Abstract
Polymer therapeutics is a successful branch of nanomedicine, which is now established in several facets of everyday practice. However, to our knowledge, no literature regarding the application of the underpinning principles, general safety, and potential of this versatile class to the perioperative patient has been published. This study provides an overview of polymer therapeutics applied to clinical surgery, including the evolution of this demand‐oriented scientific field, cutting‐edge concepts, its implications, and limitations, illustrated by products already in clinical use and promising ones in development. In particular, the effect of design of polymer therapeutics on biophysical and biochemical properties, the potential for targeted delivery, smart release, and safety are addressed. Emphasis is made on principles, giving examples in salient areas of demand in current surgical practice. Exposure of the practising surgeon to this versatile class is crucial to evaluate and maximise the benefits that this established field presents and to attract a new generation of clinician–scientists with the necessary knowledge mix to drive highly successful innovation.
Keywords: nanomedicine, nanotechnology, polymer
Background
Polymer therapeutics represents a highly successful nanomedicine class that has enjoyed extensive success from aesthetic surgery to neoadjuvant oncology, features in the top 10 US pharmaceutical sales lists, is arguably the most successful first‐generation nanomedicine class, and is well established in perioperative use (Duncan, 2014). Polymer therapeutics offer substantially different properties to conventional counterparts including passive accumulation at target sites and bioresponsive activation and lend themselves extensively to custom‐engineered solutions for specific clinical demands. A working knowledge of this burgeoning scientific field is imperative, for the surgeon to evaluate the significantly different properties compared with conventional therapy, safeguard perioperative patient safety, and contribute to the development process (Duncan and Gaspar, 2011; Gaspar and Duncan, 2009).
This study provides an overview of polymer therapeutics applied to clinical surgery in its several subspecialties, including the evolution of this demand‐oriented scientific field, cutting‐edge concepts, its implications, and drawbacks, illustrated by an extensive review of products already in clinical use and promising ones in advanced stages of development.
Method
A comprehensive database search of MEDLINE, EMBASE, and Pubmed Central was performed. The search was limited to studies in English, using the Boolean search string “polymer and therapeutic” and “surgery” (all subspecialties). Seminal works underpinning the principal tenets of polymer therapeutics were included. Title and abstract of the primary literature were reviewed for relevance and included in the review. This literature was back and forward referenced using the Web of Knowledge™ database.
Literature Review
History and working definitions
Evolution towards the microscale has represented a paradigm shift in surgical practice since Carrel's studies on vessel anastomosis (Carrel, 1902). Jacobson and Suarez introduced the benefits of the operating microscope in the 1960s, followed by the first successful free muscle transplants in the 1970s and super microsurgery (Kriss and Kriss, 1998; Tamai, Komatsu, Sakamoto, et al., 1970). The adoption of these techniques across surgical specialties within coronary vessel repair, otolaryngology, and head and neck surgery bears witness to their success. The continuous surgical drive towards the infinitesimal and its attendant benefits necessarily demanded an exploration of the next frontier represented by the nanometre scale.
Nanomedicine uses nanosized (between 1 and 100 nm) tools addressed toward the diagnosis, prevention, and treatment of disease – molecules that are of much larger size than conventional drugs (European Science Foundation, 2005). Modern awareness of the benefits of nanomedicine dates back to Paul Ehrlich's first low‐molecular‐weight synthetic chemical entities (Duncan, 2014). Since then, nanomedicine has expanded into five overlapping subdisciplines (Fig. 1). Polymer therapeutics are nanoscale therapeutics and drug delivery systems, pioneered by Herman Staudinger's ground‐breaking work on covalently linked macromolecules, popularised and expanded through the work of Kopeck, Ringsdorf, and Duncan (Duncan and Gaspar, 2011; Duncan and Vicent, 2013). The term “polymer therapeutics” has evolved to an umbrella term encompassing a number of heterogeneous entities including polymeric drugs, polymer–drug conjugates, and polymer–protein conjugates (Fig. 2) (Duncan, 2003). Polymer therapeutics lends itself particularly well to the concept of demand‐to‐supply research and therefore presents an important opportunity for the surgeon–scientist to translate a clinical demand to a custom‐engineered product. This demands a working knowledge of the major differences and designs distinguishing a polymer therapeutic from a conventional alternative.
Figure 1.
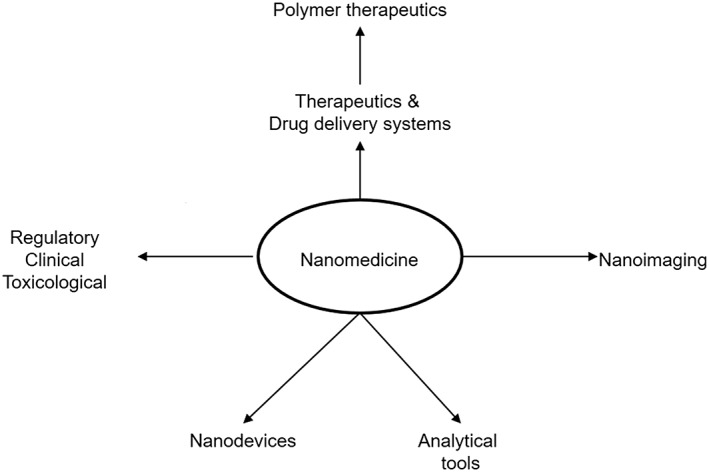
The five main subdisciplines of nanomedicine (European forward look consensus conference, 2004, and the relationship of polymer therapeutics to these subdisciplines).
Figure 2.
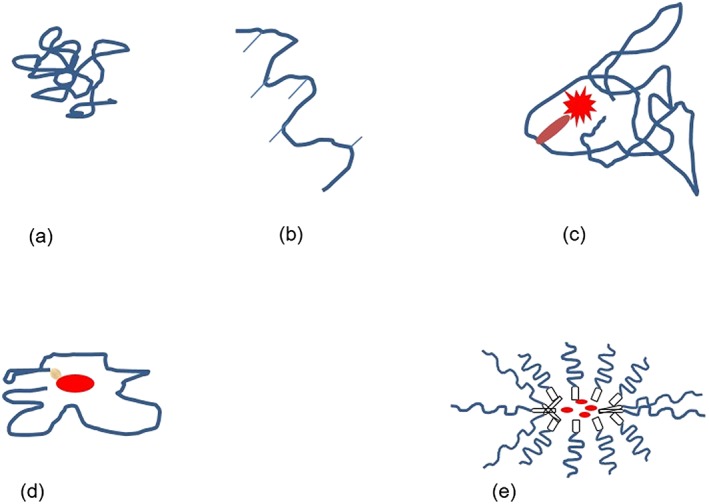
Diagrammatic representation of different types of polymer therapeutics. The presence of a water‐soluble polymer is the common denominator. Examples are given in parentheses. (a) Polymeric drug; (b) polymeric drug modified by the addition of pendant groups; (c) polymer–protein conjugate; (d) polymer–drug conjugate; and (e) PEGylated micelles.
Design: custom engineering from bench to bedside and back
The common denominator for all polymer therapeutics is the possession of a water‐soluble polymer. Polymers consist of repeating component units (monomers) to produce a macromolecular structure with unique physicochemical characteristics. Several types of polymers are employed, and a simple working classification is provided in Table 1. Polymeric drugs represent the simplest form of polymer therapeutic (Fig. 2a). Here, the polymer itself is the active drug. Applications include structural tissue support, increasing lubrication, structural volume expansion such as hyaluronic acid (HA), and plasma volume expansion such as dextran.
Table 1.
Classification of polymers and prominent examples in clinical use.
| Classification | Subclasses | Polymer example | Clinical example | Surgical specialty |
|---|---|---|---|---|
| Biodegradability | Nonbiodegradable | PEG | PEG recombinant | Gastrointestinal |
| IFN α2b (hepatitis C) | Hepatobiliary | |||
| PEG‐IFNα‐2b | Melanoma surgery | |||
| Biodegradable | HA | Dermal fillers (aesthetic surgery) | Aesthetic (Bray et al., 2010; Madani et al., 2011; Pavelka and Uebelhart, 2011) | |
| Orthopaedic (Duncan and Vicent, 2013) | ||||
| Monomer | Homopolymer | PEG | PEG–doxorubicin (ovarian cancer) | Gynaecology |
| Pelvic | ||||
| PEG‐Erythropoietin | Anaemia (Duncan and Vicent, 2013) | |||
| PEG‐anti‐TNF Fab | Rheumatoid arthritis | |||
| Copolymer | Random amino acid copolymer* | Glatiramer acetate (multiple sclerosis) | Neurosurgery | |
| Polyvinylpyrrolidone | Povidone iodide | Antiseptic (Ascher, Bayerl, Brun, et al., 2011) | ||
| Ubiquitous | ||||
| Dressings | ||||
| Hand scrub | ||||
| Shape (examples) | Linear | Dextrin | Dextrin | Nephrology |
| Branched | PEG (branched) | PEG‐IFN α2a (hepatitis C) | Gastrointestinal | |
| Hepatobiliary | ||||
| Dendrimeric | Lysine‐based dendrimer 7013 | 3% Carbopol formulation (intravaginal viricide versus HIV) | Obstetrics | |
| Gynaecology |
PEG, poly(ethyl glycol); HA, hyaluronic acid; IFN α2a, interferon alpha 2a; IFN α2b, interferon alpha 2b.
Glutamic acid, lysine, alanine, and tyrosine.
The polymer may be customised to a clinical niche by the induction of pendant branches along the main backbone chain. In the natural form, injectable HA is locally degraded within 48 h (Fakhari and Berkland, 2013). However, HA cross‐linking increases resistance to biodegradability by endogenous human hyaluronidase enzyme (further applications of HA are considered in Macromolecular Status and the Enhanced Permeability Retention Effect section). Conjugation to 1,4‐butanediol diglycidal ether and divinyl sulphone results in very predictable degradation over time. 1,4‐Butanediol diglycidal ether and divinyl sulphone are currently the only two cross‐linking agents licenced for dermal injectable use (Bray, Hopkins, and Roberts, 2010; Fullana and Wnek, 2013). Dextrin is another example of a Food and Drug Administration (FDA)‐approved, largely linear polymer, which is rapidly digested by naturally occurring amylase at physiological concentrations. However, it can be modified by increasing amounts of succinoylation (Fig. 2b). In the presence of physiological amylase activity, this provides a highly predictable and custom‐engineered degradation rates and differentially, in microenvironments within the body, where amylase might accumulate (Azzopardi, Camilleri, Moseley, et al., 2013a).
“Pendant groups” can additionally serve as “linkers” enabling chemical bonding of a polymer to a bioactive molecule of interest, a process termed conjugation. For example, HA has been conjugated to various molecules of interest including antiinterleukin‐1β and antitumour necrosis growth factor‐α monoclonal antibodies (Duncan and Vicent, 2013). Polymer–drug conjugates are arguably the commonest forms of polymer therapeutics and, like other polymer therapeutic subclasses, have properties substantially distinct from the conventional alternatives (Table 2). Additionally, conjugation creates new, original macromolecules, affording intellectual property space, which is crucial for successful commercialisation (Pinter, Horvath, Bujdoso, et al., 2009).
Table 2.
Examples of properties conferred by conjugation in polymer therapeutics.
| Polymer therapeutic | Conventional alternative |
|---|---|
| Improved biological efficacy (Duncan, 2003) | Conventional biologic efficacy |
| Extended plasma circulation time (Koburger, Hübner, Braun, et al., 2010) | Conventional plasma residence times, clearance, and degradation |
| Shielding from immunogenicity and premature biofouling and clearance (Werle and Bernkop‐Schnürch, 2006) | Conventional risk of immunological reaction, sequestration, and clearance |
| Enhanced permeability and retention effect (macromolecule) (Maeda, 2010; Maeda, Bharate, and Daruwalla, 2009) | Indiscriminate distribution (conventional small molecule) |
| Potential for “masking/unmasking” and locally triggered reinstatement of bioactivity (biodegradable polymers) | N/A |
Macromolecular status and the enhanced permeability retention effect
Conjugating a conventional “small molecule” to a polymer of adequate size creates a macromolecule. As molecular size increases, filtration at the glomerulus decreases, a happy state of affairs if the newly synthesised molecule is to avoid perioperative nephrotoxicity whilst simultaneously increasing plasma residence time (Azzopardi, Ferguson, and Thomas, 2014a). Colistin, for example, is increasingly used to treat multidrug‐resistant surgical infection. Its use is limited by its reputation for nephrotoxicity (Azzopardi, Boyce, Thomas, et al., 2013b). However, conjugation to dextrin, using succinoyl linking groups, resulted in substantial reduction of kidney clearance and absence of any observed clinical toxicity in vivo within Sprague Dawley rats (Azzopardi et al., 2014a).
Conversion of a conventional “small molecule” antibiotic into a macromolecule may benefit from passive, size‐based targeting to an inflamed area (Azzopardi, Ferguson, and Thomas, 2013c). The enhanced permeability and retention (EPR) effect refers to the ability of macromolecules to passively accumulate at the site of enhanced vascular permeability and be selectively retained therein (Maeda, 2012). This principle has been extensively applied to the design of clinically successful antineoplastic and neoadjuvant drugs, inflammatory diseases such as rheumatoid arthritis, and other chronic conditions (Duncan, 2011; Duncan and Gaspar, 2011; Duncan and Vicent, 2013; Hardwicke, Hart, Bell, et al., 2011). This concept has been extensively exploited in the design of next generation of chemotherapeutic agents entering clinical practice, such as polyethyleneglycol (PEG)‐asparaginase (OncasparR, PEG‐L‐asparaginase, Sigma‐Tau Pharmaceuticals, Inc., Gaithersburg, MD, USA) (Duncan, 2011).
It is worth noting that whilst EPR has been demonstrated to be highly successful in preclinical species, it has been less successful clinically. This could be accounted for by several possibilities including failure of the construct to address a specific niche, including real‐life drug interactions that might diminish the efficacy of EPR and does highlight the need for closer clinician collaboration in husbanding the strategy for construction of a particular polymer therapeutic to address a particular clinical niche. Meanwhile, targeting that is augmented by either specific ligands or radiotherapy may be more prerequisite for treatment of diseases that are otherwise not terminal.
Bioresponsive polymer therapeutics and smart release
Classically, nonbiodegradable synthetic polymers, including PEG, N‐(2‐hydroxypropyl) methacrylamide (HPMA), and poly(lactic‐co‐glycolic) acid, comprise the majority of clinical success stories (Pasut and Veronese, 2009; Duncan, 2009). PEG conjugates are clinically well tolerated and extensively used. The surgical community is well versed with applications of some of these polymers in suture materials (Najibi, Banglmeier, Matta, et al., 2010). More recently, however, the advantages of biodegradable polymers, including the ability to respond to biological stimuli, have been intensively studied.
The degradation of bioresponsive polymers can be custom engineered to suit particular clinical demands (Design: Custom Engineering from Bench to Bedside and Back section), based on either exogenous enzymes co‐administered to the patient (in their native form, or even themselves as polymer–enzyme conjugates) (Duncan, Gac‐Breton, Keane, et al., 2001). More recently, an elegant approach for “shielding” the bioactive payload in transit, followed by localised enzymatic controlled release and restitution of bioactivity, has been described (Duncan, Gilbert, Carbajo, et al., 2008). This depends on conjugation to biodegradable polymers. The polymer masking–unmasking protein therapy principle involves a multifunctional biodegradable polymer to envelope the payload of interest whilst allowing locally triggered polymer degradation and reinstatement of the masked bioactive's activity (Fig. 3) (Duncan et al., 2008). The masked conjugate offers improved biological efficacy, extended plasma circulation time, and reduced proteolytic degradation and protein immunogenicity (Roberts, Bentley, and Harris, 2002; Werle and Bernkop‐Schnürch, 2006). Locally triggered unmasking at the intended site allows controlled reinstatement of bioactivity. Triggered release can be effectively controlled and predicted by the length of the polymer and the amount of linker modification (Azzopardi, 2013b).
Figure 3.
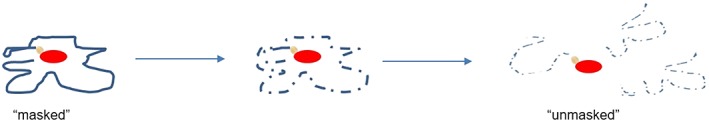
The polymer mask–unmask protein therapy principle. During transit, the polymer “masks” the bioactive from the body, at the same time shielding the body from potential toxicity. At the target site, the bioresponsive polymer is degraded (using various approaches) to release back the bioactive molecule, with its activity reinstated.
Physicochemical customisation
Conjugation can also radically alter mechanical, tensile, and viscoelastic properties. The effect of HA conjugation on its stability and potential as controlled release mechanism has already been referred to in Design: Custom Engineering from Bench to Bedside and Back section. Additionally, HA enjoys a central role in reconstructive surgery and regenerative medicine. It is present in most body fluids and tissues, including dermis, vitreous humour, and hyaline cartilage (Azzopardi, Ferguson, and Thomas, 2013d; Fakhari and Berkland, 2013; Griffith, 2000; Zheng Shu, Liu, Palumbo, et al., 2004). Its avidity to water molecules provides viscoelastic properties, but HA also simultaneously acts as mechanical support, biological scaffold, making it a prime target for cosmetic and facial rejuvenation applications (Garg and Hales, 2004). At a cellular level, its roles are multiple and complex. It is binding other matrix molecules, guiding cell proliferation and differentiation, and guides morphogenesis, wound repair, and inflammation (Zheng Shu et al., 2004). Recent studies on naked mole rats report that HA plays an important role in mediating its remarkable resistance to cancer (Tian, Azpurua, Hine, et al., 2013). It is therefore discussed as an elegant example of the versatile potential for polymer therapeutics to custom‐engineered solutions to complex clinical problems.
Photocross‐linked HA affords mechanical stability and has found favour in cartilage tissue engineering, cardiac repair, molecule delivery, valvular engineering, control of stem cell behaviour, and microdevices (Burdick and Prestwich, 2011). Chondrocytes within these modified HA hydrogels also resulted in cartilage production within the porous network (Allison and Grande‐Allen, 2006). Use of HA for nonsurgical facial rejuvenation is well established (Greco, Antunes, and Yellin, 2012).
In the Pipeline
There are a number of promising polymer therapeutics in advanced stages of experimentation in vivo relevant to the practising surgeon, and salient examples are summarised in Table 3. Examples serve to expose the reader to the opportunities presented by this field in several aspects for future surgical practice.
Table 3.
Examples of promising polymer therapeutics in development.
| Polymer | Polymer therapeutic | Examples and development |
|---|---|---|
| Dextran | 99Tc tilmanocept | Identification of sentinel lymph nodes in breast cancer and melanoma (phase III) |
| HPMA | HPMA–copolymer–diaminocyclohexyl (DACH) platinate | Malignant melanoma in phase 2 clinical trial (Maeda, Fang, Inutsuka, et al., 2003) |
| Succinoylated dextrin | Succinoylated dextrin–colistin | In vivo preclinical phase (Azzopardi et al., 2011a) |
| Succinoylated dextrin‐recombinant EGF | In vivo preclinical phase (Duncan and Vicent, 2010; Hardwicke et al., 2010, 2011; Serbest et al., 2005) | |
| Succinoylated dextrin‐phospholipase‐A2 | In vitro breast cancer (Hardwicke, Ferguson, Moseley, et al., 2008b) | |
| Poloxamer | Various | In vivo preclinical phase (Medina et al., 2011; Sikkink et al., 2009) |
HA, hyaluronic acid; HPMA, N‐(2‐hydroxypropyl) methacrylamide.
Pharmacosurgery: the potential of modality combination treatment
Polymer therapeutics offers the exciting ability to custom‐designed molecules for specific perioperative demands. Such pharmacosurgical therapy may have the potential to increase the scope and magnitude of therapy beyond the conventional. The following section lists some common examples of polymer therapeutics in surgical practice as well, high‐profile candidates for clinical entry, and specific areas of development including management of surgical site infection, oncological surgery, and radiotherapy.
Localised lymphatic distortion frequently accompanies surgically treated conditions in oncological surgery, such as lymphatic dissection. It is rational to entertain the notion that this significant lymphatic distortion may serve to amplify and prolong an EPR effect, making it possible to specifically engineer bioactive entities to selectively target the affected region. Moreover, the EPR (Maeda, 2012) effect is enhanced by radiotherapy, and targeting of polymer–drug conjugates by radiotherapy has been clinically observed (Ke, Milas, Charnsangavej, et al., 2001). These examples postulate the emergence of “Pharmacosurgery” as the synergistic perioperative combination of surgery and bioactive pharmacological agents, more efficacious in simultaneous administration than the individual therapeutic modality, as an avenue of significant interest in perioperative diagnosis and treatment.
Targeted antibiotic delivery to surgical site infections
Several macromolecular entities have been reported to accumulate at the infected site, despite the absence of specific receptors or transport mechanisms (Evans, Evans, and Gorbach, 1973; Laverman, Boerman, Oyen, et al., 1999, 2001; Laverman, Dams, Storm, et al., 2001b; Melendez‐Alafort, Nadali, Pasut, et al., 2009). Several studies report that the notion of rapid, passive, size‐based accumulation around foci of acute infection is feasible (Azzopardi 2013a). It presents the ability to target a dose of antibiotic selectively and rapidly towards surgical site infection. Such examples include PEG‐ubiquicidin, 99Tc‐labelled poly(ethylene glycol)‐coated liposomes (99mTc‐PEG‐liposomes), and gallium‐transferrin (Dams, Reijnen, Oyen, et al., 1999; Laverman et al., 1999, 2001; Oyen, Boerman, Storm, et al., 1996). 99Tc‐labelled proteins including aprotinin (6512 g/mol) allow rapid localisation around infected foci induced in vivo animal models (2 h), and their concentration was up to 6.5 times higher than control tissue (Komarek, Kleisner, Komarkova, et al., 2005). A correlation between intraabdominal abscesses and the magnitude of effect of EPR has also been reported with some compounds (Sikkink, Reijnen, Laverman, et al., 2009). Polymer therapeutics therefore presents the potential for a clinically feasible avenue to the management of multidrug‐resistant surgical site infection.
Polymer therapeutics in tissue regeneration
Poloxamers are nonionic triblock copolymers composed of a central hydrophobic chain of polyoxypropylene (poly(propylene oxide)) flanked by two hydrophilic chains of polyoxyethylene (poly(ethylene oxide)). It has been recently shown that improvement in apoptosis and cell viability mediated by poloxamer 188 may lead to increased fat graft viability (Medina, Nguyen, Kirkham, et al., 2011) and recovery of neuronal tissue from mechanical injury (Serbest, Horwitz, and Barbee, 2005).
Recently, succinoylated dextrin conjugated to recombinant EGF has shown promise as a controlled release approach with excellent results on in vivo animal models of chronic wounds (Hardwicke, Moseley, Stephens, et al., 2010; Hardwicke, Schmaljohann, Boyce, et al., 2008a; Hardwicke et al., 2011). This product combines the aim of protecting the growth factor from the harsh chronic wound fluid environment but allows reinstatement of its activity when exposed to α‐amylase using the polymer masking–unmasking protein therapy concept.
Polymer therapeutics in oncological surgery
Despite its widespread acceptance, literature reports that injection of blue dye at the site of primary tumour for the identification of dye sentinel lymph node biopsy remains largely nonstandardised (Sondak, King, Zager, et al., 2013). Isosulfan blue or Patent Blue V dye followed by methylene blue dye has been used for sentinel lymph node mapping, despite controversy about their comparability and safety (Liu, Truini, and Ariyan, 2008; Neves, Reynolds, Hazard, et al., 2011). The combined use of radiolabelled colloid is also widespread, and recently, the FDA approved 99Technetium–sulphur colloid for sentinel lymph node identification in breast cancer (Pharmalucence press release, n.d.). However, this approval was based on retrospective data showing the noninferiority of the latter to the blue dye method. Tilmanocept is a recently described mannosylated dextran‐based polymer therapeutic for sentinel lymph node imaging, which may offer an innovative solution for melanoma and breast cancer patients and requires no manipulation before injection. It is reported to bind tightly to CD 206 mannose receptors on the surface of reticuloendothelial cells resident in lymph nodes for up to 30 h (Sondak et al., 2013).
More recently, superparamagnetic iron oxide contrast agent injected subcutaneously into the breast rather than intravenously has gained FDA approval for the purpose of sentinel lymph node identification and has demonstrated an identification rate in humans that is noninferior to the standard technique (Duncan and Gaspar, 2011).
The EPR effect has been widely adopted to target biologically active payloads to solid tumours. In 2011, PEG‐interferon α‐2b has recently been approved as an adjuvant therapy for the treatment of high‐risk melanoma. An open‐label randomised study of resectable stage III melanoma reported a significantly increased median recurrence‐free survival. However, overall survival was not significantly different to controls (Ditrolio, Simeone, DI Lorenzo, et al., 2012). HPMA–copolymer–DACH platinate has recently entered phase II trials for melanoma. Similarly, first clinical studies with transferrin‐targeted polymer–cyclodextrin nanoparticle small interfering ribonucleic acid delivery systems have demonstrated nanoparticle localisation to melanoma tissue (Davis, 2009; Galanski and Keppler, 2012).
Safety of Polymer Therapeutics
The clinical success of several polymers as plasma expanders, and egregious fall from grace of others, serves to illustrate the imperative for clinical knowledge of this field in the interest of patient safety. This section summarises current controversies and cautions towards polymer therapeutics in surgical practice (Table 4).
Table 4.
Examples of salient safety issues with particular polymers.
| Polymer | Polymer therapeutic |
|---|---|
| Nondegradable polymers | Theoretical risk of toxic accumulation in lysosomal storage‐like disorders |
| Dextrans | May generate an immunoglobulin‐M response (Azzopardi, Ferguson, and Thomas, 2011b) |
| Degraded slowly (Azzopardi et al., 2011b; Battisto and Pappas, 1973) | |
| Tend to form nondegradable products during chemical modification (Battisto and Pappas, 1973) | |
| HES | HES fractions may cause hypersensitivity and interfere with coagulation processes causing haemorrhage (Vercauteren, Bruneel, Schacht, et al., 1990; Zarychanski et al., 2013) |
| Metabolites | As with all drugs, it should be ensured that the metabolites are assessed for any adverse/toxicological reaction (Duncan and Vicent, 2013; Gaspar and Duncan, 2009) |
HES, hydroxyethyl starch.
Hydroxyethyl starch was used until recently to expand the volume of circulating plasma but carried an increased risk of renal dysfunction and mortality over a 90‐day follow‐up in patients who received hydroxyethyl starch compared with crystalloids. Increased mortality in patients with sepsis was also observed prompting their UK‐wide recall (Brunkhorst, Engel, Bloos, et al., 2008; MHRA, 2013; Myburgh, Finfer, Bellomo, et al., 2012; Perner, Haase, Guttormsen, et al., 2012; Zarychanski, Abou‐Setta, Turgeon, et al., 2013). Clinical success of dextran has been mixed. Its use as volume expander has been limited by a reported aptitude for causing renal impairment (Bhatt, Neppalli, Kelley, et al., 2011). Its anticoagulant properties have, however, found clinical purpose in some quarters in microvascular surgery, although this is presently contended (Djohan, Gage, and Bernard, 2010; Riva, Chen, Tan, et al., 2012) rather than the limited quantities associated with administration of medicinals (Azzopardi, McWilliams, Iyer, et al., 2009; Boussekey, Darmon, Langlois, et al., 2010; Gattas, Dan, Myburgh, et al., 2012). It is likely that these effects are clinically relevant with the significant amounts used as volume expanders rather than the limited quantities that would be used in their role as polymers for drug conjugation. However, these cautionary tales underscore the importance of the surgeon's familiarity with potential polymer therapeutic side, as the ultimate custodian of patients' safety.
Finally, renal failure is a frequent perioperative complication (Mitchell, 2013). An advantage of macromolecular constructs is increased plasma residence time by way of avoiding filtration at the kidney, thereby decreasing unwanted nephrotoxicity. The use of biodegradable polymers like dextrin and HA present the potential for degradation and metabolism into normally produced metabolites such as glucose, maltose, and isomaltose (dextrin, dextran, and starch) or amino acids (HA). However, nonbiodegradable polymers such as PEG do present the theoretical risk of toxic accumulation lysosomal storage‐like diseases, and other metabolic aberrations become theoretically possible, especially when chronic administration would be anticipated (Gaspar and Duncan, 2009).
The US Nanotechnology Characterisation Laboratory's work is of interest to this section in having defined an assay cascade with which to assess, in a timely and rationalised manner, the physical attributes, in vitro biological properties, and in vivo compatibility (in animal models) of submitted requests, through which it standardises the preclinical characterisation of nanomaterials intended for cancer therapeutics and diagnostics (Gaspar and Duncan, 2009). To the same end, a sister European institution, the European counterpart facility, EU‐Nanotechnology Characterisation Laboratory, has been recently launched (Carrel, 1902).
Discussion
Polymer therapeutics has evolved into a clinically successful branch of nanomedicine. The extensive versatility of the building blocks themselves (polymer, linker, and drug) and the custom‐engineering strategies available open up exciting innovative and sustainable horizons to pressing surgical problems such as surgical site infection, tissue regeneration, reconstruction, and oncology.
However, transferring the benefits to the bedside requires dual expertise in surgery and polymer therapeutics. Central to the success of polymer therapeutics, therefore, is that the demand‐to‐supply ethos of the field is nourished by the dual training of surgeon–scientists. Clinical academics are ideally placed between demand and supply ends of the research translational chain, and it is essential that a new generation of clinician scientists is attracted to the field if the success stories of polymer therapeutics in other clinical areas are to be replicated across the surgical specialties. The surgical community does not afford to be left out from being intimately involved in the development of this technology and underlying paradigms, and this is being pioneered by clinical–academic programmes in the UK to some extent, and nascent training programmes geared toward the dual clinical–academic development have yielded interesting results (Azzopardi, Ferguson, and Thomas, 2011a, 2013c, 2014b; Azzopardi et al., 2013, 2013a; Madani, Naderi, Dissanayake, et al., 2011). More importantly, exposure to this ongoing revolution may help maintain and develop a clinically oriented, demand‐driven ethos in this specialism. It is essential to attract a new generation of clinician–scientists with the necessary knowledge mix to drive highly successful translational innovation whilst preserving the surgeon's role as the ultimate guardian of patient safety.
This study has provided an overview of polymer therapeutics applied to clinical surgery, including the evolution of this demand‐oriented scientific field, cutting‐edge concepts, its implications, and drawbacks, illustrated by an overview of products already in clinical use and promising ones in advanced stages of development. This journal encourages and welcomes manuscripts situated at the interface between the disciplines of clinical surgery regenerative medicine, cell biology, and pharmaceutical sciences. It is time for the surgical community to step up to the plate.
Conflict of Interest
The authors explicitly declare no conflict of interest whatsoever in this paper.
Acknowledgments
Work by the authors illustrated in this manuscript is the recipient of the Royal College of Surgeons in Edinburgh 2014 for small research grant. This work was supported by the Biotechnology and Biological Sciences Research Council grant (BB/I015426/1) to RSC.
Azzopardi, E. A. , Conlan, R. S. , and Whitaker, I. S. (2016) Polymer therapeutics in surgery: the next frontier. Journal of Interdisciplinary Nanomedicine, 1: 19–29. doi: 10.1002/jin2.6.
References
- Allison, D. D. , and Grande‐Allen K. J.. 2006. Review. Hyaluronan: a powerful tissue engineering tool. Tissue Eng. 12(8):2131–2140. [DOI] [PubMed] [Google Scholar]
- Ascher, B. , Bayerl C., Brun P. et al. 2011. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines – 6‐month interim results of a randomized, evaluator‐blinded, intra‐individual comparison study. J. Cosmet. Dermatol. 10(2):94–98. [DOI] [PubMed] [Google Scholar]
- Azzopardi, EA . Bioresponsive dextrin–colistin conjugates as antimicrobial agents for the treatment of Gram‐negative infection. Cardiff University, 2013a.
- Azzopardi, EA . Bioresponsive dextrin–colistin conjugates as antimicrobial agents for the treatment of Gram‐negative infection. Wound Biology Group, College of Medicine, Vol. PhD. Cardiff: Cardiff University, 2013b. pp. 250.
- Azzopardi, E. A. , Boyce D. E., Thomas D. W. et al. 2013. Colistin in burn intensive care: back to the future? Burns 39(1):7–15. [DOI] [PubMed] [Google Scholar]
- Azzopardi, E. A. , Camilleri L., Moseley R. et al. 2013. Statistical characterization of succinoylated dextrin degradation behavior in human α‐amylase. J. Carbohydr. Chem. 32(7):438–449. [Google Scholar]
- Azzopardi, E. , Ferguson E., and Thomas D.. 2011a. Polymer therapeutics for effective antimicrobial targeting in burn injury (Abstract). Br. J. Surg. 98(S2). [Google Scholar]
- Azzopardi, EA , Ferguson EL, Thomas DW. Polymer therapeutics for safe and effective antibiotic targeting: a novel strategy (poster presentation). British Society for Oral and Dental Research Annual Conference. Sheffield, 2011b.
- Azzopardi, E. A. , Ferguson E. L., and Thomas D. W.. 2013a. The enhanced permeability retention effect: a new paradigm for drug targeting in infection. J Antimicrob. Chemoth. 68(2):257–274. [DOI] [PubMed] [Google Scholar]
- Azzopardi, E. A. , Ferguson E. L., and Thomas D. W.. 2013b. A new class of nanoantibiotics directed at multidrug resistant infection – ex vivo kinetics and targeting potential. BP 15. Society of Academic Research Surgery. British Journal of Surgery in press, London. [Google Scholar]
- Azzopardi, E. , Ferguson E., and Thomas D.. 2013c. A new class of nanoantibiotics directed at multidrug resistant surgical infection – ex vivo release kinetics and targeting potential. Br. J. Surg. 100 WILEY‐BLACKWELL 111 RIVER ST, HOBOKEN 07030‐5774, NJ USA:74–74. [Google Scholar]
- Azzopardi, E. , Ferguson E., and Thomas D.. 2014a. A novel class of bioresponsive nanomedicines for localised reinstatement of bioactivity and specific targeting. Lancet 383:S9. [Google Scholar]
- Azzopardi, E. , Ferguson E., and Thomas D.. 2014b. A novel class of bioresponsive nanomedicines capable of localised reinstatement of bioactivity and specific targeting (Abstract). Lancet 383(9918 S1):9. [Google Scholar]
- Azzopardi, E. A. , McWilliams B., Iyer S. et al. 2009. Fluid resuscitation in adults with severe burns at risk of secondary abdominal compartment syndrome – an evidence based systematic review. Burns 35(7):911–920. [DOI] [PubMed] [Google Scholar]
- Battisto, J. , and Pappas F.. 1973. Regulation of immunoglobulin synthesis by dextran. J. Exp. Med. 138(1):176–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt, A. P. , Neppalli V. T., Kelley E. A. et al. 2011. Dextran removal by plasmapheresis in a kidney–pancreas transplant recipient with dextran 40‐induced osmotic nephrosis. Am. J. Kidney Dis. 57(4):621–623. [DOI] [PubMed] [Google Scholar]
- Boussekey, N. , Darmon R., Langlois J. et al. 2010. Resuscitation with low volume hydroxyethylstarch 130 kDa/0.4 is not associated with acute kidney injury. Crit. Care 14(2):R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray, D. , Hopkins C., and Roberts D. N.. 2010. A review of dermal fillers in facial plastic surgery. Curr. Opin. Otolaryngol. Head Neck Surg. 18(4):295–302. [DOI] [PubMed] [Google Scholar]
- Brunkhorst, F. M. , Engel C., Bloos F. et al. 2008. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N. Engl. J. Med. 358(2):125–139. [DOI] [PubMed] [Google Scholar]
- Burdick, J. A. , and Prestwich G. D.. 2011. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 23(12):H41–H56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel, A. La technique opératoire des anastomoses vasculaires et de la transplantation des viscères: Association typographique, 1902.
- Dams, E. T. , Reijnen M. M., Oyen W. J. et al. 1999. Imaging experimental intra abdominal abscesses with 99mTc‐PEG liposomes and 99mTc‐HYNIC IgG. Ann. Surg. 229(4): 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M. E. 2009. Design and development of IT‐101, a cyclodextrin‐containing polymer conjugate of camptothecin. Adv. Drug Deliv. Rev. 61(13):1189–1192. [DOI] [PubMed] [Google Scholar]
- Ditrolio, R. , Simeone E., DI Lorenzo G. et al. 2012. Update on PEG‐interferon α‐2b as adjuvant therapy in melanoma. Anticancer Res 32(9):3901–3909. [PubMed] [Google Scholar]
- Djohan, R. S. , Gage E., and Bernard S. L.. 2010. Pp. 89–100 Microsurgical techniques. Plastic and reconstructive surgery. Springer. [Google Scholar]
- Duncan, R. 2003. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2(5):347–360. [DOI] [PubMed] [Google Scholar]
- Duncan, R. 2009. Development of HPMA copolymer‐anticancer conjugates: clinical experience and lessons learnt. Adv. Drug Deliv. Rev. 61(13):1131–1148. [DOI] [PubMed] [Google Scholar]
- Duncan, R. 2011. Polymer therapeutics as nanomedicines: new perspectives. Curr. Opin. Biotechnol. 22(4):492–501. [DOI] [PubMed] [Google Scholar]
- Duncan, R. 2014. Polymer therapeutics: top 10 selling pharmaceuticals – what next? J. Control. Release 190:371–380. [DOI] [PubMed] [Google Scholar]
- Duncan, R. , Gac‐Breton S., Keane R. et al. 2001. Polymer‐drug conjugates, PDEPT and PELT: basic principles for design and transfer from the laboratory to clinic. J. Control. Release 74(1‐3):135–146. [DOI] [PubMed] [Google Scholar]
- Duncan, R. , and Gaspar R.. 2011. Nanomedicine (s) under the microscope. Mol. Pharm. 8(6):2101–2141. [DOI] [PubMed] [Google Scholar]
- Duncan, R. , Gilbert H., Carbajo R. et al. 2008. Polymer masked–unmasked protein therapy. 1. Bioresponsive dextrin–trypsin and –melanocyte stimulating hormone conjugates designed for α‐amylase activation. Biomacromolecules 9(4):1146–1154. [DOI] [PubMed] [Google Scholar]
- Duncan, R. , and Vicent M. J.. 2010. Do HPMA copolymer conjugates have a future as clinically useful nanomedicines? A critical overview of current status and future opportunities. Adv. Drug Deliv. Rev. 62(2):272–282. [DOI] [PubMed] [Google Scholar]
- Duncan, R. , and Vicent M. J.. 2013. Polymer therapeutics‐prospects for 21st century: the end of the beginning. Adv. Drug Deliv. Rev. 65(1):60–70. [DOI] [PubMed] [Google Scholar]
- European Science Foundation . Scientific forward look on nanomedicine. 2005.
- Evans, D. J., Jr. , Evans D. G., and Gorbach S. L.. 1973. Production of vascular permeability factor by enterotoxigenic Escherichia coli isolated from man. Infect. Immun. 8(5):725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhari, A. , and Berkland C.. 2013. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 9(7):7081–7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullana, M , and Wnek GE. 2 History of techniques and materials used in volume enhancement, 2013.
- Galanski, M. , and Keppler B. K.. 2012. Tumor‐targeting strategies with anticancer platinum complexes: From basic research to cancer therapy. Drug Deliv. Oncol.:1605–1629. [Google Scholar]
- Garg, HG , and Hales CA. Chemistry and biology of hyaluronan: access online via Elsevier, 2004.
- Gaspar, R. , and Duncan R.. 2009. Polymeric carriers: preclinical safety and the regulatory implications for design and development of polymer therapeutics. Adv. Drug Deliv. Rev. 61(13):1220–1231. [DOI] [PubMed] [Google Scholar]
- Gattas, D. J. , Dan A., Myburgh J. et al. 2012. Fluid resuscitation with 6% hydroxyethyl starch (130/0.4) in acutely ill patients: an updated systematic review and meta‐analysis. Anesth. Analg. 114(1):159–169. [DOI] [PubMed] [Google Scholar]
- Greco, T. M. , Antunes M. B., and Yellin S. A.. 2012. Injectable fillers for volume replacement in the aging face. Facial Plast. Surg. 28(01):08–20. [DOI] [PubMed] [Google Scholar]
- Griffith, L. 2000. Polymeric biomaterials. Acta Mater. 48(1):263–277. [Google Scholar]
- Hardwicke, J. , Ferguson E., Moseley R. et al. 2008. Dextrin–rhEGF conjugates as bioresponsive nanomedicines for wound repair. J. Control. Release 130(3):275–283. [DOI] [PubMed] [Google Scholar]
- Hardwicke, J. T. , Hart J., Bell A. et al. 2011. The effect of dextrin–rhEGF on the healing of full‐thickness, excisional wounds in the (db/db) diabetic mouse. J. Control. Release 152(3):411–417. [DOI] [PubMed] [Google Scholar]
- Hardwicke, J. , Moseley R., Stephens P. et al. 2010. Bioresponsive dextrin–rhEGF conjugates: in vitro evaluation in models relevant to its proposed use as a treatment for chronic wounds. Mol. Pharm. 7(3):699–707. [DOI] [PubMed] [Google Scholar]
- Hardwicke, J. , Schmaljohann D., Boyce D. et al. 2008. Epidermal growth factor therapy and wound healing – past, present and future perspectives. Surgeon 6(3):172–177. [DOI] [PubMed] [Google Scholar]
- Ke, S. , Milas L., Charnsangavej C. et al. 2001. Potentiation of radioresponse by polymer–drug conjugates. J. Control. Release 74(1):237–242. [DOI] [PubMed] [Google Scholar]
- Koburger, T. , Hübner N.‐O., Braun M. et al. 2010. Standardized comparison of antiseptic efficacy of triclosan, PVP–iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J. Antimicrob. Chemoth. 65(8):1712–1719. [DOI] [PubMed] [Google Scholar]
- Komarek, P. , Kleisner I., Komarkova I. et al. 2005. Accumulation of radiolabelled low molecular peptides and proteins in experimental inflammation. Int. J. Pharm. 291(1):119–125. [DOI] [PubMed] [Google Scholar]
- Kriss, T. C. , and Kriss V. M.. 1998. History of the operating microscope: from magnifying glass to microneurosurgery. Neurosurgery 42(4):899–907. [DOI] [PubMed] [Google Scholar]
- Laverman, P. , Boerman O. C., Oyen W. J. et al. 1999. Liposomes for scintigraphic detection of infection and inflammation. Adv. Drug Deliv. Rev. 37(1‐3):225–235. [DOI] [PubMed] [Google Scholar]
- Laverman, P. , Boerman O. C., Oyen W. J. G. et al. 2001. In vivo applications of PEG liposomes: unexpected observations. Crit. Rev. Ther. Drug Carrier Syst. 18(6):551–566. [PubMed] [Google Scholar]
- Laverman, P. , Dams E. T., Storm G. et al. 2001. Microscopic localization of PEG‐liposomes in a rat model of focal infection. J. Control. Release 75(3):347–355. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Truini C., and Ariyan S.. 2008. A randomized study comparing the effectiveness of methylene blue dye with lymphazurin blue dye in sentinel lymph node biopsy for the treatment of cutaneous melanoma. Ann. Surg. Oncol. 15(9):2412–2417. [DOI] [PubMed] [Google Scholar]
- Madani, S. Y. , Naderi N., Dissanayake O. et al. 2011. A new era of cancer treatment: carbon nanotubes as drug delivery tools. Int. J. Nanomedicine 6:2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, H. 2010. Tumor‐selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjugate Chem. 21(5):797–802. [DOI] [PubMed] [Google Scholar]
- Maeda, H. 2012. Vascular permeability in cancer and infection as related to macromolecular drug delivery, with emphasis on the EPR effect for tumor‐selective drug targeting. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 88(3):53–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, H. , Bharate G. Y., and Daruwalla J.. 2009. Polymeric drugs for efficient tumor‐targeted drug delivery based on EPR‐effect. Eur. J. Pharm. Biopharm. 71(3):409–419. [DOI] [PubMed] [Google Scholar]
- Maeda, H. , Fang J., Inutsuka T. et al. 2003. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int. Immunopharmacol. 3(3):319–328. [DOI] [PubMed] [Google Scholar]
- Medina, M. A. I. , Nguyen J. T., Kirkham J. C. et al. 2011. Polymer therapy: a novel treatment to improve fat graft viability. Plast. Reconstr. Surg. 127(6):2270–2282. DOI: 10.1097/PRS.0b013e3182139fc1. [DOI] [PubMed] [Google Scholar]
- Melendez‐Alafort, L. , Nadali A., Pasut G. et al. 2009. Detection of sites of infection in mice using 99mTc‐labeled PN(2)S‐PEG conjugated to UBI and 99mTc‐UBI: a comparative biodistribution study. Nucl. Med. Biol. 36(1):57–64. [DOI] [PubMed] [Google Scholar]
- MHRA . 2013. Hydroxyethyl starch intravenous infusion: suspension of licences. Drug Safety Update 6(11):S1. [Google Scholar]
- Mitchell, K. J. 2013. Pp. 135 Preoperative Evaluation, The Perioperative Medicine Consult Handbook. [Google Scholar]
- Myburgh, J. A. , Finfer S., Bellomo R. et al. 2012. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N. Engl. J. Med. 367(20):1901–1911. [DOI] [PubMed] [Google Scholar]
- Najibi, S. , Banglmeier R., Matta J. et al. 2010. Material properties of common suture materials in orthopaedic surgery. Iowa Orthop. J. 30:84. [PMC free article] [PubMed] [Google Scholar]
- Neves, R. I. , Reynolds B. Q., Hazard S. W. et al. 2011. Increased post‐operative complications with methylene blue versus lymphazurin in sentinel lymph node biopsies for skin cancers. J. Surg. Oncol. 103(5):421–425. [DOI] [PubMed] [Google Scholar]
- Oyen, W. , Boerman O., Storm G. et al. 1996. Detecting infection and inflammation with technetium‐99 m‐labeled Stealth (R) liposomes. J. Nucl. Med. 37(8):1392. [PubMed] [Google Scholar]
- Pasut, G. , and Veronese F.. 2009. PEG conjugates in clinical development or use as anticancer agents: an overview. Adv. Drug Deliv. Rev. 61(13):1177–1188. [DOI] [PubMed] [Google Scholar]
- Pavelka, K. , and Uebelhart D.. 2011. Efficacy evaluation of highly purified intra‐articular hyaluronic acid (Sinovial®) vs hylan G‐F20 (Synvisc®) in the treatment of symptomatic knee osteoarthritis. A double‐blind, controlled, randomized, parallel‐group non‐inferiority study. Osteoarthritis Cartilage 19(11):1294–1300. [DOI] [PubMed] [Google Scholar]
- Perner, A. , Haase N., Guttormsen A. B. et al. 2012. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N. Engl. J. Med. 367(2):124–134. [DOI] [PubMed] [Google Scholar]
- Pharmalucence press release. nd. Available at: http://www.pharmalucence.com/content.php?cont=129. Accessed 08/07/2014.
- Pinter, G. , Horvath P., Bujdoso S. et al. 2009. Synthesis and antimicrobial activity of ciprofloxacin and norfloxacin permanently bonded to polyethylene glycol by a thiourea linker. J. Antibiot. (Tokyo) 62(2):113–116. [DOI] [PubMed] [Google Scholar]
- Riva, F. M. , Chen Y. C., Tan N. C. et al. 2012. The outcome of prostaglandin‐E1 and dextran‐40 compared to no antithrombotic therapy in head and neck free tissue transfer: analysis of 1,351 cases in a single center. Microsurgery 32(5):339–343. [DOI] [PubMed] [Google Scholar]
- Roberts, M. , Bentley M., and Harris J.. 2002. Chemistry for peptide and protein PEGylation. Adv. Drug Deliv. Rev. 54(4):459–476. [DOI] [PubMed] [Google Scholar]
- Serbest, G. , Horwitz J., and Barbee K.. 2005. The effect of poloxamer‐188 on neuronal cell recovery from mechanical injury. J. Neurotrauma 22(1):119–132. [DOI] [PubMed] [Google Scholar]
- Sikkink, C. , Reijnen M., Laverman P. et al. 2009. Tc‐99 m‐PEG‐liposomes target both adhesions and abscesses and their reduction by hyaluronate in rats with fecal peritonitis. J. Surg. Res. 154(2):246–251. [DOI] [PubMed] [Google Scholar]
- Sondak, V. K. , King D. W., Zager J. S. et al. 2013. Combined analysis of phase III trials evaluating [99mTc] tilmanocept and vital blue dye for identification of sentinel lymph nodes in clinically node‐negative cutaneous melanoma. Ann. Surg. Oncol. 20(2):680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai, S. , Komatsu S., Sakamoto H. et al. 1970. Free muscle transplants in dogs, with microsurgical neurovascular anastomoses. Plast. Reconstr. Surg. 46(3):219–225. [DOI] [PubMed] [Google Scholar]
- Tian, X. , Azpurua J., Hine C. et al. 2013. High‐molecular‐mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 499(7458):346–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercauteren, R. , Bruneel D., Schacht E. et al. 1990. Effect of the chemical modification of dextran on the degradation by dextranase. J. Bioact. Com. Polym. 5(1):4–15. [Google Scholar]
- Werle, M. , and Bernkop‐Schnürch A.. 2006. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids 30(4):351–367. [DOI] [PubMed] [Google Scholar]
- Zarychanski, R. , Abou‐Setta A. M., Turgeon A. F. et al. 2013. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta‐analysis hydroxyethyl starch and outcomes in critically ill. JAMA 309(7):678–688. [DOI] [PubMed] [Google Scholar]
- Zheng Shu, X. , Liu Y., Palumbo F. S. et al. 2004. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials 25(7):1339–1348. [DOI] [PubMed] [Google Scholar]


