Abstract
Objective: To determine the role of a disintegrin and metalloproteinase with thrombospondin type 1 motif (ADAMTS1) and fragmented versican in the myocardial infarction (MI) process in humans and to evaluate the diagnostic efficacy of ADAMTS1 for postmortem diagnosis of MI.
Methods: Thirty autopsied individuals were allocated into 2 groups, namely, a study group of individuals who died of myocardial infarction (n = 20), and a control group who died of trauma (n = 10). We performed standard immunohistochemical staining on myocardial tissue specimens, studying anti-ADAMTS1, anti-versican, and anti-versican C terminal peptide sequence (DPEAAE) fragments.
Results: Strong, diffuse staining was observed throughout myocardial tissue for ADAMTS1 in the 2 groups. However, in the study group, we observed no expression for ADAMTS1 around fibrotic areas but detected slight staining in coagulative and necrotic zones.
Conclusion: Similar localizations of ADAMTS and fragmented versican in human heart tissue indicate that versican presumably is cleaved by ADAMTS1. Hence, ADAMTS1 can be regarded as a new marker for postmortem differential diagnosis of MI.
Keywords: ADAMTS1, myocardial infarction, versican, fragmented versican, postmortem, diagnosis
According to the World Health Organization (WHO), an estimated 17.5 million people died from cardiovascular diseases in 2012; of these deaths, an estimated 7.4 million occurred due to coronary heart disease. Acute myocardial infarction (AMI) generally occurs because of severe coronary artery occlusion and is a common cause of sudden, unexpected death.1 The postmortem diagnosis of early myocardial infarction (MI), especially when death occurs within minutes to approximately 6 hours or less after the ischemic insult, poses a challenge to forensic and clinical pathologists. This challenge is present because myocardial cell death does not occur instantaneously at the onset of ischemia: myocardial necrosis cannot be identified by standard macroscopic or microscopic postmortem examination until at least 6 hours after the ischemic injury, depending on the sensitivity of the cardiomyocytes.2 Postmortem diagnosis of early MI using routine hematoxylin and eosin (H&E) staining is neither specific nor sensitive enough if the death of the patient occurred at least 6 hours after the onset of the ischemic injury. To our knowledge, there is neither a marker nor a technique in routine medicolegal use that can solve this problem in a satisfactory manner.2,3
In contrast, immunohistochemical markers such as fatty acid–binding protein (FABP), myoglobin, fibronectin, and C5b-9 can be detected as soon as a few minutes after the onset of the ischemic injury. In the immunohistochemical detection of early myocardial damage, myoglobin is at least of the same rank as or superior to FABP and troponin. However, none of these markers give definitive proof of a myocardial infarction.4 Postmortem diagnosis of early MIs is currently a problem in forensic and clinical pathology.
The extracellular matrix (ECM) in the myocardium plays an important role in the pathogenesis and healing process after MI.5 Left ventricular (LV) enlargement frequently develops after MI. Myocardial loss initiates a vicious cycle of contractile dysfunction and progressive LV dilation, referred to as ventricular remodeling, that leads to increased mortality.6 ECM degradation requires the activation of extracellular proteases, which in turn facilitates ventricular remodeling.7 The results of recent studies have shown that matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin type 1 motif (ADAMTS1) play an important role in matrix degradation, atherosclerotic plaques, and ventricular remodeling after MI.8‐13
The de novo formation of microvessels, or angiogenesis, has beneficial effects on healing after infarction and on the potential to salvage ischemic myocardial tissue at early stages after MI. Also, angiogenesis is essential for ventricular remodeling to prevent the transition to heart failure.14,15 In our experiments, the ADAMTS1 protein suppressed fibroblast growth factor-2–induced vascularization in the cornea and inhibited vascular endothelial growth factor (VEGF)–induced angiogenesis.16
One of the biological functions of ADAMTS is proteolytic activity against extracellular matrix proteins, including proteoglycans such as versican.17‐19 Studies have reported that ADAMTS1 plays an important role in atherosclerosis by degrading versican in the cardiovascular system13,18,19 and that a polymorphism of ADAMTS1 is closely associated with the occurrence of ischemic heart disease.20 Nakamura et al10 reported that ADAMTS1 messenger RNA (mRNA) was induced in the early phase of AMI and that ADAMTS1 mRNA was expressed weakly in healthy heart tissue in rats. These previous studies led us to hypothesize that ADAMTS1 may also cleave versican in the myocardium. However, to our knowledge, no available study data examine ADAMTS1 and fragmented versican expressions in myocardial tissue after MI in humans. To this end, we examined the possible role of ADAMTS1 and fragmented versican in biological processes after MI in humans, seking a new possible marker for MI that can be used in differential diagnosis.
Materials and Methods
Thirty autopsied individuals from unknown ethnicity, aged between 16 years and 84 years, of whom 25 were male and 5 were female, were included in this study. Autopsies had been performed on those individuals between the years 2012 and 2014, with 24- to 48-hour postmortem intervals. We retrieved the bodies of the study subjects from the Morgue Department, Council of Forensic Medicine, Bursa, Turkey. To find out the cause of death for each individual, we subjected tissue specimens to histopathological examinations and examined the internal organs from the bodies, macroscopic and microscopically.
Autopsy Materials
For the study group, we have selected 20 individuals for whom the original autopsy team had determined MI to be the primary cause of death. Acute MI and infarction were determined by gross evidence at the time of autopsy and after histopathological examination had determined that these individuals had experienced AMI and infarction. These cases featured macroscopic or microscopic evidence of MI, such as cardiac rupture, hemorrhage, coronary occlusion, coagulation necrosis, and inflammatory infiltration. The age, sex, and causes of death reported for each individual are summarized in Table 1. We selected positive control individuals (those who had experienced actual MIs) and negative control individuals (those with healthy myocardia) for comparison; then, we transferred cardiac tissue specimens from their bodies onto immunohistochemical slides.
Table 1.
Age and Sex Distribution and Myocardium Histopathological Findings of Case Individuals in Whom Myocardial Infarction Was Primarily Determined as the Cause of Death
| Case Number | Age, y/Sex | Heart Weight (g) | Macroscopic Findings | Microscopic Findings | Coronary Arteries |
|---|---|---|---|---|---|
| Case 1 | 52/M | 435 | Rupture of the LVPW | CN with loss of cross-striations, hemorrhage, and early neutrophilic infiltrate | Gross yellowish atherosclerotic plaques |
| Case 2 | 52/M | 327 | Rupture of the LVFW | CN with loss of cross-striations, hemorrhage, and early neutrophilic infiltrate | Nearly complete lumenal occlusion of LAD |
| Case 3 | 78/M | 672 | Rupture of the LVPW | CN with loss of cross-striations, CB, edema, hemorrhage, and early neutrophilic infiltrate | Nearly complete lumenal occlusion of CA |
| Case 4 | 74/M | 358 | Widespread pale area on the endocardium of LV | CN with loss of cross-striations, CB, edema, hemorrhage, and early neutrophilic infiltrate | Severe atherosclerosis of CA |
| Case 5 | 65/M | 467 | Rupture of the LVFW | CN with loss of cross-striations, hypereosinophilic cells, hemorrhage, and early neutrophilic infiltrate | Thrombus severely occluded the lumen of RCA |
| Case 6 | 82/M | 422 | Hyperemic border on the septum, white fibrosis on the LV | CN with loss of cross-striations, hemorrhage, and early neutrophilic infiltrate | RCA occlusive atherosclerosis complicated by calcification |
| Case 7 | 50/M | 430 | Pallor with some hyperemia on the septum | CN with loss of cross-striations, and early neutrophilic infiltrate | LAD lumen is about 50% occluded and thrombus severely narrowed at the RCA |
| Case 8 | 80/F | 390 | Hyperemic border with central yellowing area on the LV | Continuing CN, hypereosinophilic cells, loss of nucleus, and marginal CB | LAD and LCA coronary arteries with severe atherosclerosis |
| Case 9 | 44/M | 485 | Hyperemic border on the LV, white fibrosis on the septum | CN with loss of cross-striations, hemorrhage, hypereosinophilic cells, and early neutrophilic infiltrate | LAD coronary artery with atherosclerotic plaques and bridging, thrombus severely occluded the lumen of RCA |
| Case 10 | 26/M | 250 | Pallor with some hyperemia on the LV | CN with loss of cross-striations, CB, edema, hemorrhage, and early neutrophilic infiltrate | Normal CA |
| Case 11 | 52/M | 732 | Pallor with some hyperemia on the LV and septum | Heavy neutrophilic infiltrate, macrophage and mononuclear infiltration begins, fibrovascular response begins | Thrombus severely occluded the lumen of LAD, LCA lumen is about 70% occluded, severe calcification at the RCA |
| Case 12 | 73/F | 500 | Rupture of the LVFW | CN with loss of cross-striations, hemorrhage, and early neutrophilic infiltrate | RCA occlusive atherosclerosis complicated by calcification |
| Case 13 | 68/M | 540 | Pallor with some hyperemia on the LV, white fibrosis on the septum | Heavy neutrophilic infiltrate, macrophage and mononuclear infiltration begins, fibrovascular response begins | LCA lumen is about 90% occluded, and coronary stent at the RCA |
| Case 14 | 84/F | 523 | White fibrosis on the LV and septum | Macrophage and mononuclear infiltration begins, fibrovascular response begins | Nearly complete lumenal occlusion of LAD and LCA |
| Case 15 | 60/M | 378 | Hyperemic border on the septum | CN with loss of cross-striations, hypereosinophilic cells, and neutrophilic infiltrate | Thrombus severely narrowed at the LAD, LCA lumen is about 50% occluded |
| Case 16 | 56/M | 380 | Hyperemic border on the LV | Total loss of nuclei and cross-striations along with heavy neutrophilic infiltrate | LCA occlusive atherosclerosis complicated by calcification |
| Case 17 | 58/M | 450 | White fibrosis on the septum | CN with loss of cross-striations, hypereosinophilic cells, and neutrophilic infiltrate | RCA lumen is about 80% occluded, and bridging at the LAD |
| Case 18 | 65/M | 414 | Hyperemic border on the LV | CN with loss of cross-striations, and early neutrophilic infiltrate | Gross yellowish atherosclerotic plaques with calcification |
| Case 19 | 56/F | 666 | Pallor with hyperemia and white fibrosis on the LV and septum | Macrophage and mononuclear infiltration begins, fibrovascular response begins | Thrombus severely occluded the lumen of LCA, and nearly complete lumenal occlusion of LAD |
| Case 20 | 71/M | 698 | White fibrosis on the septum, rupture of the LVFW | CN with loss of cross-striations, hemorrhage, and early neutrophilic infiltrate | Nearly complete lumenal occlusion of LAD |
M, male; F, female; LAD, left anterior descending artery; LCA, left circumflex artery; RCA, right coronary artery; LV, left ventricle; LVPW, left ventricular posterior wall; LVFW, left ventricular free wall; CN, coagulation necrosis; CA, coronary arteries; CB, contraction bands
The control group included 10 individuals who had died of acute trauma, with neither MI nor varying periods of suffering. We selected case individuals among those who had died of suicide by firearm with immediate, lethal craniocerebral injuries. None of these latter individuals had macroscopic or microscopic evidence of MI, or any signs of relevant cardiac diseases. Also, these people had no or minimal signs of coronary atherosclerosis. The age, sex, and coronary-artery macroscopic findings reported for each case individual are summarized in Table 2.
Table 2.
Age and Sex Distribution of Control Individuals in Whom Macroscopic Findings for the Coronary Arteries Were Normal
| Control Group Individual Number | Age/Sex |
|---|---|
| Control 1 | 17/M |
| Control 2 | 16/F |
| Control 3 | 31/M |
| Control 4 | 27/M |
| Control 5 | 36/M |
| Control 6 | 26/M |
| Control 7 | 25/M |
| Control 8 | 24/M |
| Control 9 | 18/M |
| Control 10 | 25/M |
F, female; M, male.
Myocardial tissue specimens were fixed in 10% neutral buffered formalin, processed routinely, embedded in paraffin wax, sectioned at 5 µm, and stained routinely with hematoxylin and eosin (H&E). For histochemical studies, replicate sections were mounted on poly-L-lysine–coated glass slides. After 2 hours of incubation at 40 °C, sections were dewaxed in xylene, hydrated through graded alcohol, and endogenous peroxidase blocked with 3% H2O2 in 70% methanol.
Immunohistochemistry
Myocardial tissue specimens were fixed in 10% neutral buffered formalin, routinely processed, embedded in paraffin wax, and sectioned at 5 µm. Subsequently, the sections were exposed to 3% H2O2 for 20 minutes to bleach endogenous peroxidases, followed by rinsing 3 times in phosphate-buffered saline (PBS) for 10 minutes. Sections were respectively incubated with anti-human ADAMTS1, antiversican human antibody, and antiversican C-terminal peptide sequence (DPEAAE) fragment antibody (all 1:250 in BSA) for 1 hour at 37 °C, washed 3 times in PBS, and incubated in a biotinylated goat secondary antimouse polyclonal antibody (Abcam plc) for 15 minutes at 37 °C. We examined the specificity of the antibodies by omitting the primary antibodies. After we washed the tissues in PBS, we visualized them with 3,3′-diaminobenzidine tetrahydrochloride, DAB chromogen (Abcam plc) and counterstained them with H&E. Finally, the sections were dehydrated in graded ethanol, immersed in xylene, and coverslipped. We acquired all images using a (×40, ×100, ×200, ×400) microscope (Nikon Digital Sight DS-UЗ; Nikon Instruments Inc.) and evaluated these images via the staining intensity using a semiquantitative scoring system.
Statistical Analysis
Regarding the extensity of antibody expression, we determined the staining score for each section using semiquantitative scoring: - (0<10%) if the section exhibited no staining; + (10%-25%), occasional staining, with most fields showing slightly negative results; ++ (25%-50%), focally abundant staining with most fields showing positive staining; or +++ (>50%), prominent staining throughout the section. Reactivity degree of the other histological structures was classified on a scale as negative, slightly positive, moderately positive, or strongly positive.
Results
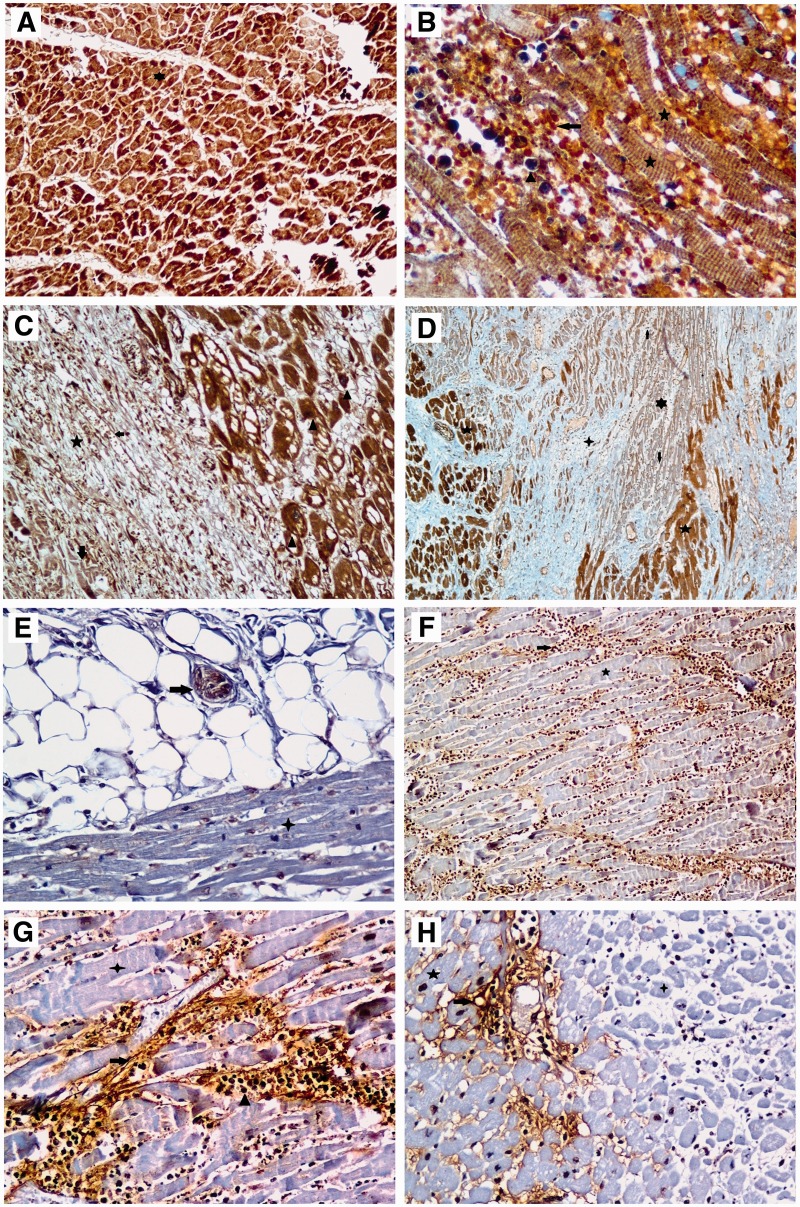
Our evaluation of immunostaining results for ADAMTS1 and fragmented versican in the control group is summarized in Table 3. Strong (+++) and diverse staining were observed throughout myocardial tissue for ADAMTS1 in the control group (Image 1A) and study group. We observed coagulation necroses, with loss of nuclei in the myocytes, in areas affected by MI. Reaction intensity for fragmanted versican in the myocardial tissue of controls was very weak or there was no staining.
Table 3.
Immunostaining Results of ADAMTS1 and Fragmented Versican in Control Group
| Control Group Individual Number | ADAMTS1 | Fragmented Versican |
|
|---|---|---|---|
| Myocardial Tissue | Pericardial Fat Tissue | ||
| Case 1 | +++ | – | NA |
| Case 2 | +++ | – | NA |
| Case 3 | +++ | – | (+) in the NS |
| Case 4 | +++ | – | NA |
| Case 5 | +++ | – | NA |
| Case 6 | +++ | – | (+) in the NS |
| Case 7 | +++ | – | (+) in the NS |
| Case 8 | +++ | – | NA |
| Case 9 | +++ | – | NA |
| Case 10 | +++ | – | (+) in the NF |
+++, prominent staining throughout the section; -, section exhibited no staining; +, occasional staining, with most fields showing slightly negative results; NA, not applicable; NS; nerve sheath; NF, nerve fibers.
Image 1.
ADAMTS1 and fragmented versican protein expressions in postmortem human myocardial tissues by immunochemistry. A, Star indicates (+++) staining for ADAMTS1 in myocardial fibers of all control cases (magnification ×100). B, Star indicates (+) and (++) staining in infarcted areas of muscles, triangle indicates (++) staining in neutrophils, and arrow indicates hemorrhage shown in myocardium sections (Magnification ×400). C, Triangle indicates (+++) staining for ADAMTS1 in adjacent healthy tissue in the myocardial infarcted area. Stars indicate marked early fibrovascular granulation tissue formation. Arrow indicates (+) staining for ADAMTS1 which shows granulation tissue formation in the remaining few necrotic myofibrils and macrophages. Small arrow indicates (+) staining for ADAMTS1 in vascular wall (Magnification ×100). D, 5-pointed star indicates (+++) staining in healthy myocardial fibers adjacent to true necrosis area in myocardial sections, 6-pointed star indicates (+) staining in myofibrils where coagulation necrosis continues, and arrows indicate expressions of ADAMTS1 in neutrophils. Four-pointed star indicates dense collagen accumulation which shows a previous myocardial infarcted area having no ADAMTS1 expression. However, ADAMTS1 expression is observed in the vascular walls seen in the whole tissue section (magnification ×40). E, Star indicates negative staining for fragmented versican in myocardial fibers of all control cases. The arrow indicates (+) staining for fragmented versican in nerve fibers of pericardial fat tissue (magnification ×200). F, Star indicates negative staining for fragmented versican in myocardial fibers and arrow indicates (+) staining in neutrophils in myocardium section (magnification ×100). G, Star indicates negative staining for fragmented versican in myocardial fibers in the areas of coagulation necrosis. Arrow indicates (+) or (++) staining of fibrils, triangle indicates (++) staining of neutrophils in focal areas of interstitial space (magnification ×200). H, 4-pointed star indicates negative staining for fragmented versican in the myocardial infarcted area, and 5-pointed star indicates in healthy myocardial fibers adjacent to infarcted area while arrow indicates (+) staining in the fibers located in the interstitial space (magnification ×200).
Our evaluation of the immunostaining results for ADAMTS1 and fragmented versican in the study group is summarized in Table 4. The staining intensity and localization for ADAMTS1 and fragmented versican in the study group were similar to the staining pattern of these proteins in control tissues. In coagulative and necrotic zones, as well as in fibrovascular proliferative areas of the study group, we detected slight staining (+) for ADAMTS1 and fragmented versican. This staining was not detected in control tissues.
Table 4.
Immunostaining Results of ADAMTS1 and Fragmented Versican in Study Group
| Case Individual Number | Adjacent Healthy Tissue |
Areas of Coagulation Necrosis |
Fibrosis Area | Fibrovascular Proliferation |
|||
|---|---|---|---|---|---|---|---|
| ADAMTS1 | Fragmented Versican | ADAMTS1 | Fragmented Versican | ADAMTS1 | ADAMTS1 | Fragmented Versican | |
| Case 1 | +++ | – | + weakly staining | + staining in the surrounding area | NA | NA | NA |
| Case 2 | +++ | – | + | + staining in the interstitial focal areas | NA | NA | NA |
| Case 3 | +++ | – | + | NA | NA | NA | NA |
| Case 4 | +++ | – | + weakly and- staining areas | + staining in the interstitial focal areas | |||
| Case 5 | ++ | – | + weakly staining in the focal areas | + staining in the interstitial focal areas | NA | NA | NA |
| Case 6 | ++ | – | – | + staining in the interstitial areas | NA | NA | NA |
| Case 7 | +++ | – | – | – | – | – | – |
| Case 8 | +++ | – | + weakly staining in the focal areas | + | – | NA | + |
| Case 9 | +++ | – | + weakly staining in the focal areas | + staining in the interstitial focal areas | NA | NA | NA |
| Case 10 | +++ | – | – | NA | NA | + | NA |
| Case 11 | +++ | – | + | + staining in the interstitial areas | NA | + staining in the macrophages | NA |
| Case 12 | +++ | – | + weakly staining | NA | NA | NA | + |
| Case 13 | +++ | – | + weakly staining in the focal areas | – | NA | NA | + |
| Case 14 | +++ | – | + | + staining in the interstitial areas | – | NA | NA |
| Case 15 | +++ | – | – | NA | NA | (+); (+) staining in the macrophages | + |
| Case 16 | +++ | – | + | + staining in the interstitial focal areas | NA | (+; (+) staining in the macrophages | ++ |
| Case 17 | +++ | – | + | + staining in the interstitial areas | – | NA | NA |
| Case 18 | +++ | – | + weakly staining in the focal areas | + staining in the interstitial focal areas | NA | + staining in the macrophages | + |
| Case 19 | +++ | – | + weakly staining | + | NA | NA | + (widespread) |
| Case 20 | +++ | – | + | + staining in the interstitial focal areas | NA | NA | NA |
+++, prominent staining throughout the section; -, section exhibited no staining; +, occasional staining, with most fields showing slightly negative results; ++, focally abundant staining with most fields showing positive staining; NA, not applicable.
The neutrophilic infiltrate and hemorrhage were evident in the interstitium (Image 1B). These findings were compatible with features of myocardial infarction at 1 to 3 days. One specimen that stained positive for ADAMTS1 displayed this positivity in the neutrophil and fibers in the necrosis area. We observed reversible ischemic changes, such as vacuolar degeneration and myocytolysis, in the live muscle fibers; the area adjacent to the myocardial infarcted area displayed ( +++) staining for ADAMTS1 (Image 1C). Focal staining that revealed the weak (+) presence of ADAMTS1 appeared in myocardial muscle necrosis in areas with true infarction (during a period of 1 to 3 days). Well-developed phagoctosis of dead myofibrils and early formation of fibrovascular granulation tissue were revealed in areas that had undergone MI (ischemia findings from approximately a 5-day to 10-day period). For ADAMTS1, weak (+) staining was present in the remaining few dead myofibrils in the fibrovascular tissue.
Two to 3 positive expressions of ADAMTS1 were apparent in macrophages and lymphocytes. Relatively strong staining ( ++) for ADAMTS1 was present in the vascular endothelium of neovascularization zones. More extensive collagen deposition occurred in the region of myocardium that underwent infarction. We observed no expression of ADAMTS1 in this area (according to ischemia findings during a period of approximately 2 to 8 weeks). Expression of fragmented versican was not observed in the control cases. However, in several cases, a positive result for fragmented versican was revealed in the nerve fibers of pericardial fatty tissue. In particular, fragmented versican staining was not present in the myofibrils in the ischemia area. However, a single weakly positive expression of fragmented versican was present in the focal area of the interstitium between necrotic fibers. One or 2 stainings for fragmented versican showed positive results in the focal area in the interstitum between live myofibrils at the marginally infarcted area (Images 1D-1H).
Discussion
We observed strong ( +++), diffuse staining throughout myocardial tissue for ADAMTS1 in the control and study groups. However, in the study group, no expression for ADAMTS1 was observed around fibrotic areas, although slight staining (+) in coagulative and necrotic zones was detected. Likewise, macrophages in fibrovascular proliferative areas reacted slightly (+) with the antibody against ADAMTS1. In the early stages of MI, Nakamura et al10 demonstrated the expression of ADAMTS1 mRNA in endothelial cells and myocites around infarcted areas in animal models. However, these researchers could not detect ADAMTS1 mRNA in healthy tissues. Contrary to their findings, we detected the expression and localization of ADAMTS1 in healthy myocardial tissue. This discrepancy needs to be elucidated.
According to the classical data in acute MI, biochemical parameters in plasma have been detected as follows: creatine kinase muscle/brain isoform (CK-MB; rises within 4 to 6 hours and peaks at 24 hours) and lactate dehydrogenase (LDH; rises within 12 to 18 hours and peaks at 48 to 72 hours). The diagnosis is performed based on the levels of the aforementioned cardiac enzymes, electrocardiographic results, physical examination results, preliminary case history, and other diagnostic tools. However, use of these parameters for postmortem diagnosis of MI has huge limitations. Instead of these parameters, forensic pathologists prefer postmortem histopathological examination of affected cardiac tissue to diagnose the cause of death. Still, there is a tremendous need for the differential-diagnosis tools of postmortem MI, which provide more-specific data for forensic pathologists. Studies performed previously indicated that ADAMTS1 in the human heart and blood vessels cleaves versican and that ADAMTS1 has a close relationship with MI in humans.13,18 Moreover, Lee et al13 detected fragmented versican and ADAMTS1 at the same spot within atheroma plaques, although those researchers did not find any ADAMTS1 mRNA in healthy myocytes. In addition, Hatipoglu et al21 theorized that transcription of ADAMTS1 mRNA is induced under hypoxic conditions. Strong expression and localization of ADAMTS1 in healthy areas of myocardial tissue, as well as weak reaction of ADAMTS1 and fragmented versican in coagulative and necrotic zones, all of which we obtained in the present study, contradict the findings of the aforementioned studies.
Throughout the inflammation period after MI, ADAMTS1 is produced by macrophages stimulated by cytokines such as tumor necrosis factor–α (TNF-α) and interleukin 1 (IL-1).22‐24 Also, a concomitant area localization of ADAMTS1, fragmented versican, and macrophages was observed.13 Similary, the results of the present study indicate that in the fibrovascular proliferative areas, slight or moderate staining for fragmented versican was visible. Also, macrophages that displayed slight or moderate ADAMTS1 expression were distributed in the same proliferative areas. Based on these findings, we postulate that fragmented versican, which we observed in fibrovascular proliferative areas in this study, is cleaved by the ADAMTS1 produced by macrophages.
This study has several limitations. First, the study was conducted on only a small number of individuals. Specifically, we studied 30 autopsied individuals divided into 2 groups, namely, 20 case subjects who died of MI and 10 control subjects who died of trauma. Because we had difficulty finding available postmortem individuals suitable for such a study, we were only able to study 30 deceased people. Second, only 5 members of the cohort were women, so our results may not be fully applicable to the female population. Third, members of the control group were much younger than their counterparts who had experienced MI. The fact that the aging process can be associated with atherosclerosis and parameters of myocardium status represents a problem, to some extent, with interpretation of our results. The control group should be comparable to the patient group in all aspects except MI, so we have preferred to have a control group of individuals in an age range closer to that of the patients. However, this age difference between groups also can be regarded as an advantage. The findings in our younger population can also yield some estimation of the original condition of the cardiac tissue in the older study individuals might have been.
Overall, some results of the present study are in line with the data presented by previous studies but others are not. Expression and localization of fragmented versican in the fibrovascular proliferative areas with ADAMTS1 indicates a close relationship between ADAMTS1 and fragmented versican. Further studies need to be performed to discover whether using ADAMTS1 antagonists is useful in preventing MI, as well as to elucidate discrepancies between different results. In conclusion, ADAMTS1 could be a candidate for postmortem differential diagnosis of MI in other medical/forensic situtations.
Glossary
Abbreviations
- WHO
World Health Organization
- AMI
acute myocardial infarction
- MI
myocardial infarction
- H&E
hematoxylin and eosin
- FABP
fatty acid-binding protein
- ECM
extracellular matrix
- LV
left ventricular
- MMPs
matrix metalloproteinases
- ADAMTS1
a disintegrin and metalloproteinase with thrombospondin type 1 motif
- VEGF
vascular endothelial growth factor
- mRNA
messenger RNA
- PBS
phosphate-buffered saline
- DPEAAE
C terminal peptide sequence having 6 amino acids
- CK-MB
creatine kinase–muscle/brain isoform
- LDH
lactate dehydrogenase
- TNF-α
tumor necrosis factor–α
- IL-1
interleukin 1
- M
male
- F
female
- LAD
left anterior descending artery
- LCA
left circumflex artery
- RCA
right coronary artery
- LV
left ventricle
- LVPW
left ventricular posterior wall
- LVFW
left ventricular free wall
- CN
coagulation necrosis
- CA
coronary arteries
- CB
contraction bands
- +++
prominent staining throughout the section
- -
section exhibited no staining
- +
occasional staining, with most fields showing slightly negative results
- NA
not applicable
- NS
nerve sheath
- NF
nerve fibers
- ++
focally abundant staining with most fields showing positive staining
References
- 1.Chugh SS. Early identification of risk factors for sudden cardiac death. Nat Rev Cardiol. 2010;7:318-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campobasso CP, Dell’Erba AS, Addante A, Zotti F, Marzullo A, Colonna MF. Sudden cardiac death and myocardial ischemia indicators: a comparative study of four immunohistochemical markers. Am J Forensic Med Pathol. 2008;29:154-161. [DOI] [PubMed] [Google Scholar]
- 3.Jia JZ, Shen YW, Xue AM, et al. Immunohistochemical analysis of cardiac troponin inhibitor in an experimental model of acute myocardial infarction experimental model and in human tissues. Pathol Res Pract. 2015:211:456-461. [DOI] [PubMed] [Google Scholar]
- 4.Ortmann C, Pfeiffer H, Brinkmann B. A comparative study on the immunohistochemical detection of early myocardial damage. Int J Legal Med. 2000;113:215-220. [DOI] [PubMed] [Google Scholar]
- 5.Lindsey ML, Mann DL, Entman ML, et al. Extracellular matrix remodeling following myocardial injury. Ann Med. 2003;35:316-326. [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990;81:1161-1172. [DOI] [PubMed] [Google Scholar]
- 7.Chan W, Duffy SJ, White DA, et al. Acute left ventricular remodeling following myocardial infarction: coupling of regional healing with remote extracellular matrix expansion. J Am Coll Cardiol Img. 2012;5:884-893. [DOI] [PubMed] [Google Scholar]
- 8.Creemers EE, Cleutjens JP, Smits JF, et al. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res. 2001;89:201-210. [DOI] [PubMed] [Google Scholar]
- 9.Fedak PWM, Altamentova SM, Weisel RD, et al. Matrix remodeling in experimental and human heart failure: a possible regulatory role for TIMP-3. Am J Physiol Heart Circ Physiol. 2003;284:H626-H634. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura K, Hirohata S, Murakami T, et al. Dynamic induction of ADAMTS1 gene in the early phase of acute myocardial infarction. J Biochem. 2004;136:439-446. [DOI] [PubMed] [Google Scholar]
- 11.Wu W, Zhou Y, Li Y, et al. Association between plasma ADAMTS-7 levels and ventricular remodeling in patients with acute myocardial infarction. Eur J Med Res. 2015;20:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CW, Hwang I, Park CS, et al. Expression of ADAMTS-2, -3, -13, and -14 in culprit coronary lesions in patients with acute myocardial infarction or stable angina. J Thromb Thrombolysis. 2012;33:362-370. [DOI] [PubMed] [Google Scholar]
- 13.Lee CW, Hwang I, Park CS, et al. Comparison of ADAMTS-1, -4 and -5 expression in culprit plaques between acute myocardial infarction and stable angina. J Clin Pathol. 2011;64:399-404. [DOI] [PubMed] [Google Scholar]
- 14.Shah AM, Mann DL. In search of new therapeutic targets and strategies for heart failure: recent advances in basic science. Lancet. 2011;378:704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Laan AM, Piek JJ, van Royen N. Targeting angiogenesis to restore the microcirculation after reperfused MI. Nat Rev Cardiol. 2009;6:515-523. [DOI] [PubMed] [Google Scholar]
- 16.Vázquez F, Hastings G, Ortega MA, et al. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem. 1999;274:23349-23357. [DOI] [PubMed] [Google Scholar]
- 17.Apte SS. A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family. Int J Biochem Cell Biol. 2004;36:981-985. [DOI] [PubMed] [Google Scholar]
- 18.Sandy JD, Westling J, Kenagy RD, et al. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem. 2001;276:13372-13378. [DOI] [PubMed] [Google Scholar]
- 19.Jönsson-Rylander AC, Nilsson T, Fritsche-Danielson R, et al. Role of ADAMTS-1 in atherosclerosis: remodeling of carotid artery, immunohistochemistry, and proteolysis of versican. Arterioscler Thromb Vasc Biol. 2005;25:180-185. [DOI] [PubMed] [Google Scholar]
- 20.Sabatine MS, Ploughman L, Simonsen KL, et al. Association between ADAMTS1 matrix metalloproteinase gene variation, coronary heart disease, and benefit of statin therapy. Arterioscler Thromb Vasc Biol. 2008;28:562-567. [DOI] [PubMed] [Google Scholar]
- 21.Hatipoglu OF, Hirohata S, Cilek MZ, et al. ADAMTS1 is a unique hypoxic early response gene expressed by endothelial cells. J Biol Chem. 2009;284:16325-16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008;130:147-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K. Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem. 1997;272:556-562. [DOI] [PubMed] [Google Scholar]
- 24.Norata GD, Björk H, Hamsten A, Catapanoa AL, Eriksson P. High-density lipoprotein subfraction 3 decreases ADAMTS-1 expression induced by lipopolysaccharide and tumor necrosis factor-α in human endothelial cells. Matrix Biol. 2004;22:557-560. [DOI] [PubMed] [Google Scholar]