Abstract
BACKGROUND
In response to rising rates of opioid abuse and overdose, U.S. states enacted laws to restrict the prescribing and dispensing of controlled substances. The effect of these laws on opioid use is unclear.
METHODS
We tested associations between prescription-opioid receipt and state controlled-substances laws. Using Medicare administrative data for fee-for-service disabled beneficiaries 21 to 64 years of age who were alive throughout the calendar year (8.7 million person-years from 2006 through 2012) and an original data set of laws (e.g., prescription-drug monitoring programs), we examined the annual prevalence of beneficiaries with four or more opioid prescribers, prescriptions yielding a daily morphine-equivalent dose (MED) of more than 120 mg, and treatment for nonfatal prescription-opioid overdose. We estimated how opioid outcomes varied according to eight types of laws.
RESULTS
From 2006 through 2012, states added 81 controlled-substance laws. Opioid receipt and potentially hazardous prescription patterns were common. In 2012 alone, 47% of beneficiaries filled opioid prescriptions (25% in one to three calendar quarters and 22% in every calendar quarter); 8% had four or more opioid prescribers; 5% had prescriptions yielding a daily MED of more than 120 mg in any calendar quarter; and 0.3% were treated for a nonfatal prescription-opioid overdose. We observed no significant associations between opioid outcomes and specific types of laws or the number of types enacted. For example, the percentage of beneficiaries with a prescription yielding a daily MED of more than 120 mg did not decline after adoption of a prescription-drug monitoring program (0.27 percentage points; 95% confidence interval, −0.05 to 0.59).
CONCLUSIONS
Adoption of controlled-substance laws was not associated with reductions in potentially hazardous use of opioids or overdose among disabled Medicare beneficiaries, a population particularly at risk.
States have responded to rising rates of prescription-opioid overdose by adopting laws that restrict the prescribing and dispensing of controlled substances. In 2010, after the adoption of many new controlled-substance restrictions, rates of prescription-opioid overdose dipped slightly before reaching a historic high in 2014.1–3 The relationship between legal restrictions and prescription-opioid use remains unclear, because previous research evaluated one or two laws, short time periods, or few states.4–6 Comprehensive national analyses of controlled-substance restrictions and prescription-opioid use do not yet exist.
Successful regulation of prescription opioids involves a difficult balance. Well-designed laws may reduce misuse and overdose. However, laws may also obstruct compassionate pain management and increase provider burden.7 Moreover, heavily promoted strategies, such as prescription-drug monitoring programs (PDMPs), are expensive to implement.8 Understanding the correlates of controlled-substance laws may help to promote safe, effective use of opioid analgesics and inform state investments.
To understand the relationship between state controlled-substance restrictions and the behaviors that they target, we examined associations between prescription-opioid outcomes and eight types of controlled-substance restrictions over a period of 7 years in a large national sample of patients. Our population consisted of disabled Medicare beneficiaries younger than 65 years of age, half of whom use opioids in a given year.2
METHODS
STUDY POPULATION
For each calendar year 2006 through 2012, we created cohorts from a random 40% sample of all Medicare beneficiaries (see the Supplementary Appendix, available with the full text of this article at NEJM.org). Cohorts included beneficiaries 21 to 64 years of age, from 50 U.S. states and the District of Columbia, who were enrolled in fee-for-service Medicare Parts A, B, and D (inpatient, outpatient, and prescription benefits) and were alive throughout the calendar year. Because controlled-substance restrictions are not designed to curb prescriptions for pain at the end of life, we excluded patients with cancer diagnoses or end-stage renal disease or who were receiving hospice care (Table S1 in the Supplementary Appendix).
OUTCOMES — FILLING OF OPIOID PRESCRIPTIONS AND NONFATAL OVERDOSE EVENTS
Using Medicare claims, we created annual measures indicating whether a beneficiary filled an opioid analgesic prescription in every calendar quarter in a given year (long-term receipt) or in one to three calendar quarters (non–long-term receipt), filled prescriptions from four or more prescribers, and filled prescriptions that resulted in a daily morphine-equivalent dose (MED) of more than 120 mg in any calendar quarter (for opioid conversion factors used to compute the MED, see Table S2 in the Supplementary Appendix).2 The last two measures are associated with opioid overdose.9–14 We measured nonfatal prescription-opioid overdose on the basis of primary or secondary diagnosis codes in emergency department and inpatient claims, excluding heroin overdose (Table S3 in the Supplementary Appendix).10 To address possible correlation across outcomes and to increase statistical precision, we developed a measure — potentially adverse opioid outcomes — that was equal to the sum of long-term receipt, a daily MED of more than 120 mg, four or more prescribers, and nonfatal prescription-opioid overdose (range, 0 to 4).
STATE LAWS
Using state statutes, regulations, and existing surveys, the two authors at the UCLA School of Law constructed a data set of legal restrictions of controlled substances in all states and the District of Columbia from 2006 through 2012.15–19 (A spreadsheet of data on controlled-substance laws by state and year as used in this study and information on each opioid-related outcome used in our study summarized by state and year are available at www.dartmouthdiffusion.org/opioids.php.) We included laws governing patients, prescribers, or dispensing pharmacists that involve quantitative prescription limits, patient identification requirements, requirements with respect to physician examination or pharmacist verification, doctor-shopping restrictions, PDMPs, requirements related to tamper-resistant prescription forms, and pain-clinic regulations (Table S4 in the Supplementary Appendix). We coded states with an authorizing statute but no active PDMP as not having a PDMP.20 We measured state controlled-substance laws according to whether each of eight types of laws was operating throughout the year in that state; according to legislative intensity, the number of types of laws added since baseline (2006) in each year and state, coded as indicators (1, 2, or ≥3 types of laws added; 0 is the reference) to allow for nonlinear effects; and according to the number of types of laws (as a single continuous variable) in place in a given state and year (range, 0 to 8).
COVARIATES
Control variables included each beneficiary’s demographic characteristics in each year: sex, race, ethnic group,21 Medicaid enrollment, receipt of any Part D low-income subsidy as an indicator of poverty, and 5-year age categories. We used inpatient and outpatient claims to identify diagnoses of depression, serious mental illness, and alcohol abuse (Table S1 in the Supplementary Appendix).22–24 We calculated individual-level prescription-drug Hierarchical Condition Category (RxHCC) risk scores25; this measure of expected prescription-drug spending is derived from administrative claims and is used by the Centers for Medicare and Medicaid Services to calculate risk-adjusted payments to prescription-drug plans.26 Given reported negative associations between deaths from prescription-opioid overdose and medical-marijuana laws, we controlled for state-level medical-marijuana laws.27,28
STATISTICAL ANALYSIS
To quantify the magnitude of hazardous use of prescription opioids, we used National Death Index Plus data for 2008 (the latest available year) to compute 1-year rates of death due to prescription-opioid overdose, excluding deaths involving heroin (for the codes used to identify fatal opioid-related overdoses, see Table S5 in the Supplementary Appendix). We compared these rates with the 2008 rate of death from prescription-opioid overdose in the U.S. population.29
We computed the annual unadjusted percentage of beneficiaries with each prescription-opioid outcome. We fit logistic regressions of each opioid measure as a function of time-varying individual types of laws operating in that year within a beneficiary’s state of residence, hypothesizing that state laws would be associated with lower values of each prescription-opioid outcome. Control variables included patient characteristics, year (indicators for each year 2007 to 2012) to account for time trends in opioid outcomes, and an indicator for state of residence. We used similar models, replacing individual types of laws with three variables indicating the number of types of laws added since 2006 (1, 2, or ≥3 types of laws added; the reference group was 0 types added) in a given state and year.
We present 95% confidence intervals based on Huber–White adjusted standard errors, with the assumption that observations are independent across, but not within, states over time. Although this method does not explicitly account for beneficiary-level autocorrelation within states, accounting for individual autocorrelation yields similar findings (see the Supplementary Appendix for information on alternative models to account for correlation within individuals nested within states).
We performed two secondary analyses (results of these analyses are shown in the Supplementary Appendix). First, we fitted the logistic-regression models described above to the subpopulation of long-term users of opioids, hypothesizing that associations with laws would be stronger in this group. Second, we plotted state-level changes in each opioid measure from 2006 through 2012 and the number of types of laws added from 2006 through 2012, reporting the slope of the fitted line for each graph.
We report confidence intervals using two-tailed tests and a 0.05 significance level. For all tests of the primary hypothesis, we also report P values based on Benjamini–Hochberg adjustments for multiple comparisons; this accounts for the chance of a false positive, which increases with the number of hypotheses examined (for details of this adjustment, see the Supplementary Appendix).30,31
RESULTS
POPULATION CHARACTERISTICS AND PRESCRIPTION-OPIOID RECEIPT
In our sample of 2.2 million beneficiaries (8.7 million person-years), representing all 50 states and the District of Columbia, the mean age was 49 years, and 49.7% were women (Table 1). On average, 45.4% of beneficiaries filled one or more opioid prescriptions in a given calendar year; 20.1% had long-term receipt of opioids and 25.3% had non–long-term receipt. In comparisons of beneficiaries with long-term receipt of opioids versus those with non–long-term receipt, a diagnosis of depression was more common (35.1% vs. 29.2%) and serious mental illness was less common (5.8% vs. 8.0%). The percentage of beneficiaries with four or more opioid prescribers was 7.5%, and 5.6% of the beneficiaries had a daily MED of more than 120 mg in one calendar quarter or more. In 2008, the rate of death from prescription-opioid overdose in our sample was nearly 10 times the U.S. rate (46.6 vs. 4.8 per 100,000).
Table 1.
Characteristics of Disabled Medicare Beneficiaries and Prescription-Opioid Use, 2006–2012.*
| Characteristic | Full Sample (N = 8,693,212 person-yr) |
Non–Long-Term Receipt of Opioids (N = 2,195,095 person-yr) |
Long-Term Receipt of Opioids (N = 1,749,141 person-yr) |
|---|---|---|---|
| Age (yr) | 48.9±10.5 | 48.8±10.6 | 51.2±8.7 |
| Female sex (% of person-yr) | 49.7 | 56.0 | 57.8 |
| Race or ethnic group (% of person-yr)† | |||
| White non-Hispanic | 67.5 | 66.3 | 75.9 |
| Black non-Hispanic | 20.2 | 21.6 | 15.9 |
| Other | 12.3 | 12.1 | 8.2 |
| Medicare Part D low-income subsidy (% of person-yr)‡ |
87.6 | 87.8 | 86.5 |
| Medicaid enrollment for ≥1 mo (% of person-yr) | 74.6 | 75.6 | 73.1 |
| RxHCC risk score§ | 1.1±0.6 | 1.2±0.6 | 1.3±0.6 |
| Behavioral health diagnoses (% of person-yr)¶ | |||
| Depression | 23.6 | 29.2 | 35.1 |
| Serious mental illness | 7.2 | 8.0 | 5.8 |
| Alcohol abuse | 3.2 | 4.3 | 3.2 |
| Opioid measures (% of person-yr) | |||
| Filling of ≥1 opioid prescription | 45.4 | 100 | 100 |
| Non–long-term receipt of opioids | 25.3 | 100 | 0 |
| Long-term receipt of opioids | 20.1 | 0 | 100 |
| ≥4 Unique opioid prescribers | 7.5 | 7.8 | 27.4 |
| Daily MED >120 mg in any quarter‖ | 5.6 | 2.2 | 25.2 |
| Any nonfatal prescription-opioid overdose (% of person-yr)** |
0.28 | 0.32 | 0.85 |
Plus–minus values are means ±SD. Long-term receipt was defined as the filling of one or more opioid prescriptions in each calendar quarter in a given year, and non–long-term receipt was defined as receipt in one, two, or three quarters. Our sample consisted of 2,227,610 unique beneficiaries. A total of 1,140,276 unique beneficiaries had non–long-term receipt of opioids during at least 1 year of our study period, and 611,955 unique beneficiaries had long-term receipt of opioids during at least 1 year of our study period. Because opioid-receipt status was measured at the person-year level, any given person could fall into more than one category (no receipt of opioids, non–long-term receipt of opioids, and long-term receipt of opioids).
Race or ethnic group was determined from the race-ethnicity variable in the Medicare Master Beneficiary Summary File.
The Medicare Part D low-income subsidy is a proxy measure of poverty, because recipients have income below 150% of the federal poverty level. Subsidy status was dichotomized as any versus none for this study.
Prescription-drug Hierarchical Condition Category (RxHCC) risk scores are based on patient demographic characteristics and patient claims and are used to predict Part D prescription-drug spending for payment of prescription-drug plans. Higher values correspond to higher Part D capitated payments. In our sample, the minimum risk score was 0, and the maximum was 6.5.
Shown is the percentage of patients with one or more diagnosis in any year of full enrollment. Serious mental illness denotes bipolar disorder, schizophrenia, schizoaffective disorder, or other nonorganic psychoses.
A daily morphine-equivalent dose (MED) of more than 120 mg equals 1 when the daily MED (total quarterly MED divided by 91) exceeds 120 mg in any calendar quarter in that year.
Excluded are beneficiaries with any observed heroin overdose in the calendar year.
ADOPTION OF CONTROLLED-SUBSTANCE RESTRICTIONS
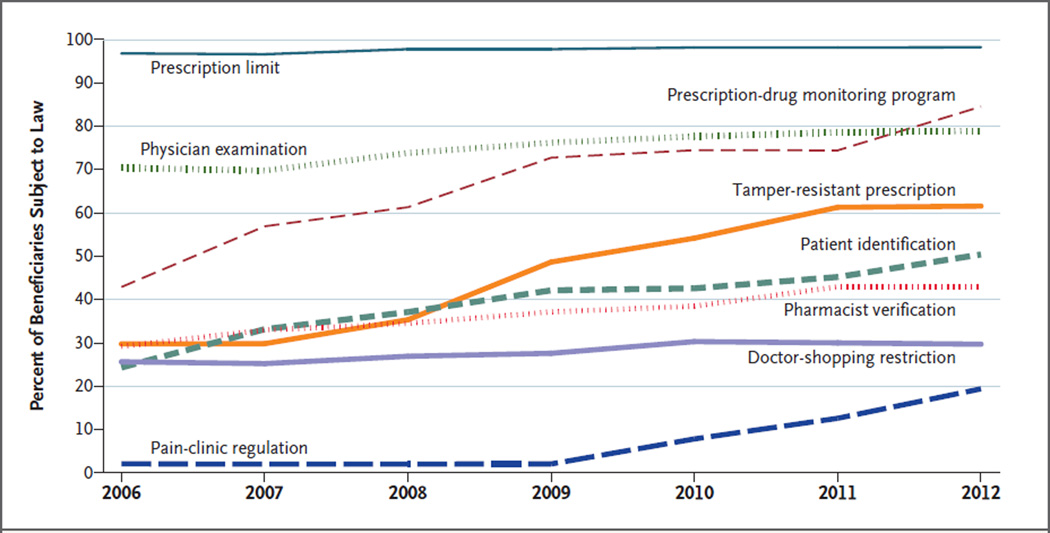
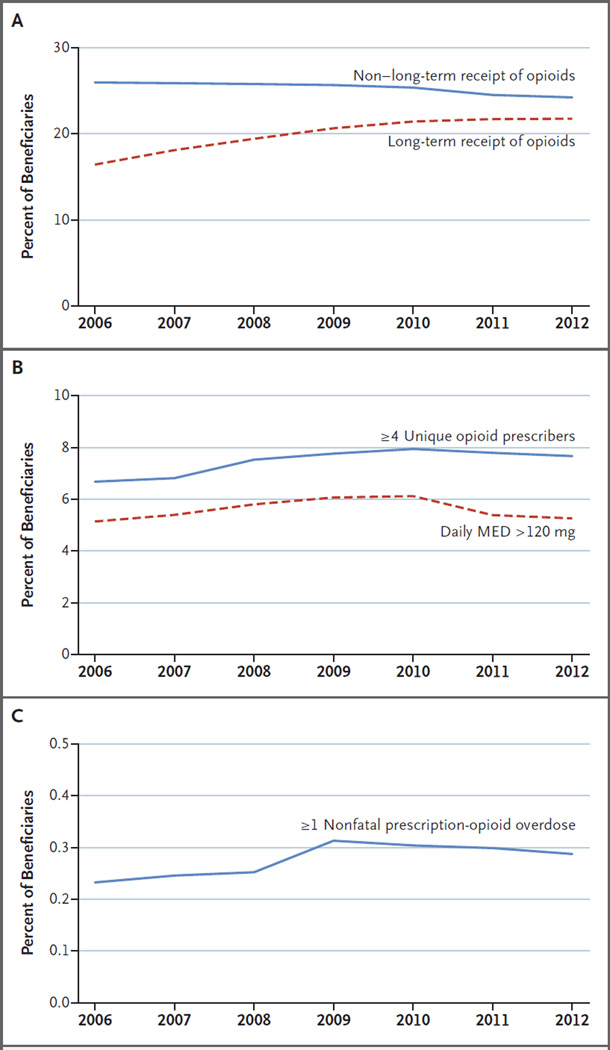
State restrictions governing opioid prescribing and dispensing proliferated between 2006 and 2012 (Fig. 1). Collectively, states added 81 controlled-substance laws; the mean (±SD) types of laws per state increased from 2.7±1.5 to 4.3±1.4. By 2012, all states had at least one type of laws and Florida had all eight types of laws. Some laws spread rapidly after 2006; for example, the percentage of beneficiaries governed by PDMPs doubled (from 43% to 85%), as did the percentage subject to requirements related to tamper-resistant prescription forms (from 30% to 62%). In contrast, only Vermont and Iowa added quantitative prescription limits, and four states each added doctor-shopping laws and pain-clinic regulations. Maps of controlled-substance restrictions in 2006 and 2012 show intense legislative activity in some states, such as Tennessee and Vermont (Fig. S1 in the Supplementary Appendix). Figure 2 displays the unadjusted prevalence of prescription-opioid measures and nonfatal overdose in each year. Except for non–long-term receipt of opioids, the prevalence of each measure increased between 2006 and 2012. The prevalence of a daily MED of more than 120 mg peaked in 2010 at 6.1% before falling to 5.3% in 2012.
Figure 1. Percentage of Sample Medicare Beneficiaries 21 to 64 Years of Age Who Were Living in a State with Controlled-Substance Prescribing and Dispensing Laws, 2006–2012.
With respect to 8 types of controlled-substance laws, the mean (±SD) types of laws per state increased from 2.7±1.5 (range, 0 to 6) in 2006 to 4.3±1.4 (range, 1 to 8) in 2012. Prescription limits, added by 2 states between 2006 and 2012, restrict the quantity dispensed. Prescription-drug monitoring programs, added by 19 states, collect data on controlled-substance dispensing for use by authorized personnel. Physician-examination restrictions, added by 12 states, require an established physician–patient relationship or physical examination before the prescription of controlled substances. Laws requiring tamper-resistant prescription forms were added by 19 states. Patient identification requirements, added by 13 states, mandate or permit pharmacists to ask for identification. Pharmacist-verification requirements, added by 8 states, prohibit pharmacists from dispensing controlled substances if there is suspicion that no doctor–patient relationship exists. Doctor-shopping restrictions, added by 4 states, prohibit patients from obtaining controlled substances through fraudulent behavior. Pain-clinic regulations, added by 4 states, affect the licensing and registration of prescribers.
Figure 2. Trends in Annual Prescription-Opioid Measures, 2006–2012.
Long-term receipt was defined as the filling of one or more opioid prescriptions in each calendar quarter in a given year, and non–long-term receipt was defined as receipt in one, two, or three quarters (Panel A). A daily morphine-equivalent dose (MED) of more than 120 mg (Panel B) equals 1 when the daily MED (total quarterly MED divided by 91) exceeds 120 mg in any quarter during the calendar year. Nonfatal prescription-opioid overdose (Panel C) excludes beneficiaries with any observed heroin overdose in the calendar year.
ESTIMATED ASSOCIATIONS BETWEEN OPIOID RECEIPT AND CONTROLLED-SUBSTANCE RESTRICTIONS
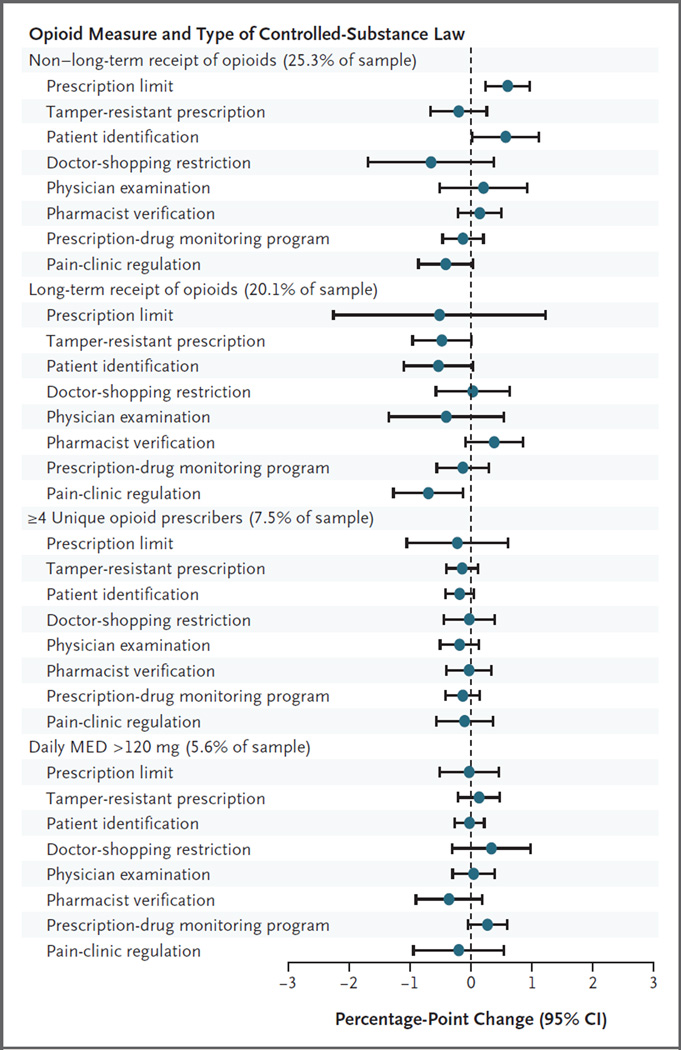
There was little systematic association between individual types of controlled-substance laws and each of the opioid-related outcomes (Fig. 3, and Tables S6 through S10 in the Supplementary Appendix). For example, none of the four opioid-prescribing outcomes had significant associations with the two types of laws that were most broadly adopted during this period, PDMPs and requirements related to tamper-resistant prescription forms. In comparisons between years with operational PDMPs and those without them, the percentage of beneficiaries with four or more opioid prescribers declined little (−0.14 percentage points; 95% confidence interval [CI], −0.42 to 0.14), and the percentage of beneficiaries with a daily MED of more than 120 mg did not decline (0.27 percentage points; 95% CI, −0.05 to 0.59). After adjustment for multiple comparisons, P values were 0.80 and 0.45, respectively.
Figure 3. Estimated Difference in Opioid Measures Associated with Individual Types of Controlled-Substance Laws.
This figure shows adjusted estimates of the association between state-level controlled-substance laws and measures of opioid receipt. We estimated marginal effects (the change in the predicted probability of the dependent variable was multiplied by 100 for ease of presentation) on the basis of individual-level logistic regressions of annual prescription-opioid measures on time-varying individual types of laws in place in a given state in a given year, controlling for the state of residence, year indicators, and beneficiary characteristics. Variance estimates account for autocorrelation of observations within states over time with the use of Huber–White sandwich estimators. Confidence intervals were estimated with the use of the delta method and were not adjusted for multiple comparisons.
In comparisons between years with requirements related to tamper-resistant prescription forms and years without such requirements, the change in the percentage of beneficiaries with four or more opioid prescribers was −0.15 percentage points (95% CI, −0.41 to 0.12), and the change in the percentage of beneficiaries with a daily MED of more than 120 mg was 0.13 percentage points (95% CI, −0.22 to 0.48); P values were 0.68 and 0.82, respectively, after adjustment for multiple comparisons. The lack of change in each opioid-related outcome as laws proliferated is also evident in the smooth trend lines before and after law implementation (Fig. S2 and S3 in the Supplementary Appendix). The associations between individual types of laws and rates of nonfatal prescription-opioid overdose were not significant for any type of laws. For example, the percentage of beneficiaries with one or more events of nonfatal prescription-opioid overdose declined little (−0.02 percentage points; 95% CI, −0.08 to 0.04; adjusted P = 0.89) after adoption of prescription-limit laws. This estimate and all other associations between nonfatal overdose and individual types of laws were not significant even without adjustment for multiple comparisons (Table S10 in the Supplementary Appendix).
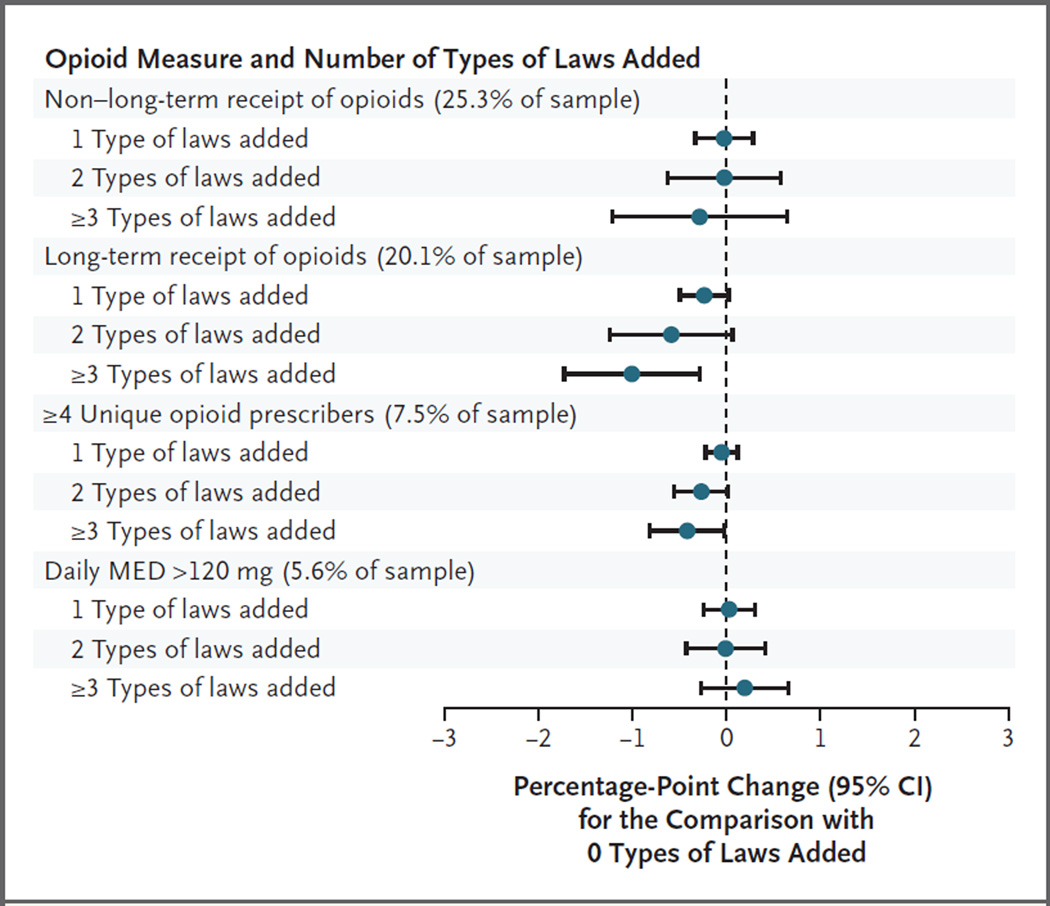
In secondary analyses of beneficiaries with long-term receipt of prescription opioids, none of the individual types of laws had significant associations with the opioid measures (Fig. S4 in the Supplementary Appendix). Opioid outcomes also varied little according to state legislative intensity (Fig. 4, and Tables S11 through S15 in the Supplementary Appendix). Two measures were slightly lower in states adding three or more types of controlled-substance laws after 2006, as compared with states adding no types of laws: long-term receipt of opioids (−1.01 percentage points; 95% CI, −1.75 to −0.27) and the percentage of beneficiaries with four or more opioid prescribers (−0.42 percentage points; 95% CI, −0.83 to −0.01), although estimates were not significant after adjustment for multiple comparisons (P = 0.17 and P = 0.55, respectively). Rates of nonfatal prescription-opioid overdose were no lower in states with more legislative activity than in other states (Table S15 in the Supplementary Appendix). The number of potentially hazardous opioid outcomes varied little according to the number of types of laws in a state, with estimated associations close to zero (−0.004; 95% CI, −0.008 to 0.0; adjusted P = 0.50) (Table S16 in the Supplementary Appendix).
Figure 4. Estimated Difference in Opioid Measures Associated with Number of Types of State Controlled-Substance Laws Added since 2006.
This figure shows adjusted estimates of the association between the number of types of state-level controlled-substance laws added since 2006 and measures of opioid receipt. We estimated marginal effects (the change in the predicted probability of the dependent variable was multiplied by 100 for ease of presentation) on the basis of individual-level logistic regressions of prescription-opioid measures on the time-varying number of types of laws added since 2006 (1, 2, or ≥3; 0 is the reference) in a beneficiary’s state of residence in a given year, controlling for the state of residence, year indicators, and beneficiary characteristics. Variance estimates account for autocorrelation of observations within states over time with the use of Huber–White sandwich estimators. Confidence intervals were estimated with the use of the delta method and were not adjusted for multiple comparisons.
Finally, to assess state-level variation during our study period, we plotted the state-level change in each opioid-related outcome against the number of types of laws added, measuring changes from 2006 to 2012 (Fig. S5 in the Supplementary Appendix). Variation in opioid outcomes within a group of states (e.g., all states adding two types of laws) was greater than the difference in opioid measures across groups.
DISCUSSION
Laws that restrict the prescribing and dispensing of controlled substances showed few meaningful associations with the receipt of prescription opioids by disabled Medicare beneficiaries in our sample. States that adopted multiple laws between 2006 and 2012 (≥3 types) had lower growth in long-term receipt of opioids and multiple opioid prescribers than states that adopted no laws. However, these associations were not significant after adjustment for the large number of hypotheses examined in this study. In addition, legislative restrictions showed no measurable association with the percentage of beneficiaries filling prescriptions that yield high daily opioid doses or the percentage treated for nonfatal prescription-opioid overdose.
The estimated rate of death due to prescription-opioid overdose in our sample, 46.6 per 100,000, suggests that disabled Medicare beneficiaries accounted for nearly 1 in 4 deaths from prescription-opioid overdose nationwide in 2008. The scale of this estimate, combined with our finding of no significant association between legislative activity and nonfatal prescription-opioid overdose, should motivate renewed innovation to address misuse of prescription opioids.
Differences between our results and those of previous studies that showed negative associations between laws and prescription-opioid use may be explained by our comprehensive data and analyses. Although we, like others, used observational data, individual-level data permitted adjustments for patient characteristics. Furthermore, we considered multiple controlled-substance restrictions, examined a national rather than a regional population, and used a longitudinal study design to account for fixed differences across states and national trends in prescription-opioid use.4,5,8,32–36
These results are likely to disappoint state officials who are implementing laws to mitigate the unintended consequences of opioid analgesic use, but they also show the benefits of rigorous analyses. Administrative data such as those used here, paired with robust analysis, may reveal promptly the relationship between legislative efforts on targeted prescribing and dispensing behaviors and can be used to alter course and advance efforts to protect public health effectively and efficiently.
Indeed, efforts to leverage data promptly for the protection of the public are under way; the retail pharmacy company CVS CareMark has used data on prescription filling to establish specialty-specific benchmarks for controlled-substance prescribing. This approach permits identification of outlier prescribers who are worthy of examination and who may be excluded from CVS pharmacies if their prescribing behavior cannot be justified.37 Other payers may scrutinize prescriptions with real-time analyses of claims data. The Prescription Behavior Surveillance System launched in 2012, which collects data from participating state PDMPs to track prescription dispensing across states and time, offers another resource to monitor opioid prescribing.38
Our study has limitations. First, disabled fee-for-service Medicare beneficiaries who were alive throughout the calendar year have higher rates of opioid use, poverty, and coexisting complex medical conditions than the general U.S. population; our findings may not apply to other populations, among whom legal restrictions may be associated with patterns of prescription-opioid use and overdose.6 Yet, because disabled Medicare beneficiaries account for so many deaths from opioid overdose, this population is particularly in peril and could benefit from effective regulation.
Second, substantial legislative activity occurred after our study period, when more than 20 states began requiring prescribers to consult the PDMP before prescribing controlled substances to new patients and at regular intervals thereafter.16,39 New laws and increased enforcement of existing laws may have improved the effectiveness of controlled-substance regulation. Third, the laws may become more effective over a longer period. Indeed, some laws, such as pain-clinic regulations, were new during our study period, and PDMPs are being adapted by states to enhance effectiveness. However, on average we observed laws for 3.5 years after implementation, which suggests that legal solutions may influence opioid misuse and overdose slowly, if at all, in this population.
Fourth, our overdose data depend on the reliability of hospital and emergency department claims data. Although we cannot observe untreated overdoses, the ratio of nonfatal to fatal opioid overdose (6:1 in 2008) in our sample is similar to that found in previous research based on electronic medical records.10 Fifth, given a lack of significant associations, it is natural to question whether these analyses, driven by state-level changes in laws, had sufficient statistical power to yield meaningful insights. For conventional significance levels of 5%, the study had 90% power to detect declines in each measure of potentially hazardous opioid prescriptions and nonfatal overdose as small as 0.1 standard deviation. In other words, we had the ability to definitively rule out large changes in opioid measures associated with these laws. We cannot, however, rule out moderate-sized associations, because the lower boundary of the 95% confidence intervals sometimes reached 5 to 10% of the mean. In a few cases (e.g., estimated associations of opioid measures with prescription-limit laws), the lower boundary of the confidence intervals included estimated associations as large as 20% of the mean, in relative terms.
Misuse of and overdose from opioid analgesics threaten public health. Yet we found that state laws that impose costly requirements on prescribers, pharmacists, and patients did not have meaningful associations with opioid use or adverse outcomes, at least in the vulnerable population that we studied. Financially stressed states may wish to invest resources in rigorous evaluation of current legislation. Existing administrative data could be used to develop opioid-prescribing measures that permit active monitoring and identify hazardous patterns early. Effective and safer alternatives for chronic pain management are needed, as is a comprehensive response to opioid addiction.40
Supplementary Material
Acknowledgments
Supported by grants (P01AG019783 and U01AG046830) from the National Institute on Aging; mapping was supported by the Norris Cotton Cancer Center GeoSpatial Resource, funded by a National Institutes of Health Cancer Center Support Grant (5P30CA023108).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 2.Morden NE, Munson JC, Colla CH, et al. Prescription opioid use among disabled Medicare beneficiaries: intensity, trends, and regional variation. Med Care. 2014;52:852–859. doi: 10.1097/MLR.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374:154–163. doi: 10.1056/NEJMra1508490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutkow L, Chang HY, Daubresse M, Webster DW, Stuart EA, Alexander GC. Effect of Florida’s prescription drug monitoring program and pill mill laws on opioid prescribing and use. JAMA Intern Med. 2015;175:1642–1649. doi: 10.1001/jamainternmed.2015.3931. [DOI] [PubMed] [Google Scholar]
- 5.Haegerich TM, Paulozzi LJ, Manns BJ, Jones CM. What we know, and don’t know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend. 2014;145:34–47. doi: 10.1016/j.drugalcdep.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilby A. Opioids for the masses: welfare tradeoffs in the regulation of narcotic pain medications. Cambridge: Massachusetts Institute of Technology; 2015. [Google Scholar]
- 7.Arlotta CJ. Have prescription drug abuse regulations gone too far? Forbes. 2015 Jan 12; [Google Scholar]
- 8.Report to the Subcommittee on Oversight and Investigations, Committee on Energy and Commerce. Washington, DC: General Accounting Office; 2002. May, Prescription drugs: state monitoring programs provide useful tool to reduce diversion. [Google Scholar]
- 9.Jena AB, Goldman D, Weaver L, Karaca-Mandic P. Opioid prescribing by multiple providers in Medicare: retrospective observational study of insurance claims. BMJ. 2014;348:g1393. doi: 10.1136/bmj.g1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larrick AK. Request for comments: enhancements to the Star Ratings for 2016 and beyond: letter to all Medicare Advantage organizations, prescription drug plan sponsors, and other interested parties. Baltimore: Centers for Medicare and Medicaid Services; 2014. [Google Scholar]
- 12.Franklin GM, Mai J, Wickizer T, Turner JA, Fulton-Kehoe D, Grant L. Opioid dosing trends and mortality in Washington State workers’ compensation, 1996–2002. Am J Ind Med. 2005;48:91–99. doi: 10.1002/ajim.20191. [DOI] [PubMed] [Google Scholar]
- 13.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 14.Braden JB, Russo J, Fan MY, et al. Emergency department visits among recipients of chronic opioid therapy. Arch Intern Med. 2010;170:1425–1432. doi: 10.1001/archinternmed.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.2014 NASCSA survey of controlled substance authorities. Quincy, MA: National Association of State Controlled Substances Authorities; 2014. ( http://www.nascsa.org/Surveys/2014CSauthoritiesSurvey.pdf) [Google Scholar]
- 16.States that require prescribers and/or dispensers to access PMP database in certain circumstances. Charlottesville, VA: National Alliance for Model State Drug Laws; 2014. [Google Scholar]
- 17.Prescription Drug Monitoring Program Center of Excellence. Mandating medical provider participation in PDMPs. Waltham, MA: Brandeis University; 2014. [Google Scholar]
- 18.Davis C, Chang S. Legal interventions to reduce overdose mortality: naloxone access and overdose good Samaritan laws. St. Paul, MN: The Network for Public Health Law; 2015. [Google Scholar]
- 19.Prevention of prescription drug overdose and abuse. Washington, DC: National Conference of State Legislatures; 2016. ( http://www.ncsl.org/research/health/prevention-of-prescription-drug-overdose-and-abuse.aspx) [Google Scholar]
- 20.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Division of Unintentional Injury Prevention. Prescription drug overdose: state laws — selected state legislative strategies governing prescription drug abuse and diversion in the U.S. Atlanta: Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 21.Bonito A, Bann C, Eicheldinger C, Carpenter L. Creation of new race-ethnicity codes and socioeconomic status (SES) indicators for Medicare beneficiaries. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 24.Panis C, Euller R, Grant C, et al. SSA program data user’s manual. Washington, DC: Social Security Administration; 2000. Jun, [Google Scholar]
- 25.2006–2011 RxHCC model software/ICD-9-CM mappings. Baltimore: Centers for Medicare and Medicaid Services; 2012. ( http://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Risk-Adjustors-Items/Risk2006-2011.html?DLPage=1&DLSort=0&DLSortDir=descending) [Google Scholar]
- 26.Robst J, Levy JM, Ingber MJ. Diagnosis-based risk adjustment for Medicare prescription drug plan payments. Health Care Financ Rev. 2007;28:15–30. [PMC free article] [PubMed] [Google Scholar]
- 27.Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern Med. 2014;174:1668–1673. doi: 10.1001/jamainternmed.2014.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacula RL, Powell D, Heaton P, Sevigny EL. Assessing the effects of medical marijuana laws on marijuana use: the devil is in the details. J Policy Anal Manage. 2015;34:7–31. doi: 10.1002/pam.21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Multiple cause of death 1999–2013 on CDC WONDER online database. Atlanta: Centers for Disease Control and Prevention, National Center for Health Statistics; 2015. [Google Scholar]
- 30.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 31.McDonald JH. Multiple comparisons. In: McDonald JH, editor. Handbook of biological statistics. 3rd. Baltimore: Sparkey House Publishing; 2015. pp. 254–260. [Google Scholar]
- 32.Johnson H, Paulozzi L, Porucznik C, Mack K, Herter B. Decline in drug overdose deaths after state policy changes — Florida, 2010–2012. MMWR Morb Mortal Wkly Rep. 2014;63:569–574. [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Opioid painkiller prescribing: where you live makes a difference. Vital Signs. 2014 Jul [Google Scholar]
- 34.Baehren DF, Marco CA, Droz DE, Sinha S, Callan EM, Akpunonu P. A state-wide prescription monitoring program affects emergency department prescribing behaviors. Ann Emerg Med. 2010;56(1):19–23.e1. doi: 10.1016/j.annemergmed.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Reifler LM, Droz D, Bailey JE, et al. Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med. 2012;13:434–442. doi: 10.1111/j.1526-4637.2012.01327.x. [DOI] [PubMed] [Google Scholar]
- 36.Prescription Drug Monitoring Program Center of Excellence. Briefing on PDMP effectiveness. Waltham, MA: Brandeis University; 2014. Sep, [Google Scholar]
- 37.Betses M, Brennan T. Abusive prescribing of controlled substances — a pharmacy view. N Engl J Med. 2013;369:989–991. doi: 10.1056/NEJMp1308222. [DOI] [PubMed] [Google Scholar]
- 38.Paulozzi LJ, Strickler GK, Kreiner PW, Koris CM. Controlled substance prescribing patterns — Prescription Behavior Surveillance System, eight states, 2013. MMWR Surveill Summ. 2015;64(SS-9):1–14. doi: 10.15585/mmwr.ss6409a1. [DOI] [PubMed] [Google Scholar]
- 39.Haffajee RL, Jena AB, Weiner SG. Mandatory use of prescription drug monitoring programs. JAMA. 2015;313:891–892. doi: 10.1001/jama.2014.18514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313:1636–1644. doi: 10.1001/jama.2015.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.