M, Munisamy M, et al. 25-Hydroxy vitamin D levels in chronic urticaria and its correlation with disease severity from a tertiary care centre in South India. Clin Chem Lab Med. 2014;52(6):e115–8. doi: 10.1515/cclm-2013-1014. [DOI] [PubMed] [Google Scholar]
- 59.Grzanka A, Machura E, Mazur B, et al. Relationship between vitamin D status and the inflammatory state in patients with chronic spontaneous urticaria. J Inflam (Lond) 2014;11(2):1–5. doi: 10.1186/1476-9255-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heine G, Anton K, Henz BM, Worm M. 1α,25-Dihydroxyvitamin D3 inhibits anti-CD40 plus IL-4-mediated IgE production in vitro. Eur J Immunol. 2002;32(12):3395–404. doi: 10.1002/1521-4141(200212)32:12<3395::AID-IMMU3395>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 61.Sindher S, Jariwala S, Gilbert J, Rosenstreich D. Resolution of chronic urticaria coincident with vitamin D supplementation. Ann Allergy Asthma Immunol. 2012;109(5):359–60. doi: 10.1016/j.anai.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 62.Goetz D. Idiopathic itch, rash, and urticaria/angioedema merit serum vitamin D evaluation: a descriptive case series. W VA Med J. 2011;107(1):14–20. [PubMed] [Google Scholar]
- 63.Boonpiyathad T, Pradubpongsa P, Sangasapaviriya A. Vitamin D supplements improve urticaria symptoms and quality of life in chronic spontaneous urticaria patients. Dermatoendocrinol. 2014 doi: 10.4161/derm.29727. published online 16 July 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rorie A, Goldner WS, Lyden E, Poole JA. Beneficial role for supplemental vitamin D3 treatment in chronic urticaria: a randomized study. Ann Allergy Asthma Immunol. 2014;112(4):376–82. doi: 10.1016/j.anai.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 65.Oguz Topal I, Kocaturk E, Gungor S, et al. Does replacement of vitamin D reduce the symptom scores and improve quality of life in patients with chronic urticaria? J Dermatolog Treat. 2016;27(2):163–6. doi: 10.3109/09546634.2015.1079297. [DOI] [PubMed] [Google Scholar]
- 66.Kaplan DH, Igyarto BZ, Gaspari AA. Early immune events in the induction of allergic contact dermatitis. Nat Rev Immunol. 2012;12(2):114–24. doi: 10.1038/nri3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Röse L, Schneider C, Stock C, et al. Extended DNFB-induced contact hypersensitivity models display characteristics of chronic inflammatory dermatoses. Exp Dermatol. 2012;21(1):25–31. doi: 10.1111/j.1600-0625.2011.01395.x. [DOI] [PubMed] [Google Scholar]
- 68.Shi D, Li X, Li D, et al. Oral administration of paeoniflorin attenuates allergic contact dermatitis by inhibiting dendritic cell migration and Th1 and Th17 differentiation in a mouse model. Int Immunopharmacol. 2015;25(2):432–9. doi: 10.1016/j.intimp.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 69.Fonacier L, Dreskin S, Leung D. Allergic skin diseases. J Allergy Clin Immuol. 2010;125(2):S138–49. doi: 10.1016/j.jaci.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 70*.Malley RC, Muller HK, Norval M, Woods GM. Dietary vitamin D alters the response of the skin to UVB-irradiation depending on the genetic background of the mice. Photochem Photobiol Sci. 2013;12(3):536–45. doi: 10.1039/c2pp25211b. This is the only study involving the role of vitamin D in ACD, and it involved mice. [DOI] [PubMed] [Google Scholar]
- 71.Hata TR, Audish D, Kotol P, et al. A randomized controlled double-blind investigation of the effects of vitamin D dietary supplementation in subjects with atopic dermatitis. JEADV. 2013;28(6):781–9. doi: 10.1111/jdv.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72*.Heine G, Hoefer N, Franke A, et al. Association of vitamin D receptor gene polymorphisms with severe atopic dermatitis in adults. Br J Dermatol. 2013;168(4):855–8. doi: 10.1111/bjd.12077. Differences in VDR genotypes may help explain the conflicting results about the role of vitamin D in AD. [DOI] [PubMed] [Google Scholar]
- 73*.Hallau J, Hamann L, Schumann RR, et al. A Promoter Polymorphism of the Vitamin D Metabolism Gene Cyp24a1 is Associated with Severe Atopic Dermatitis in Adults. Acta Derm Venereol. 2015 doi: 10.2340/00015555-2226. published online 28 August 2015. Differences in vitamin D synthesizing and inactivating enzymes may help explain the conflicting results about the role of vitamin D in AD. [DOI] [PubMed] [Google Scholar]