Abstract
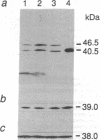
Studies of chloroplast DNA variations, and several direct experimental observations, indicate the existence of recombination ability in algal and higher plant plastids. However, no studies have been done of the biochemical pathways involved. Using a part of a cyanobacterial recA gene as a probe in Southern blots, we have found homologous sequences in total DNA from Pisum sativum and Arabidopsis thaliana and in a cDNA library from Arabidopsis. A cDNA was cloned and sequenced, and its predicted amino acid sequence is 60.7% identical to that of the cyanobacterial RecA protein. This finding is consistent with our other results showing both DNA strand transfer activity and the existence of a protein of the predicted molecular mass crossreactive with antibodies to Escherichia coli RecA in the stroma of pea chloroplasts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein H., Byerly H. C., Hopf F. A., Michod R. E. Genetic damage, mutation, and the evolution of sex. Science. 1985 Sep 20;229(4719):1277–1281. doi: 10.1126/science.3898363. [DOI] [PubMed] [Google Scholar]
- Cedergren R., Gray M. W., Abel Y., Sankoff D. The evolutionary relationships among known life forms. J Mol Evol. 1988 Dec;28(1-2):98–112. doi: 10.1007/BF02143501. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N., Clark A. J. Deletions generated by the transposon Tn10 in the srl recA region of the Escherichia coli K-12 chromosome. Genetics. 1979 Oct;93(2):321–343. doi: 10.1093/genetics/93.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutreix M., Moreau P. L., Bailone A., Galibert F., Battista J. R., Walker G. C., Devoret R. New recA mutations that dissociate the various RecA protein activities in Escherichia coli provide evidence for an additional role for RecA protein in UV mutagenesis. J Bacteriol. 1989 May;171(5):2415–2423. doi: 10.1128/jb.171.5.2415-2423.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols H., Goodman M. F. Mutation induced by DNA damage: a many protein affair. Mutat Res. 1990 Sep-Nov;236(2-3):301–311. doi: 10.1016/0921-8777(90)90013-u. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Mulligan J. T., Ramer S. W., Spottswood M., Davis R. W. Lambda YES: a multifunctional cDNA expression vector for the isolation of genes by complementation of yeast and Escherichia coli mutations. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1731–1735. doi: 10.1073/pnas.88.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C. Eukaryotic DNA repair: glimpses through the yeast Saccharomyces cerevisiae. Bioessays. 1991 Jun;13(6):295–302. doi: 10.1002/bies.950130607. [DOI] [PubMed] [Google Scholar]
- Gantt J. S., Baldauf S. L., Calie P. J., Weeden N. F., Palmer J. D. Transfer of rpl22 to the nucleus greatly preceded its loss from the chloroplast and involved the gain of an intron. EMBO J. 1991 Oct;10(10):3073–3078. doi: 10.1002/j.1460-2075.1991.tb07859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavel Y., von Heijne G. A conserved cleavage-site motif in chloroplast transit peptides. FEBS Lett. 1990 Feb 26;261(2):455–458. doi: 10.1016/0014-5793(90)80614-o. [DOI] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M. Codon frequencies in 119 individual genes confirm consistent choices of degenerate bases according to genome type. Nucleic Acids Res. 1980 May 10;8(9):1893–1912. doi: 10.1093/nar/8.9.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham R. Viral, prokaryote and eukaryote genes contrasted by mRNA sequence indexes. FEBS Lett. 1978 Nov 1;95(1):1–11. doi: 10.1016/0014-5793(78)80041-6. [DOI] [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Howe C. J., Barker R. F., Bowman C. M., Dyer T. A. Common features of three inversions in wheat chloroplast DNA. Curr Genet. 1988 Apr;13(4):343–349. doi: 10.1007/BF00424430. [DOI] [PubMed] [Google Scholar]
- Kindle K. L., Richards K. L., Stern D. B. Engineering the chloroplast genome: techniques and capabilities for chloroplast transformation in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1721–1725. doi: 10.1073/pnas.88.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiec E., Holloman W. K. Homologous pairing of DNA molecules promoted by a protein from Ustilago. Cell. 1982 Jun;29(2):367–374. doi: 10.1016/0092-8674(82)90153-2. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Evans D. H., Morrison P. T. Purification and characterization of an activity from Saccharomyces cerevisiae that catalyzes homologous pairing and strand exchange. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5560–5564. doi: 10.1073/pnas.84.16.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczykowski S. C. Biochemical and biological function of Escherichia coli RecA protein: behavior of mutant RecA proteins. Biochimie. 1991 Feb-Mar;73(2-3):289–304. doi: 10.1016/0300-9084(91)90216-n. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Lowenhaupt K., Sander M., Hauser C., Rich A. Drosophila melanogaster strand transferase. A protein that forms heteroduplex DNA in the absence of both ATP and single-strand DNA binding protein. J Biol Chem. 1989 Dec 5;264(34):20568–20575. [PubMed] [Google Scholar]
- Medgyesy P., Fejes E., Maliga P. Interspecific chloroplast recombination in a Nicotiana somatic hybrid. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6960–6964. doi: 10.1073/pnas.82.20.6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogen B. D., MacDonald M. H., Graybosch R., Hunt A. G. Upstream sequences other than AAUAAA are required for efficient messenger RNA 3'-end formation in plants. Plant Cell. 1990 Dec;2(12):1261–1272. doi: 10.1105/tpc.2.12.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. P., Fishel R. Purification and characterization of a protein from human cells which promotes homologous pairing of DNA. J Biol Chem. 1990 Jul 5;265(19):11108–11117. [PubMed] [Google Scholar]
- Murphy R. C., Gasparich G. E., Bryant D. A., Porter R. D. Nucleotide sequence and further characterization of the Synechococcus sp. strain PCC 7002 recA gene: complementation of a cyanobacterial recA mutation by the Escherichia coli recA gene. J Bacteriol. 1990 Feb;172(2):967–976. doi: 10.1128/jb.172.2.967-976.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. L., Marín A., Martínez-Zapater J. M. Chloroplast genes transferred to the nuclear plant genome have adjusted to nuclear base composition and codon usage. Nucleic Acids Res. 1990 Jan 11;18(1):65–73. doi: 10.1093/nar/18.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. D. Comparative organization of chloroplast genomes. Annu Rev Genet. 1985;19:325–354. doi: 10.1146/annurev.ge.19.120185.001545. [DOI] [PubMed] [Google Scholar]
- Palmer J. D. Contrasting modes and tempos of genome evolution in land plant organelles. Trends Genet. 1990 Apr;6(4):115–120. doi: 10.1016/0168-9525(90)90125-p. [DOI] [PubMed] [Google Scholar]
- Pang Q., Hays J. B., Rajagopal I. A plant cDNA that partially complements Escherichia coli recA mutations predicts a polypeptide not strongly homologous to RecA proteins. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8073–8077. doi: 10.1073/pnas.89.17.8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca A. I., Cox M. M. The RecA protein: structure and function. Crit Rev Biochem Mol Biol. 1990;25(6):415–456. doi: 10.3109/10409239009090617. [DOI] [PubMed] [Google Scholar]
- Sassanfar M., Roberts J. W. Nature of the SOS-inducing signal in Escherichia coli. The involvement of DNA replication. J Mol Biol. 1990 Mar 5;212(1):79–96. doi: 10.1016/0022-2836(90)90306-7. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R. Mechanism and control of homologous recombination in Escherichia coli. Annu Rev Genet. 1987;21:179–201. doi: 10.1146/annurev.ge.21.120187.001143. [DOI] [PubMed] [Google Scholar]
- Sugino A., Nitiss J., Resnick M. A. ATP-independent DNA strand transfer catalyzed by protein(s) from meiotic cells of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3683–3687. doi: 10.1073/pnas.85.11.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz M., Lipman R., Chowdary D., Pawlak J., Verdine G. L., Nakanishi K. Isolation and structure of a covalent cross-link adduct between mitomycin C and DNA. Science. 1987 Mar 6;235(4793):1204–1208. doi: 10.1126/science.3103215. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Inducible DNA repair systems. Annu Rev Biochem. 1985;54:425–457. doi: 10.1146/annurev.bi.54.070185.002233. [DOI] [PubMed] [Google Scholar]
- Wang W. B., Tessman E. S. Location of functional regions of the Escherichia coli RecA protein by DNA sequence analysis of RecA protease-constitutive mutants. J Bacteriol. 1986 Nov;168(2):901–910. doi: 10.1128/jb.168.2.901-910.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M., Roegner-Maniscalco V., Sweasy J. B., McCall J. O. Recovery from ultraviolet light-induced inhibition of DNA synthesis requires umuDC gene products in recA718 mutant strains but not in recA+ strains of Escherichia coli. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6805–6809. doi: 10.1073/pnas.84.19.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Nishikawa K. Chloroplast transit peptides. The perfect random coil? FEBS Lett. 1991 Jan 14;278(1):1–3. doi: 10.1016/0014-5793(91)80069-f. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]