Abstract
Purpose
To investigate the relationship between serum neutrophil/lymphocyte ratio and pseudoexfoliation syndrome.
Materials and methods
This study was designed as a retrospective analysis. Patients were divided into three groups: 55 patients with pseudoexfoliation syndrome (group 1), 19 patients with pseudoexfoliation glaucoma (group 2), and 48 control subjects without pseudoexfoliation syndrome or pseudoexfoliation glaucoma (group 3). The levels of neutrophils and lymphocytes were measured by ABX Pentra DF120/USA biochemical analyzer. The neutrophil/lymphocyte ratio was measured by dividing neutrophil count by lymphocyte count.
Results
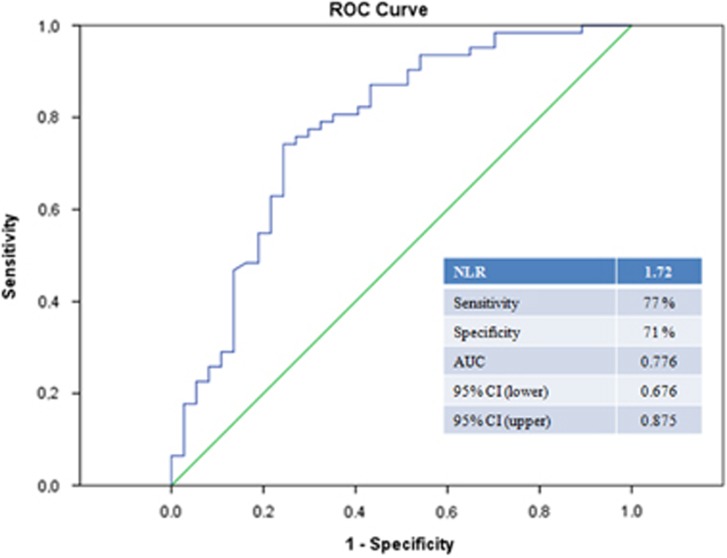
The mean ages of patients were 67.9±8.1 in group 1, 70.6±7.4 in group 2, and 67.3±7.6 in group 3 (P=0.172). Baseline clinical characteristics, such as diabetes and hypertension, were similar among groups. The mean neutrophil/lymphocyte ratio values were 2.08±0.61 in group 1, 2.20±0.58 in group 2, and 1.51±0.57 in group 3. The neutrophil/lymphocyte ratio was significantly higher in group 1 and 2 than the group 3 (P<0.001). In receiver operating characteristics analysis, the area under the curve for neutrophil/lymphocyte ratio was 0.776, and a neutrophil/lymphocyte ratio of >1.72 predicted pseudoexfoliation syndrome with a sensitivity of 77% and specificity of 71%.
Conclusion
The elevated neutrophil/lymphocyte ratio is significantly associated with pseudoexfoliation syndrome. This novel and low-cost parameter can provide useful information for the relevant risk evaluation in these patients.
Introduction
Pseudoexfoliation syndrome (PEXS) is known as an age-related, systemic, multifactorial disorder. PEXS is characterized by the production and accumulation of a gray-white, abnormal fibrillogranular material in and around the anterior segment of the eye. The pathogenesis of PEXS includes genetic and nongenetic factors as age, race, vascular status, ultraviolet rays, autoimmune diseases, trauma, viral infections, inflammation and oxidative stress.1, 2, 3 Inflammatory biomarkers will become an increasingly important issue in clinical practice. Both increased neutrophil levels and lymphopenia as proper biomarkers of acute inflammation has been started to be used with the other markers like C-reactive protein.4, 5 The inflammation is an important component of various age-related diseases such as: Alzheimer's disease and atherosclerosis.6 The role of neutrophil/lymphocyte ratio (NLR) in cardiovascular and cancer diseases has been reported 4, 5 but the relation with the ocular diseases is unclear.
In this study, based on the role of inflammation in damage and pathogenesis of PEXS; we aimed to evaluate the relation between serum NLR and PEXS.
Materials and methods
This retrospective study was undertaken at a training and research hospital, ophthalmology department, from September 2012 to November 2014. This submission has received Review Board/Ethics Committee approval. The study adhered to the tenets of the Declaration of Helsinki.
Fifty-five patients who had PEXS diagnosed during routine ophthalmology examination were assigned to group 1, 19 patients with pseudoexfoliative glaucoma (PEXG) were determined as group 2, and 48 patients who did not have PEXS or PEXG were included randomly to the group 3. The detailed systemic disorders were reported including diabetes and hypertension. The patients with hematologic disorder, acute or chronic infection, inflammatory ocular diseases, any ocular medication (except topical medications because of PEXG), chronic obstructive pulmonary disease, current steroid therapy and/or history of steroid use 3 month before the admission and a history of cancer and/or treatment with radiation or chemotherapy were excluded from the study.
The diagnosis of PEXS was based on the presence of typical exfoliation material on the anterior lens capsule or pupillary margin after the pupillary dilatation by pharmacological agents in one or both eyes, with a normal optic disc and visual field findings in patients with an intraocular pressure (IOP) <21 mm Hg. Control subjects had no history of ocular disease (except for refractive error and cataract) and elevated IOP (>21 mm Hg), and no evidence of exfoliation material on the anterior lens capsule or pupillary margin. Presence of exfoliation material in the anterior chamber with IOP over 21 mm Hg, an open anterior chamber angle determined with gonioscopy, and visual field and optic nerve changes established the diagnosis of PEXG.
The serum neutrophil and lymphocyte values were evaluated by Horiba ABX Pentra DF120/MA-USA (Holliston, MA, USA) biochemical analyze. The complete blood counts of patients were gained from the hospital laboratory archive. The NLR was calculated as the ratio of the neutrophil count to the lymphocyte count.
Statistical analysis
All analyses were performed using SPSS for Windows 18.0 (version 18.0, SPSS, Chicago, IL, USA). Quantitative variables were expressed as mean value±SD. Continuous variables were analyzed for normal distribution using the Kolmogorov–Smirnov test and analyzed for homogeneity using the Levene tests. Comparisons of parametric values among groups were performed by one-way ANOVA. Comparisons of non-parametric values among groups were performed by the Kruskal–Wallis test. Tukey HSD (for parametric variables) and Bonferroni adjustment Mann–Whitney U test (for non-parametric variables) were used as a post hoc test for multiple comparisons among the groups. A two-tailed P-value<0.05 was considered as significant. The receiver operating characteristics (ROCs) curve analysis was performed to demonstrate the sensitivity and specificity of admission NLR, optimal cutoff value for predicting PEXS.
Results
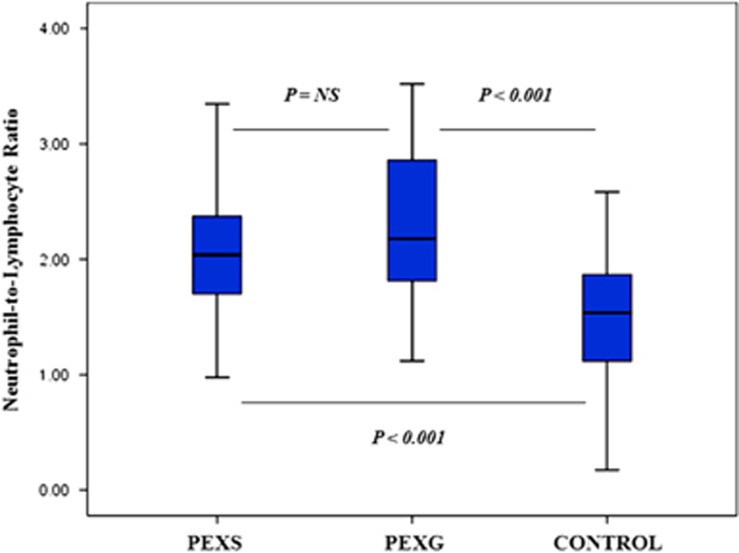
A total of 130 patients were screened. Eight of them were not included in the study because of exclusion criteria (acute or chronic infection (n=3), inflammatory ocular diseases (n=2), current steroid therapy (n=2), and history of cancer (n=1)). Thirty-eight patients had unilateral and 17 patients had bilateral PEXS in group 1. Ten patients had unilateral and 9 patients had bilateral PEXG in group 2. The mean NLR values were 2.08±0.61 in group 1, 2.20±0.58 in group 2, and 1.51±0.57 in group 3. The NLR values were significantly higher in group 1 and 2 than the group 3 (P<0.001). Baseline clinical features and laboratory measurements of subjects were shown in Table 1. The clinical characteristics such as age, diabetes, and hypertension were similar among groups. There was not a significant difference between group 1 and 2 in terms of all clinical parameters (Figure 1). The area under the ROCs curve for NLR was 0.776, and a NLR of 1.72 or higher predicted PEXS with a sensitivity of 77% and specificity of 71% (Figure 2).
Table 1. Comparison of baseline characteristics and laboratory measurements among the groups.
| Variable | Group 1 (PESX; n =55) | Group 2 (PEXG; n =19) | Group 3 (Control; n =48) | P-value |
|---|---|---|---|---|
| Age, years±SD | 67.9±8.1 | 70.6±7.4 | 67.3±7.6 | 0.172 |
| Gender (female/male) | 30/25 | 7/12 | 31/17 | 0.216 |
| Diabetes mellitus | 14 (25.5%) | 5 (26.3%) | 15 (31.3%) | 0.222 |
| Hypertension | 30 (54.5%) | 12 (63.2%) | 22 (45.8%) | 0.529 |
| Neutrophil count (× 103 μl)±SD | 3.52±0.80 | 3.68±0.67 | 3.16±0.91 | 0.06 |
| Lymphocyte count (× 103 μl)±SD | 1.76±0.46a | 1.72±0.31a | 2.12±0.53 | 0.002 |
| Neutrophil-to-lymphocyte ratio±SD | 2.08±0.61a | 2.20±0.58a | 1.51±0.57 | <0.001 |
Abbreviations: PEXS, pseudoexfoliation syndrome; PEXG, pseudoexfoliative glaucoma; SD, standard deviation.
P<0.05 in group 1 and 2 versus group 3.
Figure 1.
Comparison of neutrophil-to-lymphocyte ratio among groups.
Figure 2.
ROCs curve analyses of neutrophil-to-lymphocyte ratio for predicting PEXS. AUC, area under the curve.
Discussion
This report demonstrates the association between NLR and PEXS. In our study, we have found an association of elevated NLR with PEXS and PEXG.
There is a relation between PEXS with ocular ischemia, increased inflammatory activity, iris hypoperfusion, anterior chamber hypoxia, and with a decreased ocular and retroocular micro and macrovascular blood flow.6 The cellular stress conditions such as oxidative stress, inflammation, ischemia and hypoxia play a role in the pathophysiology of PEXS and PEXG.7, 8
A clear role for inflammatory mediators and cells has been established in recent years. Yildirim et al suggested that inflammation is a factor that can be involved in etiopathogenesis of PEXS.9 Several inflammatory biomarkers were studied in patients with PEXS. Cumurcu et al10 emphasized the elevated serum alpha 1-antitrypsin activity as an inflammation biomarker in patients with PEXS. The relationship between PEXS and serum YKL-40, a proinflammatory protein that has been demonstrated to have a role in the pathogenesis of endothelial dysfunction was shown.11 Proinflammatory cytokines in eyes with early and late stages of PEX syndrome/glaucoma were studied.12 The authors support a role for a stress-induced, spatially, and temporally restricted subclinical inflammation in the onset of the fibrotic matrix process characteristic of PEXS/PEXG.12 Sorkhabi et al studied high-sensitivity C-reactive protein and tumor necrosis factor alpha in PEXS and mentioned that increased levels of these markers, as signs of inflammation and peripheral endothelial dysfunction, may be risk factors for systemic and ocular manifestations of PEXS.13 In contrast, high-sensitivity C-reactive protein had not been found as a predictive marker of inflammation and peripheral endothelial dysfunction in PEXS and PEXG.14
The inflammation is an important component of other ocular diseases such as age-related macular degeneration (AMD) and vascular diseases of retina.15, 16, 17 The relationship between NLR and diabetic retinopathy was shown.11 The assessment of NLR in patients with AMD was studied by Ilhan N et al.17 In their study, patients with AMD have higher NLR compared with controls and NLR correlates with age and disease severity.
The number of patients, especially of patients with PEXG, was small. Therefore, the statistical power to detect differences between patients with PEXS and PEXG was limited. This is the limitation criteria of our study.
In conclusion, based on the role of inflammation in damage and pathogenesis of PEXS, NLR values may be evaluated as a simple and a reliable biomarker of an increased inflammatory activity in patients with PEXS. Further investigations including more patients are needed to investigate the possible role of serum NLR levels in PEXS.
The authors declare no conflict of interest.
References
- Scharfenberg E, Schlötzer-Schrehardt U. PEX syndrome: clinical diagnosis and systemic manifestations. Ophthalmologe 2012; 109(10): 952–961. [DOI] [PubMed] [Google Scholar]
- Gartaganis SP, Georgakopoulos CD, Patsoukis NE, Gotsis SS, Gartaganis VS, Georgiou CD. Glutathione and lipid peroxide changes in pseudoexfoliation syndrome. Curr Eye Res 2005; 30(8): 647–651. [DOI] [PubMed] [Google Scholar]
- Faschinger C, Schmut O, Wachswender C, Mossböck G. Glaucoma and oxidative stress. Determination of malondialdehyde—a product of lipid peroxidation. Ophthalmologe 2006; 103(11): 953–959. [DOI] [PubMed] [Google Scholar]
- Kaya MG. Inflammation of coronary artery disease: as a new biomarker neutrophil/lymphocyte ratio. Arch Turk Soc Cardiol 2013; 41: 191–192. [Google Scholar]
- Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit Rev Oncol Hematol 2013; 88(1): 218–230. [DOI] [PubMed] [Google Scholar]
- Ocakoglu O, Koyluoglu N, Kayiran A, Tamcelik N, Ozkan S. Microvascular blood flow of the optic nerve head and peripapillary retina in unilateral exfoliation syndrome. Acta Ophthamol 2004; 82(1): 49–53. [DOI] [PubMed] [Google Scholar]
- Sorkhabi R, Ghorbanihaghjo A, Ahoor MH. Oxidative stress in psudoexfoliationsyndrome. Indian J Ophthalmol 2011; 23: 27–32. [Google Scholar]
- Sorkhabi R, Ghorbanihaghjo A, Javadzadeh A, Rashtchizadeh N, Moharrery M. Oxidative DNA damage and total antioxidant status in glaucoma patients. Mol Vis 2011; 17: 41–46. [PMC free article] [PubMed] [Google Scholar]
- Yildirim Z, Yildirim F, Ucgun NI, Sepici-Dincel A. The role of the cytokines in the pathogenesis of pseudoexfoliation syndrome. Int J Ophthalmol 2013; 6(1): 50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumurcu T, Ozyurt H, Demir HD, Yardim H. Serum alpha-1-antitriypsin levels in patients with pseudoexfolative syndrome. Curr Eye Res 2008; 33(2): 159–162. [DOI] [PubMed] [Google Scholar]
- Turkyılmaz K, Öner V, Kırbas A, Sevim MS, Sekeryapan B, Özgür G et al. Serum YKL-40 levels as a novel marker of inflammation and endothelial dysfunction in patients with pseudoexfoliation syndrome. Eye (Lond) 2013; 27(7): 854–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenkel M, Lewczuk P, Jünemann A, Kruse FE, Naumann GO, Schlötzer-Schrehardt U. Proinflammatory cytokines are involved in the initiation of the abnormal matrix process in pseudoexfoliation syndrome/glaucoma. Am J Pathol 2010; 176(6): 2868–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkhabi R, Ghorbanihaghjo A, Ahoor M, Nahaei M, Rashtchizadeh N. High-sensitivity C-reactive protein and tumor necrosis factor alpha in pseudoexfoliation syndrome. Oman Med J 2013; 28(1): 16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yüksel N, Pirhan D, Altintaş O, Caglar Y. Systemic high-sensitivity C-reactive protein level in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. J Glaucoma 2010; 19(6): 373–376. [DOI] [PubMed] [Google Scholar]
- Ulu SM, Dogan M, Ahsen A, Altug A, Demir K, Acartürk G et al. Neutrophil-to-lymphocyte ratio as a quick and reliable predictive marker to diagnose the severity of diabetic retinopathy. Diabetes Technol Ther 2013; 15(11): 942–947. [DOI] [PubMed] [Google Scholar]
- Rodrigues EB. Inflammation in dry age-related macular degeneration. Ophthalmologica 2007; 221(3): 143–152. [DOI] [PubMed] [Google Scholar]
- Ilhan N, Daglioglu MC, Ilhan O, Coskun M, Tuzcu EA, Kahraman H et al. Assessment of Neutrophil/Lymphocyte Ratio in Patients with Age-related Macular Degeneration. Ocul Immunol Inflamm 2014; 2: 1–4. [DOI] [PubMed] [Google Scholar]