Abstract
Mutation of the Golgi Ca2+-ATPase ATP2C1 is associated with deregulated calcium homeostasis and altered skin function. ATP2C1 mutations have been identified as having a causative role in Hailey-Hailey disease, an autosomal-dominant skin disorder. Here, we identified ATP2C1 as a crucial regulator of epidermal homeostasis through the regulation of oxidative stress. Upon ATP2C1 inactivation, oxidative stress and Notch1 activation were increased in cultured human keratinocytes. Using RNA-seq experiments, we found that the DNA damage response (DDR) was consistently down-regulated in keratinocytes derived from the lesions of patients with Hailey-Hailey disease. Although oxidative stress activates the DDR, ATP2C1 inactivation down-regulates DDR gene expression. We showed that the DDR response was a major target of oxidative stress-induced Notch1 activation. Here, we show that this activation is functionally important because early Notch1 activation in keratinocytes induces keratinocyte differentiation and represses the DDR. These results indicate that an ATP2C1/NOTCH1 axis might be critical for keratinocyte function and cutaneous homeostasis, suggesting a plausible model for the pathological features of Hailey-Hailey disease.
Epidermal homeostasis depends on the continuous balance between the proliferative and differentiation potential of keratinocytes, which exit the cell cycle and differentiate as they move outward from the proliferative basal layer through the suprabasal layers1,2,3. Several signaling pathways regulate the morphological and functional changes associated with epidermal differentiation. These signals and changes are important for epidermal barrier function and responses to external environmental insults. In keratinocytes, complex and fine-tuned calcium signaling gives rise to a number of signaling pathways involved in differentiation. A crucial aspect of calcium signaling is the maintenance of the cytoplasmic calcium level by an array of calcium pumps and exchangers located on the surfaces of intracellular membranes4,5. The orthologue of a yeast gene encoding a calcium ATPase, ATP2C1, is thought to play a critical role in the pathogenesis of Hailey-Hailey disease (HHD)6,7,8,9. HHD is a rare autosomal-dominant inherited disease with high penetrance. It is characterized by suprabasal cell separation (acantholysis) of the epidermis. Although HHD is a rare disorder, it is a very good model by which to study the different key regulatory systems of the skin, including keratinocyte proliferation, differentiation and adhesion. Indeed, the information obtained for HHD can be translated into other diseases with similar underlying pathogenesis. We showed an increase in oxidative stress in keratinocytes derived from cutaneous lesions of HHD patients10,11,12. Interestingly, we showed that Notch1 expression is negatively regulated by ATP2C1 deficiency-mediated reactive oxygen species (ROS) induction in keratinocytes, both ex vivo and in vitro10,11,12. Perhaps the best evidence underscoring the importance of ROS and NOTCH1 is the recently established role of this axis in the process of keratinocyte differentiation13. ROS generated by the mitochondria are important regulators of epidermal differentiation. A failure to generate mitochondria-derived ROS impaired epidermal differentiation by preventing the transmission of Notch signals that are essential for epidermal differentiation13. Although terminal differentiation of keratinocytes includes cell death, it has become clear during the past decade that this pathway also employs pro-survival mechanisms14. Recent studies have determined that stem cells can undergo cell differentiation upon DNA damage in an ATM (protein kinase ataxia-telangiectasia mutated)-dependent manner15. ATM, best known for its role as an apical activator of the DNA damage response (DDR), mobilizes and orchestrates one of the most extensive signaling networks in response to the induction of DNA damage and directly or indirectly modifies a broad range of targets16. Oxidative DNA damage caused by ROS generated during metabolism makes significant contributions to genomic instability, carcinogenesis and cellular aging16,17,18. ATM has also been directly implicated in the regulation of oxidative stress, and several reports have indicated that oxidative stress was not properly controlled in cells from patients with ataxia-telangiectasia and in tissues of ATM-deficient mice16,17,18. Notably, genotoxic stress induces an increase in DNA damage that blocks transcription and might down-regulate DNA repair itself. Notch is a direct negative regulator of the DDR that counteracts ATM signaling19. Interestingly, the DDR is activated upon oxidative DNA damage that results from ROS. It has been shown that MYC hyperactivity causes the accumulation of DNA damage and terminal squamous differentiation20.
Much about the molecular mechanisms regulating the complex interactions among resident skin cells and the additional extrinsic signals involved in HHD manifestation remains unknown. In this study, RNA-seq was used to identify the differentially expressed genes in lesioned (LS) and non-lesioned (NL) HHD skin samples from the same individual. Three pairs of LS and NL skin biopsies were taken and analyzed. RNA-seq data analysis identified a substantial number of differentially expressed genes in lesioned skin. These changes in gene expression were clearly shown by the down-regulation of genes involved in the DDR and the up-regulation of genes related to the inflammatory response. Dysregulation of inflammatory genes contributes to altered cutaneous homeostasis and to the pathogenesis of inflammatory skin diseases21. Our data raise the possibility that keratinocyte-intrinsic pathways have key roles in regulating immune homeostasis and inflammation in HHD-lesioned skin. We have shown that the differentiation of HHD-derived keratinocytes is impaired, suggesting that ROS may play an early, causal role in the altered HHD-derived keratinocyte differentiation10,11,12. Our hypothesis is that the disturbance of calcium homeostasis in Hailey-Hailey keratinocytes promotes skin lesions by causing DNA damage and that Notch1 might mediate this response. This signaling can lead to a level of DNA damage that overwhelms the defenses of the keratinocyte cells and causes their death. Our results may contribute to a better characterization of a subset of human skin diseases and guide new therapeutic treatments.
Results
Differential gene expression between the lesioned and non-lesioned skin of individuals with Hailey-Hailey disease
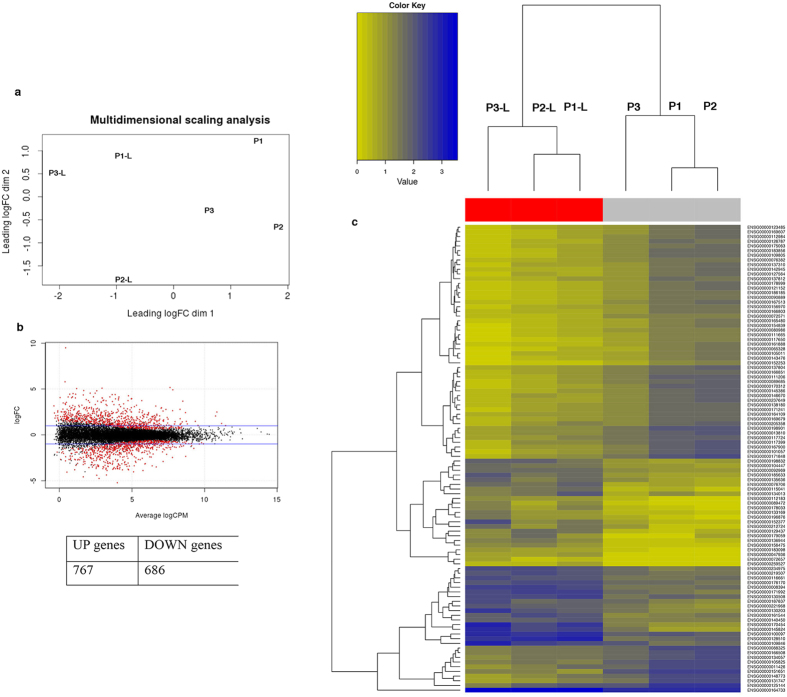
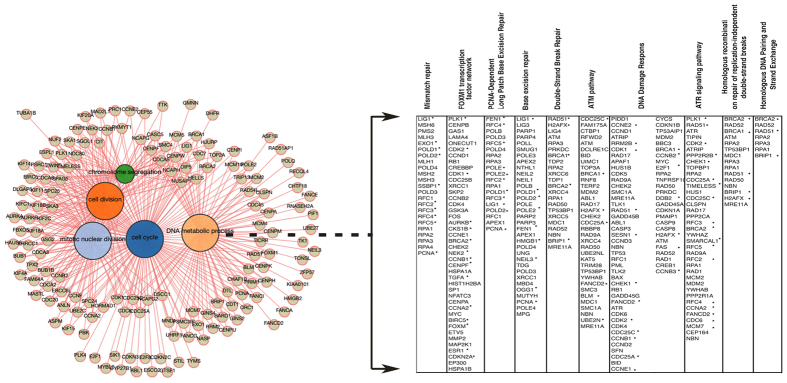
HHD is a chronic, rare inherited disease of the skin characterized by red scaly areas that can be painful and itchy and can lead to superficial blisters and eroded areas of the skin. The disease often has a remission and recurrence pattern, which may be constant in some patients. HHD is associated with the loss of a single copy of ATP2C1, a gene that is likely essential in humans, as more severe phenotypes are found in patients who suffer clonal loss of both copies of the gene22. Consistently, mice embryos homozygous for null mutations in ATP2C1 die due to defects in neural tube closure, whereas heterozygotes show susceptibility to squamous cell tumors, a phenotype that is rarely observed in humans with HHD (ref. 23,24 and our personal observations). ATP2C1 is localized in the Golgi, where it transports both Ca2+ and Mn2+ and plays important roles in the homeostasis of both molecules4,25,26,27. It remains an enigma why individuals with defects in the ATP2C1 gene develop a skin-specific disease, highlighting the need to elucidate how genetics might contribute to the tissue specificity of HHD. Accordingly, we performed whole-genome expression profiling using RNA-seq data from lesioned (P1-L, P2-L, and P3-L) and unaffected skin (P1, P2, and P3) of three individuals with HHD to identify the differentially expressed genes. For each sample, an average of ~50 million 100-bp reads was generated. The sequenced reads were mapped to the human reference genome (hg19), with an average of 46.5 million mapped reads per sample. An average of ~85% of all the aligned reads were uniquely mapped on the genome and were considered for read counting, with respect to the Ensembl transcriptome annotation28. Normalization and the analysis of differential gene expression were performed using the EdgeR package29,30. The multidimensional scaling analysis supported the partition of the non-lesioned vs lesioned samples into two groups (Fig. 1a). A total of 1,453 genes were significantly differentially expressed between the normal and lesion-derived keratinocytes at a false discovery rate (FDR) < 0.05. The expression of 686 genes was down-regulated and the expression of 767 genes was up-regulated in the lesions compared with the unaffected skin controls (Fig. 1b). Hierarchical clustering of gene expression clearly showed separation between the keratinocytes from the non-lesioned and lesioned keratinocytes; the corresponding heatmap of the top 100 differentially expressed genes is shown in Fig. 1c. A functional annotation was performed to identify the biological processes that were significantly enriched in the differentially expressed genes. An enrichment map was generated to visualize the functional clusters of the enriched biological processes, including cell cycle, DDR and metabolic process (Fig. 2). DNA damage/DNA repair was the main cluster in the enrichment map, and the genes involved in the process were predominantly down-regulated in the lesioned skin of HHD patients compared with the unaffected controls (Fig. 2 and Supplementary Fig. 1). We found that the HHD lesion-derived keratinocytes had a higher level of oxidative stress than the non-lesion-derived keratinocytes10,11,12. Oxidative stress can result in oxidative DNA damage; if the DNA-damage remains unrepaired, the cells may enter cellular senescence or programmed cell death31,32,33. Interestingly, we found that HHD lesion-derived keratinocytes were hypoproliferative compared to the non-lesion-derived keratinocytes11. Therefore, it is possible that persistent DDR repression is causally associated with HHD lesion manifestation. We performed whole-exome sequencing of two lesion-derived keratinocytes using human all-exon targeted capture (Agilent) followed by massively parallel sequencing (Illumina) to address this important question. The mean sequencing coverage across the targeted bases was 150X, and 85% of the bases had more than 100X coverage. After some variant filtering steps (see Methods), we identified a total of 1,520 missense mutations that were shared between the lesions derived from the two patients (Supplementary Fig. 2), most of which (~400) were novel non-synonymous variants that had not already been annotated in dbSNP (Build 142)34. The mutation burden we identified in the HHD lesions was greater than those of most other common genetic diseases, as previously described35; for example, the mutation burden was comparable to the burden observed in Seckel Syndrome, in which the functional loss of the centrosomal protein CEP152 results in an impairment of DDR pathways35,36. We also identified 45 null mutations that were common to both lesions (Supplementary Fig. 2a,b and Supplementary Fig. 3a,b). Nonsense mutations were identified in IRS1 (insulin receptor substrate), which is essential for skin formation and development37. The lesions harbored an additional nonsense mutation that disrupted PRIM2, a DNA primase that plays a key role in DNA replication and in the DDR38. We also identified nonsense mutations targeting the CROCC (ciliary rootlet coiled-coil or Rootletin) gene, which forms centriole-associated filaments and functions in centrosome cohesion39. Conceivably, these lesions harbor mutations in genes whose loss would be deleterious to skin cells. Overall, the results show that the DDR is strongly repressed in HHD lesions. Consequently, the keratinocytes derived from the HHD lesions tend to be lost, likely as a result of the sequential acquisition of mutations in genes involved in the cellular stress response.
Figure 1.
(a) Multidimensional scaling (MDS) plot generated using the edgeR package. The distance between the sample labels indicates similarity. Lesioned samples are named P1-L through P3-L. Non-lesioned samples are named P1 through P3. Dimension 1 separates the lesioned samples from the non-lesioned samples, whereas dimension 2 roughly corresponds to the patient number. This result confirms the paired nature of the samples. (b) Plot showing the relationship between the log2-fold change versus the average log2-counts-per-million (CPM) of the genes; the horizontal lines indicate a fold change of two. Red font was used to indicate the differentially expressed genes at an FDR of 0.05; in the table the corresponding numbers of up- and down-regulated genes. (c) Unsupervised hierarchical clustering of the expression profiles of the non-lesion- and lesion-derived keratinocyte samples from HHD patients was performed using the top 100 differentially expressed genes. The color scale from yellow to blue corresponds to their expression values. The Ensembl gene names are labeled on the dendrogram. Lesion-derived samples are named P1-L through P3-L. Control samples are named P1 through P3.
Figure 2. Network graphic output of the BioMart enrichment tool, which reports the Gene Ontology (GO) enrichment analysis performed using 686 down-regulated genes from the keratinocytes derived from the HHD lesions (indicated by * in the table).
The functional annotations were further analyzed using the Consensus Path Database (cpdb.molgen.mpg.de).
ATP2C1 loss in keratinocytes is associated with Notch1 activation and ATM down-regulation
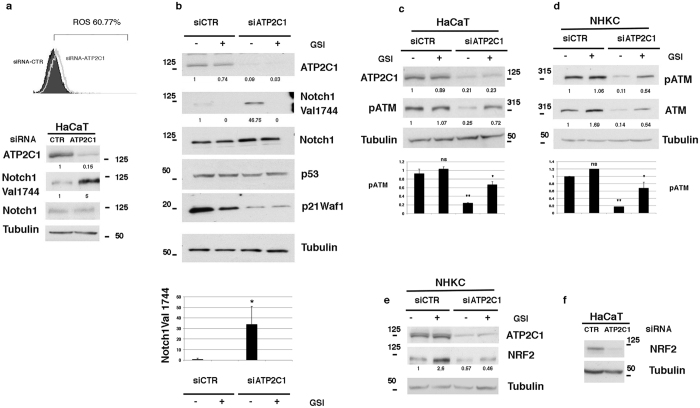
Because HHD is associated with ATP2C1 loss, we examined the consequences of ATP2C1 inactivation in the DDR response in human primary keratinocytes and keratinocyte-derived cell lines. HHD lesion-derived keratinocytes are characterized by increased oxidative stress and decreased expression levels of both Notch1 and NRF211. Interestingly, Nrf2 activation directly regulates DDR gene transcription40; thus, the loss of Nrf2 in lesioned HHD skin may play a role in the down-regulation of the transcription of DDR genes. Therefore, we first confirmed that ATP2C1 loss increased oxidative stress (Fig. 3a). The percentage of DFCA-positive cells in siATP2C1 cells reached 60% at 48 hrs after transfection, whereas only 15% of the siRNA-CTR control cells were DFCA-positive. Unexpectedly, inhibition of ATP2C1 expression in keratinocytes resulted in increased levels of activated Notch1 in both HaCaT cells and primary keratinocytes (Fig. 3a lower panel and 3b). Conversely, NRF2 expression was down-regulated in both HaCaT cells and primary keratinocytes, as previously shown in primary keratinocytes derived from the HHD lesions (Fig. 3e,f). Recently, it has been shown that Notch inactivation of ATM kinase activity represents an evolutionarily conserved mechanism that impairs the DDR response19. Notch1 binds to ATM and directly inhibits its kinase activity. Therefore, we tested whether Notch1 signaling is involved in the control of ATM/DDR in keratinocytes. Interestingly, the level of the activated Notch1 protein was increased upon transfection with an siRNA for the ATP2C1 gene (Fig. 3b). Strikingly, cells expressing activated Notch1 showed reductions in both phosphorylated and total ATM compared to the control cells (Fig. 3b–d). However, when we inhibited Notch1 activation by adding a γ-secretase inhibitor (GSI), the ATM levels were no longer reduced in the siATP2C1-treated cells (Fig. 3b–d).
Figure 3. Decreased ATM levels by knockdown of ATP2C1 expression.
(a) In the upper panel, the HaCaT keratinocyte-derived cell line was transfected with either siRNA-CTR or siRNA-ATP2C1, and the cells were analyzed by flow cytometry. The percentage of positive cells is also shown. Overlay of the FACS profile of the siRNA-CTR-transfected keratinocytes (filled histogram) onto the profile of the siRNA-ATP2C1-transfected keratinocytes (empty histogram). In the lower panel, the HaCaT cells were treated as in the upper panel and were analyzed by western blotting for ATP2C1, Notch1, and Notch1Val1744. Tubulin blots are shown as a control for equal loading. (b) Primary human keratinocytes were transfected with either siRNAs targeting ATP2C1 or a scrambled siRNA control. The cells were analyzed by western blotting for ATP2C1 and the indicated proteins at 48 h after transfection. Twenty-four hrs after transfection, the cells were treated with GSI (10 μΜ), incubated for an additional 24 h, and analyzed by immunoblotting with the indicated antibodies. Each data point in the graph represents the mean ± SEM of three independent experiments. *P < 0.05; lanes 1 vs lane 3. (c,d) Both NHKCs and HaCaT cells were transfected with the control (siRNA-CTR) or ATP2C1-specific siRNAs; 24 h later, the cells were treated with GSI (10 μΜ) for 24 h and analyzed by immunoblotting with the indicated antibodies. In panels b and d, the same cell extracts were analyzed on separated gels. In panels c and d, each data point in the graph represents the mean ± SEM of three independent experiments. ns lane 1 vs lane 2; **P < 0.005, lane 1 vs lane 3; *P < 0.05, lane 3 vs lane 4; (e) NHKCs were treated as in panel b and analyzed by western blotting for ATP2C1 and NRF2. Tubulin is shown as a control for equal loading. (F) HaCaT cells were treated as in panel a and were analyzed by Western blotting for NRF2. Tubulin is shown as a control for equal loading.
Notch1 down-regulated ATM during keratinocyte differentiation
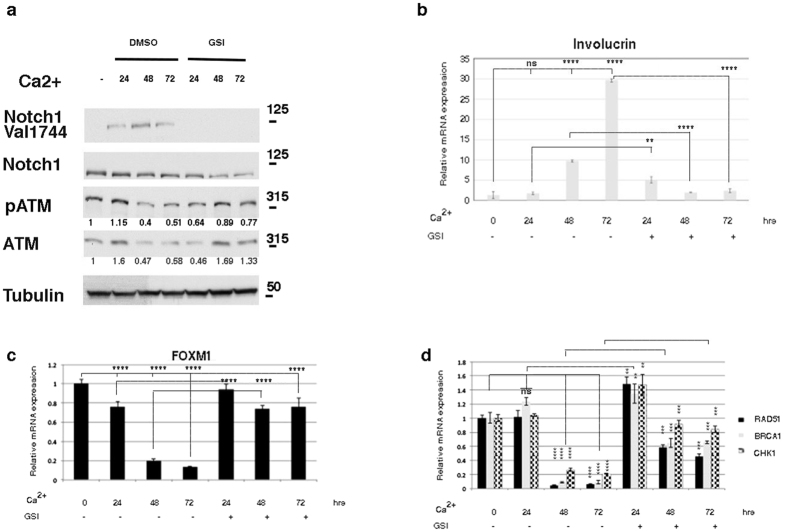
The Notch pathway plays an important role in regulating epidermal differentiation41,42,43,44. Genetic ablation or activation of the pathway reveals that Notch signaling promotes the differentiation of the interfollicular epidermis. Using a calcium-induced keratinocyte differentiation model, we investigated the interaction between Notch1/ATM and DDR signaling during keratinocyte differentiation. Ca2+ treatment induces cell cycle arrest and morphological changes typical of keratinocyte differentiation in cultured cells (Supplementary Fig. 6a,b). Upon further investigation by real-time PCR, the analysis revealed that calcium-induced keratinocyte differentiation specifically increased expression of specific suprabasal markers and Notch1 signaling, changes that were inhibited by GSI treatment (Fig. 4a,b). We analyzed the mRNA expression levels of the DDR factors FoxM1, RAD51, BRCA1 and CHK1 by quantitative RT-PCR (qRT-PCR) in Ca2+-differentiated keratinocytes to determine whether DDR signaling is modulated by keratinocyte differentiation. We found that the transcription levels of all these genes were strongly down-regulated in the Ca2+-treated keratinocytes (Fig. 4c,d). We next analyzed the expression levels of all investigated genes upon treatment with a Notch inhibitor. As shown in Fig. 4c,d, the mRNA levels of DDR genes were slightly or unaffected by GSI treatment. Notably, the same expression profile was observed for ATM protein expression (Fig. 4a). Our analysis of the DDR factors provided evidence that DDR gene expression was significantly decreased in keratinocytes after calcium treatment. The Notch inhibitor blocked the calcium-induced repression of the DDR genes and the induction of keratinocyte differentiation. These data indicate that calcium decreases ATM/DDR by increasing Notch1 signaling, indicating that the inactivation of both ATM and DDRs might also be involved in driving the cell toward differentiation. However, although ATM down-regulation was correlated with the activation of Notch1 signaling during calcium-induced keratinocyte differentiation, ATM inhibition was not sufficient to clearly promote keratinocyte differentiation. Thus, although ATM inhibition results in DDR down-regulation (Supplementary Fig. 4a,b), the analysis of the KU55933-treated keratinocytes displayed strikingly decreased K1 and K10 expression and increased Involucrin expression (Supplementary Fig. 4c). Consistent with our observation, it has been shown that normal human skin is characterized by a more prominent pATM/ATM staining in the basal layers of the epidermis, indicating that ATM expression is down-regulated upon the initiation of epidermal differentiation45. The analysis of the KU55933-treated keratinocytes showed that K1 and K10 expression was decreased and that Involucrin expression was increased; this pattern of marker expression was observed at the transition from the spinous to the granular layer. This observation indicates that ATM down-regulation might play a role in the transition from the spinous to the granular layer; however, the exact stage at which ATM is involved in keratinocyte differentiation remains to be clarified.
Figure 4. Calcium down-regulates the DNA damage response.
(a) Cell extracts were prepared from undifferentiated NHKC cells and cells that were differentiated with high calcium concentrations for 24, 48 and 72 hours. Calcium-induced differentiation was performed in the presence of dimethyl sulfoxide (DMSO) as a vehicle control or GSI (10 μΜ). Lysates were analyzed with the indicated antibodies. For the ATM analysis, the membrane was first analyzed with a pATM antibody and was then re-probed with an antibody against the total ATM protein (b–d) NHKCs were treated as in (a) and expression levels of the differentiation marker Involucrin and genes involved in the DDR pathways were determined by qRT-PCR. All graphs are representative of three independent experiments, and the values are expressed as the means ± SD of experiments conducted in triplicate. The significance of the differences was calculated using two-tailed Student’s t test, with the vehicle-treated sample as the reference, and one-way ANOVA by comparing the mean of each indicated column. In panel b, ****P < 0.0001 and **P = 0.002. In panel c, ****P < 0.0001. In panel d, ****P < 0.0001, **P = 0.001 and ***P = 0.0001.
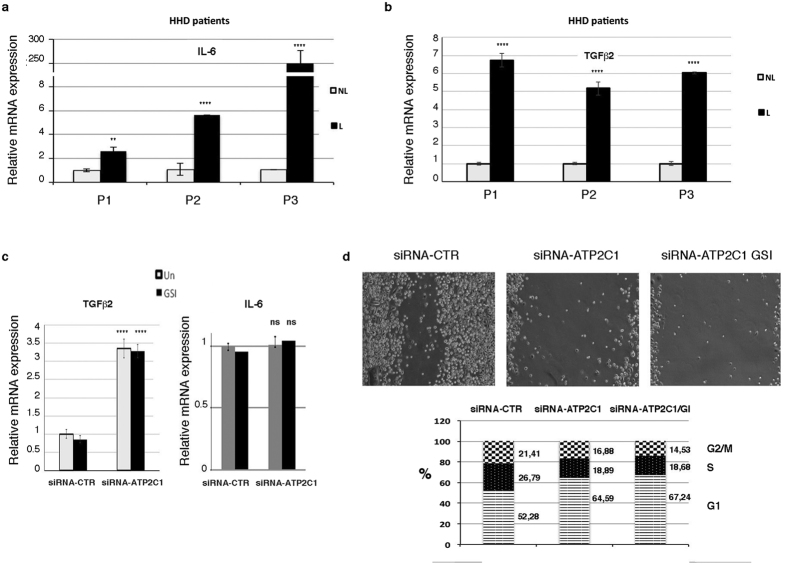
ATP2C1 RNA interference enhances Notch1 expression, promotes keratinocyte differentiation, and suppresses cell migration
HHD is characterized by skin lesions that do not heal and by recurrent skin infections, indicating that HHD keratinocytes might not respond well to challenges such as wounding or infection. After injury to both mouse and human skin, an increase in local cytokine production from keratinocytes occurs46,47,48. In skin wounds, interleukin (IL)-6, the IL-1 family and transforming growth factor (TGF)-beta, crucial cytokines that regulate the re-epithelialization process, are produced locally49,50,51,52,53,54,55. We examined whether HHD lesion-derived keratinocytes were defective in wound-induced cytokine production. In our RNA-seq analysis, we found that several factors involved in the wound response were up-regulated, including IL1-θ, TGF-beta2, Toll-like receptor (TLR)2, TLR1, TLR6, IL-6, IL-32, IL-34, and Serum Amyloid A1 and A2. Interestingly, the IL1R2 receptor, which may serve to terminate IL-1-driven inflammation56, was down-regulated in HHD lesions. IL-6 and TGF-beta-2 are very important for skin repair. Thus, we further analyzed the expression levels of these cytokines by qRT-PCR. The increases in IL-6 and TGFbeta-2 were confirmed by qRT-PCR in the three patients analyzed (Fig. 5a,b). In primary keratinocytes treated with siRNA-ATP2C1 and the siRNA control, TGF-beta-2 (but not IL-6) expression was increased, indicating that ATP2C1 loss had a direct effect on TGF-beta-2 expression (Fig. 5c). Therefore, these data indicate that keratinocytes derived from the HHD lesions are not defective in the production of the wound signal but that the poor healing of HHD lesions may result from other aspects of wound repair. Notch signaling controls a number of cellular functions in keratinocytes. Notch down-regulation in the epidermis appears to contribute to tissue regeneration during wound healing57. In our analysis, we found that ATP2C1 inhibition resulted in Notch1 activation (Fig. 3), which would alter the wound repair process. Thus, we first analyzed whether ATP2C1 inhibition might affect wound repair using a scratch wound healing assay of primary keratinocyte monolayers. Interestingly, ATP2C1 down-regulation played a role in regulating keratinocyte migration in the scratch wound (Fig. 5d). However, Notch1 inhibition did not rescue the migration properties of keratinocytes in the scratch assay, suggesting that the induction of Notch1 activity by ATP2C1 down-regulation does not play a role in regulating the wound repair of HHD keratinocytes. Additionally, we observed that the scratch wound defect might be the result of compromised proliferation as a consequence of the loss of ATP2C1 function (Fig. 5d, lower panel). However, the proliferation rate of siRNA-ATP2C1-treated cells was not affected by Notch1 inhibition, indicating that neither the proliferation nor the wound healing of ATP2C1-defective keratinocytes require Notch1.
Figure 5.
(a,b) qRT-PCR analysis of IL-6 and TGF-beta-2 mRNA expression levels in the non-lesioned and lesioned skin of HHD patients. The values are expressed as the fold changes of the indicated samples vs the siRNA-CTR-treated, GSI-treated, and untreated samples. (c) qRT-PCR of TGF-beta-2/IL-6 in the siRNA-ATP2C1- or siRNA-CTR-transfected NEHKs. (d-Upper panel) A scratch assay was performed to assess the migration rates of keratinocytes transfected with the control siRNA-CTR or ATP2C1-specific siRNAs for 48 hrs. Photographs were taken at the indicated time points after scratch injury. Where indicated, the scratch assay was performed in the presence of DMSO as a vehicle control or GSI (10 μΜ), which was added to the cells after the scratch injury (10X magnification). (d-Lower panel) Analysis of the cell cycle phases in NHKCs transfected with siRNA-ATP2C1 or siRNA-CTR. Cells stained with propidium iodide were subjected to flow cytometry analysis to determine the cell cycle distribution. The stacked bars represent the mean percentage of cells in a given phase. All graphs are representative of at least three independent experiments, and the values are expressed as the means ± SD of experiments conducted in triplicate. The significance of the differences was calculated using two-tailed Student’s t test, with non-lesioned skin samples as the reference. ****P < 0.01; **P < 0.05.
Discussion
Role of ATP2C1 in human keratinocytes
Previous studies have shown that ATP2C1 plays an essential role in the maintenance of skin homeostasis, and the loss of ATP2C1 function has a causative role in HHD6,7,26,58. However, it is unclear how ATP2C1 loss affects keratinocyte homeostasis. DNA damage is a crucial stage of MYC-mediated replication and stress-induced keratinocyte differentiation20, and DNA damage induced by genotoxic agents triggers squamous differentiation20,59,60. In this report, our results show that human keratinocytes respond to the loss of ATP2C1 function in a manner consistent with DNA damage-induced differentiation. This process is paralleled by increased Notch1 activation. Notch signaling is an essential regulatory determinant of keratinocyte growth and differentiation44. Human cells expressing Notch1 show inactivation of ATM and other DDR components19,40. Interestingly, down-regulation of the DDR has been proposed to constitute part of a mechanism associated with astrocyte differentiation61. Consistent with this model, it has been shown that the DDR is down-regulated upon the initiation of epidermal differentiation; furthermore, Human Papillomavirus actives the ATM/DNA damage pathway for viral genome amplification upon differentiation62. Thus, the loss of ATP2C1 may allow Notch1 activation to trigger the differentiation response. This response would be augmented by Notch1-mediated inhibition of the DNA repair/ATM pathway in cells that accumulate irreparable DNA damage (Fig. 6). One keratinocyte-specific function of ATP2C1 might be to protect the epidermal cells from a temporally inappropriate activation of Notch1, as HHD is a skin-specific disease.
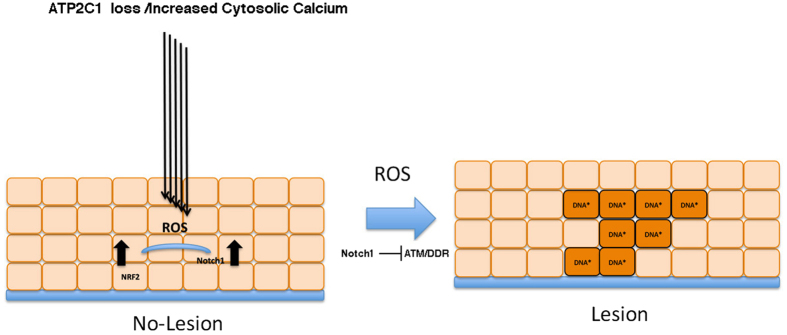
Figure 6. Proposed model of the mechanism involved in the manifestation of Hailey-Hailey disease.
The loss of one functional ATP2C1 allele alone is not sufficient to cause clinically overt HHD and skin lesions; additional alterations, e.g., infections or lesions, disrupt the compensatory mechanisms, resulting in deregulated calcium homeostasis. Therefore, our model of the mechanism of HHD pathogenesis is that altered calcium homeostasis produces oxidative stress and subsequent Notch1 activation. Notch1 activation can down-regulate ATM, leading to DNA damage that would then trigger terminal differentiation. However, the shift toward the generation of differentiated cells can irreversibly impact either ESCs or transit amplifying cells, resulting in compromised skin repair.
Implications for Hailey-Hailey disease
HHD patients exhibit skin blistering from acantholysis, indicating that cell-cell adhesion is compromised in ATP2C1-deficient keratinocytes. Given the key role for desmosomes in maintaining tissue integrity and epidermal organization, we analyzed the expression levels of adhesion molecules in the lesioned tissue from HHD patients to clarify the potential involvement of the deregulation of adhesion factors in the acantholysis of HHD lesions. An examination of our RNA-seq data revealed the there was no obvious difference in the adhesion molecule levels in any of the lesions from the analyzed patients. Therefore, although substantial evidence indicates that ATP2C1 loss compromises adhesion, cellular signaling directly or indirectly regulates desmosomal adhesion. Desmosome adhesion is calcium-independent but reverts to calcium dependence upon wounding in both cultured cell sheets and the epidermis63. Remarkably, the mRNA levels of cytokines involved in the wound response were up-regulated in the lesioned skin of HHD patients, indicating that HHD keratinocytes activate repair signaling to maintain skin homeostasis, as shown in other chronic inflammatory diseases21,64. In particular, the mRNA levels of the proinflammatory cytokines IL-6, IL-1 and IL-32-34 were up-regulated. Among these cytokines, IL-32 produced by keratinocytes has been shown to increase KC apoptosis in inflammatory skin diseases. Interestingly, IL-32 is expressed by human primary keratinocytes and modulates keratinocyte apoptosis in atopic dermatitis, whereas it was not present in either skin biopsy specimens from healthy donors or lesioned skin from patients with psoriasis65. Conversely, IL-33 levels are decreased in keratinocytes derived from HHD lesions; IL-33 may play pivotal roles in the maintenance of cutaneous homeostasis and the acceleration of normal wound healing. IL-33 promotes the healing of Staphylococcus aureus-infected wounds in mice66,67; one of the most common causes of skin infections in the HHD lesions is Staphylococcus aureus. Together, these findings reveal that keratinocytes derived from the HHD lesions are characterized by deregulated cytokine expression and decreased repair properties. Thus, it is likely that the chronic wounds present in ATP2C1-defective keratinocytes might influence cellular adhesion, possibly through an effect on the intracellular calcium concentrations by shifting cell adhesion from a calcium-independent to a calcium-dependent process.
Conclusions
Taken together, our findings indicate that ATP2C1 is involved in skin homeostasis by participating in various cellular processes. The keratinocytes derived from the clinically symptom-less skin of HHD patients behave like keratinocytes derived from healthy donors. We show that ATP2C1 expression and proliferation did not differ significantly between the non-lesion and healthy donor keratinocytes11,12. However, ATP2C1 expression was reduced in the keratinocytes from the lesioned skin, indicating that an unknown mechanism is responsible for its reduced expression level in the lesioned skin from HHD patients, thus initiating the chain of the molecular events that lead to lesion development. Our hypothesis is that the deregulation of calcium homeostasis resulting from the loss of ATP2C1 function produces ROS-induced DNA damage. ATP2C1 loss would then trigger a mechanism that results in Notch1 activation and subsequent ATM down-regulation. Increased ROS levels and ATM loss would produce DNA damage up to a threshold that keratinocytes cannot repair, which would then promote terminal differentiation. HHD is a complex condition characterized by poor wound healing. In the classical model of wound healing, the regenerative capacity of the skin relies on epidermal stem cells (ESCs)52. Under normal homeostatic conditions, disruption of epidermal integrity triggers a wound response that induces the recruitment of ESCs to replenish the lost cells. Efficient wound repair relies on the maintenance of both the ESC niche and the production of cells that undergo a limited number of divisions before differentiating as they replenish the damaged tissue. Our hypothesis is that the loss of ATP2C1 leads to the premature differentiation and exhaustion of the transit amplifying keratinocytes, resulting in compromised skin repair. However, there are still many unanswered questions; it is not clear to what extent the alterations in gene expression observed in the lesioned HHD skin represent a direct response to ATP2C1 loss and reflect downstream alterations. The discrepancy in Notch1 expression between the lesion-derived and siRNA-ATP2C1-treated keratinocytes in our in vitro model may result from the pathways that were altered prior to lesion formation that likely are causative and other alterations in the lesioned area that are likely secondary and associated with tissue damage. However, it might also be possible that these alterations underlie the signaling pathways that are responsible for the initiation and progression of the lesions. A future comparison of the gene expression profiles between lesion-derived and siRNA-ATP2C1-treated keratinocytes may provide more insights into the fundamental biological processes that underlie lesion manifestation.
Methods
Patients with Hailey-Hailey disease
This study was conducted according to principles of the Declaration of Helsinki and was approved by the S. Gallicano Institute Ethical Committee. “Written informed consent” was obtained from all subjects. Three familial HHD cases were included in the study. In each patient, diagnosis was established based on the clinical features and the medical and familial histories. Genomic DNA was extracted from whole blood using a Qiagen Blood mini kit (Qiagen, Milan, Italy). All translated ATP2C1 exons and exon-intron boundaries were amplified using 28 primer pairs10. The amplification products were then sequenced in both directions using an automatic sequencing system (310; Applied Biosystems, Foster City, CA, USA). The sequencing results were analyzed with reference to cDNA ATP2C1 sequence (ENST00000508532). ATP2C1 mutations in these patients, P1, P2 and P3, were previously described11.
RNA-seq and whole exome sequencing data
The RNA-seq data discussed in this publication have been deposited in the NCBI Gene Expression Omnibus and are accessible through GEO Series accession number GSE77446 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE77466). The Whole Exome sequencing Biosample records are accessible at http://www.ncbi.nlm.nih.gov/biosample/4450420.
Additional Information
How to cite this article: Cialfi, S. et al. The loss of ATP2C1 impairs the DNA damage response and induces altered skin homeostasis: Consequences for epidermal biology in Hailey-Hailey disease. Sci. Rep. 6, 31567; doi: 10.1038/srep31567 (2016).
Supplementary Material
Acknowledgments
This work was supported by the Fondazione Telethon GGP12264 and by the Italian Association for Cancer Research (AIRC) IG-15218.
Footnotes
Author Contributions C.T. designed the research, analyzed the data and wrote the paper; L.L.P. performed the NGS analysis; S.C., G.M., R.P., C.D.B. and A.Z. performed the experiments; L.B. and G.B. provided HHD biopsies; I.S., D.U. and C.P. commented on the paper. C.T. and S.C. assembled the figures.
References
- Chuong C. M. et al. What is the ‘true’ function of skin? Exp Dermatol 11, 159–187 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. & Nowak J. A. Building epithelial tissues from skin stem cells. Cold Spring Harbor symposia on quantitative biology 73, 333–350 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F. M. Mammalian skin cell biology: at the interface between laboratory and clinic. Science 346, 937–940 (2014). [DOI] [PubMed] [Google Scholar]
- Missiaen L., Dode L., Vanoevelen J., Raeymaekers L. & Wuytack F. Calcium in the Golgi apparatus. Cell calcium 41, 405–416 (2007). [DOI] [PubMed] [Google Scholar]
- Tu C. L., Oda Y., Komuves L. & Bikle D. D. The role of the calcium-sensing receptor in epidermal differentiation. Cell calcium 35, 265–273 (2004). [DOI] [PubMed] [Google Scholar]
- Hu Z. et al. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nature genetics 24, 61–65 (2000). [DOI] [PubMed] [Google Scholar]
- Sudbrak R. et al. Hailey-Hailey disease is caused by mutations in ATP2C1 encoding a novel Ca(2+) pump. Human molecular genetics 9, 1131–1140 (2000). [DOI] [PubMed] [Google Scholar]
- Uccelletti D. et al. The Golgi Ca2+-ATPase KlPmr1p function is required for oxidative stress response by controlling the expression of the heat-shock element HSP60 in Kluyveromyces lactis. Mol Biol Cell 16, 4636–4647 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisset C., Garcia-Rodriguez N., Birkmire A., Blondel M. & Wellinger R. E. Using yeast to model calcium-related diseases: example of the Hailey-Hailey disease. Biochim Biophys Acta 1843, 2315–2321 (2014). [DOI] [PubMed] [Google Scholar]
- Biolcati G. et al. Efficacy of the melanocortin analogue Nle4-D-Phe7-alpha-melanocyte-stimulating hormone in the treatment of patients with Hailey-Hailey disease. Clinical and experimental dermatology 39, 168–175 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cialfi S. et al. Complex multipathways alterations and oxidative stress are associated with Hailey-Hailey disease. Br J Dermatol 162, 518–526 (2010). [DOI] [PubMed] [Google Scholar]
- Manca S. et al. Oxidative stress activation of miR-125b is part of the molecular switch for Hailey-Hailey disease manifestation. Exp Dermatol 20, 932–937 (2011). [DOI] [PubMed] [Google Scholar]
- Hamanaka R. B. et al. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Science signaling 6, ra8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart L., Lippens S., Tschachler E. & Declercq W. Cell death by cornification. Biochim Biophys Acta 1833, 3471–3480 (2013). [DOI] [PubMed] [Google Scholar]
- Sherman M. H., Bassing C. H. & Teitell M. A. Regulation of cell differentiation by the DNA damage response. Trends in cell biology 21, 312–319 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh Y. & Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nature reviews. Molecular cell biology 14, 197–210 (2013). [PubMed] [Google Scholar]
- Bernstein C., Nfonsam V., Prasad A. R. & Bernstein H. Epigenetic field defects in progression to cancer. World journal of gastrointestinal oncology 5, 43–49 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbine L., Brunton H., Goodarzi A. A., Shibata A. & Jeggo P. A. Endogenously induced DNA double strand breaks arise in heterochromatic DNA regions and require ataxia telangiectasia mutated and Artemis for their repair. Nucleic acids research 39, 6986–6997 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermezovic J. et al. Notch is a direct negative regulator of the DNA-damage response. Nature structural & molecular biology 22, 417–424 (2015). [DOI] [PubMed] [Google Scholar]
- Freije A. et al. Inactivation of p53 in Human Keratinocytes Leads to Squamous Differentiation and Shedding via Replication Stress and Mitotic Slippage. Cell reports 9, 1349–1360 (2014). [DOI] [PubMed] [Google Scholar]
- Pasparakis M., Haase I. & Nestle F. O. Mechanisms regulating skin immunity and inflammation. Nature reviews. Immunology 14, 289–301 (2014). [DOI] [PubMed] [Google Scholar]
- Poblete-Gutierrez P. et al. Allelic loss underlies type 2 segmental Hailey-Hailey disease, providing molecular confirmation of a novel genetic concept. The Journal of clinical investigation 114, 1467–1474 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr M. R. et al. Two patients with Hailey-Hailey disease, multiple primary melanomas, and other cancers. Archives of dermatology 147, 211–215 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okunade G. W. et al. Loss of the Atp2c1 secretory pathway Ca(2+)-ATPase (SPCA1) in mice causes Golgi stress, apoptosis, and midgestational death in homozygous embryos and squamous cell tumors in adult heterozygotes. The Journal of biological chemistry 282, 26517–26527 (2007). [DOI] [PubMed] [Google Scholar]
- Behne M. J. et al. Human keratinocyte ATP2C1 localizes to the Golgi and controls Golgi Ca2+ stores. The Journal of investigative dermatology 121, 688–694 (2003). [DOI] [PubMed] [Google Scholar]
- Foggia L. et al. Activity of the hSPCA1 Golgi Ca2+ pump is essential for Ca2+-mediated Ca2+ response and cell viability in Darier disease. Journal of cell science 119, 671–679 (2006). [DOI] [PubMed] [Google Scholar]
- Lissandron V., Podini P., Pizzo P. & Pozzan T. Unique characteristics of Ca2+ homeostasis of the trans-Golgi compartment. Proc Natl Acad Sci USA 107, 9198–9203 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flicek P. et al. Ensembl 2014. Nucleic acids research 42, D749–D755 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. D., McCarthy D. J. & Smyth G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. D. & Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome biology 11, R25 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccaldi R., Rondinelli B. & D’Andrea A. D. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends in cell biology (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S. P. & Bartek J. The DNA-damage response in human biology and disease. Nature 461, 1071–1078 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos W. P. & Kaina B. DNA damage-induced cell death by apoptosis. Trends in molecular medicine 12, 440–450 (2006). [DOI] [PubMed] [Google Scholar]
- Sherry S. T. et al. dbSNP: the NCBI database of genetic variation. Nucleic acids research 29, 308–311 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilissen C., Hoischen A., Brunner H. G. & Veltman J. A. Disease gene identification strategies for exome sequencing. European journal of human genetics: EJHG 20, 490–497 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalay E. et al. CEP152 is a genome maintenance protein disrupted in Seckel syndrome. Nature genetics 43, 23–26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagurski M. et al. Insulin receptor substrate 1 (IRS-1) plays a unique role in normal epidermal physiology. J Cell Physiol 213, 519–527 (2007). [DOI] [PubMed] [Google Scholar]
- Fuchs F. et al. Clustering phenotype populations by genome-wide RNAi and multiparametric imaging. Molecular systems biology 6, 370 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahe S., Stierhof Y. D., Wilkinson C. J., Leiss F. & Nigg E. A. Rootletin forms centriole-associated filaments and functions in centrosome cohesion. J Cell Biol 171, 27–33 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. B. et al. Targeting of Nrf2 induces DNA damage signaling and protects colonic epithelial cells from ionizing radiation. Proc Natl Acad Sci USA 109, E2949–E2955 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demehri S., Turkoz A. & Kopan R. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer cell 16, 55–66 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell S., Jones P., Le Roux I., Dunne J. & Watt F. M. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Current biology: CB 10, 491–500 (2000). [DOI] [PubMed] [Google Scholar]
- Nicolas M. et al. Notch1 functions as a tumor suppressor in mouse skin. Nature genetics 33, 416–421 (2003). [DOI] [PubMed] [Google Scholar]
- Rangarajan A. et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J 20, 3427–3436 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail F. et al. Cutaneous squamous cell carcinoma (SCC) and the DNA damage response: pATM expression patterns in pre-malignant and malignant keratinocyte skin lesions. PLoS One 6, e21271 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff B. J. & Naidu Y. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. Journal of the American Academy of Dermatology 30, 535–546 (1994). [DOI] [PubMed] [Google Scholar]
- Wood L. C., Jackson S. M., Elias P. M., Grunfeld C. & Feingold K. R. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. The Journal of clinical investigation 90, 482–487 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood L. C. et al. Barrier disruption increases gene expression of cytokines and the 55 kD TNF receptor in murine skin. Exp Dermatol 6, 98–104 (1997). [DOI] [PubMed] [Google Scholar]
- Doi H., Shibata M. A., Kiyokane K. & Otsuki Y. Downregulation of TGFbeta isoforms and their receptors contributes to keratinocyte hyperproliferation in psoriasis vulgaris. Journal of dermatological science 33, 7–16 (2003). [DOI] [PubMed] [Google Scholar]
- Groves R. W. et al. Inflammatory and hyperproliferative skin disease in mice that express elevated levels of the IL-1 receptor (type I) on epidermal keratinocytes. Evidence that IL-1-inducible secondary cytokines produced by keratinocytes in vivo can cause skin disease. The Journal of clinical investigation 98, 336–344 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z. Q., Kondo T., Ishida Y., Takayasu T. & Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. Journal of leukocyte biology 73, 713–721 (2003). [DOI] [PubMed] [Google Scholar]
- Pastar I. et al. Epithelialization in Wound Healing: A Comprehensive Review. Advances in wound care 3, 445–464 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomic-Canic M., Komine M., Freedberg I. M. & Blumenberg M. Epidermal signal transduction and transcription factor activation in activated keratinocytes. Journal of dermatological science 17, 167–181 (1998). [DOI] [PubMed] [Google Scholar]
- Turksen K., Kupper T., Degenstein L., Williams I. & Fuchs E. Interleukin 6: insights to its function in skin by overexpression in transgenic mice. Proc Natl Acad Sci USA 89, 5068–5072 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. P. et al. The interleukin-6 cytokine system regulates epidermal permeability barrier homeostasis. The Journal of investigative dermatology 123, 124–131 (2004). [DOI] [PubMed] [Google Scholar]
- Saxena A. et al. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. Journal of immunology 191, 4838–4848 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazawa Y. et al. Notch down-regulation in regenerated epidermis contributes to enhanced expression of interleukin-36alpha and suppression of keratinocyte differentiation during wound healing. Journal of dermatological science 79, 10–19 (2015). [DOI] [PubMed] [Google Scholar]
- Yoshida M., Yamasaki K., Daiho T., Iizuka H. & Suzuki H. ATP2C1 is specifically localized in the basal layer of normal epidermis and its depletion triggers keratinocyte differentiation. Journal of dermatological science 43, 21–33 (2006). [DOI] [PubMed] [Google Scholar]
- Gandarillas A. The mysterious human epidermal cell cycle, or an oncogene-induced differentiation checkpoint. Cell cycle 11, 4507–4516 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandarillas A. & Freije A. Cycling up the epidermis: reconciling 100 years of debate. Exp Dermatol 23, 87–91 (2014). [DOI] [PubMed] [Google Scholar]
- Schneider L., Fumagalli M. & d’Adda di Fagagna F. Terminally differentiated astrocytes lack DNA damage response signaling and are radioresistant but retain DNA repair proficiency. Cell death and differentiation 19, 582–591 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody C. A. & Laimins L. A. Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation. PLoS pathogens 5, e1000605 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod D. R., Berika M. Y., Bardsley W. F., Holmes D. & Tabernero L. Hyper-adhesion in desmosomes: its regulation in wound healing and possible relationship to cadherin crystal structure. Journal of cell science 118, 5743–5754 (2005). [DOI] [PubMed] [Google Scholar]
- Suarez-Farinas M. et al. RNA sequencing atopic dermatitis transcriptome profiling provides insights into novel disease mechanisms with potential therapeutic implications. The Journal of allergy and clinical immunology 135, 1218–1227 (2015). [DOI] [PubMed] [Google Scholar]
- Meyer N. et al. IL-32 is expressed by human primary keratinocytes and modulates keratinocyte apoptosis in atopic dermatitis. The Journal of allergy and clinical immunology 125, 858–865 e810 (2010). [DOI] [PubMed] [Google Scholar]
- Yin H. et al. IL-33 accelerates cutaneous wound healing involved in upregulation of alternatively activated macrophages. Molecular immunology 56, 347–353 (2013). [DOI] [PubMed] [Google Scholar]
- Yin H. et al. IL-33 promotes Staphylococcus aureus-infected wound healing in mice. International immunopharmacology 17, 432–438 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.