Abstract
Objective: To examine glutathione S-transferase (GST) deletion polymorphisms in development of early-onset severe mental disorders, with the hypothesis that patients with GSTM1-null and GSTT1-null genotypes will develop psychotic disorders at a younger age.
Methods: We identified GSTM1 and GSTT1 deletion polymorphisms by multiplex polymerase chain reaction (PCR) in 93 patients with early onset severe mental disorders and 278 control individuals. The diagnoses were confirmed by Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version and Schedule for Affective Disorders and Schizophrenia–Life-Time Version (K-SADS-PL) interviews.
Results: Individuals with the GSTM1-null genotype were at 3.36-fold higher risk of developing early-onset severe mental disorders than carriers of a corresponding active genotype. The risk of those disorders was increased by 6.59-fold in patients with GSTM1-null/GSTT1-active genotype. Patients with the GSTM1-null genotype were at approximately 2-fold increased risk for developing early-onset schizophrenia-spectrum disorder (EOS), early-onset bipolar disorder (EOBD) with psychotic symptoms, or early-onset first-episode psychosis (EOFEP), compared with patients with the GSTM1-active genotype.
Conclusion: The GSTM1-null genotype might be associated with higher risk for early onset severe mental disorders.
Keywords: schizophrenia, bipolar disorder, psychotic disorders, glutathione transferases, genetic polymorphisms, risk factor
Identification and evaluation of early-onset psychotic and bipolar disorders is an important health concern. Because these disorders may be transient, intermittent, and short-term, or part of a long-term psychiatric condition, recognition of risk factors, early detection, and intervention might improve the outcome in patients with these conditions.
Schizophrenia is a chronic, degenerative disorder of the brain characterized by positive (hallucinations, delusions) and/or negative symptoms (decreased motivation, socialization, cognitive dysfunction). The high heritability of schizophrenia has been well established in twin and adoption studies.1 The onset of schizophrenia typically occurs in early adulthood. Childhood onset is relatively rare, with approximately 1 child in 10,000 affected in the general population; the prevalence of schizophrenia increases in adolescence and peaks in early adulthood. The definition of early-onset schizophrenia (EOS), or adolescent-onset schizophrenia, varies among studies and most commonly refers to schizophrenia with onset before the age of 17 years to 21 years. EOS is associated with higher familial risk, a higher ratio of males affected, and fewer offspring per proband.2-4 Another important early-onset entity, which is often the first of multiple episodes in chronic schizophrenia, is early-onset first-episode psychosis (EOFEP). Further, early-onset bipolar disorders (EOBPs) also share common features with schizophrenia, including cognitive characteristics, environmental factors, premorbid developmental impairments, neurological signs and heritability, and changes in antioxidant status.5,6
Several lines of evidence suggest increased oxidative stress in patients with schizophrenia.7,8 The findings of some genetic association and gene expression studies suggest that, due to polymorphisms in genes encoding antioxidant enzymes, patients with schizophrenia might have altered ability to enact antioxidant mechanisms.9-12 Several promising candidate genes, including those that encode enzymes involved in glutathione (GSH) metabolism, have been suggested.10-14 GSH is the key element of antioxidant defense mechanisms in the brain.15 Gawryluk and associates found decreased levels of GSH in postmortem samples of prefrontal cortex tissue in patients with bipolar disorder and schizophrenia.16 It has been suggested that these decreased levels may occur due to the reduction in glutathione S-transferase (GST) activity in these brain regions.17 GST family members play a dual role because they are able to detoxify numerous xenobiotics and possess strong antioxidant activity towards reactive oxygen species (ROSs).18 Human GSTs consist of 3 families: cytosolic, mitochondrial, and membrane-associated proteins in eicosanoid and glutathione metabolism (membrane-associated proteins in eicosanoid and glutathione metabolism [MAPEG] family). Cytosolic GSTs are further categorized into 7 major classes according to their amino-acid sequence: alpha (5 members), mu (5 members), pi (1 member), theta (2 members), zeta (1 member), omega (2 members), and sigma (1 member) subfamilies. Although members of the same class possess more than 40% amino-acid sequence identity, less than 25% of sequence identity exists among classes.19 Almost all members of the GST family exhibit genetic polymorphism, resulting in the complete lack or lowering of enzyme activity.20 Due to the homozygous deletion of the GSTM1 gene (NM_000561; GSTM1-null genotype) or GSTT1 gene (NM_0008553.3; GSTT1-null genotype), approximately 50% or 20% of white individuals lack GSTM1 or GSTT1 enzyme activity, respectively.21 Specifically, the null genotypes have both alleles missing, causing the gene to become nonfunctional.
Apart from Gawryluk et al, who report that GSTM1 activity was significantly reduced in patients with schizophrenia compared with control individuals, several other population studies have implicated GST gene polymorphisms in the pathogenesis of schizophrenia.22‐27 The contradictory findings that researchers have obtained so far indicate that other factors, such as ethnicity, should also be considered when assessing risk factors in schizophrenia.28
Apart from patients with SCH, the data on GST polymorphism in patients with psychotic or bipolar disorder in whites are scarce. Thus, in this study, we examined the role of deletion polymorphisms in GSTM1 and GSTT1 genes in the development of early-onset psychotic and bipolar disorders. To our knowledge, this study is among the first to examine GST polymorphisms in early-onset mental disorders. We hypothesize that patients with GSTM1-null and GSTT1-null genotypes will be more susceptible to oxidative stress and prone to developing psychotic disorders at a younger age, compared with their peers who possess other genotypes.
Materials and Methods
Study Subjects
The study was conducted with 93 patients (31 female, 62 male; average age, 21.18 [6.68] years) diagnosed with early-onset severe mental disorders. Our sample included 3 different entities: EOS-spectrum disorder, EOBD with psychotic symptoms, and EOFEP. In the EOS and EOBD groups, we included adult patients with diagnoses verified before the age of 18 years. For the EOFEP group, we included patients younger than 18 years whose disorder had manifested for the first time.
Subjects were recruited as consecutive referrals from the Institute of mental health, University psychiatry clinic, Belgrade, Serbia between June 1, 2009 and December 31, 2011. The inclusion criterion was the presence of schizophrenia, bipolar disorder with psychotic symptoms, and first psychotic episode that had started before the age of 18 years. The diagnosis was verified by the International Classification of Diseases, Revision 10 (ICD-10)29 and the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV)30 criteria confirmed by a referring pediatric/adolescent psychiatrist or adult psychiatrist, through clinical interview with the parent and examination of the child or adolescent, or interviewing only the patient if he or she was older than 18 years.
The control group consisted of 278 healthy control subjects (95 female, 183 male; average age, 31.60 [4.46] years), matched for sex, age, ethnicity, and geographic origin. Although the average age in the control group seems to be higher compared with the study group, statistical significance was not reached (P> .05); this lack of statistical significance, however, is unimportant because EOS, EOBP, and EOFEP are all early-onset disorders. On a practical level, this means that controls who did not have a psychiatric diagnosis up to the moment of their inclusion in the study did not develop any of the examined entities. After informed consent was obtained, subjects were interviewed using a standard questionnaire to collect information, including demographic characteristics, presence of mental disorders, previous hospitalizations, and pharmacotherapy undergone. Only subjects without any previous mental disorders were included in the control group.
The Institutional Ethics Committee of Institute of Mental Health approved this study, and the research was carried out in accordance with the International Code of Medical Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Before participating, all participants gave written informed consent. For participants younger than 18 years, written informed consent was obtained from their parent(s) or legal guardian(s).
Assessment Tools
We assessed the participants younger than 18 years by using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime version (K-SADS–PL), which generates reliable and valid psychiatric diagnoses for children and adolescents. This tool is a semistructured diagnostic interview designed to assess current and past episodes of psychopathologic manifestations in children and adolescents, according to the Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition–Revised (DSM-III-R) and DSM-IV criteria.31
For participants older than 18 years but for whom there was no clear evidence in their medical records that their illness had started before the age of 18 years, we assessed them using the Schedule for Affective Disorders and Schizophrenia Lifetime (SADS-L). This tool is a semistructured diagnostic interview that assesses current and lifetime history of Axis I disorders.25,31
DNA Extraction and Genotyping
We extracted DNA from blood leucocytes using the QIAGEN QIAamp Mini Kit (Qiagen, Inc.). The DNA purification is performed in 4 steps, using spin columns in a standard microcentrifuge. First, the lysate buffering conditions are adjusted to allow optimal binding of the DNA to the spin-column membrane. After loading the specimen, DNA is adsorbed onto the silica membrane during a brief centrifugation, followed by 2 washing steps undertaken to remove residual contaminants. In the final step, purified DNA in a concentrated form is eluted from the spin column using an elution buffer; the resulting product is suitable for direct use in polymerase chain reaction (PCR).
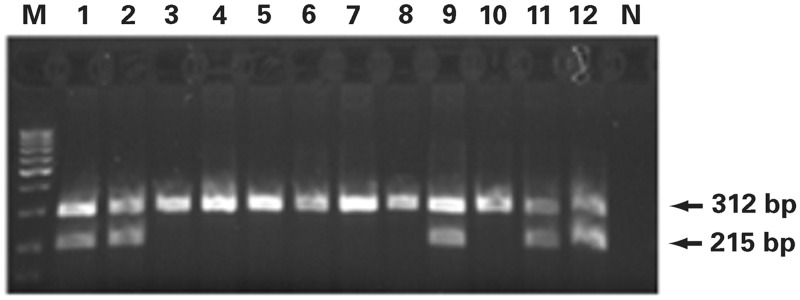
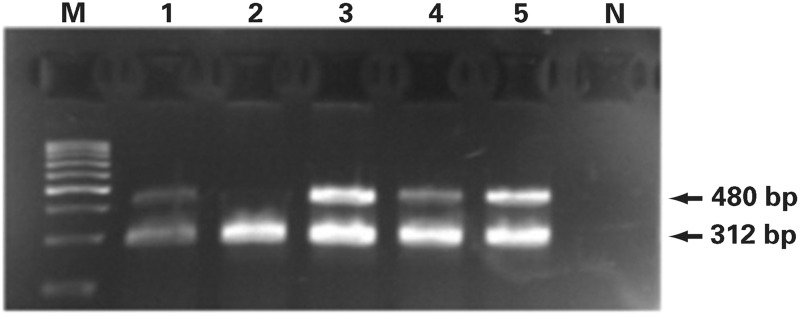
We performed GSTM1 and GSTT1 genotyping via PCR (Image 1), as described by Garcia-Closas32 and Pemble and Taylor,33 respectively. GSTM1 genotyping was performed by multiplex PCR; the primers we used were as follows: GSTM1 forward: 5′-CTGCCCTACTTGATTGATGGG-3′ and GSTM1 reverse: 5′-CTGGATTGTAGCAGATCATGC-3 (isolated DNA (100-150 ng) was amplified in a total volume of 25-μl reaction mixture containing 20 pmol of primers, commercial Master Mix, and water (Thermo Fisher Scientific, Inc) and subjected to initial denaturation at 94 °C for 4 minutes, followed by 33 cycles at 94° C for 2 minutes, 59 °C for 1 minute, and 72 °C for 1 minute. We performed the final extension at 72 °C for 10 minutes. The PCR products were analyzed in 2% agarose gels, electrophoresed for approximately 20 minutes (125V constant, 0.27A, 50W) at room temperature, and visualized using ethidium bromide staining (Image 2). The assay does not distinguish heterozygous or homozygous wild type genotypes and therefore detects the presence (at least 1 allele present, homozygote or heterozygote) or the absence (complete deletion of both alleles, homozygote) of the genotype. As a result, the GSTM1-active genotype was detected by the band at 215 bp, and the absence of this particular band was indicative of the GSTM1-null genotype. GSTT1 genotyping also was performed by multiplex PCR, using GSTT1-forward: 5′-TTCCTTACTGGTCCTCACATCTC-3′ and GSTT1-reverse: 5′-TCACCGGATCATGGCCAGCA-3′ primers (Figure 1) under the same thermal cycler and gel conditions as for GSTM1 genotyping. Similarly, because the assay does not distinguish between heterozygous or homozygous wild-type genotypes, the presence of 480-bp bands was indicative for the GSTT1-active genotype and the absence of the GSTT1-null genotype (Figure 2). Exon 7 of the CYP1A1 housekeeping gene was coamplified in GSTM1 and GSTT1 genotyping and was used as an internal control for the presence of the amplifiable DNA. We used the following primers: CYP1A1 forward: 5′-GAACTGCCACTTCAGCTGTCT-3′ and CYP1A1 reverse: 5′-CAGCTGCATTTGGAAGTGCTC-3′; the CYP1A1 PCR product corresponded to 312 bp.
Image 1.
A 2% agarose gel electrophoretogram. PCR products of the GSTM1 gene. Lanes 1, 2, 9, 11 and 12 are patients with the GSTM1 active genotype (215 bp band) and lanes 3 through 8 with lane 10 represent the GSTM- null genotype; 312bp band represents the CYP1A1 housekeeping gene, used as internal control for amplifiable DNA; M, DNA Q2 marker; N, negative control without a DNA content.
Image 2.
A 2% agarose gel electrophoretogram. PCR products of the GSTT1 gene. Lanes 1, 3, 4 and 5 are patients with the GSTT1 active genotype (480bp band) and lane 2 represents the GSTT1- null genotype; 312bp band represents the CYP1A1 housekeeping gene, used as internal control for amplifiable DNA; M, DNA Q2 marker; N, negative control without DNA content.
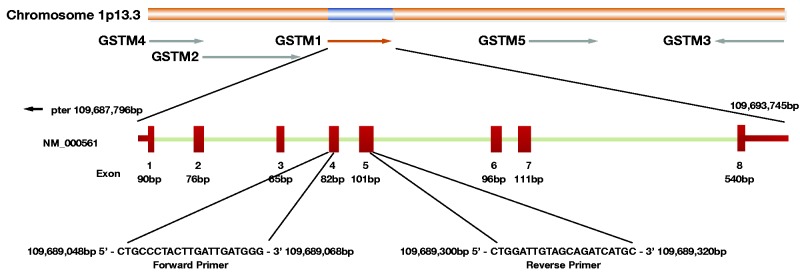
Figure 1.
The GSTM1 gene is situated at chromosome 1 (1p13.3), composed of 8 exons, spanning a region of 21,244 bases. GSTM1 null allele arose from a recombination event resulting in deletion of a 20-kb segment. This deletion produces a novel 7.4-kb HindIII fragment with the loss of 10.3- and 11.4-kb HindIII fragments, hence homozygotes for GSTM1 null allele produce no GSTM1 protein. The end points of the polymorphic GSTM1 deletion are: the left repeated region 5 kb downstream from the 3’-end of the GSTM2 gene and 5 kb upstream from the beginning of the GSTM1 gene; the right repeated region 5 kb downstream from the 3’-end of the GSTM1 and 10 kb upstream from the 5’-end of the GSTM5 gene.
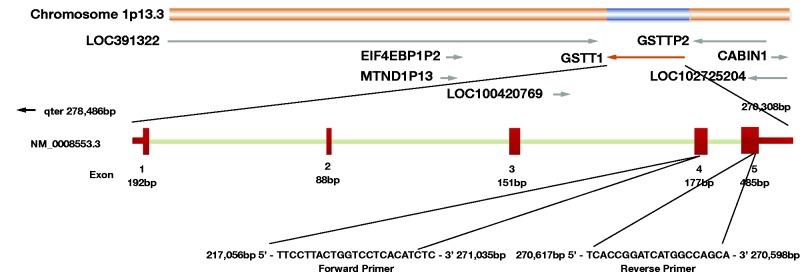
Figure 2.
The GSTT1 gene is situated at chromosome 22 (22q11.23), and composed of 5 exons, spanning a region of 8,179 bases. PCR mapping and sequencing revealed a 54251 bp fragment including GSTT1 to be deleted from chromosome 22, most likely by a homologous recombination event between 2 highly homologous sequence stretches that flank GSTT1.
Statistical Analysis
We performed statistical analysis using Statistical Package for the Social Sciences software, version 20.0 (SPSS Inc). In descriptive statistics, we summarized continuous variables by mean (SD). The relative associations between the studied genotypes and early-onset severe mental disorders were evaluated by multinomial logistic regression to calculate odds ratios (ORs) and 95% confidence intervals (CIs). We compared the distributions of GSTM1 and GSTT1 genotypes between case individuals and controls, as well as different types of early onset severe mental disorders, using the χ2 test.
Results
Baseline characteristics of controls and patients with early-onset severe mental disorders are presented in Table 1. There was no statistically significant difference between case and control subjects regarding age and sex distribution (P> .05 for each). Among 93 patients included in the study, 49 had EOS (53%), 12 had EOBP (13%), and 32 EOFEP (34%). To avoid diagnostic instability, the diagnostic spectra were confirmed after 1 year of follow-up. Most of the patients were hospitalized and treated by pharmacotherapy (96% and 98%, respectively).
Table 1.
Baseline Characteristics of Control Individuals and Patients With Early Onset Severe Mental Disorders
| Characteristic | Casesa | Controlsb |
|---|---|---|
| Age (y), mean (SD) | 21.18 (6.68) | 31.60 (4.46) |
| Sex, no. (%) | ||
| Male | 62 (66) | 183 (66) |
| Female | 31 (33) | 95 (34) |
| Age at diagnosis (y), mean (SD) | 16.07 (4.73) | NA |
| Disorder type, no. (%) | ||
| EOS | 49 (53) | NA |
| EOBD | 12 (13) | NA |
| EOFEP | 32 (34) | NA |
| Hospitalized | ||
| Yes | 89 (96) | 0 |
| No | 4 (4) | 278 (100) |
| Pharmacotherapy | ||
| Yes | 91 (98) | 0 |
| No | 2 (2) | 278 (100) |
| Epilepsy | ||
| Yes | 1 (1) | 0 |
| No | 92 (99) | 278 (100) |
EOS, early-onset schizophrenia spectrum disorder; EOBD, early-onset bipolar disorder; EOFEP, early-onset first-episode psychosis; NA, not applicable.
an = 93.
bn = 278.
We conducted genotyping all recruted patients and controls. Distribution of individual GSTM1 and GSTT1 genotypes, as well as combined GST genotypes in controls and patients with early-onset psychotic mental disorders, are shown in Table 2. The GSTM1-null genotype was more frequent among patients (57%) compared with controls (49%), with an adjusted OR of 3.36 (95% CI, 0.99-11.33; P> .046). However, no significant difference was observed in the distribution of GSTT1 gene variants between patients and controls (P> .05).
Table 2.
Distribution of Individual and Combined GSTM1 and GSTT1 Genotypes in Patients and Control Individuals
| GST Genotype | Patients, No. (%)a | Controls, No. (%)b | OR | CI (95%) | P Value |
|---|---|---|---|---|---|
| GSTM1 | |||||
| activec | 40 (43) | 141 (51) | 1.00 [reference] | ||
| nulld | 53 (57) | 137 (49) | 3.36 | .99-11.33 | .046 |
| GSTT1 | |||||
| nulld | 22 (24) | 65 (23) | 1.00 [reference] | ||
| activec | 71 (76) | 213 (77) | 2.96 | .71-12.22 | .13 |
| Combined | |||||
| GSTM1 active/GSTT1 null | 8 (9) | 42 (15) | 1.00 [reference] | ||
| GSTM1 active/GSTT1 active | 32 (34) | 99 (36) | 2.62 | .32-11.18 | .37 |
| GSTM1 null/GSTT1 null | 14 (15) | 23 (8) | 9.41 | .52-24.94 | .12 |
| GSTM1 null/GSTT1 activec | 39 (42) | 114 (41) | 6.59 | .95-25.65 | >.05 |
GST, glutathione S-transferase; CI, confidence interval; OR, odds ratio, adjusted for age and sex.
an = 93.
bn = 278.
cActive (present) if at least one active allele is present.
dInactive (null) if no active alleles are present.
When we analyzed the association of combined GSTM1/GSTT1 gene variants with the risk for early-onset severe mental disorders, the risk of developing those disorders was increased 6.59-fold in patients with GSTM1-null/GSTT1-active genotype; this result was found to be marginally statistically insignificant (OR, 6.59; 95% CI, .95-25.65; P> .05). The risk was also increased 9.41-fold in patients with the GSTM1-null/GSTT1-null genotype, although it was statistically insignificant (OR, 9.41; 95% CI, .52-24.94; P = .12) (Table 2).
In an attempt to verify whether GST polymorphisms might be considered a risk factor for any group of disorders individually, we analyzed distribution of individual GSTM1 and GSTT1 genotypes, as well as combined GST genotypes, in patients with EOS, EOBP, and EOFEP compared with controls (Table 3). The results of our analysis showed that patients with the GSTM1-null genotype were at approximately 2-fold increased risk for developing EOS, EOBP, and EOFEP (OR = 1.86, 95% CI = .74-4.66, P = .18; 2.33, .58-9.37, P = .23; and 2.24, .72-6.95, P = .16, respectively), compared with patients with GSTM1-active genotype, although these results did not reach statistical significance. However, similar effects were not observed in case of GSTT1 polymorphism.
Table 3.
Individual and Combined GSTM1 and GSTT1 Genotypes as Risk Factors for EOS, EOBP, and EOFEP
|
Disorder
Type |
|||
|---|---|---|---|
| GST Genotype | EOS | EOBP | EOFEP |
| GSTM1 active a | |||
| Ca/Co | 22/141 | 5/141 | 13/141 |
| OR (95% CI) | 1.00 [reference] | 1.00 [reference] | 1.00 [reference] |
| P value | |||
| GSTM1 nullb | |||
| Ca/Co | 28/137 | 7/137 | 18/137 |
| OR (95% CI) | 1.86 (.74-4.66) | 2.33 (.58-9.37) | 2.24 (.72-6.95) |
| P value | .18 | .23 | .16 |
| GSTT1 nullb | |||
| Ca/Co | 9/65 | 4/65 | 9/65 |
| OR (95% CI) | 1.00 [reference] | 1.00 [reference] | 1.00 [reference] |
| P value | |||
| GSTT1 activea | |||
| Ca/Co | 41/213 | 8/213 | 22/213 |
| OR (95% CI) | 1.65 (.53-5.09) | .42 (.90-2.05) | 1.00 (.27-3.69) |
| P value | .38 | .28 | .99 |
| GSTM1 active/GSTT1 null | |||
| Ca/Co | 4/42 | 2/42 | 2/42 |
| OR (95% CI) | 1.00 [reference] | 1.00 [reference] | 1.00 [reference] |
| P value | |||
| GSTM1 active/GSTT1 active | |||
| Ca/Co | 18/99 | 3/99 | 11/99 |
| OR (95% CI) | 1.43 (.32-6.43) | .75 (.08-6.08) | 2.10 (.33-13.34) |
| P value | .63 | .80 | .42 |
| GSTM1 null/GSTT1 null | |||
| Ca/Co | 5/23 | 2/23 | 7/23 |
| OR (95% CI) | 2.52 (.29-22.17) | 2.36 (.24-24.32) | 5.16 (.51-51.53) |
| P value | .39 | .47 | .16 |
| GSTM1 null/GSTT1 active | |||
| Ca/Co | 23/114 | 5/114 | 11/114 |
| OR (95% CI) | 2.9 (.65-12.90) | .67 (.11-4.05) | 4.16 (.60-28.68) |
| P value | .160 | .67 | .14 |
GST, glutathione S-transferase; EOS, early-onset schizophrenia spectrum disorder; EOBD, early-onset bipolar disorder; EOFEP, early-onset first-episode psychosis; Ca/Co, no. of case vs control individuals; OR, odds ratio, adjusted for age and sex; CI, confidence interval
aActive (present) if at least one active allele is present.
bInactive (null) if no active alleles present.
When we analyzed the effect of combined GST genotypes, the highest risk was observed in the group of patients with EOFEP carrying a combination of GSTM1-null/GSTT1-active genotypes (OR, 4.16; 95% CI, .60-28.68; P = .14) and GSTM1-null/GSTT1-null genotypes (OR, 5.16; 95% CI, 0.51-51.53; P = .16), although these results were statistically insignificant. However, patients with EOS who are carriers of GSTM1-null/GSTT1-active and GSTM1-null/GSTT1-null exhibited more than 2.5-fold increased risk for disease development, whereas in patients with EOBP, increased risk was observed only in carriers of both null genotypes (Table 3).
Discussion
The results of this study showed that the GSTM1-null genotype is more frequent among patients compared with controls. Apart from the independent association of GSTM1-null genotype with the risk of early-onset mental disorders, the risk of those disorders was also increased in patients with the combined GSTM1-null/GSTT1-active genotype. Further, patients with GSTM1-null genotype exhibited higher risk for developing EOS, EOBP, and EOFEP, suggesting a possible association of the GSTM1-null genotype with higher risk for developing psychotic disorders at a young age.
It has been suggested that oxidative stress might be implicated in the pathophysiology of schizophrenia.2,7 Apart from the antioxidant-enzyme defense mechanisms, which seem to be decreased in patients with EOS, glutathione deficit might also be implicated in the pathogenesis of this type of psychotic disorder.16,17,22,34 GSH is the main nonenzymatic cellular antioxidant and it plays a critical role in cellular protection from ROS-induced damage.35 Further, this tripeptide is involved in the disposal of peroxides by brain cells and protection against ROS, although its content strongly depends on the availability of its precursors.36 The cytosolic GST family catalyzes the conjugation of electrophilic compounds, including products of oxidative stress, with GSH.18 Although several types of allelic variations have been identified within GST classes, GSTM1 gene has received the most attention in genetic epidemiologic studies in patients with schizophrenia.24,28,37‐39 Some study results suggested increased risk associated with the GSTM1-null genotype,26,37,39 whereas other studies found no association.12,25 To our knowledge, our results on the independent contribution of the GSTM1 polymorphism towards the risk of early-onset schizophrenia are among the first in the literature because most previous studies on similar topics were conducted in adult subjects. Also, evaluation of the contribution of GSTM1 common copy number variation (CNV) to vulnerability towards schizophrenia also received a lot of attention.28,40 Although Tam et al have indicated that CNVs confer an increased susceptibility to schizophrenia,41 obtained results on the contribution of GSTM1 that preceded and followed these findings have been inconsistent.12,28,37‐39
Genetic polymorphism of another widely investigated GST polymorphism, GSTT1, was found to be associated with decreased or increased risk of schizophrenia, or in some cases showed lack of any effect. We have shown that individual GSTT1 polymorphism does not independently contribute to the risk of schizophrenia. These findings are in agreement with those of Matsuzawa et al12 However, Raffa et al suggested the GSTT1 gene as a candidate gene for susceptibility to schizophrenia in a Tunisian population.25 This finding was supported by the findings of Kashani et al, who suggested that lack of GSTT1 function, meaning the GSTT1-null genotype, might increase the risk of schizophrenia.26 Also, Saadat et al have found that the GSTT1-null genotype was associated with significantly reduced risk of developing schizophrenia, proposing the GSTT1-active genotype as a candidate gene for susceptibility to schizophrenia.42
Results obtained in analysis of the combined effect of GSTM1 and GSTT1 genotypes are, again, inconsistent. Our study has shown, however, with marginal statistical significance, that the risk of developing early-onset mental disorders was increased 6.59-fold in patients with the GSTM1-null/GSTT1-active genotype. This result is in agreement with the findings of Kashani et al, who suggested that the impairment in the function of GSTs may increase the risk of schizophrenia.26 The role of GSTs as phase II biotransformation enzymes may provide a possible explanation for this finding—specifically, under physiological conditions, GST Mu-class catalysis conjugation of catechol o-quinones with glutathione.37 Reduced or lacking activity of these enzymes may lead to an excess of neurotoxic compounds of catecholamine o-quinones, which may contribute to the development of certain forms of schizophrenia.37
However, not all reactions catalyzed by GSTs will result in detoxification. In particular, the GST Theta class is responsible for bioactivation of certain toxic and mutagenic compounds and drugs.43 However, the role of GSTs in antioxidant defense should not be forgotten because oxidative stress has been suggested as a potential mechanism underlying the development of schizophrenia. Gravina et al have indicated that combination of different GST polymorphisms has a role in predisposition to schizophrenia, probably due to affecting the capacity of the cell to detoxify the oxidized metabolites of catecholamine. In other words, genetic polymorphism of GSTs results in the complete lack or lowering of the enzyme activity. Hence, Gravina et al found that combination of the GSTM1-null, GSTA1 low activity, and GSTT1-active genotypes represents a risk factor for schizophrenia.23
Micó et al have suggested that patients with more severe and chronic diseases, such as schizophrenia and bipolar disorder, have higher markers of oxidative stress than patients with first early-onset psychotic episode.44 However, our results on the distribution of GST genotypes in patients with EOS, EOBP, and EOFEP showed that the GSTM1-null genotype was more frequent in all 3 types of mental disorders, although the GSTT1-active genotype was more frequent in the EOS group and the GSTT1-null genotype in the EOBP and EOFEP groups of patients. Still, no significant difference regarding the distribution of GST genotypes was observed among different types of early-onset severe mental disorders.
Apart from the possible role of GST polymorphisms in susceptibility for developing various mental disorders, GSTs might also be evaluated from a different perspective. It has recently been suggested that oxidative stress might mediate vascular damage in patients with psychosis, and it has been established that patients with psychosis are at higher risk for developing cardiovascular diseases45 and diabetes mellitus.46 GSTM1-null and GSTT1-null genotypes have already been recognized as potential determinants of higher risk for coronary artery disease47 and even cardiovascular death,48 as well as an increased susceptibility to advanced atherosclerosis49 in patients without mental psychiatric diseases. As a result, it seems reasonable to assume that these genetic polymorphisms might make certain contributions to the development and progression of mental disorders.
Some limitations in the present study should be considered. First, it is well known that in case-control studies, selection bias might influence the results. Our control group was hospital-based; therefore, the use of population controls may have been more appropriate. Also, future researchers on this topic should strongly consider assessing a larger sample. A larger sample size might verify the difference in the effect of GST polymorphisms among various types of early-onset severe mental disorders. Regarding ethnic specificity, because it has already been suggested that ethnicity might affect antioxidant defense mechanisms, further genetic association studies of GST genes in patients with severe mental disorders from different ethnic populations (eg, whites, ethnic Japanese, and African Americans) might lead to less inconsistent results.
Acknowledgments
The study was performed as a part of The Seventh Framework Programme (FP7)–European Union project Copy number variations conferring the risk of psychiatric disorders in children—PsychCNVs grant agreement no. 223423.
Glossary
Abbreviations
- EOS
early-onset schizophrenia
- EOFEP
early-onset first-episode psychosis
- EOBPs
early-onset bipolar disorders
- GSH
glutathione
- GST
glutathione S-transferase
- ROSs
reactive oxygen species
- MAPEG
membrane-associated proteins in eicosanoid and glutathione metabolism
- K-SADS-PL
Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version
- ICD-10
International Classification of Diseases, Revision 10
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th Edition
- DSM-III-R
Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition–Revised
- SADS-L
Schedule for Affective Disorders and Schizophrenia Lifetime
- PCR
polymerase chain reaction
- ORs
odds ratios
- CIs
confidence intervals
- CNV
copy number variation
- NA
not applicable
References
- 1.Sadock BJ, Sadock VA. Kaplan and Sadock's Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. Philadelphia: Lippincot Williams & Wilkins; 2007. [Google Scholar]
- 2.Veling W. Ethnic minority position and risk for psychotic disorders. Curr Opin Psychiatry. 2013;26:166–171. [DOI] [PubMed] [Google Scholar]
- 3.Clemmensen L, Vernal DL, Steinhausen HC. A systematic review of the long-term outcome of early onset schizophrenia. BMC Psychiatry 2012;12:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillberg C, Harrington R, Steinhausen HC. A Clinician’s Handbook of Child and Adolescent Psychiatry. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- 5.Payá B, Rodríguez-Sánchez JM, Otero S. et al. Premorbid impairments in early-onset psychosis: differences between patients with schizophrenia and bipolar disorder. Schizophr Res. 2013;146:103–110. [DOI] [PubMed] [Google Scholar]
- 6.Welham JL, Thomis RJ, McGrath JJ. Age-at-first-registration for affective psychosis and schizophrenia. Schizophr Bull. 2004;30:849–853. [DOI] [PubMed] [Google Scholar]
- 7.Wood SJ, Yucel M, Pantelis C, Berk M. Neurobiology of schizophrenia spectrum disorders: the role of oxidative stress. Ann Acad Med Singapore. 2009;38:296–396. [PubMed] [Google Scholar]
- 8.Chowdari KV, Bamne MN, Nimgaonkar VL. Genetic association studies of antioxidant pathway genes and schizophrenia. Antioxid Redox Signal. 2011;15:2037–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–230. [DOI] [PubMed] [Google Scholar]
- 10.Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tosic M, Ott J, Barral S. et al. Schizophrenia and oxidative stress: glutamate cysteine ligase modifier as a susceptibility gene. Am J Hum Genet. 2006;79:586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuzawa D, Hashimoto K, Hashimoto T. et al. Association study between the genetic polymorphisms of glutathione-related enzymes and schizophrenia in a Japanese population. Am J Med Genet B Neuropsychiatr Genet. 2009;150:86–94. [DOI] [PubMed] [Google Scholar]
- 13.Hayes JD, Strange RC. Glutathione S-transferase polymorphismsand their biological consequences. Pharmacology. 2000;61:154–166. [DOI] [PubMed] [Google Scholar]
- 14.Ma J, Li DM, Zhang R. et al. Genetic analysis of glutamate cysteine ligase modifier (GCLM) gene and schizophrenia in Han Chinese. Schizophr Res. 2010;119:273–274. [DOI] [PubMed] [Google Scholar]
- 15.Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267:4904–4911. [DOI] [PubMed] [Google Scholar]
- 16.Gawryluk JW, Wang J-F, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–30. [DOI] [PubMed] [Google Scholar]
- 17.Gawryluk JW, Wang JF, Andreazza AC, Shao L, Yatham LN, Young LT. Prefrontal cortex glutathione S-transferase levels in patients with bipolar disorder, major depression and schizophrenia. Int J Neuropsychopharmacol. 2011;14:1069–1074. [DOI] [PubMed] [Google Scholar]
- 18.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. [DOI] [PubMed] [Google Scholar]
- 19.Wu B, Dong D. Human cytosolic glutathione transferases: structure, function, and drug discovery. Trends in Pharmacological Sciences. 2012; 33:656–668. [DOI] [PubMed] [Google Scholar]
- 20.Board P, Coggan M, Johnston P, Ross V, Suzuki T, Webb G. Genetic heterogeneity of the human glutathione transferases: a complex of gene families. Pharmacol Ther. 1990;48:357–369. [DOI] [PubMed] [Google Scholar]
- 21.Wiencke JK, Pemble S, Ketterer B, Kelsey KT. Gene deletion of glutathiones-transferase theta: correlation with induced genetic damageand potential role in endogenous mutagenesis. Cancer Epidemiol Biomarkers Prev. 1995;4:253–259. [PubMed] [Google Scholar]
- 22.Spalletta G, Piras F, Gravina P, Bello ML, Bernardini S, Caltagirone C. Glutathione S-transferase alpha 1 risk polymorphism and increased bilateral thalamus mean diffusivity in schizophrenia. Psychiatry Res. 2012;203:180–183. [DOI] [PubMed] [Google Scholar]
- 23.Gravina P, Spoletini I, Masini S. et al. Genetic polymorphisms of glutathione S-transferases GSTM1, GSTT1, GSTP1 and GSTA1 as risk factors for schizophrenia. Psychiatry Res. 2011;187:454–456. [DOI] [PubMed] [Google Scholar]
- 24.Pae CU, Kim JJ, Lee SJ. et al. Association study between glutathione S-transferase P1 polymorphism and schizophrenia in the Korean population. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:519–523. [DOI] [PubMed] [Google Scholar]
- 25.Raffa M, Lakhdar R, Ghachem M. et al. Relationship between GSTM1 and GSTT1 polymorphisms and schizophrenia: a case-control study in a Tunisian population. Gene. 2013;512:282–285. [DOI] [PubMed] [Google Scholar]
- 26.Kashani FL, Kordi-Tamandani DM, Sahranavard R, Hashemi M, Kordi-Tamandan F, Torkamanzehi A. Analysis of glutathione S-transferase genes polymorphisms and the risk of schizophrenia in a sample of Iranian population. Neuron Glia Biol. 2011;7:199–203. [DOI] [PubMed] [Google Scholar]
- 27.Hezova R, Bienertova-Vasku J, Sachlova M. et al. Common polymorphisms in GSTM1, GSTT1, GSTP1, GSTA1 and susceptibility to colorectal cancer in the Central European population. Eur J Med Res. 2012;17(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe Y, Nunokawa A, Kaneko N, Someya T. A case-control study and meta-analysis of association between a common copy number variation of the glutathione S-transferase mu 1 (GSTM1) gene and schizophrenia. Schizophr Res. 2010;124:236–237. [DOI] [PubMed] [Google Scholar]
- 29. World Health Organisation. ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organisation; 1992. [Google Scholar]
- 30.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 31.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–844. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Closas M, Kelsey KT, Hankinson SE. et al. . Glutathione S-transferase mu and theta polymorphisms and breast cancer susceptibility. J Natl Cancer Inst. 1999;91:1960–1964. [DOI] [PubMed] [Google Scholar]
- 33.Pemble SE, Taylor JB. An evolutionary perspective on glutathione transferases inferred from class-theta glutathione transferase cDNA sequences. Biochem J. 1992;287:957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahadik SP, Mukherjee S, Scheffer R, Correnti EE, Mahadik JS. Elevated plasma lipid peroxides at the onset of nonaffective psychosis. Biol Psychiatry. 1998;43:674–679. [DOI] [PubMed] [Google Scholar]
- 35.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res. 1999;31:273–300. [DOI] [PubMed] [Google Scholar]
- 36.Dringen R. Metabolism and function of glutathione in brain. Prog Neurobiol. 2000;62:649–671. [DOI] [PubMed] [Google Scholar]
- 37.Harada S, Tachikawa H, Kawanishi Y. Glutathione S-transferase M1 gene deletion may be associated with susceptibility to certain forms of schizophrenia. Biochem Biophys Res Commun. 2001;281:267–271. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez Santiago B, Brunet A, Sobrino B. et al. Association of common copy number variants at the glutathione S-transferase genes and rare novel genomic changes with schizophrenia. Mol Psychiatry. 2010;15:1023–1033. [DOI] [PubMed] [Google Scholar]
- 39.Pae CU, Yu HS, Kim JJ. et al. Glutathione S-transferase M1 polymorphism may contribute to schizophrenia in Korean population. Psychiatr Genet. 2004;14:147–150. [DOI] [PubMed] [Google Scholar]
- 40.Seidegård J, Vorachek WR, Pero RW, Pearson WR. Hereditary differences in the expression of the glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proc Natl Acad Sci U S A. 1988;85:7293–7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tam GW, Redon R, Carter NP, Grant SG. The role of DNA copy number variation in schizophrenia. Biol Psychiatry. 2009;66:1005–1012. [DOI] [PubMed] [Google Scholar]
- 42.Saadat M, Mobayen F, Farrashbandi H. Genetic polymorphism of glutathione S-transferase T1: a candidate genetic modifier of individual susceptibility to schizophrenia. Psychiatry Res. 2007;153:87–91. [DOI] [PubMed] [Google Scholar]
- 43.Dhanani Y, Awasthi YC. Glutathione S-transferase isozyme composition of human tissues In: Awasthi YC, Taylor, Fransis, editor Toxiciology of Glutathione Transferases. Boca Raton, FL: CRC Press; 2006. p. 321–331. [Google Scholar]
- 44.Micó JA, Rojas-Corrales MO, Gibert-Rahola J. et al. Reduced antioxidant defense in early onset first-episode psychosis: a case-control study. BMC Psychiatry. 2011;11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Do KQ, Trabesinger AH, Kirsten-Krüger M. et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. [DOI] [PubMed] [Google Scholar]
- 46.Medved V, Jovanović N, Knapić VP. The comorbidity of diabetes mellitius and psychiatric disorders. Psychiatr Danub. 2009;21:585–588. [PubMed] [Google Scholar]
- 47.Nomani H, Mozafari H, Ghobadloo SM. et al. The association between GSTT1, M1, and P1 polymorphisms with coronary artery disease in Western Iran. Mol Cell Biochem. 2011;354:181–187. [DOI] [PubMed] [Google Scholar]
- 48.Suvakov S, Damjanovic T, Pekmezovic T. et al. Associations of GSTM1*0 and GSTA1*A genotypes with the risk of cardiovascular death among hemodialyses patients. BMC Nephrol. 2014;15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramprasath T, Senthil Murugan P. et al. Potential risk modifications of GSTT1, GSTM1 and GSTP1 (glutathione-S-transferases) variants and their association to CAD in patients with type-2 diabetes. Biochem Biophys Res Commun. 2011;407:49–53. [DOI] [PubMed] [Google Scholar]