Abstract
Given the increasing emergence of antimicrobial resistant microbes and the near absent development of new antibiotic classes, innovative new therapeutic approaches to address this global problem are necessary. The use of predatory bacteria, bacteria that prey upon other bacteria, is gaining interest as an “out of the box” therapeutic treatment for multidrug resistant pathogenic bacterial infections. Before a new antimicrobial agent is used to treat infections, it must be tested for safety. The goal of this study was to test the tolerability of bacteria on the ocular surface using in vitro and in vivo models. Predatory bacteria Bdellovibrio bacteriovorus and Micavibrio aeruginosavorus were found to be non-toxic to human corneal stromal keratocytes in vitro; however, they did induce production of the proinflammatory chemokine IL-8 but not IL-1β. Predatory bacteria did not induce inflammation on the ocular surface of rabbit eyes, with and without corneal epithelial abrasions. Unlike a standard of care antibiotic vancomycin, predatory bacteria did not inhibit corneal epithelial wound healing or increase clinical inflammatory signs in vivo. Together these data support the safety of predatory bacteria on the ocular surface, but future studies are warranted regarding the use predatory bacteria in deeper tissues of the eye.
Emerging antibiotic resistance by pathogenic bacteria is considered a global threat and is responsible for thousands of deaths and millions of dollars spent on health care each year1,2,3,4. With few new antibiotic classes being developed5, novel methods to treat bacterial infections are becoming necessary. Alternative methods such as bacteriophage therapy show promise, but can be hampered by narrow host range and rapid development of resistance by bacteria6. Like bacteriophage, predatory bacteria, such as Bdellovibrio bacteriovorus may be useful as a therapeutic7,8,9. Predatory bacteria generally have a broader host-range than phage10,11,12,13, and genetically stable resistance of bacteria to predatory bacteria has yet to be described14. Importantly, multidrug resistant bacteria are killed by predatory bacteria, just as readily as their non-resistant kin15,16.
If predatory bacteria are to be used as therapeutic agents, thorough testing must be performed to demonstrate a lack of toxicity. Previous studies have shown a lack of toxicity of B. bacteriovorus and Micavibrio aeruginosavorus to a number of cell types in vitro16,17,18. Similarly, animal studies supported the safety of predatory bacteria in dosed per os with B. bacteriovorus10 and mice treated intravenously and in the respiratory tract with B. bacteriovorus and M. aeruginosavorus19. Furthermore, B. bacteriovorus was found in the gut microflora of healthy humans by genetic analysis20.
In addition to the use of predatory bacteria as therapeutic agents in the oral cavity or gut, topical application of these microbes on skin or the ocular surface may be feasible8. Whether or not the ocular surface tolerates aggressive application of predatory bacteria has yet to be examined. Additionally, the impact of predatory bacteria on wound healing has not been examined. The goal of this study is to test the safety of B. bacteriovorus and M. aeruginosavorus for topical application using the eye and ocular cells as models. Ocular clinical signs of inflammation and wound healing were measured. The results of this study are consistent with predatory bacteria being non-toxic to the ocular surface.
Materials and Methods
Tissue culture, cytotoxicity analysis, and ELISA
De-identified corneas from organ donors were obtained from the National Disease Research Interchange (Philadelphia, PA) or from Center for Organ Recovery and Education (Pittsburgh, PA). The use of de-identified tissue from non-living individuals is not human subject research under DHHS regulation 45CFR46, and exemption from human subjects regulation was recognized by the Institutional Review Board of the University of Pittsburgh. Ethical aspects of the research protocols were approved by and performed in accordance with guidelines of the Committee for Oversight of Research Involving the Dead. Human stromal keratocytes were derived from adult human corneal stromal stem cells as previously described21. Monolayers of stem cells were grown to confluence, transferred to keratocyte differentiation medium (KDM) containing FGF2 and TGF-ß322 for 3 days, then challenged by predatory bacteria, Pseudomonas aeruginosa, triton X-100 (0.25%), or lysogeny broth (LB)23,24 as previously described16.
P. aeruginosa strain PA1425 was grown to stationary phase in LB medium, washed with saline supplemented with glucose (0.1%), and suspended in KDM medium (50 μl). P. aeruginosa was added to wells with keratocytes covered with 450 μl of KDM medium, to create a multiplicity of infection (MOI) of 200 bacteria per keratocyte. Predatory bacteria were cultured and purified as previously described19. Predatory bacteria were added at an MOI of 594 for B. bacteriovorus HD100, 516 for B. bacteriovorus 109J, and 161 for M. aeruginosavorus ARL-13.
Following 4–24 h exposure to challenges, the cell layers were washed gently with saline with glucose (0.1%) to remove bacteria and suspended in KDM with Presto Blue viability reagent (Life Technologies) with amikacin (40 μg/ml) to prevent growth of remaining bacteria. Presto Blue was used as previously described26. KDM medium was used as a control (mock) to determine the maximum viability value, and Triton X-100 (0.25%) was used to kill the keratocytes to determine the minimum viability value. The experiment was performed four times.
ELISA experiments were performed using supernatants from the above cytotoxicity experiments as previously described16. The limit of detection for the CXCL8/IL-8 ELISA kit was 31.2 pg/ml (R&D Systems) and 2 pg/ml for TNFα (ThermoFisher). Each experiment was performed twice with independent samples.
Ocular tolerability and wound healing experiments
New Zealand White (NZW) female rabbits weighing 1.1–1.4 kg were purchased from Charles River (Wilmington, MA, USA). Rabbits were housed under specific pathogen-free conditions at the University of Pittsburgh animal facility. Animal use protocols were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh (Protocol #15025331) and the Animal Care and Use Review Office of the US. Army Medical Research and Material Command, and were performed in accordance with the approved guidelines. All studies conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Following general anesthesia with 40 mg/kg ketamine (Ketathesia, Henry Schein Animal Health, Dublin OH) and 4 mg/kg xylazine (AnaSed Injection, Lloyd Laboratories, Shenandoah, IA) administered intramuscularly and topical anesthesia with two drops of 0.5% proparacaine (Proparacaine Hydrochloride Ophthalmic Solution, USP, Sandoz, Princeton, NJ), epithelial defects were introduced onto corneas on the right eyes of the rabbits using an Amoils epithelial scrubber (Innovative Excimer Solutions, Inc, Toronto, ON, Canada) with a sterile 6.5 mm brush, which makes a ~7 mm abrasion. The rabbits were immediately treated with analgesia in the form of intramuscular injections of 1.5 mg/kg of ketoprofen (Ketofen, Zoetis Inc., Kalamazoo, MI). Nothing was done to the left eyes.
The rabbits were then divided into 5 groups, (n = 3 per group): (1) Saline; (2) Vancomycin (5% w/v); (3) Micavibrio aeruginosavorus strain ARL-13 (Mica); (4) Bdellovibrio bacteriovorus strain HD100 (BD HD100); (5) Bdellovibrio bacteriovorus strain 109J (BD 109J). Ocular clinical signs were obtained using a slit lamp and evaluated following a modified MacDonald-Shadduck scoring system27 by a skilled observer (EGR). The scoring system has a maximum score of 26, and evaluates corneal opacity (up to 6 points), area of corneal opacity (up to 4 points), corneal vascularization (up to 2 points), conjunctival congestion (up to 3 points), conjunctival chemosis and swelling (up to 4 points), conjunctival discharge (up to 3 points), and area of cornea staining with topical fluorescein (FLUORESOFT-0.35%, Alden Optical, Lancaster, NY), a dye that reveals defects in the corneal epithelium when illuminated with a cobalt blue light (up to 4 points). Both eyes of each rabbit were dosed with one 50 μl drop of predatory bacteria, saline, or vancomycin at 1 hour intervals, 5 times per day, for five days, and were evaluated on the first day before treatment (day 0), and each day after treatment. Drops contained either 1.30 × 107 PFU of M. aeruginosavorus, or 4.80 × 107 PFU of B. bacteriovorus HD100, or 4.13 × 107 PFU of B. bacteriovorus 109J. Evaluations continued at intervals over an additional week.
The study was repeated on three occasions, with one animal removed from the saline group due to injury incurred during shipping. For two experiments, the largest diameters of the fluorescein stained epithelial defects were measured using a ruler and the slit lamp to a resolution of 0.5 mm.
Topical vancomycin 5% was prepared by reconstituting 500 mg of vancomycin hydrochloride (Fresenius Kabl USA LLC, Lake Zurich, IL) in 10 ml of sterile water. Pharmaceutical grade saline (0.9% Sodium Chloride Injection USP, Baxter Healthcare Corp. Deerfield, IL) was purchased from the inpatient pharmacy at the University of Pittsburgh Medical Center. The evaluations on day 1 and 3 were omitted for one iteration of the experiment.
Statistical analysis
Kruskal-Wallis with Dunn’s multicomparison and two-tailed Fisher Exact tests were performed with Graphpad Prism software, and two-tailed Monte Carlo randomization test with 1000 shuffles was performed with True Epistat software. Significance was set at p < 0.05.
Results
Susceptibility of human stromal keratocytes to predatory bacteria
Stromal keratocytes play an important role in wound healing and in maintenance of corneal clarity, which is required for vision28. Here we tested whether predatory bacteria were cytotoxic to monolayers of human keratocytes in vitro as a primary step in determining their safety for ocular use. Monolayers were challenged for 4 and 24 hours with predatory bacteria. Compared to the mock control and positive controls for cytotoxicity, detergent and P. aeruginosa PA14, a cytotoxic strain, were significantly more damaging to keratocytes than the PBS negative control and predatory bacteria at both 4 and 24 h (p < 0.001, ANOVA with Tukey’s post-test) (Fig. 1A,B). Predatory bacteria were not significantly different than the mock treatment group (PBS) for cytotoxicity (p > 0.5, ANOVA with Tukey’s post-test), (Fig. 1A,B).
Figure 1. Predatory bacteria are non-cytotoxic to human keratocytes in vitro.
Mean and standard deviations from four independent experiments are shown. Asterisks indicate significant differences using ANOVA with Tukey’s post-hoc test (***p < 0.001). There was no difference in cytotoxicity between Mock and predatory bacteria. PA14 indicates P. aeruginosa strain PA14, Mica = M. aeruginosavorus, HD100 and 109J indicate B. bacteriovorus strains. (A) 4 h, (B) 24 h.
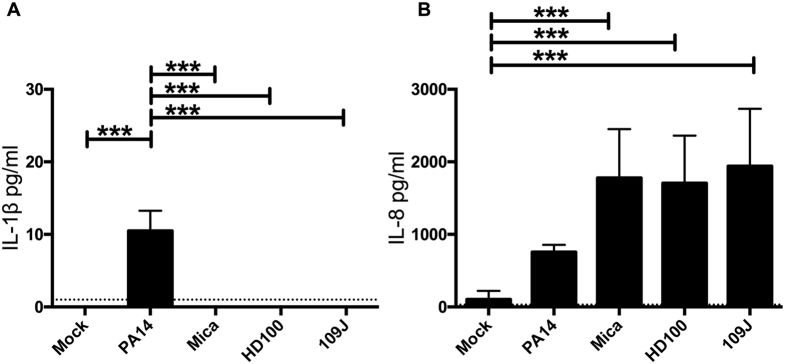
Pro-inflammatory cytokines IL-1β and IL-8 were measured from the human stromal keratocyte supernatants after exposure to P. aeruginosa and predatory bacteria by ELISA. After 4 hours of exposure to P. aeruginosa there was a significant increase in IL-1 β, but not IL-8 levels in the supernatants of human keratocytes (Fig. 2A). Predatory bacteria did not induce IL-1β, but did induce IL-8 levels (Fig. 2B).
Figure 2. Predatory bacteria induce an IL-8, but not IL-1β response from corneal stromal keratocytes in vitro.
Pro-inflammatory cytokines IL-1β (A) and IL-8 (B) were measured by ELISA. Cell supernatants taken from keratocytes after 4 hours of incubation with medium only negative control (Mock), positive control Pseudomonas aeruginosa strain PA14 (MOI = 200), and experimental strains M. aeruginosavorus (Mica, MOI = 161), B. bacteriovorus strain HD100 (MOI = 594), and B. bacteriovorus strain 109J (MOI = 516). The average and standard deviation from n = 7 independent data points from 2 experiments are shown. Asterisks indicate significant differences (*p < 0.05, **p < 0.01, ***p < 0.001 determined using ANOVA with Tukey’s post-test). Limits of assay detection are denoted by the dotted horizontal lines.
In vivo ocular tolerability of predatory bacteria
Since the predatory bacteria were non-cytotoxic to ocular epithelial cells16 and keratocytes (Fig. 1A,B), their safety in an animal model was tested.
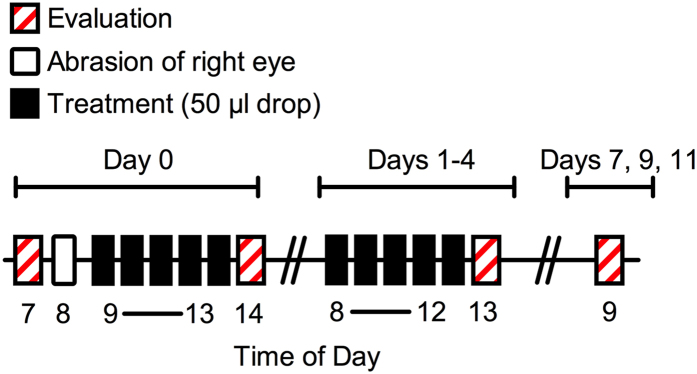
Corneas on the left eyes of New Zealand White Rabbits were exposed to predatory bacteria (≥107 PFU) five times a day for five days and evaluated for an additional week (Fig. 3). Eyes were stained with fluorescein and graded for ocular clinical signs of inflammation using a slit-lamp and quantified according to a modified MacDonald-Shadduck scoring system. Vancomycin (5%) was used as a positive control for ocular surface toxicity. Compared to the saline negative control, vancomycin was clearly more toxic to the ocular surface, inducing inflammation and swelling (chemosis) of the conjunctiva and nictitating membrane along with the production of ocular mucous (Fig. 4). Eyes exposed to each of the predatory bacteria were indistinguishable from the saline treatment group (Fig. 4).
Figure 3. Time line and experimental outline for in vivo ocular tolerability experiments.
The corneas of NZW rabbits were abraded (right eye) or left intact (left eye), and exposed to drops of saline (negative control for toxicity), vancomycin (positive control for toxicity), or predatory bacteria (>107 PFU per drop). Drops were administered at 1 hour intervals, five times per day for the first 5 days. Each day the eyes were treated with fluorescein and evaluated for clinical signs of inflammation and toxicity using a MacDonald-Shadduck scoring system. The diameter of the corneal defect on the right eye was measured with a ruler.
Figure 4. Predatory bacteria do not cause ocular toxicity in the intact (left) of rabbits.
Eyes of NZW rabbits were photographed using a slit-lamp camera at time points indicated above. Inflammatory signs are observed in the vancomycin treatment group, but not the other groups. The eye of a representative rabbit for each group is shown.
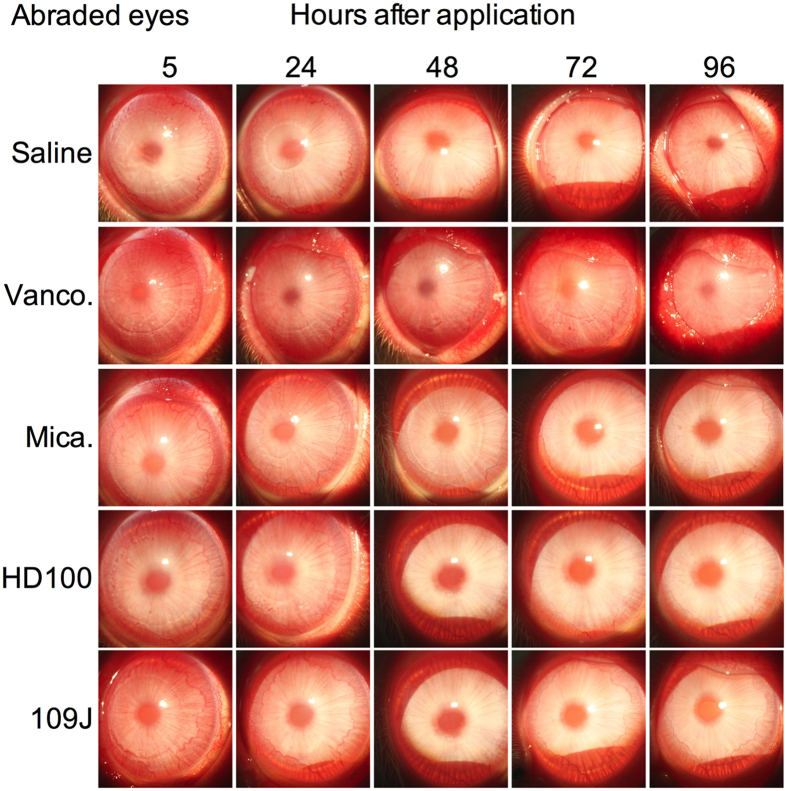
Because microbial keratitis is generally associated with erosion of the corneal epithelium and wounded or inflamed eyes can be more sensitive to topical therapeutic approaches, the above in vivo tolerability study was also performed using eyes with epithelial defects. An epithelial scrubber, used by ophthalmologists on human eyes, was used to produce 7 mm diameter zones free of corneal epithelium in the right eyes of the above rabbits (abraded eye). As expected, the baseline clinical scores were higher in the saline treatment of the wounded eyes than the non-wounded eyes for the first several days; afterwards there was no quantitative difference (Fig. 5). Vancomycin caused clear toxicity, in some cases for the entire 12 day course of the experiment. Predatory bacteria mirrored the saline treatment group, suggesting that they are well tolerated even by eyes with damaged surface epithelia in both intact and abraded eyes (Figs 5 and 6).
Figure 5. Predatory bacteria do not cause ocular toxicity in abraded (right) rabbit eyes.
A 7 mm diameter region of the cornea of NZW rabbits was abraded of epithelial cells. Subsequently, eyes were treated with saline, vancomycin, and predatory bacteria, and photographed with a slit-lamp camera. Clear inflammatory signs are observed in all groups at the 5 hour time point, that persisted only in the vancomycin treatment group. The eye of a representative rabbit for each group is shown.
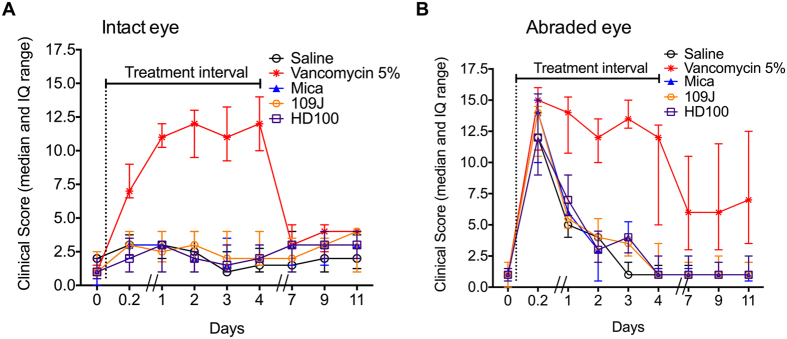
Figure 6. Clinical signs of rabbit eyes exposed to predatory bacteria were similar to those exposed to saline.
The intact eye (A) and abraded eye (B) of NZW rabbits were treated with saline, vancomycin, and predatory bacteria 5 times a day for 5 days (treatment interval). Eyes were stained with fluorescein and graded using a modified MacDonald-Shadduck grading system. The median and interquartile range (IQ) are shown (n ≥ 8 animals per group).
Statistical analysis (Kruskal-Wallis analysis with Dunn’s multicomparision test) of combined clinical scores for individual time points indicate no statistical difference between the saline and other groups of the intact left eyes before treatment (p = 0.63). However, there was a statistical difference between saline and vancomycin (p < 0.001) but not saline and predatory bacteria groups (t = 5 hrs) (p > 0.05).
Similar statistical analysis of total clinical scores for the abraded right eyes indicate no statistical difference between the saline and other groups before treatment (p = 0.91), or just after abrasion and initial treatment (t = 0.2) (p = 0.45). At all subsequent evaluation days (1–11) there was no significant difference between saline and the predatory bacteria, but there was a significant difference between the saline group and the vancomycin group (p < 0.01). The significant difference between the vancomycin and saline groups continued even after treatment was finished (p < 0.01), see days 7–11.
Impact of predatory bacteria on corneal wound healing
Wound healing following infection or trauma is essential for tissue function and to prevent infection. It is generally thought that bacteria play an important role in preventing wound healing associated with chronic wounds, and experimental studies support this hypothesis29,30,31. The epithelial defect, in the right eyes of the above noted rabbits, was used to evaluate whether predatory bacteria inhibit corneal wound healing. Wound closure of the Amoils scrubber treated corneas described above was recorded. With the saline treatment group, the ~7 mm wound was closed by the 48 hour post-wounding evaluation (Fig. 7). Vancomycin, an antibiotic used to treat microbial keratitis caused by methicillin-resistant Staphylococcus aureus, strongly inhibited corneal epithelial wound healing. Wound healing by predatory bacteria treated eyes closely mimicked the healing pattern of the saline treatment group (Fig. 8).
Figure 7. Vancomycin, but not predatory bacteria, inhibited corneal wound healing in vivo.
A 7 mm diameter region of the cornea of NZW rabbits was abraded of epithelial cells. Eyes were treated with saline, vancomycin, and predatory bacteria. Fluorescein was used to reveal epithelial defects, and eyes were photographed with a slit-lamp camera. The eye of a representative rabbit for each group is shown.
Figure 8. Predatory bacteria do not inhibit corneal wound healing in vivo.

NZW rabbits with epithelial defects were treated with saline, vancomycin, and predatory bacteria five times a day for five days. Eyes were stained with fluorescein and graded using a modified MacDonald-Shadduck grading system. The average and standard deviation are shown (n = 5 rabbits for the saline group, and 6 for the remaining groups).
When eyes were grouped into healed versus not healed epithelial defect categories, the differences were quite striking. All of the saline and M. aeruginosavorus treated epithelial defects healed by 48 h, 5 out of the 6 eyes treated with each Bdellovibrio strain had completely healed, and 0 out of 6 of the vancomycin eyes were healed. By 72 h, all of the predatory bacteria treated epithelial defects had completely healed, whereas only one of the vancomycin treated defects had healed by 96 h. Monte Carlo analysis of healed versus non-healed eyes at 48 hours revealed a significant difference (p = 0.003), and Fisher exact tests showed a significant difference between the vancomycin group and all other groups (p < 0.016). At 48 hours, there was no difference between the predatory bacteria groups and the saline group by Fisher Exact test (p = 1).
Discussion
This study expanded upon a previous in vitro study designed to examine whether predatory bacteria could be safely used as an alternative antimicrobial therapy for ocular surface infections16. Here, we used cells from a deeper layer of the cornea that would not normally experience microbial assault. Stromal keratocytes were unperturbed by predatory bacteria in cytotoxicity assays lasting up to 24 hours, whereas P. aeruginosa caused significant reduction in ocular cell viability. This result supports our previous finding in which predatory bacteria were found to be non-toxic to human limbal corneal epithelial (HCLE) cells16,32, thus completely different cell type, keratocytes versus epithelial cells, retains their viability when exposed to high MOI of predatory bacteria.
It was suggested that predatory bacteria might bring about a reduced inflammatory response compared to other Gram-negative bacteria. This is attributed to the unusual structure of the lipid A portion of their lipopolysaccharide, which does not interact with TLR433, as well as their membrane encased flagella34, which should not interact with TLR5. Unlike a previous study wherein proinflammatory cytokines were not induced from corneal epithelial cells exposed to predatory bacteria16, in this study IL-8 was induced from corneal stromal keratocytes by the tested predatory bacteria. We also observed relatively mild induction of IL-8 by P. aeruginosa relative to the predatory bacteria; this may be as a result of P. aeruginosa killing the keratocytes before the cells could mount a full immune response. Unlike IL-8, predatory bacteria did not induce IL-1β production in exposed corneal stromal keratocytes cells, supporting the non-inflammatory attributes of the predators. In a recent study, mice were exposed to high concentrations of the predatory bacteria, B. bacteriovorus 109J, HD100 and M. aeruginosavorus, which were delivered via inhalation and direct tail vein injection. It was reported that although inflammatory responses were detected at early time points of 1–3 hours after exposure, the inflammatory effects were not sustainable and were found to return to baseline levels 18 hours after exposure19. Furthermore, histological analysis showed no pathological changes in tissue following exposure to the predators, thus it was concluded that any inflammatory response caused by the predator did not impact animal well being or cause tissue inflammation19.
Predatory bacteria were well tolerated on the rabbit ocular surface in vivo regardless of whether the corneal epithelium was present or removed. This is especially important because wounded eyes can display highly elevated inflammatory responses to certain therapeutics such as antimicrobial peptides35. Furthermore, predatory bacteria appeared to have no impact upon corneal wound healing, unlike other bacteria such as P. aeruginosa and Serratia marcescens that can robustly inhibit corneal epithelial wound healing in vitro and ex vivo31,36. The lack of ocular surface toxicity caused by predatory bacteria is especially clear given the toxicity of vancomycin (5%), which is used by clinicians to treat ocular infections. Although, some clinicians are now using a lower concentration of vancomycin (2.5%) to treat ocular infections.
There has been mixed reports on the effect of topical vancomycin on corneal wound healing. Petroutsos and colleagues demonstrated that vancomycin (3.1%) as well as kanamycin and penicillin inhibited corneal wound healing in vivo using a rabbit model37. In contrast, Lin and Boehnke used an ex vivo pig corneal model, and reported that amphotericin B and gentamicin both inhibited corneal wound healing, but vancomycin (5%) did not38. The differences between the outcomes of these studies may be due to the use of different organisms, the intact immune system in the in vivo model, or the different dosing regimens (3 doses for the Lin study, versus 6 doses per day for four days). Our study continued longer than the previous studies and demonstrated that even after topical vancomycin treatment had ended for several days, the epithelial defects of some of the eyes failed to resolve. The impact on wound healing should be considered with fortified vancomycin, especially when this antibiotic is used for prophylaxis as is commonly done for patients with keratoprostheses39.
In summary, this study demonstrates that predatory bacteria are not toxic to ocular cells in vitro, are well tolerated on the ocular surface in vivo, and do not prohibit corneal epithelial wound healing, and adds to the growing body of studies supporting that predatory bacteria are non-toxic to mammalian tissues.
Additional Information
How to cite this article: Romanowski, E. G. et al. Predatory bacteria are nontoxic to the rabbit ocular surface. Sci. Rep. 6, 30987; doi: 10.1038/srep30987 (2016).
Acknowledgments
The authors thank Jake Callaghan for technical assistance. The research was sponsored by the U.S. Army Research Office and the Defense Advanced Research Projects Agency and accomplished under Cooperative Agreement Number W911NF-15-2-0036 (DEK and RMQS). The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the Army Research Office, DARPA, or the U.S. Government. Additional funding was provided by NIH grants RO1-EY016415 (J.L.F.), P30-EY08098, RO1-AI085570 (R.M.Q.S), and F32-EY024785 (K.B) and from an unrestricted grant from Research to Prevent Blindness Inc. This work was supported by the U.S. Army Research Office and the Defense Advanced Research Projects Agency and accomplished under Cooperative Agreement Number W911NF-15-2-0036, unrestricted funds from Research to Prevent Blindness, the Eye and Ear Foundation of Pittsburgh, and National Institute of Health grants AI085570, EY016415, EY024785, and EY08098.
Footnotes
Author Contributions J.L.F., D.E.K., E.G.R. and R.M.Q.S. wrote the manuscript. K.M.B., S.D., M.L.F., S.G., E.G.R., N.A.S. and K.A.Y. performed the experiments. E.G.R., R.M.Q.S. and D.E.K. conceived of the experiments. All authors reviewed the manuscript.
References
- Carlet J., Pulcini C. & Piddock L. J. Antibiotic resistance: a geopolitical issue. Clin Microbiol Infect 20, 949–953, 10.1111/1469-0691.12767 (2014). [DOI] [PubMed] [Google Scholar]
- Shallcross L. J., Howard S. J., Fowler T. & Davies S. C. Tackling the threat of antimicrobial resistance: from policy to sustainable action. Philos Trans R Soc Lond B Biol Sci 370, 20140082, 10.1098/rstb.2014.0082 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zowawi H. M. et al. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat Rev Urol 12, 570–584, 10.1038/nrurol.2015.199 (2015). [DOI] [PubMed] [Google Scholar]
- Morrill H. J., Caffrey A. R., Jump R. L., Dosa D. & LaPlante K. L. Antimicrobial Stewardship in Long-Term Care Facilities: A Call to Action. J Am Med Dir Assoc 17, 183 e181–183 e116, 10.1016/j.jamda.2015.11.013 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Projan S. J. Whither antibacterial drug discovery? Drug Discov Today 13, 279–280, 10.1016/j.drudis.2008.03.010 (2008). [DOI] [PubMed] [Google Scholar]
- Roach D. R. & Donovan D. M. Antimicrobial bacteriophage-derived proteins and therapeutic applications. Bacteriophage 5, e1062590, 10.1080/21597081.2015.1062590 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwidar M., Monnappa A. K. & Mitchell R. J. The dual probiotic and antibiotic nature of Bdellovibrio bacteriovorus. BMB Rep 45, 71–78, 10.5483/BMBRep.2012.45.2.71 (2012). [DOI] [PubMed] [Google Scholar]
- Shanks R. M. & Kadouri D. E. Predatory prokaryotes wage war against eye infections. Future Microbiol 9, 429–432, 10.2217/fmb.14.19 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockett R. E. & Lambert C. Bdellovibrio as therapeutic agents: a predatory renaissance? Nat Rev Microbiol 2, 669–675, 10.1038/nrmicro959 (2004). [DOI] [PubMed] [Google Scholar]
- Atterbury R. J. et al. Effects of orally administered Bdellovibrio bacteriovorus on the well-being and Salmonella colonization of young chicks. Appl Environ Microbiol 77, 5794–5803, AEM.00426-11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashiff A., Junka R. A., Libera M. & Kadouri D. E. Predation of human pathogens by the predatory bacteria Micavibrio aeruginosavorus and Bdellovibrio bacteriovorus. J Appl Microbiol 110, 431–444, 10.1111/j.1365-2672.2010.04900.x (2011). [DOI] [PubMed] [Google Scholar]
- Richards G. P. et al. Predatory bacteria as natural modulators of Vibrio parahaemolyticus and Vibrio vulnificus in seawater and oysters. Appl Environ Microbiol 78, 7455–7466, 10.1128/AEM.01594-12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnappa A. K., Dwidar M., Seo J. K., Hur J. H. & Mitchell R. J. Bdellovibrio bacteriovorus inhibits Staphylococcus aureus biofilm formation and invasion into human epithelial cells. Sci Rep 4, 3811, srep03811 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh Y. & Jurkevitch E. Plastic phenotypic resistance to predation by Bdellovibrio and like organisms in bacterial prey. Environ Microbiol 6, 12–18, 10.1046/j.1462-2920.2003.00530.x (2004). [DOI] [PubMed] [Google Scholar]
- Kadouri D. E., To K., Shanks R. M. & Doi Y. Predatory bacteria: a potential ally against multidrug-resistant Gram-negative pathogens. PLoS One 8, e63397, 10.1371/journal.pone.0063397 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks R. M. et al. An eye to a kill: using predatory bacteria to control Gram-negative pathogens associated with ocular infections. PLoS One 8, e66723, 10.1371/journal.pone.0066723 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz R. W. & Hespell R. B. Attempts to grow Bdellovibrios micurgically-injected into animal cells. Arch Microbiol 119, 245–248 (1978). [Google Scholar]
- Boileau M. J., Clinkenbeard K. D. & Iandolo J. J. Assessment of Bdellovibrio bacteriovorus 109J killing of Moraxella bovis in an in vitro model of infectious bovine keratoconjunctivitis. Can J Vet Res 75, 285–291 (2011). [PMC free article] [PubMed] [Google Scholar]
- Shatzkes K. et al. Examining the safety of respiratory and intravenous inoculation of Bdellovibrio bacteriovorus and Micavibrio aeruginosavorus in a mouse model. Sci Rep 5, 12899, 10.1038/srep12899 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iebba V. et al. Higher prevalence and abundance of Bdellovibrio bacteriovorus in the human gut of healthy subjects. PLoS One 8, e61608, 10.1371/journal.pone.0061608 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med 6, 266ra172, 10.1126/scitranslmed.3009644 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. et al. Bioengineering organized, multilamellar human corneal stromal tissue by growth factor supplementation on highly aligned synthetic substrates. Tissue Eng Part A 19, 2063–2075, 10.1089/ten.TEA.2012.0545 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62, 293–300 (1951). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani G. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J Bacteriol 186, 595–600 10.1128/JB.186.3.595-600 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahme L. G. et al. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268, 1899–1902 10.1126/science.7604262 (1995). [DOI] [PubMed] [Google Scholar]
- Brothers K. M., Stella N. A., Romanowski E. G., Kowalski R. P. & Shanks R. M. EepR mediates secreted protein production, desiccation survival, and proliferation in a corneal infection model. Infect Immun , 10.1128/IAI.00466-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann S. et al. A quantitative rabbit model of vaccinia keratitis. Invest Ophthalmol Vis Sci 51, 4531–4540, 10.1167/iovs.09-5106 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. C., Bergmanson J. P. & Doughty M. J. Keratocytes: no more the quiet cells. J Am Optom Assoc 69, 180–187 (1998). [PubMed] [Google Scholar]
- de Bentzmann S. et al. Pseudomonas aeruginosa virulence factors delay airway epithelial wound repair by altering the actin cytoskeleton and inducing overactivation of epithelial matrix metalloproteinase-2. Lab Invest 80, 209–219 10.1038/labinvest.3780024 (2000). [DOI] [PubMed] [Google Scholar]
- Loryman C. & Mansbridge J. Inhibition of keratinocyte migration by lipopolysaccharide. Wound Repair Regen 16, 45–51, 10.1111/j.1524-475X.2007.00290.x (2008). [DOI] [PubMed] [Google Scholar]
- Brothers K. M. et al. Putting on the brakes: Bacterial impediment of wound healing. Sci Rep 5, 14003, 10.1038/srep14003 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Brothers K. M., Shanks R. M. & Kadouri D. E. Visualizing Bdellovibrio bacteriovorus by using the tdTomato fluorescent protein. Appl Environ Microbiol 82, 1653–1661, 10.1128/AEM.03611-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwudke D. et al. The obligate predatory Bdellovibrio bacteriovorus possesses a neutral lipid A containing alpha-D-Mannoses that replace phosphate residues: similarities and differences between the lipid As and the lipopolysaccharides of the wild type strain B. bacteriovorus HD100 and its host-independent derivative HI100. J Biol Chem 278, 27502–27512, 10.1074/jbc.M303012200 (2003). [DOI] [PubMed] [Google Scholar]
- Thomashow M. F. & Rittenberg S. C. Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: solubilization of Escherichia coli peptidoglycan. J Bacteriol 135, 998–1007 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon Y. J., Romanowski E. G. & McDermott A. M. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr Eye Res 30, 505–515, 10.1080/02713680590968637 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslani M., Movahedan A., Afsharkhamseh N., Sroussi H. & Djalilian A. R. The role of toll-like receptor 4 in corneal epithelial wound healing. Invest Ophthalmol Vis Sci 55, 6108–6115, 10.1167/iovs.14-14736 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroutsos G., Guimaraes R. & Pouliquen Y. The effect of concentrated antibiotics on the rabbit’s corneal epithelium. Int Ophthalmol 7, 65–69 10.1007/BF00165106 (1984). [DOI] [PubMed] [Google Scholar]
- Lin C. P. & Boehnke M. Effect of fortified antibiotic solutions on corneal epithelial wound healing. Cornea 19, 204–206 10.1097/00003226-200003000-00014 (2000). [DOI] [PubMed] [Google Scholar]
- Jassim S. H. et al. Bacteria colonizing the ocular surface in eyes with Boston Type 1 Keratoprosthesis: analysis of biofilm-forming capability and vancomycin tolerance. Invest Ophthalmol Vis Sci 56, 4689–4696, 10.1167/iovs.15-17101 (2015). [DOI] [PubMed] [Google Scholar]