Abstract
c-Kit tyrosine kinase receptor has been identified as a regulator of bone homeostasis. The c-Kit loss-of-function mutations in WBB6F1/J-KitW/W-v mice result in low bone mass. However, these mice are sterile and it is unclear whether the observed skeletal phenotype is secondary to a sex hormone deficiency. In contrast, C57BL/6J-KitW-sh/W-sh (Wsh/Wsh) mice, which carry an inversion mutation affecting the transcriptional regulatory elements of the c-Kit gene, are fertile. Here, we showed that Wsh/Wsh mice exhibited osteopenia with elevated bone resorption and bone formation at 6- and 9-week-old. The c-Kit Wsh mutation increased osteoclast differentiation, the number of committed osteoprogenitors, alkaline phosphatase activity and mineralization. c-Kit was expressed in both osteoclasts and osteoblasts, and c-Kit expression was decreased in Wsh/Wshosteoclasts, but not osteoblasts, suggesting an indirect effect of c-Kit on bone formation. Furthermore, the osteoclast-derived coupling factor Wnt10b mRNA was increased in Wsh/Wsh osteoclasts. Conditioned medium from Wsh/Wsh osteoclasts had elevated Wnt10b protein levels and induced increased alkaline phosphatase activity and mineralization in osteoblast cultures. Antagonizing Wnt10b signaling with DKK1 or Wnt10b antibody inhibited these effects. Our data suggest that c-Kit negatively regulates bone turnover, and disrupted c-Kit signaling couples increased bone resorption with bone formation through osteoclast-derived Wnt 10 b.
c-Kit, a receptor tyrosine kinase belonging to the platelet-derived growth factor (PDGF) and the colony-stimulating factor 1 (CSF-1) receptor family, is a product of the gene at the Dominant White Spotting (W) locus1,2. The ligand for c-Kit is the gene product of the Steel (Sl) locus and is known as mast cell growth factor, stem cell factor, steel factor, and Kit ligand (KL)3,4. c-Kit and KL are essential for normal development and maintenance of three stem cell populations: germ cells, neural crest–derived melanocytes, and hematopoietic stem cells. c-Kit is present in primordial germ cells, spermatogonia, primordial oocytes, growing oocytes, melanocytes5, mast cells6, and osteoclasts7. Homozygotes carrying mutations at the W and Sl loci are erythrocyte- and mast cell-deficient, infertile, and lack pigmented coats8.
Several naturally occurring loss-of-function mutations of c-Kit have been identified in mice and humans. The W mutation is a null mutation causing deletion of the transmembrane domain of the c-Kit receptor, while Wv is a point mutation in the kinase domain of the receptor resulting in impaired receptor activity9. Cells expressing the Wv mutation do not respond to KL in proliferation and apoptosis assays, presumably due to the inability of the receptor to initiate signal transduction10,11,12. W-sash (Wsh), an allele of W, is an inversion mutation upstream of the c-Kit promoter region affecting a key regulatory element, resulting in cell-type-specific altered expression of the gene13,14,15,16. The Wsh mutation arises spontaneously from crossing two inbred strains of C3H/HeH and 101/H mice.
The role of KL/c-Kit signaling in the regulation of bone metabolism has been studied in vitro and in vivo. The human osteoblast-like cell line Saos-2 expresses KL on its cell surface, whereas the osteoclast progenitor-like cell line FLG 29.1 expresses c-Kit7. Based on these studies, it was concluded that the c-Kit receptor mediates cell-to-cell interactions between osteoclasts and osteoblasts/stromal cells through membrane-bound KL. Previous studies have identified skeletal changes in Sl/Sld mutants lacking the transmembrane form of KL but had normal c-Kit receptors17. Deletion of membrane-bound KL induces osteopenia. The negative bone balance observed in these mice was primarily due to increased osteoclast surface. It has been shown that 14-week-old female WBB6F1/J-KitW/W-v (W/Wv) mice carrying a compound c-Kit mutation were osteopenic18. However, these mice were infertile due to a lack of germ cells in the ovary and had reduced estrogen and progesterone levels, leading to increased FSH level19. It is unclear whether the observed skeletal phenotype in W/Wv mice resulted from cell-autonomous effects in osteoclasts or was a consequence of changes in sex hormone levels. In the present study, we focused on the skeletal phenotype of C57BL/6J-KitW-sh/W-sh (Wsh/Wsh) mice that were fully fertile and determined the mechanism by which altered c-Kit signaling affected bone turnover. Our data indicated that the c-Kit Wsh mutation resulted in decreased cancellous bone volume with an increase in bone resorption and bone formation in growing mice. Calvarial osteoblasts derived from Wsh/Wshmice showed an increase in osteoblast precursors, alkaline phosphatase (ALP) activity and mineralization in vitro. Moreover, the RANKL/OPG ratio was increased in osteoblasts derived from these mice, leading to increased number of osteoclasts in c-Kit mutants. Quantitative real-time PCR (qPCR) indicated that c-Kit expression was decreased in Wsh/Wshbone marrow macrophage (BMM) and osteoclasts but not osteoblasts, suggesting that increased bone formation in Wsh/Wsh mice was not an osteoblasts intrinsic effect. Conditioned medium derived from Wsh/Wsh osteoclasts contained increased levels of the osteoclast-derived coupling factor Wnt10b, and enhanced ALP activity and mineralization by osteoblasts. Blocking Wnt10b activity inhibited these effects. These findings demonstrate that c-Kit regulates bone turnover by suppressing osteoblast and osteoclast differentiation. Thus, c-Kit mutation increased bone formation by increasing the generation of osteoclast-derived coupling factor Wnt10b.
Results
W/W v mice exhibited osteopenic phenotype
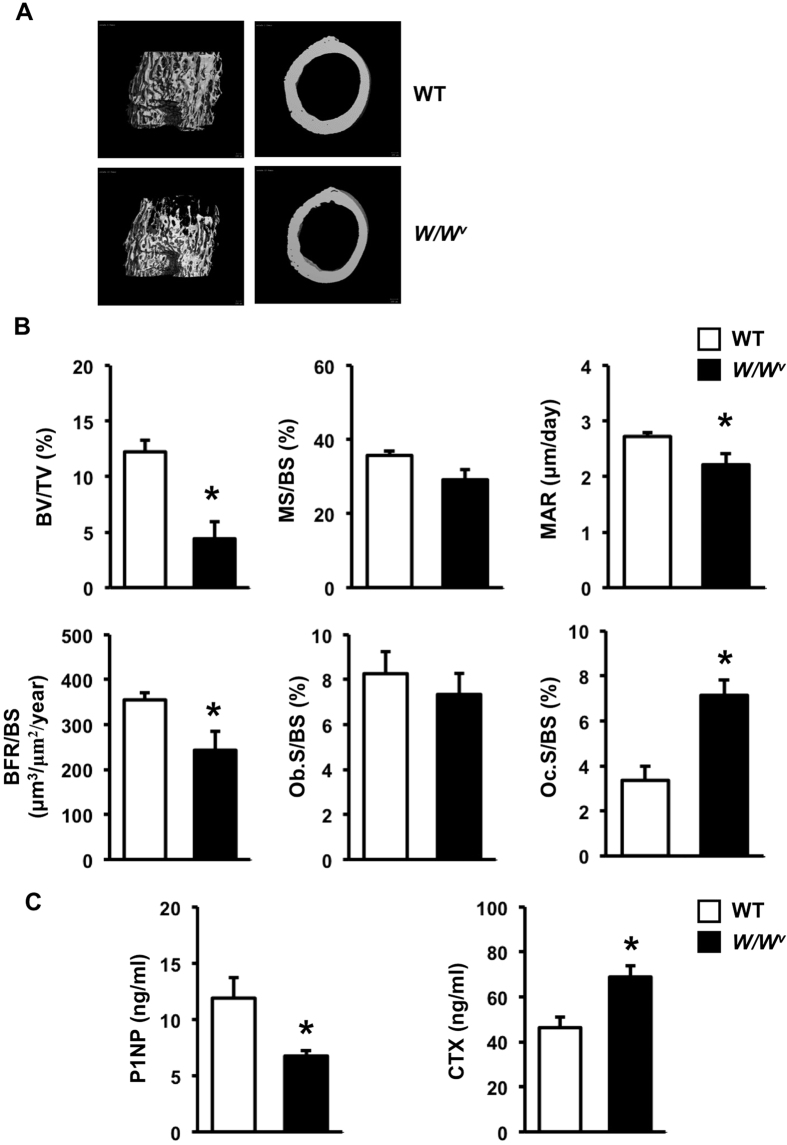
Previous studies indicated that both male and female W/Wv mice are infertile19,20. To determine whether a sex hormone deficiency contributed to changes in the skeletal phenotype of growing W/Wv mice, we first analyzed the skeletal phenotype of 6-week-old W/Wv mice. Theses mutants were smaller compared with wild type (WT) littermates and their body weight was 20% less (24.50 ± 0.88 g in WT vs 19.49 ± 0.42 g in W/Wv mice, p < 0.05). μCT analysis showed a decrease in cancellous bone volume, trabecular thickness, trabecular number and connectivity density with a concomitant increase in trabecular separation (Fig. 1A and Supplementary Table S1). Cross-sectional volume, cortical volume, and cortical thickness were also decreased in W/Wv mice compared with controls. Histomorphometric analysis confirmed a significant decrease in cancellous bone volume (Fig. 1B). The cancellous bone was less dense and thinner in W/Wv mice with decreased trabecular number and thickness and increased trabecular separation (data not shown). Bone formation was reduced due to a slight decrease in mineralizing surface per bone surface (p = 0.052) and a significant decrease in mineral apposition rate. Although osteoblast surface per bone surface was not changed in the mutants, osteoclast surface per bone surface was significantly increased. Therefore, the reduction in bone volume in the mutants was the result of decreased bone formation and increased bone resorption. As expected, W/Wv mice had decreased serum P1NP and increased serum CTX, confirming uncoupled bone turnover (Fig. 1C). Seminal vesicle weight, an index of androgen deficiency, was lower in W/Wv mice (0.122 ± 0.009 g in WT vs 0.071 ± 0.005 g in W/Wv mice, p < 0.05). Serum testosterone was significantly decreased in W/Wv mice (2.21 ± 0.30 ng/ml) compared with WT controls (5.02 ± 1.19 ng/ml).
Figure 1. Six-week-old male W/Wv mice are osteopenic.
(A) Representative μCT images of cancellous (left) and cortical bone (right) from femora of WT and W/Wv mice. (B) Histomorphometric analysis of cancellous bone in tibiae. (C) Serum concentration of P1NP and CTX (ng/ml). Results are mean ± SEM. *p < 0.05 versus WT.
Growing W sh /W sh mice are osteopenic
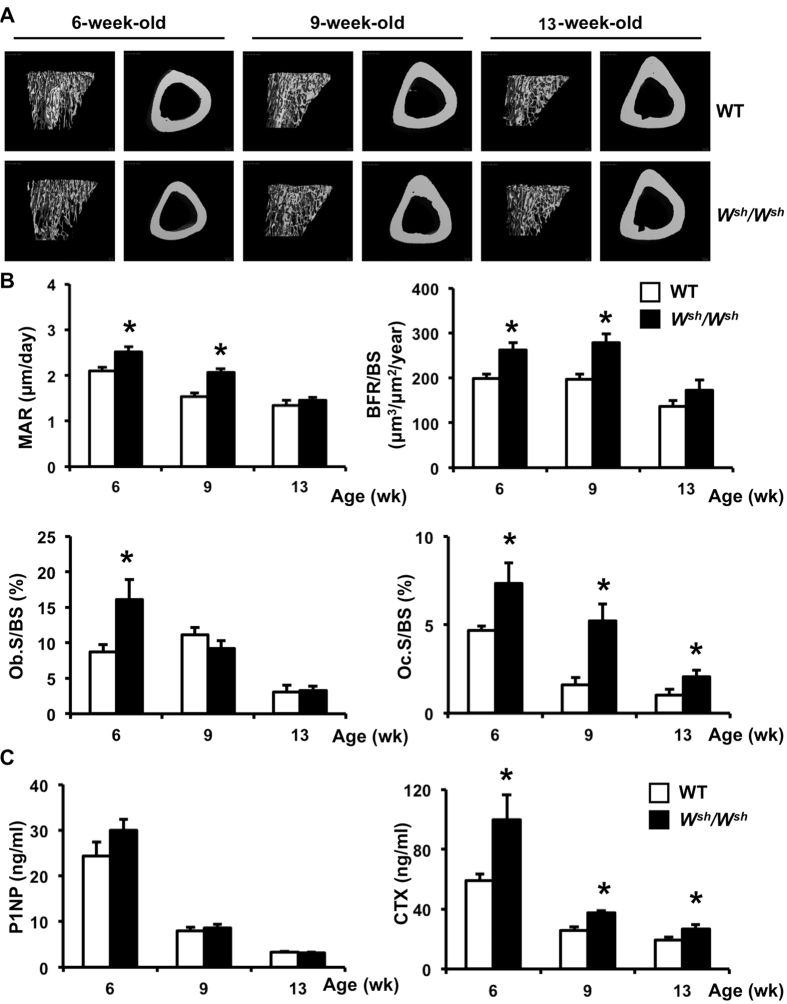
To eliminate the possible effect of sex hormones on the skeletal phenotype in c-Kit mutants, we analyzed the skeletal phenotype of male Wsh/Wsh mice. The body weight of the mutants and WT were similar (data not shown). μCT analysis of the cortical bone indicated that c-Kit mutation resulted in a significant decrease in total cross sectional volume, cortical volume, and marrow volume at 6, but not 9 and 13 weeks of age (Fig. 2A and Supplementary Table S2). A significant decrease in cancellous bone volume, trabecular number and connectivity density with a concomitant increase in trabecular separation was observed in 6- and 9-week-old Wsh/Wsh mice. Unlike the W/Wv mice, seminal vesicle weight was similar in Wsh/Wsh mice and WT controls (data not shown). The serum testosterone levels in 6-week-old mice (6.05 ± 1.08 ng/ml in WT vs 5.84 ± 1.44 ng/ml in Wsh/Wsh mice, NS) confirmed that male Wsh/Wsh mice are not androgen deficient.
Figure 2. Mutation of c-Kit increases bone formation and bone resorption in growing male mice.
(A) Representative μCT images of cancellous (left) and cortical bone (right) from tibiae of 6-, 9-, and 13-week-old WT and Wsh/Wsh mice. (B) Histomorphometric analysis of cancellous bone in tibiae. (C) Serum concentration of P1NP and CTX (ng/ml). Results are mean ± SEM. *p < 0.05 versus WT.
W sh mutation increases bone formation and bone resorption in growing mice
Histomorphometric analysis of the tibiae confirmed a decrease in cancellous bone volume at 6 weeks of age in Wsh/Wsh mice (Supplementary Table S3). Although mineralizing surface was not affected, mineral apposition rate was higher in 6- and 9-week-old Wsh/Wsh mice, leading to increased bone formation rate (Fig. 2B and Supplementary Table S3). Indices of bone formation; osteoblast surface per bone surface, osteoid surface per bone surface, osteoid volume per tissue volume, and osteoid thickness, were markedly increased at 6 weeks of age. A trend toward an increase in serum P1NP was also observed (Fig. 2C). However, the skeletal phenotype was milder in older mice. There was no statistical significant difference between control and mutant mice in all indices of bone formation at 13 weeks of age. In contrast, osteoclast surface per bone surface was dramatically increased compared with age-matched controls in all age groups. As shown in Fig. 2C, increased bone resorption in the mutants was confirmed by increased serum CTX levels.
We then examined the skeletal phenotype of 6 weeks old female mice. Similar to male Wsh/Wsh mice, female mice had increased bone turnover. As shown in Supplementary Table S4, mineral apposition rate was higher in female Wsh/Wsh mice compared with WT, leading to an increase in bone formation rate expressed per bone surface and bone volume. Osteoblast surface per bone surface, osteoblast number per tissue area and osteoblast number per bone perimeter were significantly increased in 6-week-old Wsh/Wsh mice. Osteoclast surface per bone surface, osteoclast number per tissue area and osteoclast number per bone perimeter were also increased. The magnitude of change in bone formation rate was higher in female (40–57%) compared with male mice (30–37%). Therefore, there was no net change in bone volume in female mice. Male Wsh/Wsh and their controls were selected for further investigation.
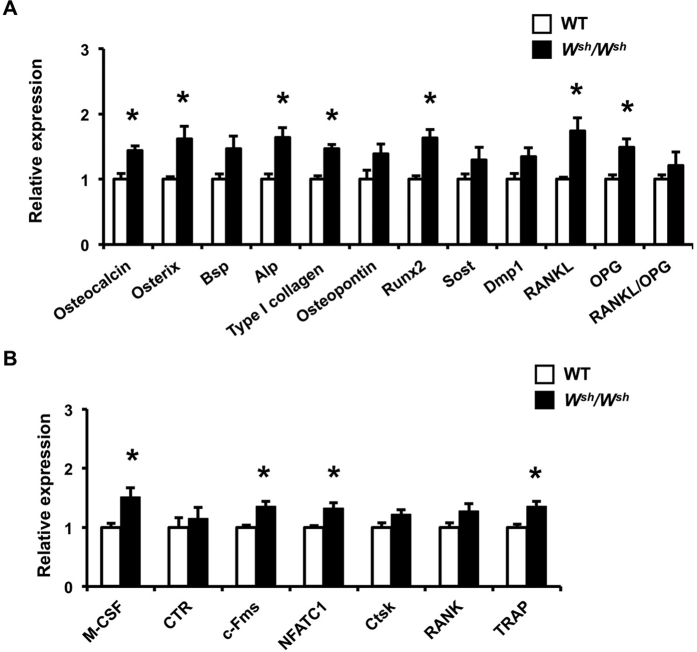
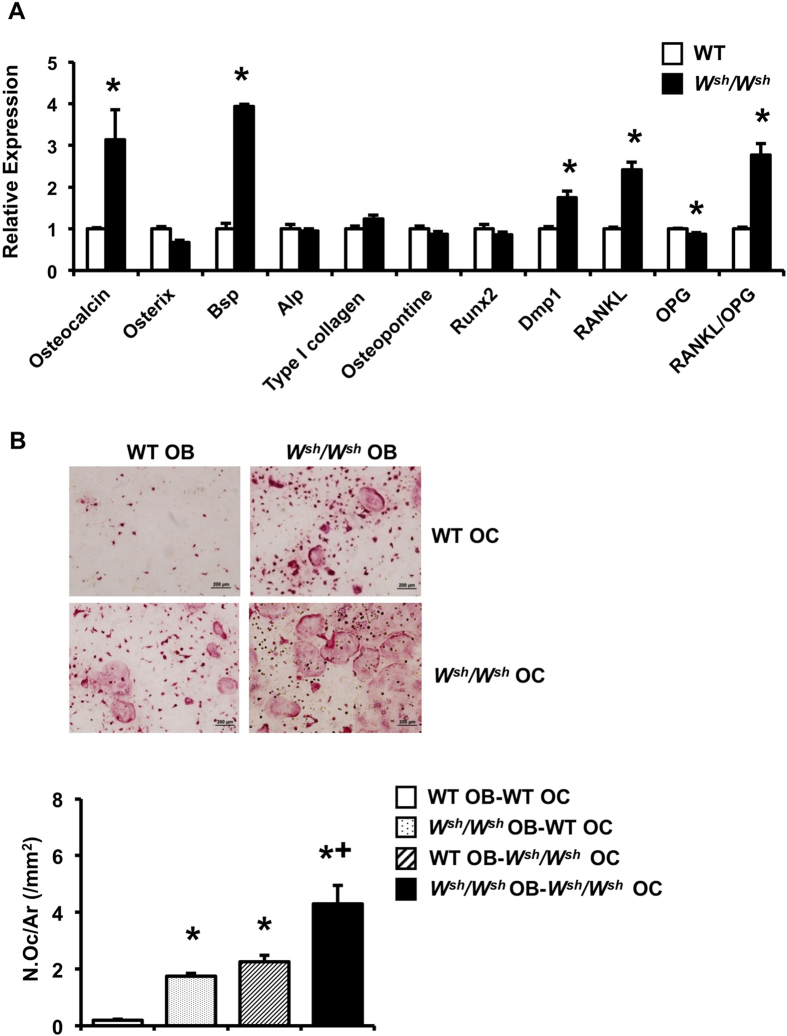
Osteoblast and osteoclast marker gene expression was examined in 6-weeks-old male Wsh/Wshmice and their controls. As shown in Fig. 3A, qPCR indicated that c-Kit mutation increased the expression of several osteoblast marker genes in femora including osteocalcin, Osterix, ALP, type I collagen and Runx2. The mRNA levels of both RANKL and OPG were increased therefore the RANKL/OPG ratio was not significantly changed. Expression profiling of osteoclast target genes showed increased expression of M-CSF, c-Fms, NFATC1 and TRAP in 6-week-old Wsh/Wsh mice (Fig. 3B). These data suggest that the increased bone turnover observed in 6-week-old Wsh/Wsh mice is likely to be due to increased bone formation and bone resorption in vivo.
Figure 3. Osteoblast and osteoclast marker genes are upregulated in the distal femoral metaphysis of 6-week-old male WT and Wsh/Wsh mice.
(A) qPCR analysis of osteoblast marker genes. (B) qPCR analysis of osteoclast marker genes. Results are mean ± SEM. *p < 0.05 versus WT.
c-Kit mutation increases osteoclast differentiation and precursors
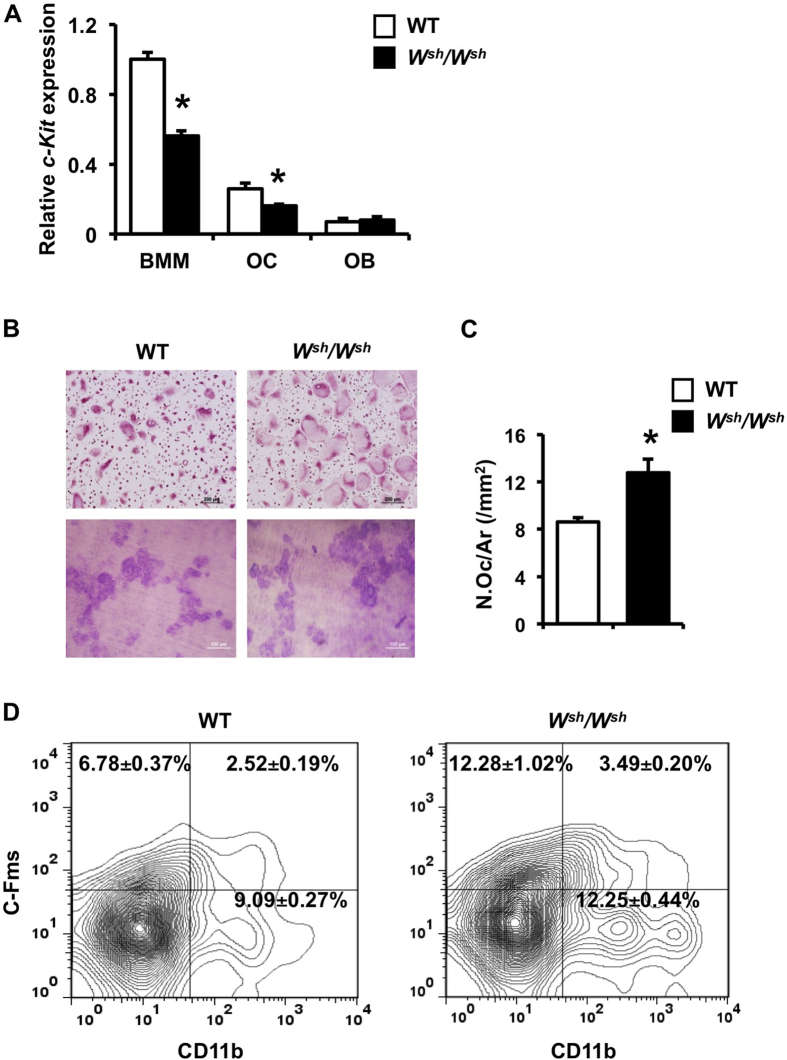
We examined the expression of c-Kit in BMM, osteoclasts, and osteoblasts. The mRNA level of c-Kit was much lower in osteoblasts compared with BMM and osteoclasts in Wsh/Wsh mice (Fig. 4A). c-Kit mutation reduced the c-Kit mRNA levels in BMM and osteoclasts by 43 and 35%, respectively, whereas the c-Kit mRNA level in osteoblasts was not altered.
Figure 4. Mutation of c-Kit increases osteoclast precursors and differentiation but not resorbing activity.
(A) qPCR analysis of c-Kit expression in BMMs, osteoclasts and osteoblasts. (B) Osteoclasts were generated on glass coverslips in the presence of M-CSF and RANKL and osteoclast differentiation was evaluated by TRAP staining (upper). Osteoclasts generated by co-culture with osteoblasts on collagen gel in media containing vitamin D3 and PGE2 were replated onto dentin slices and cultured for 48 h (lower). Resorption pits were stained with toluidine blue. (C) TRAP-positive osteoclast number per total area (/mm2) was quantified by OsteoMeasure software. (D) FACS analysis of CD11b+, c-Fms+ and CD11b+ cFms+ cells from spleen derived from WT and Wsh/Wsh mice. Results are mean ± SEM. *p < 0.05 versus WT.
Mutation of c-Kit increased osteoclast number in all age groups. We examined whether the increased number of osteoclasts in Wsh/Wsh mice was a cell-autonomous effect. Consistent with the increased in vivo bone resorption, TRAP staining showed increased osteoclast number in cultured BMM derived from Wsh/Wsh mice compared with WT (Fig. 4B,C), indicating an intrinsic role for c-Kit in osteoclast differentiation. To determine the effect of c-Kit on osteoclast resorbing ability, equal numbers of WT and mutant osteoclasts were cultured on dentin slices and the resorbed areas were quantified. No differences were observed in resorption between the mutant and WT cells (Fig. 4B).
Wsh/Wsh mice had increased osteoclast number in vivo and in vitro. To examine whether the increased osteoclast number was secondary to a greater number of osteoclast precursors, FACS analysis was performed on spleen and bone marrow cells. The results revealed that the percentages of CD11b+, c-Fms+ and CD11b+ c-Fms+ cells in Wsh/Wsh spleen cells were all increased compared with WT cells (Fig. 4D). There was an increase in the CD11b+ bone marrow cells (23.95 ± 1.25 in WT vs 31.10 ± 1.44 in Wsh/Wsh mice, p < 0.05), whereas c-Fms+ and CD11b+ c-Fms+ cells were not altered in Wsh/Wsh mice.
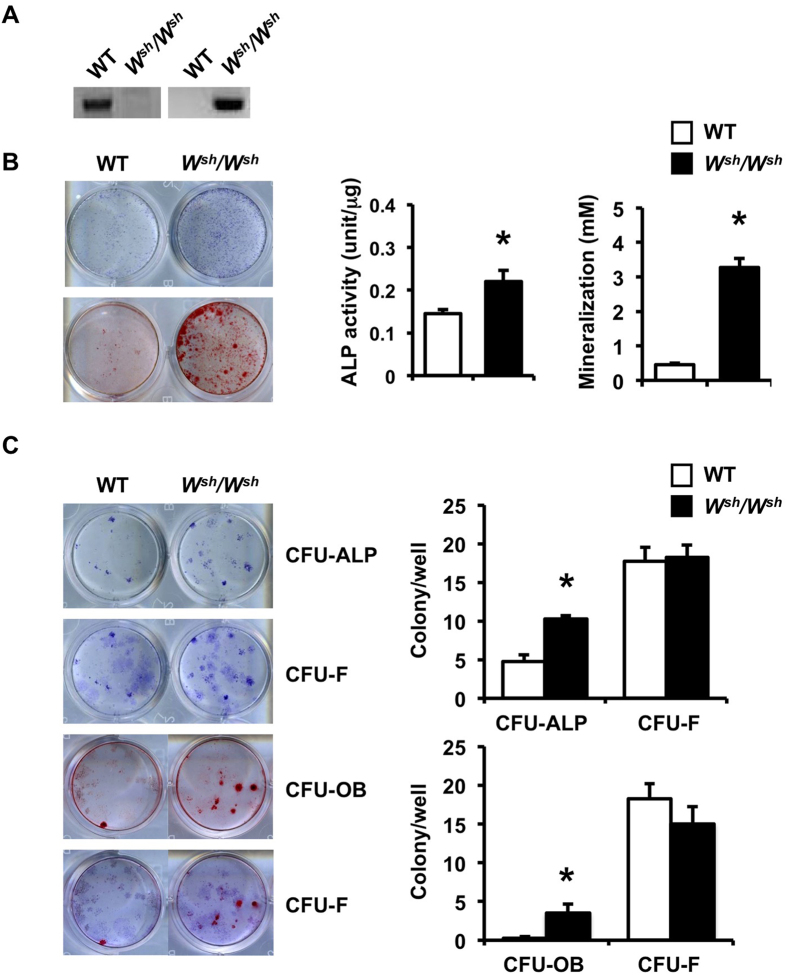
Mutation of c-Kit increases osteoblast progenitors and differentiation
To determine whether the increased bone formation observed in growing Wsh/Wsh mice was due to enhanced osteoblast proliferation and/or differentiation, we analyzed the proliferation and differentiation of calvarial osteoblasts in vitro. One-day-old pups were genotyped (Fig. 5A). Osteoblasts derived from Wsh/Wsh mice had higher ALP activity and formed more mineralized bone nodules than those from WT controls (Fig. 5B), whereas osteoblast proliferation, determined by BrdU labeling, was unchanged (data not shown). Because c-Kit expression was similar in Wsh/Wsh and WT osteoblasts, we hypothesized that an increase in the osteoblast precursor population contributed to increased osteoblast differentiation in Wsh/Wsh mice. To test this hypothesis, we analyzed the capability of calvarial osteoblasts derived from Wsh/Wsh mice and their WT littermates to form ALP-positive colony forming units (CFU-ALP) and CFU-osteoblasts (CFU-OB). We found that although CFU-ALP and CFU-OB were increased, the number of CFU-fibroblasts (CFU-F) was not changed in Wsh/Wsh osteoblasts compared with WT controls (Fig. 5C). qPCR analysis of calvarial osteoblasts derived from Wsh/Wsh mice showed increased expression of the osteoblast marker genes osteocalcin, Bsp, and Dmp1 (Fig. 6A). The mRNA level for RANKL increased, whereas OPG mRNA level decreased, leading to an increase in the RANKL/OPG mRNA ratio in Wsh/Wsh osteoblasts. To further investigate whether the increased RANKL/OPG ratio in these cells enhanced osteoclast differentiation, co-culture of Wsh/Wsh or WT osteoblasts with Wsh/Wsh or WT BMMs was performed (Fig. 6B). Compared with WT osteoblasts, Wsh/Wsh osteoblasts induced greater osteoclast differentiation regardless of the BMM genotype, suggesting that the increased RANKL/OPG ratio in Wsh/Wsh osteoblasts led to increased osteoclast differentiation.
Figure 5. Mutation of c-Kit increases osteoblast differentiation and osteoprogenitors.
(A) PCR analysis using primers to amplify product from either WT (left) or Wsh/Wsh (right) genomic DNA. (B) ALP and alizarin red staining in calvarial osteoblasts at day 7 and 21, respectively. (C) The number of ALP-positive colony-forming unit (CFU-ALP) and alizarin red-positive CFU (CFU-OB) was increased in Wsh/Wsh osteoblasts. The same tissue culture plates were stained with toluidine blue to determine CFU-fibroblasts (CFU-F). CFU-F number was not changed. Results are mean ± SEM. *p < 0.05 versus WT.
Figure 6. Mutation of c-Kit increases osteoblast marker genes in vitro.
(A) qPCR analysis of mRNA expression in calvarial osteoblasts from WT and Wsh/Wsh mice. (B) TRAP-positive osteoclast number per total area (N.Oc/Ar, /mm2) from co-culture of WT or Wsh/Wsh osteoblasts with WT or Wsh/Wsh BMMs. OB; osteoblast and OC; osteoclast. Results are mean ± SEM. *p < 0.05 versus WT, and +p < 0.05 versus Wsh/Wsh OB-WT OC and WT OB-Wsh/Wsh OC.
Osteoclast-coupling factor Wnt 10b is increased in W sh /W sh osteoclasts
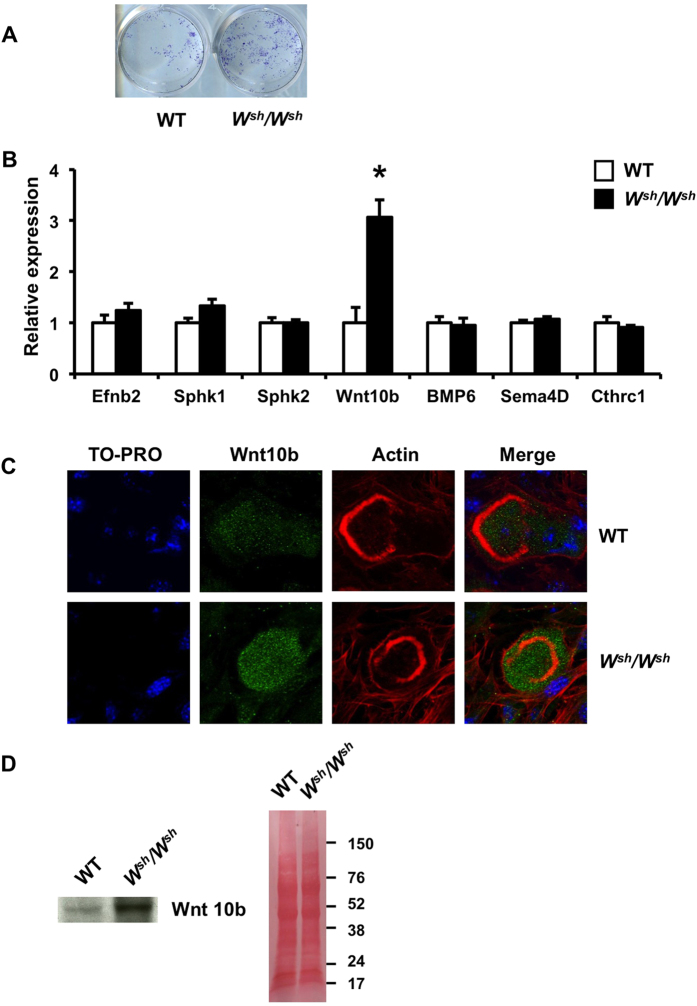
We examined whether altered local regulation between osteoblasts and osteoclasts contributed to increased osteoanabolic factors and subsequent bone formation in Wsh/Wsh mice. WT calvarial osteoblasts were cultured in conditioned medium from either Wsh/Wsh or control osteoclasts. Calvarial osteoblasts treated with conditioned medium derived from Wsh/Wsh osteoclast cultures demonstrated increased ALP staining, indicating that the conditioned medium contained more anabolic coupling factors (Fig. 7A). We then determined the expression of the known osteoclast-derived coupling factor genes, Efnb2, Sphk1, Sphk2, BMP6, Sema4D, Cthrc1 and Wnt10b21,22,23,24,25,26 from Wsh/Wsh and WT osteoclasts using qPCR. As shown in Fig. 7B, c-Kit mutants increased their expression of Wnt10b but not that of Efnb2, Sphk1, Sphk2, BMP6, Sema4D, or Cthrc1. Confocal immunofluorescence analysis of osteoclasts revealed increased Wnt10b staining in Wsh/Wsh osteoclasts cultured on dentin slices (Fig. 7C). Western blot analysis of osteoclast-conditioned media confirmed an increase in Wnt10b protein level in mutants (Fig. 7D). Before blotting, the membranes were stained with Ponceau S for protein detection. We found that the protein levels were comparable between the Wsh/Wsh and WT conditioned medium.
Figure 7. Mutation of c-Kit increases Wnt10b mRNA expression and protein level in osteoclasts.
(A) ALP staining of osteoblast cultures treated with conditioned medium derived from either Wsh/Wsh or WT osteoclasts (B) qPCR analysis of osteoclast mRNA expression. (C) Confocal immunofluorescence images of osteoclasts generated on dentin slices and immunolabeled for nuclei (TO-PRO3, blue), Wnt10b (green) and actin (rhodamine phalloidin, red). (D) Western blot analysis of conditioned medium from WT and Wsh/Wsh osteoclast was performed with an anti-Wnt10b antibody (left). The same membrane was stained with Ponceau S (right) as a loading control. Results are mean ± SEM. *p < 0.05 versus WT.
Blocking Wnt10b signaling decreased W sh /W sh osteoclast conditioned medium-induced ALP activity and mineralization
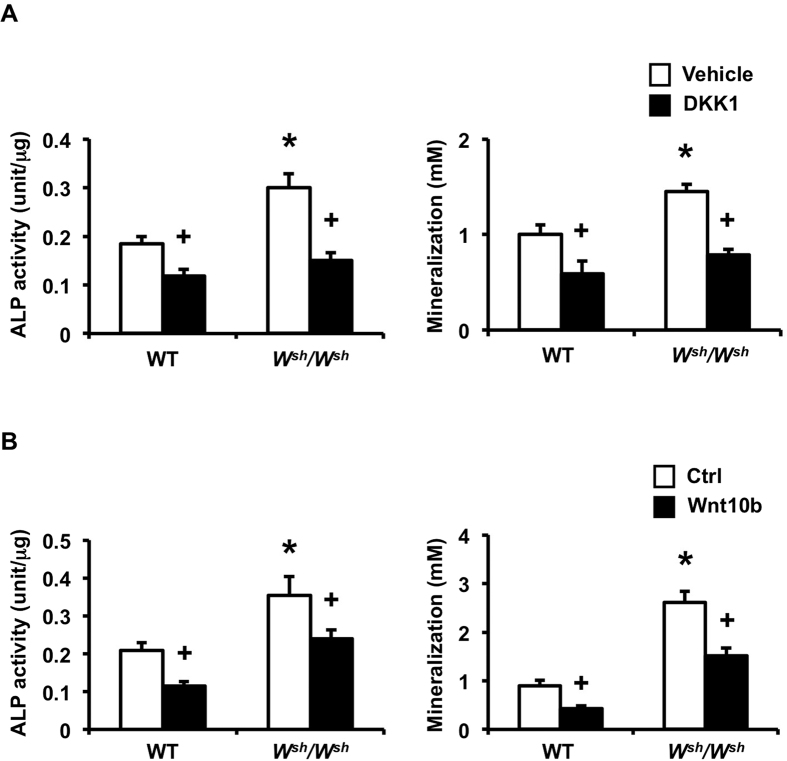
To evaluate whether osteoclast-derived Wnt10b contributed to the coupling-mediated enhancement of osteoblast formation, 0.2 μg/ml DKK1, a Wnt antagonist, was added to the WT calvarial osteoblast cultures with conditioned medium from either Wsh/Wsh or control osteoclasts. DKK1 markedly inhibited the increase in ALP activity and mineralization induced by Wsh/Wsh osteoclast conditioned medium (Fig. 8A). To further confirm the effects of the Wnt antagonist, we used antibody to neutralize the influence of Wnt10b on ALP activity and mineralization. Neutralizing Wnt10b inhibited the Wsh/Wsh osteoclast conditioned medium-induced increase in ALP activity and mineralization. Therefore, Wnt10b is the major coupling factor responsible for Wsh/Wsh osteoclast-mediated increase in bone formation.
Figure 8. Blocking Wnt10b attenuates ALP activity and mineralization induced by Wsh/Wsh osteoclast-conditioned medium.
(A) Calvarial osteoblasts were treated with either WT or Wsh/Wshosteoclast-conditioned medium with or without DKK1. ALP activity (n = 5 per group) and mineralization (n = 5 per group) were quantified. (B) Osteoblasts were cultured with either WT or Wsh/Wshosteoclast-conditioned medium pretreated with either isotype control (Ctrl) or Wnt10b antibody (Wnt10b). ALP activity (n = 5 per group) and mineralization (n = 5 per group) were quantified. Results are mean ± SEM. *p < 0.05 versus WT and + p < 0.05 versus corresponding controls.
Discussion
Our data suggest that c-Kit plays a crucial role in bone remodeling process. In the present study, we first examined the skeletal phenotype of W/Wv mice that carry a c-Kit point mutation and are sterile. W/Wv mutants had decreased cortical and cancellous bone volume. The reduction in cancellous bone volume was the result of a marked decrease in osteoblast surface and increase in osteoclast surface, indicating uncoupled bone turnover. To gain further insight into the precise role of c-Kit in bone metabolism, we used Wsh/Wsh mice that possess an inversion mutation upstream of the c-Kit region and are fertile. This c-Kit mutation, which reduced c-Kit expression in BMMs and osteoclasts but did not affect its expression in osteoblasts, resulted in osteopenia associated with increased bone formation and increased bone resorption in growing Wsh/Wsh mice. The skeletal phenotype was milder when animals were mature. The increased osteoclast number was a consequence of an increased RANKL/OPG ratio in osteoblasts. It appears that the alteration in the osteoclast-osteoblast coupling mechanism contributes to increased bone formation in Wsh/Wsh mice. Mutation of c-Kit stimulates Wnt10b secretion from osteoclasts that promotes osteoblast mineralization and subsequently bone formation. Blocking Wnt10b markedly inhibited the increased ALP activity and mineralization that were induced by Wsh/Wshosteoclast conditioned medium.
Physiologically, c-Kit expression is tightly regulated and the reduction or loss of c-Kit activity by known mutations is associated with bone loss. Osteoclasts express c-Kit receptor on their cell membrane and respond to its ligand directly through cell-to-cell contact7 or indirectly through paracrine factors. The skeletal phenotype of W/Wv mice is subtle and these mice are infertile due to germ cell depletion. It has been reported that male W/Wv mice have normal plasma testosterone levels but elevated FSH levels27. However, our data indicated that seminal vesicle weight, an index of androgen deficiency, decreased by 42% in W/Wv mice. The serum testosterone was also decreased in these mice. Male hypogonadism increases the production of osteoclasts and osteoblasts, leading to an increase in cancellous bone turnover28,29,30. These changes were quite distinct from those found in W/Wv mice. Thus, the low bone mass observed in growing W/Wv mice is unlikely to be solely the consequence of androgen deficiency. Wsh/Wsh mice that are fully fertile and have normal testosterone level also exhibited osteopenia. However, the cellular mechanism of bone loss in these mice was different from that of W/Wv mice. Increased osteoclast number and increased osteoblast function with a net increase in bone resorption contributed to the skeletal phenotype observed in Wsh/Wsh mice.
Bone undergoes renewal and repair through bone remodeling process, with no net change in bone volume when the amount of bone removed is precisely replaced by that of bone formed. Failure to elicit a corresponding increase in bone formation following a dramatic increase in osteoclasts causes net bone loss in growing male Wsh/Wsh mice. c-Kit mutation decreased the mRNA level of c-Kit in BMMs and osteoclasts leading to increased osteoclast differentiation in vitro. These results suggest a cell-autonomous effect in Wsh/Wsh mice. Osteoclast formation is driven by the key effector RANKL derived from osteoblasts or other cell lineages within the bone microenvironment. RANKL activity is moderated by a decoy receptor OPG. c-Kit mutation induced bone resorption by increasing RANKL expression in both in vivo and in vitro. Wsh/Wsh osteoblasts had an increased RANKL/OPG mRNA ratio, which has been shown to promote osteoclastogenesis31,32. Our co-culture experiments using osteoblasts and osteoclasts also confirmed that the increased RANKL/OPG ratio in Wsh/Wsh osteoblasts was responsible for the increased osteoclast differentiation observed in vitro.
According to the osteoclast commitment and differentiation pathway, CD11b is expressed during the differentiation of mononuclear early progenitor cells to mature multinucleated osteoclasts. The expression is higher in mononuclear cells and low in mature osteoclasts. It has been reported that c-Fms is a major determinant in osteoclast differentiation33,34. FACS analysis of spleen cells derived from Wsh/Wsh mice revealed an increase in the percentage of CD11b+, c-Fms+, and CD11b+ c-Fms+ cells. Although the number of c-Fms+, and CD11b+ c-Fms+ cells in Wsh/Wsh bone marrow was not altered, CD11b+ cell number was increased. These data suggest that reduced c-Kit signaling acts to expand the pool of osteoclast precursors, leading to increased osteoclast differentiation and bone resorption in Wsh/Wsh mice. Although histomorphometric analysis indicated no changes in bone architecture at 9 weeks of age, three-dimensional μCT showed decreases in cancellous bone volume, trabecular thickness and trabecular number with concomitant increase in trabecular separation. The CTX level in Wsh/Wsh mice was increased by 68, 44 and 41% at 6, 9 and 13 weeks old, respectively, indicating a reduction in osteoclast activity in the mutants as the animals grew.
The mechanism by which loss-of-function mutation of c-Kit led to osteopenia in Wsh/Wshmice remains unclear. It has been reported that c-Kit mediates cell-to-cell interactions between osteoclasts and osteoblasts/stromal cells through membrane bound KL7. Soluble KL, in concert with other factors, stimulates osteoclast formation and activity35. Gleevec, which inhibits c-Kit as well as c-Fms, c-Abl, and PDGF receptor36,37, decreases osteoclast number in rodents38 and inhibit osteoclast differentiation in vitro39. When used therapeutically to treat chronic myeloid leukemia, it sometimes induces secondary hyperparathyroidism with inconsistent reports of changes in bone formation and bone resorption40,41. The extent to which the effects of Gleevec on bone are mediated by inhibition of c-Kit has not been determined.
W/Wv and Wsh/Wsh mice have reduced numbers of mast cells in various soft tissues42,43,44. Mast cell deficiency is associated with low bone turnover, whereas excessive mast cell number induces bone loss45. Although the relationship between mast cells and osteoclasts is not completely understood, mast cells can modulate osteoclast activity through their release of granule-associated cytokines. However, it has been reported that mast cells are rarely found in mouse bone marrow18,46. Therefore, it is unlikely that mast cells contribute to the skeletal changes observed in our study.
The role of c-Kit in bone formation remains undefined. Although it has been reported that c-Kit is expressed in primary rat osteoblasts and SaOS-2 cells but not MC3T3-E1, ST2 or RAW 264.7 cells47, we found that c-Kit expression was much lower in mouse calvarial osteoblasts compared with osteoclasts. The Wsh mutation affects the tissue-specific expression of c-Kit during embryonic development and adulthood16. Although c-Kit expression in Wsh/Wsh osteoclasts was decreased by 35%, its expression in osteoblasts was not altered, indicating that the increased bone formation in Wsh/Wsh mice was not due to an intrinsic effect in osteoblasts. The fact that calvarial osteoblasts isolated from Wsh/Wsh mice had increased ALP activity and formed more bone nodules together with an increase in the number of CFU-ALP and CFU-OB without any change in total CFU-F suggests that the increased osteoblast number in bone in vivo resulted from an increase in the number of committed osteoblast precursors. Our finding indicates that c-Kit mutation leads to increased bone formation in Wsh/Wsh mice through indirect osteoclast-mediated effects. The evidence that bone resorption triggers bone formation suggests that certain coupling factors derived from osteoclasts are responsible for recruiting osteoblasts progenitors to the remodeling site and stimulating bone formation. Our finding that conditioned medium derived from Wsh/Wsh osteoclast culture increased ALP activity and mineralization in osteoblasts confirmed an increase in osteoclast-secreted osteoanabolic factors. We demonstrated that c-Kit mutation stimulated Wnt10b, a known coupling factor, production, and secretion from osteoclasts. The increase in Wnt10b production in c-Kit mutant osteoclasts, together with the increased osteoblast differentiation induced by Wsh/Wsh osteoclast-conditioned medium and the increased bone formation in vivo, strengthen the evidence that osteoclasts can enhance bone formation through secreted coupling factors. Binding of Wnt10b to Wnt receptors, LRP-5 and LRP-6, on osteoblasts stimulates new bone formation48. Antagonizing Wnt10b blunted the anabolic effects of the osteoclast-conditioned medium in vitro. Therefore, it is likely that Wnt10b is an osteoclast-derived molecule responsible for the enhancement of bone formation in Wsh/Wsh mice. However, the mechanism by which c-Kit mutation regulates Wnt10b production by osteoclasts remains to be determined. Our findings do not exclude a contribution of matrix-derived growth factors, such as TGF-β1, released from the bone matrix during bone resorption. Other investigators have shown that TGF-β1 stimulates Wnt10b production in osteoclasts that enhances the coupling of bone resorption with formation26. Further studies are required to address this question.
In conclusion, this study is the first to report the importance of c-Kit as a negative regulator of bone turnover and that Wnt10b is a physiologically important osteoclast-secreted molecule that promotes bone formation in c-Kit mutants. Targeting c-Kit may provide a new insight to develop therapeutic intervention for skeletal disorders.
Materials and Methods
Animals
Wsh/Wsh, W/Wv and WBB6F1/J-Kit+/+ wildtype (WT) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Wsh/Wsh mice were crossed to C57BL6/J (Jackson Laboratory) to produce heterozygotes. Wsh/ + mice were then crossed to generate Wsh/Wsh mice and littermate controls. W/Wv and Wsh/Wsh mice are white, and black-eyed, whereas their controls are black. Male and female mice were fed standard mouse chow ad libitum and maintained under a 12:12 h light/dark cycle. Animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The experimental protocols were approved by the Institutional Animal Care and Use Committee at the Harvard Medical School.
Mice were subcutaneously injected with 20 mg/kg calcein (Sigma, St Louis, MO, USA) and 40 mg/kg demeclocycline (Sigma) and the interlabeling periods were 4, 5, and 6 days for 6-, 9-, and 13-week-old mice, respectively. At the end of the experiment, the mice were weighed and anesthetized with isoflurane. Blood samples were collected and centrifuged and the serum was kept at −80 °C for determination of P1NP and CTX. The seminal vesicles, tibiae, and femora were removed. The right femora and tibiae of W/Wv mice were fixed in 70% alcohol for μCT analysis and bone histomorphometry, respectively. For Wsh/Wsh mice, the left tibiae were used for μCT analysis, whereas the right tibiae were analyzed for bone histomorphometry. The left femora of Wsh/Wsh mice were frozen in liquid nitrogen and stored at −80 °C until processed for RNA isolation and qPCR analysis.
Histomorphometry
The proximal metaphyses of the right tibiae were dehydrated in acetone, infiltrated, and embedded without demineralization in methyl methacrylate. Undecalcified longitudinal 5 μm thick sections were cut on a Reichert-Jung Supercut 2165 microtome (Leica) and mounted unstained for dynamic measurements. Mineralizing surface per bone surface (MS/BS, %) and mineral apposition rate (MAR) were measured. Bone formation rate (BFR) was calculated as the product of MS/BS and MAR and expressed per bone surface (BFR/BS, (μm3/μm2/year), bone volume (BFR/BV, %/year) and tissue volume referent (BFR/TV, %/year). Consecutive sections were toluidine blue stained to quantify osteoblast number and osteoclast number. Bone volume per tissue volume (BV/TV, %), trabecular thickness (Tb.Th, μm), trabecular separation (Tb.Sp, μm), and trabecular number (Tb.N, /mm) were measured. Histomorphometric measurements were carried out using the OsteoMeasure system (OsteoMetric Inc.) and all parameters were expressed according to standardized nomenclature49.
μCT
A three-dimensional reconstruction of bone microarchitecture was performed using a desktop μCT35 (Scanco Medical) following the recommended guidelines50. Cortical bone at the tibial or femoral midshaft and cancellous bone at the proximal tibial metaphysis or femoral distal metaphysis were scanned using a 7-μm isotropic voxel size, 50 kVp, and 144 μA. For cortical bone, 86 transverse μCT slices were evaluated using a threshold at 35% of the maximal gray scale value to assess the total cross-sectional volume (mm3), cortical volume (mm3), marrow volume (mm3), and cortical thickness (mm). Cancellous bone was assessed in 464 transverse slices using a fixed threshold at 29% of the maximal gray scale value. Measurements included bone volume fraction (BV/TV, −), trabecular number (Tb.N,/mm), trabecular thickness (Tb.Th, mm) and trabecular separation (Tb.Sp, mm), connectivity density (ConnD,/mm3), and structural model index (SMI, −).
Osteoblast differentiation and proliferation and CFU assays
One-day old pups were genotyped by PCR using primer pair P1 (TTTGCACGTGCTAGTTACAC) and P2 (TTAAGATGGCACCCTGCTG) for WT template and primer pair P3 (AGGCTTGCAGCGCATTAT) and P4 (GAGGATTCATAGTTGTTCAATGTCC) for Wsh/Wsh template51. Primary calvarial osteoblasts derived from Wsh/Wsh mice and their control littermates were plated at 1 × 104 cells/well in 12-well plates and cultured in differentiation medium containing α-MEM supplemented with 10% FBS, 100 unit/ml penicillin and 100 μg/ml streptomycin, 5 mM β-glycerophosphate, 10 μM dexamethasone, and 50 μg/ml ascorbic acid. The cells were fixed with 3.7% formaldehyde, and stained for ALP and bone nodules with Fast Blue Alkaline phosphatase (Sigma) and 2% alizarin red (Sigma) on days 7 and 21, respectively. ALP activity and mineralized bone nodules were quantified as previously described52. For the DKK1 experiments, WT calvarial osteoblasts were plated at 0.5 × 104 cells/well in 24-well plates and cultured with osteoclast-conditioned medium. Recombinant DKK1 (R&D systems) at 0.2 μg/ml was added to the medium and ALP activity and mineralization bone nodules were determined. For the Wnt10b neutralization experiments, osteoclast-conditioned medium was pretreated with either isotype control or Wnt10b antibody (R&D) at 4 μg/ml before addition to the osteoblast cultures. The cells were stained with ALP and alizarin red.
Calvarial osteoblasts derived from Wsh/Wsh mice and controls (7 × 103 cells/well) were cultured in 96-well plates for 24 h and exposed to BrdU labeling reagent for 18 h. Osteoblast proliferation was determined by a BrdU incorporation assay per the manufacturer’s instructions (Cell Signaling). For CFU assays, calvarial osteoblasts derived from Wsh/Wshand WT littermates were plated at 0.25 × 104 cells/well in 12-well plates and cultured in differentiation medium. CFU-ALP was determined as ALP-positive colonies on day 7. Mineralized nodules were stained with alizarin red on day 23 for the determination of CFU-OB. The cells were then stained with toluidine blue to determine CFU-F on days 7 and 23 in the same tissue culture plate.
Preparation of osteoclasts
Bone marrow cells were cultured in α-MEM containing 10%FBS, 100 unit/ml penicillin and 100 μg/ml streptomycin for 24 h to generate BMMs. The BMMs were then cultured on tissue culture plastic or coverslips in α-MEM for 2 days with 20 ng/ml M-CSF and for an additional 6 days in the same medium with 20 ng/ml M-CSF and 3.3 ng/ml RANKL. Osteoclast-conditioned medium was collected. Multinucleated cells were identified by TRAP staining. For the resorption assay, osteoclasts were generated by culturing BMMs with primary CD1 calvarial osteoblasts in media containing 10 nM 1,25-dihydroxyvitamin D3 and 1 μM prostaglandin E2 on a collagen gel. The collagen was digested with 0.1% collagenase and the osteoclasts were replated onto dentin slices for an additional 48 h to determine their bone-resorbing activity using toluidine blue staining.
For the co-culture studies, calvarial osteoblasts derived from WT and mutants were cultured in α-MEM containing 10% FBS, 100 unit/ml penicillin and 100 μg/ml streptomycin for 24 h. BMMs were added and cultured in media containing 1,25-dihydroxyvitamin D3 and prostaglandin E2 for 5 additional days. TRAP staining was performed.
qPCR
Total RNA was isolated from femora using Trizol reagent according to the manufacturer’s instructions (Invitrogen) and purified using an RNeasy Mini kit (Qiagen). The RNA yields were determined spectrophotometrically at 260 nm. One μg of total RNA was used to synthesize cDNA using SuperScript VILO (Invitrogen). The qPCR was performed at 57 °C for 40 cycles using an iCycler (Biorad) and the results were normalized to GAPDH expression. The primer sequences used are shown in Supplementary Table S5.
FACS analysis
Bone marrow and spleen cells were removed and red blood cells were lysed with lysis buffer (eBioscience). The cells were incubated with Alexa Fluor® 488-conjugated anti-mouse CD11b (eBioscience) for 30 minutes at 4 °C and washed twice with washing buffer. The cells were incubated with PE-conjugated anti-mouse c-Fms (eBioscience) for 30 minutes and washed twice. The stained cells were suspended in PBS and flow cytometry was performed using BD LSRFortessa (Becton Dickinson).
Confocal microscopy
Osteoclasts plated on dentin slices were fixed with 3.7% formaldehyde in phosphate-buffered saline (PBS) for 10 min. Cells were permeabilized in PBS containing 0.05% saponin, 0.1% BSA and 5% normal serum for 30 min, incubated with anti-mouse/rat/human Wnt10b antibody (Santa Cruz) for 1 h, washed, incubated with fluorescent secondary antibody (Alexa Fluor 488), washed again, and mounted in FluorSave (Calbiochem). For actin labeling, the cells were incubated in a 1:40 dilution of rhodamine phalloidin stock solution (Invitrogen) for 1 h and washed with PBS. The nuclei were labeled with TO-PRO-3 (1:1000) in the rhodamine phalloidin solution. Osteoclasts were visualized using a 510 Meta laser scanning confocal microscope (Carl Zeiss) and images were recorded.
Western blot analysis
Osteoclasts were generated in a 6-well-plate and conditioned medium was collected and concentrated 50-fold using Amicon ultra centrifuge filter units (Millipore). Protein concentration was determined using a BCA protein assay kit (Thermo Scientific). Samples (200 μg) were resolved using 4–12% Mini-PROTEAN TGX precast gel (Biorad) and transferred to nitrocellulose membranes. The membranes were stained with Ponceau S dye for protein detection then washed in TBST buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% Tween 20) and incubated in TBST buffer containing anti-Wnt10b antibody overnight at 4 °C. The membranes were washed in TBST and incubated with anti–rabbit IgG horseradish peroxidase–conjugated secondary antibody (Promega). The membranes were then washed and developed with an enhanced chemiluminescence detection kit (PerkinElmer, Inc.).
Serum analysis
Serum P1NP and CTX were determined using Rat/Mouse P1NP and RatLapsTM EIA kit, respectively per the manufacturer’s protocol (Immunodiagnostic systems). Serum testosterone concentration was measured by enzyme-linked immunosorbent assay (ELISA) per the manufacturer’s directions (R&D Systems).
Statistical analysis
All data are expressed as the mean ± SE. Unpaired Student’s t-test was used to compare between 2 groups. Multiple comparisons were determined using one-way ANOVA followed by Fisher’s protected least significant difference test. The in vitro experiments were repeated three times. Statistical significance was considered at p < 0.05.
Additional Information
How to cite this article: Lotinun, S. and Krishnamra, N. Disruption of c-Kit Signaling in KitW-sh/W-sh Growing Mice Increases Bone Turnover. Sci. Rep. 6, 31515; doi: 10.1038/srep31515 (2016).
Supplementary Material
Acknowledgments
We thank Drs Roland Baron and William C. Horne for their helpful discussion and suggestion during the study and Lynn Neff for immunohistochemistry. We also thank Dr. Kevin Tompkins for a careful reading of the manuscript. This work was supported by the National Institutes of Dental and Craniofacial Research grant (R03 DE019819) and the Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University (CU-56-(641)-HR) to S. Lotinun.
Footnotes
Author Contributions S.L. designed and performed the experiments. S.L. analyzed data and wrote the manuscript. S.L. and N.K. discussed the results and revised the manuscript.
References
- Chabot B., Stephenson D. A., Chapman V. M., Besmer P. & Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature 335, 88–89 (1988). [DOI] [PubMed] [Google Scholar]
- Qiu F. H. et al. Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family–oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J 7, 1003–1011 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. et al. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell 63, 225–233 (1990). [DOI] [PubMed] [Google Scholar]
- Rajaraman S. et al. An allelic series of mutations in the kit ligand gene of mice. I. Identification of point mutations in seven ethylnitrosourea-induced Kitl(Steel) alleles. Genetics 162, 331–340 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besmer P. et al. The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. Dev Suppl, 125–137 (1993). [PubMed] [Google Scholar]
- Akin C. et al. Effects of tyrosine kinase inhibitor STI571 on human mast cells bearing wild-type or mutated c-kit. Exp Hematol 31, 686–692 (2003). [DOI] [PubMed] [Google Scholar]
- Gattei V. et al. Human osteoclasts and preosteoclast cells (FLG 29.1) express functional c-kit receptors and interact with osteoblast and stromal cells via membrane-bound stem cell factor. Cell Growth Differ 7, 753–763 (1996). [PubMed] [Google Scholar]
- Morrison-Graham K. & Weston J. A. Mouse mutants provide new insights into the role of extracellular matrix in cell migration and differentiation. Trends Genet 5, 116–121 (1989). [DOI] [PubMed] [Google Scholar]
- Nocka K. et al. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J 9, 1805–1813 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocka K. et al. Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice–evidence for an impaired c-kit kinase in mutant mice. Genes Dev 3, 816–826 (1989). [DOI] [PubMed] [Google Scholar]
- Tsai M. et al. The rat c-kit ligand, stem cell factor, induces the development of connective tissue-type and mucosal mast cells in vivo. Analysis by anatomical distribution, histochemistry, and protease phenotype. J Exp Med 174, 125–131 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M. et al. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc Natl Acad Sci USA 88, 6382–6386 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle D. L., Kozak C. A., Mano H., Chapman V. M. & Bucan M. Physical mapping of the Tec and Gabrb1 loci reveals that the Wsh mutation on mouse chromosome 5 is associated with an inversion. Hum Mol Genet 4, 2073–2079 (1995). [DOI] [PubMed] [Google Scholar]
- Berrozpe G. et al. A distant upstream locus control region is critical for expression of the Kit receptor gene in mast cells. Mol Cell Biol 26, 5850–5860 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrozpe G. et al. The W(sh), W(57), and Ph Kit expression mutations define tissue-specific control elements located between -23 and -154 kb upstream of Kit. Blood 94, 2658–2666 (1999). [PubMed] [Google Scholar]
- Duttlinger R. et al. W-sash affects positive and negative elements controlling c-kit expression: ectopic c-kit expression at sites of kit-ligand expression affects melanogenesis. Development 118, 705–717 (1993). [DOI] [PubMed] [Google Scholar]
- Lotinun S., Evans G. L., Turner R. T. & Oursler M. J. Deletion of membrane-bound steel factor results in osteopenia in mice. J Bone Miner Res 20, 644–652 (2005). [DOI] [PubMed] [Google Scholar]
- Iwaniec U. T. & Turner R. T. Failure to generate bone marrow adipocytes does not protect mice from ovariectomy-induced osteopenia. Bone 53, 145–153 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. R. et al. White spotting variant mouse as an experimental model for ovarian aging and menopausal biology. Menopause 19, 588–596 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Dobrinski I., Avarbock M. R. & Brinster R. L. Transplantation of male germ line stem cells restores fertility in infertile mice. Nat Med 6, 29–34 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J. et al. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J 25, 5840–5851 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson L., Ruan M., Westendorf J. J., Khosla S. & Oursler M. J. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci USA 105, 20764–20769 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C. et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab 4, 111–121 (2006). [DOI] [PubMed] [Google Scholar]
- Takeshita S. et al. Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J Clin Invest 123, 3914–3924 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi-Koga T. et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med 17, 1473–1480 (2011). [DOI] [PubMed] [Google Scholar]
- Ota K. et al. TGF-beta induces Wnt10b in osteoclasts from female mice to enhance coupling to osteoblasts. Endocrinology 154, 3745–3752 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Franca L. R. et al. Sertoli cells in testes containing or lacking germ cells: a comparative study of paracrine effects using the W (c-kit) gene mutant mouse model. Anat Rec 240, 225–232 (1994). [DOI] [PubMed] [Google Scholar]
- Weinstein R. S., Jilka R. L., Parfitt A. M. & Manolagas S. C. The effects of androgen deficiency on murine bone remodeling and bone mineral density are mediated via cells of the osteoblastic lineage. Endocrinology 138, 4013–4021 (1997). [DOI] [PubMed] [Google Scholar]
- Weinstein R. S. et al. The skeletal effects of glucocorticoid excess override those of orchidectomy in mice. Endocrinology 145, 1980–1987 (2004). [DOI] [PubMed] [Google Scholar]
- Liu P. Y. et al. Genetic and hormonal control of bone volume, architecture, and remodeling in XXY mice. J Bone Miner Res 25, 2148–2154 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. P., Baker S. U., Eisman J. A. & Gardiner E. M. Changing RANKL/OPG mRNA expression in differentiating murine primary osteoblasts. J Endocrinol 170, 451–460 (2001). [DOI] [PubMed] [Google Scholar]
- Boyce B. F. & Xing L. The RANKL/RANK/OPG pathway. Curr Osteoporos Rep 5, 98–104 (2007). [DOI] [PubMed] [Google Scholar]
- Arai F. et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med 190, 1741–1754 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariban E., Mitchell T. & Kufe D. Expression of the c-fms proto-oncogene during human monocytic differentiation. Nature 316, 64–66 (1985). [DOI] [PubMed] [Google Scholar]
- van’t Hof R. J., von Lindern M., Nijweide P. J. & Beug H. Stem cell factor stimulates chicken osteoclast activity in vitro. FASEB J 11, 287–293 (1997). [DOI] [PubMed] [Google Scholar]
- Savage D. G. & Antman K. H. Imatinib mesylate–a new oral targeted therapy. N Engl J Med 346, 683–693 (2002). [DOI] [PubMed] [Google Scholar]
- Dewar A. L. et al. Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood 105, 3127–3132 (2005). [DOI] [PubMed] [Google Scholar]
- Ando W. et al. Imatinib mesylate inhibits osteoclastogenesis and joint destruction in rats with collagen-induced arthritis (CIA). J Bone Miner Metab 24, 274–282 (2006). [DOI] [PubMed] [Google Scholar]
- Dewar A. L. et al. Imatinib as a potential antiresorptive therapy for bone disease. Blood 107, 4334–4337 (2006). [DOI] [PubMed] [Google Scholar]
- Grey A., O’Sullivan S., Reid I. R. & Browett P. Imatinib mesylate, increased bone formation, and secondary hyperparathyroidism. N Engl J Med 355, 2494–2495 (2006). [DOI] [PubMed] [Google Scholar]
- O’Sullivan S. et al. Decreased bone turnover despite persistent secondary hyperparathyroidism during prolonged treatment with imatinib. J Clin Endocrinol Metab 94, 1131–1136 (2009). [DOI] [PubMed] [Google Scholar]
- Grimbaldeston M. A. et al. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol 167, 835–848 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tono T. et al. c-kit Gene was not transcribed in cultured mast cells of mast cell-deficient Wsh/Wsh mice that have a normal number of erythrocytes and a normal c-kit coding region. Blood 80, 1448–1453 (1992). [PubMed] [Google Scholar]
- Yamazaki M. et al. C-kit gene is expressed by skin mast cells in embryos but not in puppies of Wsh/Wsh mice: age-dependent abolishment of c-kit gene expression. Blood 83, 3509–3516 (1994). [PubMed] [Google Scholar]
- Chiappetta N. & Gruber B. The role of mast cells in osteoporosis. Semin Arthritis Rheum 36, 32–36 (2006). [DOI] [PubMed] [Google Scholar]
- Turner R. T., Iwaniec U. T., Marley K. & Sibonga J. D. The role of mast cells in parathyroid bone disease. J Bone Miner Res 25, 1637–1649 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan S. et al. Imatinib promotes osteoblast differentiation by inhibiting PDGFR signaling and inhibits osteoclastogenesis by both direct and stromal cell-dependent mechanisms. J Bone Miner Res 22, 1679–1689 (2007). [DOI] [PubMed] [Google Scholar]
- Roser-Page S., Vikulina T., Zayzafoon M. & Weitzmann M. N. CTLA-4Ig-induced T cell anergy promotes Wnt-10b production and bone formation in a mouse model. Arthritis Rheumatol 66, 990–999 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster D. W. et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28, 2–17 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouxsein M. L. et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25, 1468–1486 (2010). [DOI] [PubMed] [Google Scholar]
- Nigrovic P. A. et al. Genetic inversion in mast cell-deficient (Wsh) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol 173, 1693–1701 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotinun S. et al. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J Clin Invest 123, 666–681 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.