Abstract
Background
Fingolimod is a once-daily, orally administered therapy for relapsing forms of MS. It has been shown to reduce relapse rates significantly in all phase II and phase III clinical trials when compared with placebo and intramuscular interferon β-1a (IFNβ-1a IM).
Methods
This study compared annualized relapse rates (ARRs) associated with fingolimod, placebo and IFNβ-1a IM, in patient subgroups from the pooled FREEDOMS, FREEDOMS II, and TRANSFORMS populations. This provided a large data set in which the efficacy of fingolimod could be assessed across a range of patient subgroups, including clinically relevant subgroups not previously analysed.
Results
Compared with placebo, fingolimod was associated with significantly lower ARRs across all patient subgroups with relative reductions in ARRs ranging from 35% (patients who had previously received treatment for their MS for up to 1 year; P < 0.05) to 69% (patients with symptoms for less than 3 years before study entry; P < 0.001). Other relative reductions in ARR compared with placebo included 64% in patients aged 40 years or younger and 63% in those naïve to treatment (P < 0.001 for both). Compared with IFNβ-1a IM, the greatest benefits to ARR were seen in patients aged 40 years or younger (55% relative ARR reduction, P < 0.001) and in a small subgroup of patients who had previously received IFNβ and glatiramer acetate (55% relative ARR reduction; P < 0.05). Reductions in ARR compared with IFNβ-1a IM were not statistically significant in men (33%, P = 0.081), in patients aged over 40 years (23%, P = 0.230) and in those who had received treatment prior to the study for 1 year or less (35%, P = 0.108). Fingolimod was associated with significantly lower ARRs compared with placebo and with IFNβ-1a IM irrespective of treatment status (treatment-naïve and previously treated for MS), and regardless of type of previous therapy.
Conclusions
Fingolimod provided consistent efficacy benefits over placebo and IFNβ-1a IM across a range of subgroups of patients with relapsing MS. The magnitude of the beneficial effect of fingolimod over IFNβ-1a IM may depend on age, sex, and duration of previous treatment. These findings suggest that most benefit will be gained by patients who start fingolimod early in the disease course, but the findings also suggest that fingolimod treatment will benefit patients later in the disease course when they have already accrued disability.
Keywords: clinical efficacy, early treatment, disability
1. Introduction
Key therapeutic aims in relapsing–remitting multiple sclerosis (RRMS) are the preservation of neurological function and control of disease activity. Relapses are an important component of disease activity that impact patients’ quality of life [1], and incomplete recovery from relapses is associated with step-wise accumulation of neurological disability [2, 3]. Studies suggest that relapse frequency early in the course of MS correlates with long-term disability: follow-up of the pivotal 2-year trial of subcutaneous interferon (IFN) β-1b in patients with RRMS, found that increased annualized relapse rate (ARR) on study correlated with an increased likelihood of poor long-term physical outcomes at 16 years [4]. Similarly, breakthrough disease activity including relapses during 2 years of intramuscular (IM) IFNβ-1a therapy was associated with an increased risk of disability at 15 years [5]. In the shorter term, it has been shown that reductions in relapse rates associated with treatment contributed to reductions in disability progression at follow-up [6, 7].
Fingolimod (Gilenya; Novartis Pharma AG, Basel) is a once-daily, orally administered therapy for relapsing forms of MS. It has been shown to reduce relapse rates significantly in phase 2 and phase 3 clinical trials when compared with placebo and IFNβ-1a IM [8–11]. In the 2-year, phase III FTY720 Research Evaluating Effects of Daily Oral Therapy in MS (FREEDOMS) and FREEDOMS II studies, fingolimod significantly reduced ARR compared with placebo [9,11]. In the 1-year, phase III Trial Assessing Injectable Interferon versus FTY720 Oral in RRMS (TRANSFORMS), ARR was significantly lower in patients treated with fingolimod than among those treated with IFNβ-1a IM [8], and patients who switched from IFNβ-1a IM to fingolimod at the end of TRANSFORMS showed a significant improvement in ARR over the 1-year study extension period [12].
Further analyses of the three individual phase III studies assessed the effects of fingolimod in prespecified patient subgroups [13–15]. Subgroups were defined by age (≤ 40 or > 40 years), sex, treatment history (previously treated or treatment-naïve), and disease characteristics at baseline, including the number of previous relapses (0, 1, or ≥ 2 relapses in the previous 2 years), number of lesions (0, 1–2, or ≥ 3 gadolinium [Gd]-enhancing lesions), and the level of disability (Expanded Disability Status Scale [EDSS] score < 3 or ≥3) [13–15]. In most of these patient subgroups in the three studies, fingolimod was associated with higher efficacy assessed by clinical and magnetic resonance imaging measures of disease activity, than placebo or IFNβ-1a IM.
The aim of the current paper is to present findings from subgroup analyses in a patient population pooled from FREEDOMS, FREEDOMS II, and TRANSFORMS. Pooling these patient populations provides a large data set, analysis of which may provide more insight than could be derived from individual study populations into the efficacy of fingolimod across a range of patient subgroups. In addition to those reported from the individual studies, this new analysis includes subgroups based on duration of symptoms prior to enrolment to provide further assessment of the impact of fingolimod relatively early in the disease course. Furthermore, insights into the efficacy of fingolimod when switching treatments is provided by analyses of subgroups based on prior treatment with IFN or glatiramer acetate (GA), and the duration of any prior treatment for MS. This subgroup analysis is especially important as in most countries fingolimod is registered as second line treatment after failure of injectable therapies.
2. Methods
2.1 Study design and patient population
This pooled analysis involved patients from FREEDOMS (NCT00289978), FREEDOMS II (NCT00355134), and TRANSFORMS (NCT00340834). The methods of all three studies have been published previously [8,9,11], and the analyses in this article do not involve new patients. All studies adhered to the International Conference on Harmonization Guidelines for Good Clinical Practice [16], and were conducted in accordance with the Declaration of Helsinki [17]. Study protocols were approved by the institutional review board at each study site. All patients provided written, informed consent before any study-related procedure was performed.
The studies involved patients aged 18–55 years with RRMS, diagnosed in accordance with the 2005 McDonald criteria [18], who had an EDSS score of 0–5.5 and one or more documented relapses in the previous year or two or more documented relapses in the previous 2 years. Exclusion criteria included relapse or corticosteroid treatment in the 30 days before randomization. In FREEDOMS and FREEDOMS II, patients had to have stopped IFNβ-1a or GA therapy at least 3 months before randomization to be included; in TRANSFORMS, recent therapy with IFNβ or GA was permitted.
In the double-blind FREEDOMS and FREEDOMS II studies, patients were randomized (1:1:1) to oral fingolimod 0.5 mg or 1.25 mg or placebo once daily for 24 months [9,11]. In TRANSFORMS, a double-blind, active-controlled study, patients were randomized (1:1:1) to oral fingolimod 0.5 mg or 1.25 mg once daily or IFNβ-1a IM at a weekly dose of 30 μg for 12 months [8].
The primary efficacy endpoint in all studies was estimation of ARR, which was defined as the number of confirmed relapses during a 12-month period. Relapses were assessed by the examining neurologist within 7 days of onset. To qualify as a confirmed relapse, neurological symptoms had to be accompanied by an increase in EDSS score of ≥ 0.5 points or by an increase of 1 point in each of two EDSS functional-system scores or 2 points in one EDSS functional-system score (excluding the bowel–bladder or cerebral functional systems).
2.2 Analysis subgroups
Analysis subgroups were selected on the basis of previous studies that have shown certain factors to be potential predictors of clinical outcome in patients with RRMS [19–23]. The following subgroups were either prespecifed, or based on ones prespecified (see below), in the TRANSFORMS, FREEDOMS, and FREEDOMS II protocols: age (≤ 40 years or > 40 years; sex; treatment history (treatment-naïve or previously treated with any MS medication at any time before study entry); number of Gd-enhancing lesions (0 or ≥ 1); number of relapses in the 2 years before study entry (≤ 2 or ≥ 3); baseline disability (EDSS score 0–2.5 or ≥ 3). In these subgroups, ARR was estimated using a negative binomial model with study as a factor. The other subgroups examined were ones defined post hoc to explore in greater depth factors such as treatment history in the FREEDOMS and TRANSFORMS populations [24,25], and which have identified subpopulations that may particularly benefit from fingolimod treatment: time from first MS symptom to study entry (< 3 or ≥ 3 years) [24]; patients who had received injectable treatment for MS before study entry (IFN versus IFN-naïve, GA versus GA-naïve, IFN and GA versus naïve to either); and duration of previous treatment before randomization (0, ≤ 1, > 1 – ≤ 3, and > 3 years) [25]. In these subgroups, ARR was estimated using a negative binomial model with study, treatment, subgroup, and treatment-by-subgroup as factors.
In all three studies, modifications were made to some subgroup definitions after database lock [13,14]. The reasons for these modifications are explained in detail in a previous publication [14]. Briefly, definitions were modified to harmonize the three studies, to ensure adequate patient representation, and to enable clinically meaningful comparisons. The categories for number of relapses were changed from the predefined values (0, 1, 2–3, 4–5, and > 5 relapses) in order to combine groups with limited patient numbers, while the predefined EDSS score subgroups (≤ 3.5 and > 3.5) were altered so that they defined a group of less severely affected patients. The age cut-off was raised from 37 years to 40 years in FREEDOMS and FREEDOMS II for consistency with the predefined cut-off of 40 years in TRANSFORMS. For the Gd-enhancing lesion subgroups, the three predefined subgroups (0, 1–2, and ≥ 3 lesions) were reduced to two subgroups (0 or ≥ 1 lesion) in order to group patients according to whether they were with or without inflammation at baseline [11,13,14].
2.3 Statistical analyses
Statistical analyses were performed using data from the pooled intent-to-treat population from TRANSFORMS, FREEDOMS, and FREEDOMS II, which comprised all patients who received at least one dose of study drug. ARR was estimated using negative binomial regression models (see section 2.2 for covariates). As the FREEDOMS trials were of 2 years’ duration and the TRANSFORMS trial ran for 1-year, the logarithm of time-on-study was used as the offset variable to account for the varying lengths of time that patients spent on study; an assumption was made that each patient’s relapse rate did not vary with time on study. This assumption was validated by an analysis of truncated 1-year data (Supplementary Figure 1) that yielded similar results. ARR ratios are presented with 95% confidence intervals, and hypothesis-generated P values indicate the statistical significance of treatment differences. Each subgroup analysis was conducted independently for hypothesis generation; no adjustments were made for multiple comparisons. Here, we focus on the findings in patients treated with the approved fingolimod 0.5 mg dose versus placebo and IFNβ-1a IM. Data for patients who received fingolimod 1.25 mg are shown in the Appendix (Supplementary Figure 2).
3. Results
The baseline demographic and disease characteristics of patients included in the pooled analyses were similar across the treatment groups (Table 1), although patients in FREEDOMS II had a higher mean age and longer disease duration and more individuals had received previous therapy, compared with FREEDOMS and TRANSFORMS [8,9,11]. Overall, more than two-thirds (71.4%) of patients were women, and approximately 59% were aged 40 years or younger. Across the treatment groups, mean EDSS score ranged from 2.2 to 2.5 and mean duration of disease ranged from 4.9 to 5.7 years at baseline.
Table 1.
Demographic characteristics, treatment history and disease characteristics at baseline (randomized population).
| Fingolimod 0.5 mg (N = 1214) | Placebo (N = 773) | IFNβ-1a IM (N = 435) | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, years, mean (SD) | 37.8 (8.9) | 38.6 (8.6) | 36.0 (8.3) |
| Sex, female, n (%) | 853 (70.3) | 586 (75.8) | 295 (67.8) |
| Treatment history, n (%) | |||
| Treatment-naïve | 531 (43.7) | 345 (44.6) | 190 (43.7) |
| Previous MS treatment | |||
| Interferon-β 1a SC | 236 (19.4) | 143 (18.5) | 72 (16.6) |
| Interferon-β 1a IM | 313 (25.8) | 185 (23.9) | 118 (27.1) |
| Interferon-β 1b SC | 173 (14.3) | 120 (15.5) | 69 (15.9) |
| Glatiramer acetate | 228 (18.8) | 190 (24.6) | 67 (15.4) |
| Natalizumab | 25 (2.1) | 25 (3.2) | 1 (0.2) |
| Other | 87 (7.2) | 79 (10.2) | 16 (3.7) |
| Disease characteristics, mean (SD) | |||
| Number of Gd-enhancing lesions at baseline | 1.3 (4.1) | 1.2 (3.1) | 1.1 (2.8) |
| Number of relapses in the previous year | 1.5 (1.0) | 1.5 (0.8) | 1.5 (0.8) |
| Number of relapses in the previous 2 years | 2.2 (1.7) | 2.2 (1.3) | 2.3 (1.2) |
| EDSS score | 2.3 (1.3) | 2.5 (1.3) | 2.2 (1.3) |
| Time since MS diagnosis, years | 5.2 (5.3) | 5.7 (5.5) | 4.9 (5.4) |
EDSS, Expanded Disability Status Scale; Gd, gadolinium; IFN, interferon; IM, intramuscular; MS, multiple sclerosis; SC, subcutaneous; SD, standard deviation.
In total, 86%, 76%, and 89% of patients in the fingolimod 0.5 mg, placebo, and IFNβ-1a IM groups, respectively, completed the study with a median time on study drug (interquartile range) of 576 (363–723), 719 (497–731), and 361 (351–370) days, respectively (Table 2). ARRs were adjusted to account for the differences in treatment duration as described above.
Table 2.
Patient disposition and study duration (randomized population).
| Fingolimod 0.5 mg (N = 1214) | Placebo (N = 773) | IFNβ-1a IM (N = 435) | |
|---|---|---|---|
| Completed study, n (%) | 1039 (85.6) | 587 (75.9) | 386 (88.7) |
| On study drug | 972 ( 80.1) | 535 (69.2) | 380 ( 87.4) |
| Off study drug | 67 (5.5) | 52 (6.7) | 6 (1.4) |
| Discontinued from study, n (%) | 175 (14.4) | 186 (24.1) | 49 (11.3) |
| Reason for discontinuation, n (%) | |||
| Abnormal laboratory value | 29 (2.4) | 3 ( 0.4) | 1 (0.2) |
| Abnormal test procedure | 6 (0.5) | 2 ( 0.3) | 3 (0.7) |
| Administrative problems | 5 (0.4) | 5 ( 0.6) | 7 (1.6) |
| Adverse events | 44 (3.6) | 34 (4.4) | 9 (2.1) |
| Death | 0 (0.0) | 2 (0.3) | 0 (0.0) |
| Lost to follow-up | 19 (1.6) | 28 (3.6) | 4 (0.9) |
| Protocol violation | 7 (0.6) | 6 (0.8) | 2 (0.5) |
| Patient withdrew consent | 50 (4.1) | 63 (8.2) | 16 (3.7) |
| Patient’s condition no longer required study drug | 0 (0.0) | 1 (0.1) | 0 (0.0) |
| Unsatisfactory therapeutic effect | 15 (1.2) | 42 (5.4) | 7 (1.6) |
| Duration of drug exposure, daysa | |||
| Mean (SD) | 517 (221) | 596 (223) | 337 (81) |
| Median (interquartile range) | 576 (363–723) | 719 (497–731) | 361 (351–370) |
| Total duration of drug exposure, patient-yearsb | 1716 | 1261 | 398 |
The duration of exposure per patient is the number of days on study drug, with all interruptions excluded.
The total duration of exposure was calculated as duration of exposure for all patients in the group divided by 365.25 days.
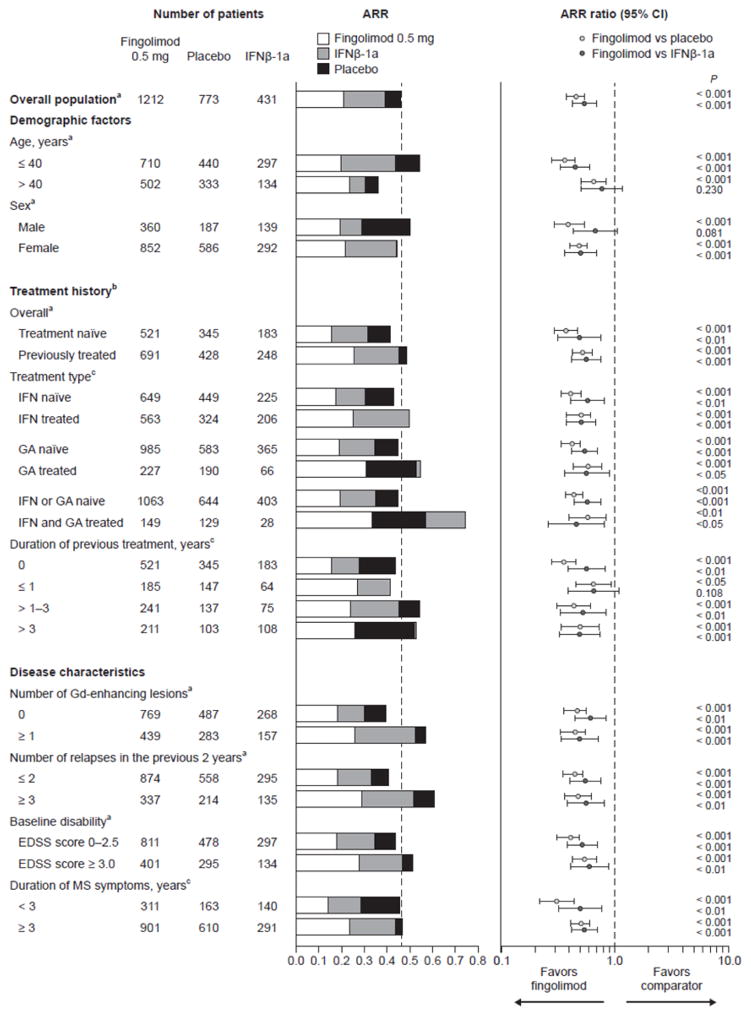
Compared with placebo, analyses showed that fingolimod 0.5 mg was associated with significantly lower ARRs across all patient subgroups (Figure 1). For the subgroups defined by demographic characteristics, fingolimod 0.5 mg showed relative reductions in ARRs of up to 64%, compared with placebo, with greatest benefit seen in those aged 40 years or younger (relative reduction 64%, n = 710, P < 0.001). The effect of fingolimod was less pronounced in patients older than 40 years, with a relative reduction in ARR of 35% (n = 502, P < 0.001), compared with placebo (Figure 1).
Figure 1.
Annualized relapse rates (ARRs) in patient subgroups defined by demographic factors, treatment history, and disease characteristics at baseline (intent-to-treat population).
ARRs and ARR ratios for fingolimod 0.5 mg versus comparators were estimated using a negative binomial regression model adjusted for study. aARR was estimated using a negative binomial model with study as a factor. bTreatment-naïve patients were defined as those not receiving any drugs for the treatment of MS, according to MS history case-report forms. cARR was estimated using a negative binomial model, with study, treatment, subgroup, and treatment-by-subgroup as factors. CI, confidence interval; EDSS, Expanded Disability Status Scale; Gd, gadolinium; IFN, interferon; MS, multiple sclerosis
Compared with IFNβ-1a IM, fingolimod 0.5 mg showed relative reductions in ARRs of up to 55% across all patient subgroups, with the greatest benefits seen in patients aged 40 years or younger (relative reduction 55%, P < 0.001, n = 710) and in patients who had previously received IFN and GA (relative reduction 55%, P < 0.05, n = 28), although the sample size in this subgroup was quite small (Figure 1). ARRs reductions did not reach statistical significance in patients older than 40 years (relative reduction 23%, P = 0.230, n = 502) men (relative reduction 33%, P = 0.081, n = 360), or patients previously treated for 1 year or less (relative reduction 35%, P = 0.108, n = 185), compared with IFNβ-1a IM (n = 134, n = 139, n = 64, respectively) (Figure 1).
Irrespective of patients’ overall treatment status (treatment-naïve; previously treated), fingolimod was associated with significantly lower ARRs than were placebo or IFNβ-1a IM. Improvements tended to be more pronounced in treatment-naïve patients (relative reductions 63%, P < 0.001, n = 521 and 51%, P = 0.001, n = 521, vs placebo and IFNβ-1a IM, respectively) than in those who previously received drug treatments for MS (relative reductions 48%, P < 0.001, n = 691 and 44%, P < 0.001, n = 691). Furthermore, fingolimod demonstrated consistent efficacy over placebo and IFNβ-1a IM regardless of the type of previous treatment. Patients previously treated with injectable IFNβ-1a had relative reductions in ARR of 49% (P < 0.001, n = 324) and 49% (P < 0.001, n = 206) for fingolimod 0.5 mg compared with placebo and IFNβ-1a IM, respectively, while IFN-naïve patients had relative reductions in ARR of 59% (P < 0.001, n = 449) and 42% (P = 0.001, n = 225) for the same comparisons. Similar results were observed for fingolimod-treated patients who had received GA before study entry (relative reduction 42–44%) compared with GA-naïve patients (relative reduction 46–58%).
Subgroup analyses by disease characteristics showed that patients with high disease activity (assessed according to number of Gd-enhancing lesions and the number of relapses in the previous 2 years), or high levels of disability (as measured by EDSS score) at baseline experienced higher ARRs than those with less disease activity or disability (Figure 1). Fingolimod 0.5 mg was associated with consistently lower ARRs across these subgroups, with relative reductions in ARR of 46–59% compared with placebo and 39–51% compared with IFNβ-1a IM.
When patients were analyzed on the basis of disease duration, treatment with fingolimod 0.5 mg led to reductions in ARR of 69% compared with placebo (P < 0.001, n = 163) and of 50% compared with IFNβ-1a IM (P = 0.002, n = 140) in patients who had first experienced symptoms less than 3 years previously. Significant, but slightly smaller, ARR reductions were demonstrated in patients who had first experienced symptoms at least 3 years previously, with reductions of 49% (P < 0.001, n = 610) and 46% (P < 0.001, n = 291) for fingolimod compared with placebo and IFNβ-1a IM, respectively. In addition, treatment with fingolimod reduced ARR in patients with 0, > 1–3, and more than 3 years of treatment before randomization compared with placebo and IFNβ-1a IM (by 64% and 56%, 50% and 44%, 47% and 51%, respectively).
4. Discussion
These subgroup analyses using data pooled from three large phase III trials showed that once-daily fingolimod therapy was associated with improvements in ARR across all subgroups when compared with placebo. Reduction in ARR with fingolimod compared with IFNβ-1a IM was also significant in most subgroups although the magnitude of the effect was to some extent dependent on age, sex, and duration of previous treatment at baseline. In the context of the known association between breakthrough disease on IFN treatment and poor long-term outcome [4,5], these findings highlight the potential benefit of choosing an appropriate therapy early in the disease course.
Improvements in ARR with fingolimod relative to the comparator groups were more obvious in patients with lower disability scores or shorter disease duration at baseline than among those who had higher disability scores or longer duration since first symptom onset. The greatest reductions in ARR associated with fingolimod relative to comparators were generally seen in younger patients (aged ≤ 40 years) and in those who were treatment-naive. Taken together, these findings suggest that young patients with less disability and shorter disease duration who start fingolimod as a first line therapy are likely to benefit most from treatment. However, reductions in ARR relative to comparators were also substantial among patients who had accrued disability, indicating that patients at a later stage of the disease course are also likely to benefit from fingolimod treatment.
Less pronounced treatment effects with fingolimod relative to IFNβ-1a IM were seen in older patients, in men and in those previously treated for no more than 1 year. The marginal treatment benefit relative to IFNβ-1a IM among older patients may simply be attributable to an inherently lower relapse rate in this subgroup. Relapse rates generally decrease with age [26] and it is noticeable that ARR in patients on placebo in this subgroup was the lowest among the placebo subgroups examined (Figure 1). Regarding the difference in effect between men and women, ARRs in both groups receiving fingolimod 0.5 mg were essentially the same (0.20 and 0.21, respectively) and were very similar among men and women on placebo (0.50 and 0.45, respectively) but ARRs were much lower among men receiving IFNβ-1a IM than they were among women (0.29 and 0.44). This runs counter to an analysis of five studies of IFNβ-1a IM that compared the responses of men and women and found no differences in treatment effects [27], but the finding is consistent with another study with a 7-year follow-up, which found that men receiving IFNβ had a lower risk of first relapse than women [28].
These analyses of pooled data support the results of subgroup analyses reported separately for FREEDOMS, FREEDOMS II, and TRANSFORMS [13–15]. As well as adjusting for study duration, bias possibly arising from combining different study populations was addressed to some degree by using patient-level data from trials with similar endpoints and by including appropriate covariates in the binomial regression analyses [29]. There are, however, certain limitations associated with pooled analyses. Direct comparison between the studies was not possible because of differences in characteristics such as patient demographics, design, methodology, geographical location, control groups, and outcome definitions. Although study effects were factored as covariates to account for the varying lengths of time that patients spent in the studies, there are numerous other hypothetically relevant differences that could not all be accounted for. Despite having similar study designs, there were noticeable differences even between the two FREEDOMS trials with regard to patient characteristics such as age, disease duration, and the proportions previously treated [11]. Furthermore, the three trials included in this analysis were not prospectively designed or powered to test formally for heterogeneity among subgroups or for treatment differences within subgroups. While the model estimates of activity within treatment arms and the estimates of between-treatment effects are valid, the P values and the 95% confidence intervals for ARR ratios should be interpreted with caution. Modifications were made to some of the predefined subgroup criteria, but these changes were made to pool groups containing few patients or to make small adjustments to cut-off values and they are unlikely to affect the study conclusions [13,14]. While there are limitations, it should be considered that the aim of the analyses was to identify subgroups of patients who respond to therapy differently than the average population, rather than to provide a definitive measure of efficacy in the various groups.
5. Conclusions
These pooled analyses showed that fingolimod 0.5 mg once daily provided consistent efficacy benefits over placebo and IFNβ-1a IM across a range of subgroups of patients with RRMS. There were consistent reductions in ARR seen with fingolimod among patients who were younger, treatment-naïve, or had lower levels of disability based on EDSS at baseline. These findings provide insight for physicians considering the long-term outcomes of disease-modifying therapies for relapsing forms of MS, particularly considering that the development of long-term disability correlates with disease activity early in the course of MS.
Supplementary Material
Supplementary Figure 1. Annualized relapse rates in patient subgroups defined by demographic factors and baseline disease characteristics: 1-year truncated analysis of fingolimod 0.5 mg patient group
Supplementary Figure 2. Annualized relapse rates in patient subgroups defined by demographic factors and baseline disease characteristics; fingolimod 1.25 mg patient group.
Highlights.
Reducing annualized relapse rates (ARRs) is a key treatment goal in relapsing MS
ARRs were examined post hoc by subgroup in three pooled phase 3 fingolimod trials
Subgroup ARRs were consistently lower with fingolimod than with comparators
Patients early in the disease course benefitted most from fingolimod treatment
Benefits were also substantial among patients who had accrued physical disability
Acknowledgments
This study was funded by Novartis Pharmaceuticals Corporation. Oxford PharmaGenesis, Oxford, UK provided writing and editorial support for this manuscript. Funding for this support was provided by Novartis Pharmaceuticals Corporation.
Footnotes
Authors’ contributions
All authors participated in data interpretation, made substantial contributions to the intellectual content of the manuscript, and were involved in manuscript preparation. All authors approve and are accountable for the final manuscript.
Conflicts of interest
T Derfuss serves on scientific advisory boards for Bayer Schering Pharma, Biogen Idec, Genzyme, GeNeuro, Merck Serono, Mitsubishi Pharma, Novartis Pharmaceuticals and Teva Pharmaceuticals; has received funding for travel and/or speaker honoraria from Bayer Schering Pharma, Biogen Idec, Genzyme, Novartis and Merck Serono; and receives research support from Biogen Idec, the European Union, Novartis Pharma, the Swiss MS Society and the Swiss National Foundation.
D Ontaneda has received research support from the US Multiple Sclerosis Society and the US National Institutes of Health; and has received consulting or speaking fees from Acorda Therapeutics, Biogen Idec, Genzyme, Novartis, QuestCor and Teva Pharmaceuticals. J Nicholas has received research support from Actelion Pharmaceuticals, Biogen Idec, Mallinckrodt Pharmaceuticals, Novartis, Roche, the US National Multiple Sclerosis Society and the US National Institutes of Health; and has received consulting or speaking fees from Biogen Idec, Genzyme, Novartis, Teva Pharmaceuticals and Vindico Medical. X Meng and K Hawker are employees of Novartis Pharmaceuticals Corporation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oleen-Burkey M, Castelli-Haley J, Lage MJ, Johnson KP. Burden of a multiple sclerosis relapse: the patient’s perspective. Patient. 2012;5:57–69. doi: 10.2165/11592160-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Lublin FD. The incomplete nature of multiple sclerosis relapse resolution. J Neurol Sci. 2007;256(Suppl 1):S14–18. doi: 10.1016/j.jns.2007.01.062. [DOI] [PubMed] [Google Scholar]
- 3.Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain. 2003;126(Pt 4):770–782. doi: 10.1093/brain/awg081. [DOI] [PubMed] [Google Scholar]
- 4.Goodin DS, Traboulsee A, Knappertz V, Reder AT, Li D, Langdon D, Wolf C, Beckmann K, Konieczny A, Ebers GC. Relationship between early clinical characteristics and long term disability outcomes: 16 year cohort study (follow-up) of the pivotal interferon beta-1b trial in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012;83(3):282–287. doi: 10.1136/jnnp-2011-301178. [DOI] [PubMed] [Google Scholar]
- 5.Bermel RA, You X, Foulds P, Hyde R, Simon JH, Fisher E, Rudick RA. Predictors of long-term outcome in multiple sclerosis patients treated with interferon beta. Ann Neurol. 2013;73(1):95–103. doi: 10.1002/ana.23758. [DOI] [PubMed] [Google Scholar]
- 6.Sormani MP, Li DK, Bruzzi P, Stubinski B, Cornelisse P, Rocak S, De Stefano N. Combined MRI lesions and relapses as a surrogate for disability in multiple sclerosis. Neurology. 2011;77(18):1684–1690. doi: 10.1212/WNL.0b013e31823648b9. [DOI] [PubMed] [Google Scholar]
- 7.Sormani MP, De Stefano N, Francis G, et al. Fingolimod effect on brain volume loss independently contributes to its effect on disability. Mult Scler. 2015;21:916–24. doi: 10.1177/1352458515569099. [DOI] [PubMed] [Google Scholar]
- 8.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 9.Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 10.Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355(11):1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 11.Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, Vollmer T, Agius MA, Kappos L, Stites T, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(6):545–556. doi: 10.1016/S1474-4422(14)70049-3. [DOI] [PubMed] [Google Scholar]
- 12.Khatri B, Barkhof F, Comi G, Hartung HP, Kappos L, Montalban X, Pelletier J, Stites T, Wu S, Holdbrook F, et al. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol. 2011;10(6):520–529. doi: 10.1016/S1474-4422(11)70099-0. [DOI] [PubMed] [Google Scholar]
- 13.Cohen JA, Barkhof F, Comi G, Izquierdo G, Khatri B, Montalban X, Pelletier J, Eckert B, Haring DA, Francis G. Fingolimod versus intramuscular interferon in patient subgroups from TRANSFORMS. J Neurol. 2013;260(8):2023–2032. doi: 10.1007/s00415-013-6932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devonshire V, Havrdova E, Radue EW, O’Connor P, Zhang-Auberson L, Agoropoulou C, Haring DA, Francis G, Kappos L. Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. Lancet Neurol. 2012;11(5):420–428. doi: 10.1016/S1474-4422(12)70056-X. [DOI] [PubMed] [Google Scholar]
- 15.Goodin D, Jeffery D, Kappos L, Lublin FD, Radue EW, Rammohan KW, Reder AT, Vollmer T, Agius MA, Stites T, et al. Fingolimod reduces annualized relapse rate in patients with relapsing-remitting multiple sclerosis: FREEDOMS II study subgroup analysis (P07.102) Neurology. 2013;80(Meeting Abstracts 1):P07.102. [Google Scholar]
- 16.ICH harmonised tripartite guidelines for good clinical practice E6(R1). International Conference on Harmonization of technical requirements for registration of pharmaceuticals for human use; Geneva. [accessed 23 September 2015]. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. [Google Scholar]
- 17.World Medical Association. [accessed 23 September 2015];Declaration of Helsinki: Ethical principles for medical research involving human subjects. www.wma.net/en/30publications/10policies/b3/index.html.
- 18.Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O’Connor PW, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58(6):840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 19.Scott TF, Schramke CJ, Novero J, Chieffe C. Short-term prognosis in early relapsing-remitting multiple sclerosis. Neurology. 2000;55(5):689–693. doi: 10.1212/wnl.55.5.689. [DOI] [PubMed] [Google Scholar]
- 20.Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006;129(Pt 3):595–605. doi: 10.1093/brain/awh714. [DOI] [PubMed] [Google Scholar]
- 21.Bove R, Chitnis T. Sexual disparities in the incidence and course of MS. Clin Immunol. 2013;149(2):201–210. doi: 10.1016/j.clim.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Losseff NA, Miller DH, Kidd D, Thompson AJ. The predictive value of gadolinium enhancement for long term disability in relapsing-remitting multiple sclerosis--preliminary results. Mult Scler. 2001;7(1):23–25. doi: 10.1177/135245850100700105. [DOI] [PubMed] [Google Scholar]
- 23.Brex PA, Ciccarelli O, O’Riordan JI, Sailer M, Thompson AJ, Miller DH. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med. 2002;346(3):158–164. doi: 10.1056/NEJMoa011341. [DOI] [PubMed] [Google Scholar]
- 24.Agius M, Meng X, Chin P, Grinspan A, Hashmonay R. Fingolimod therapy in early multiple sclerosis: an efficacy analysis of the TRANSFORMS and FREEDOMS studies by time since first symptom. CNS Neurosci Ther. 2014;20(5):446–51. doi: 10.1111/cns.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kremenchutzky M, O’Connor P, Hohlfeld R, et al. Impact of prior treatment status and reasons for discontinuation on the efficacy and safety of fingolimod: Subgroup analyses of the Fingolimod Research Evaluating Effects of Daily Oral Therapy in Multiple Sclerosis (FREEDOMS) study. Mult Scler Relat Disord. 2014;3(3):341–9. doi: 10.1016/j.msard.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Tremlett H, Zhao Y, Joseph J, Devonshire V UBCMS Clinic Neurologists. Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry. 2008;79:1368–74. doi: 10.1136/jnnp.2008.145805. [DOI] [PubMed] [Google Scholar]
- 27.Rudick RA, Kappos L, Kinkel R, et al. Gender effects on intramuscular interferon beta-1a in relapsing remitting multiple sclerosis: analysis of 1406 patients. Mult Scler. 2011;17:353–60. doi: 10.1177/1352458510384605. [DOI] [PubMed] [Google Scholar]
- 28.Trojano M, Pellegrini F, Paolicelli D, Fuiani A, Zimatore GB, Tortorella C, Simone IL, Patti F, Ghezzi A, Portaccio E, et al. Post-marketing of disease modifying drugs in multiple sclerosis: an exploratory analysis of gender effect in interferon beta treatment. J Neurol Sci. 2009;286(1–2):109–113. doi: 10.1016/j.jns.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 29.Alemayehu D. Perspectives on pooled data analysis: the case for an integrated approach. J Data Sci. 2011;9:389–97. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Annualized relapse rates in patient subgroups defined by demographic factors and baseline disease characteristics: 1-year truncated analysis of fingolimod 0.5 mg patient group
Supplementary Figure 2. Annualized relapse rates in patient subgroups defined by demographic factors and baseline disease characteristics; fingolimod 1.25 mg patient group.