Abstract
Sphingomyelin phosphodiesterase 3 (SMPD3), a lipid-metabolizing enzyme present in bone and cartilage, has been identified to be a key regulator of skeletal development. A homozygous loss-of-function mutation called fragilitas ossium (fro) in the Smpd3 gene causes poor bone and cartilage mineralization resulting in severe congenital skeletal deformities. Here we show that Smpd3 expression in ATDC5 chondrogenic cells is downregulated by parathyroid hormone-related peptide through transcription factor SOX9. Furthermore, we show that transgenic expression of Smpd3 in the chondrocytes of fro/fro mice corrects the cartilage but not the bone abnormalities. Additionally, we report the generation of Smpd3flox/flox mice for the tissue-specific inactivation of Smpd3 using the Cre-loxP system. We found that the skeletal phenotype in Smpd3flox/flox; Osx-Cre mice, in which the Smpd3 gene is ablated in both late-stage chondrocytes and osteoblasts, closely mimics the skeletal phenotype in fro/fro mice. On the other hand, Smpd3flox/flox; Col2a1-Cre mice, in which the Smpd3 gene is knocked out in chondrocytes only, recapitulate the fro/fro mouse cartilage phenotype. This work demonstrates that Smpd3 expression in both chondrocytes and osteoblasts is required for normal endochondral bone development.
INTRODUCTION
Sphingomyelin phosphodiesterase 3 (SMPD3) is a lipid-metabolizing enzyme present in the membranes of the endoplasmic reticulum and the inner leaflet of the cell membrane (1). Recently, this enzyme has been identified to be a key regulator of skeletal development (2–6). SMPD3 cleaves sphingomyelin and generates ceramides, a class of lipid second messengers, and phosphocholine, an important intermediate for a number of metabolic pathways (7–9).
Currently, there are two reported mouse models that lack functional SMPD3. These mouse models have been extensively used to study the physiological roles of this enzyme. Mice in the first model, the fro/fro mouse model, harbor a recessive mutation called fragilitas ossium (fro). It was generated by chemically inducing a deletion in the Smpd3 gene, leading to a complete loss of SMPD3 enzymatic activity (2). The second model, consisting of Smpd3−/− mice, in which Smpd3 was ablated in all the tissues, was generated by a gene-targeting method (2, 10, 11).
Stoffel et al. identified the skeletal abnormalities in Smpd3−/− mice to be a form of chondrodysplasia (10, 11). Their study suggested that neuronal SMPD3, through systemic means, regulates skeletal development. On the other hand, Aubin et al. reported that the fro mutation results in osteogenesis and dentinogenesis imperfecta (2). Although the skeletal deformities were somewhat similar in the two mouse models, they were more severe in fro/fro mice than in Smpd3−/− mice. Moreover, fro/fro mice showed bone and tooth mineralization defects, which were not investigated in Smpd3−/− mice (5, 12). The phenotypic discrepancies between these two mouse models demand further investigation of the tissue-specific roles of SMPD3 during skeletogenesis.
Our group previously reported that in the developing and adult skeletal tissues, Smpd3 is highly expressed in both cartilage and bone. We showed that the restoration of Smpd3 expression in the osteoblasts of fro/fro; Col1a1-Smpd3 mice is sufficient to correct the bone mineralization defects. However, the cartilage abnormalities, which include an expanded zone of hypertrophic chondrocyte-like cells and poor mineralization of the cartilage matrix surrounding these cells in the growth plate, persist in this model (5). These findings suggest that SMPD3 may have distinct roles in bone and cartilage development. At present, the regulation of Smpd3 expression in chondrocytes is not fully understood. Also, the relative contribution of chondrocyte-derived SMPD3 in skeletal development is still unknown.
Here we show that SOX9, a key transcription factor involved in chondrogenesis, regulates Smpd3 expression in ATDC5 chondrogenic cells (13). We performed further analyses of the fro/fro mouse developing endochondral bones to show that the expression of major chondrocyte differentiation markers was largely unaffected in the growth plates. Also, proliferation of growth plate chondrocytes was not altered in the fro/fro mice. In order to investigate the role of chondrocyte-derived SMPD3 in skeletal development, we followed two different genetic approaches. First, we generated a transgenic mouse model, fro/fro; Acan-Smpd3 mice, to restore Smpd3 expression specifically in the chondrocytes. This corrected the cartilage abnormalities but not the bone mineralization defects. Next, we generated two conditional knockout models, Smpd3flox/flox; Osx-Cre and Smpd3flox/flox; Col2a1-Cre mice, to ablate Smpd3 in both late-stage chondrocytes and osteoblasts together or only in chondrocytes, respectively (14, 15). The Osx-Cre-mediated ablation of Smpd3 resulted in a skeletal phenotype very similar to that in fro/fro mice. On the other hand, Col2a1-Cre-mediated ablation of Smpd3 resulted in a cartilage phenotype that mimicked that in the fro/fro mice, without causing any bone mineralization defects.
In the current study, we investigated the regulation of Smpd3 expression in chondrocytes and determined the relative contribution of chondrocyte-derived SMPD3 in bone development. This work will help us understand how the specific yet synergistic actions of this enzyme in two different skeletal tissues, bone and cartilage, facilitate normal skeletal development.
MATERIALS AND METHODS
DNA constructs.
The DNA construct for chondrocyte-specific expression of the Smpd3 transgene was generated using a 2.6-kb proximal promoter fragment of the aggrecan gene (Acan) (16). A full-length Smpd3 cDNA (American Type Culture Collection) preceded by the rabbit β-globin intron was inserted between the Acan promoter fragment and a simian virus 40 (SV40) polyadenylation signal. The transgene was released from the plasmid backbone by XbaI restriction digestion and was used for pronuclear injection. For Smpd3 promoter studies, a 1.9-kb mouse Smpd3 proximal promoter was cloned into the SmaI and SalI sites in the multiple-cloning site of the pGL4.10 (luc2) vector (Promega, Madison, WI). Sequences corresponding to peak 1 and peak 2, the SOX9-interacting regions found in intron 1 of the rat Smpd3 gene (17), were amplified and cloned in front of the proximal promoter sequence using the following primers (primers Peak 1/F, Peak 1/R, Peak 2/F, and Peak 2/R, respectively): 5′-CACACTACCTCTTCTTTGAGCCTG-3′, 5′CCAGTGTTCCTGACCACAGAT-3′, 5′-TAGGAGCAATCCAATCAGAGC-3′ and 5′-TGGTCTGGCCTCCATTCACTG-3′.
Screening of the ES cell clones.
The genomic DNA used to generate the Smpd3 targeting construct was amplified by PCR from R1 mouse embryonic stem (ES) cells and modified in vitro. ES cells were transfected by the NotI-linearized construct and selected in the presence of a neomycin analogue, G418 at the McIntyre Cancer Center Transgenic Core Facility at McGill University. A total of 500 G418-resistant clones were picked and screened by Southern blotting and PCR. Southern blotting was performed as described previously (18). The detection of the lone loxP site (the third loxP site) inserted within the Smpd3 3′ untranslated region was determined using the following primers (primers F1 and R1, respectively): 5′-ATGCTATACGAAGTTATGACGTC-3′ and 5′-AGAGAAGGATGCGGCTCTTAC-3′ (amplicon size, 650 bp).
Mice.
The generation and genotyping of fro/fro mice and Col1a1-Smpd3 transgenic mice were described previously (2). Genotypes were determined by PCR on genomic DNA isolated from tail biopsy specimens. The Acan-Smpd3 transgene integration was detected using the following primer pair specific for the Acan promoter region: 5′-CCAGGGTTTCCTTGATGATG-3′ and 5′-CCGGTTTGGACTCAGAGTAT-3′ (amplicon size, ~610 bp). The Col2a1-Cre and Osx-Cre mice were obtained from The Jackson Laboratory (stock numbers 003554 and 006361, respectively). The Cre transgene was detected using the following primer pair: 5′-GCCTGCATTACCGGTCGATGCAACGA-3′ and 5′-GTGGCAGATGGCGCGGCAACACCCATT-3′ (amplicon size, ∼700 bp). The presence of the floxed allele in the gene-targeted mice was confirmed by use of the following primers (primers F2 and R2, respectively): 5′-TCAGAGAACGGAGGCTGATC-3′ and 5′-AAGAGACCGTTGGGAATACC-3′ (amplicon sizes, 106 bp for the wild-type [WT] allele and 140 bp for the floxed allele). All animal procedures were reviewed and approved by the McGill University Institutional Animal Care and Use Committee following the guidelines of the Canadian Council on Animal Care.
In order to detect the Cre-mediated deletion, DNA was isolated from tissues collected from Smpd3flox/flox, Smpd3flox/flox; Col2a1-Cre, and Smpd3flox/flox; Osx-Cre mice at embryonic day 15.5 (E15.5) by using the phenol-chloroform method. A multiplexed PCR with an equal amount of template DNA was performed using the F2 and R2 primers mentioned above. The efficiency of deletion of the floxed sequence was determined by normalizing the band intensity to that of the Cre PCR.
Skeletal tissue preparation.
Skeletal tissues from newborn and adult mice were fixed overnight in 95% ethanol, stained by 0.015% alcian blue (AB) dye (Sigma-Aldrich) in a 1:4 solution of glacial acetic acid and absolute ethanol for 24 h, and treated with 2% potassium hydroxide until the soft tissues were dissolved. The mineralized tissues were stained by 0.005% alizarin red S (Sigma-Aldrich; hereafter referred to as alizarin red) solution in 1% potassium hydroxide and clarified in 1% potassium hydroxide–20% glycerol for >2 days.
Histology and imaging.
Mouse embryos were fixed in 4% paraformaldehyde (PFA)–phosphate-buffered saline (PBS) (pH 7.4) overnight and embedded in paraffin. Sections of the humeri 7 μm thick were processed for von Kossa (VK), alcian blue, and van Gieson (VG) staining. For immunohistochemistry, deparaffinized sections were blocked with 5% bovine serum albumin (BSA; Fisher, Pittsburgh, PA) in Tris-buffered saline (TBS)–Triton X-100, followed by incubation with anti-SOX9 (catalog number H-90; Santa Cruz Biotechnology), antiaggrecan (Abcam), anti-type II collagen (Abcam), anti-type X collagen (Abcam), or anti-SMPD3 (Santa Cruz Biotechnology) antibody. The samples were further incubated with horseradish peroxidase (HRP)-conjugated goat secondary antibody (1:1,000; Abcam), and then a colorimetric reaction was carried out with 3,3-diaminobenzidine and 0.02% H2O2, followed by counterstaining with hematoxylin. For plastic sectioning, vertebrae were fixed overnight in 4% PFA–PBS, embedded in methyl methacrylate, and sectioned (thickness, 7 μm), and VK-VG staining was applied. Unmineralized bone sections were analyzed using Osteomeasure software (Osteometrics Inc.).
For cell proliferation analyses, pregnant mice were injected intraperitoneally with 0.5 ml of a 50 mM solution of 5-bromo-2′-deoxyuridine (BrdU). The mice were sacrificed an hour later, and the embryos were collected at E16.5. For immunostaining, the humeri were embedded in paraffin and sectioned (thickness, 7 μm). Sections were blocked with 5% BSA in TBS–Triton X-100, followed by incubation with the biotinylated anti-BrdU antibody (Abcam). Detection was by streptavidin-HRP (1:1,000; Abcam). BrdU-positive nuclei in the proliferating region of the humeri were quantified using Infinity Analyze software.
Images were taken at room temperature using a light microscope (model DM200; Leica) with a 20× (numerical aperture, 0.40), 40× (numerical aperture, 0.65), or 5× (numerical aperture, 0.11) objective. All histological images were captured using a camera (model DP72; Olympus), acquired with DP2-BSW software (XV3.0; Olympus), and processed using Photoshop software (Adobe).
Radiographic and micro-CT analyses.
Radiography and micro-computed tomography (micro-CT) analyses of the skeletal samples were performed at the Centre for Bone and Periodontal Research Core Facility at McGill University using an Xpert X-ray imaging system (Kubtec, Milford, CT) and a micro-CT system (SkyScan), respectively. The X-ray source was operated at 21 kV and at 550 μA. Samples were scanned at a magnification of ×4 (the highest magnification). Micro-CT analyses of the tibias were performed as described previously (5). Long bones (femurs and tibias) were dissected individually and measured with a digital slide caliper (Mastercraft).
Cell culture.
ATDC5 cells were cultured in alpha minimal essential medium (alpha-MEM; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; PAA Laboratories) and 100 U/ml of penicillin-streptomycin at 37°C under 5% CO2 in a humidified incubator. To induce chondrogenic differentiation, subconfluent cultures were changed to the medium containing ascorbic acid (100 μg/ml) and β-glycerol phosphate (5 mM). After culturing in the differentiation medium for 6 days, ATDC5 cells were treated with 5 μg/ml of Indian hedgehog (IHH) (catalog number 1705-HH; R&D), 0.1 μM parathyroid hormone-related peptide (PTHrP; catalog number SRP4651; Sigma), and 25 μM forskolin (Sigma) for 1 h and then were subjected to gene expression analysis. ATDC5 cells were transfected with 500 ng of a Sox9 expression vector using a MicroPorator system (pulse voltage, 1,750, pulse width, 20; pulse number, 1; Digital Bio, Seoul, South Korea) and cultured for 48 h before the gene expression analysis. Rat chondrosarcoma (RCS) cells and HEK293 cells were cultured in Dulbecco's modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 10% FBS (PAA Laboratories) and 100 U/ml of penicillin-streptomycin at 37°C under 5% CO2 in a humidified incubator.
Transfection of siRNA.
Mission small interfering RNA (siRNA) transfection reagent, endoribonuclease-prepared siRNA (esiRNA) targeting mouse Sox9 and Renilla luciferase (control) were purchased from Sigma-Aldrich. ATDC5 cells were plated at a density of 200,000 cells per well in a 6-well plate 24 h before transfection. Six microliters of the siRNA was added to 200 μl of undiluted DMEM. Then, 10 μl of Mission siRNA transfection reagent was added to the siRNA mixture. The final concentration of siRNA was 38 nM. The remaining procedure was performed according to the supplier's instructions.
Primary osteoblast isolation and culture.
Osteoblast isolation from the calvaria and long bones of a 4-month-old fro/fro; Acan-Smpd3 mouse and its control mouse was performed as described previously (19). Briefly, the calvaria and long bones were dissected and subjected to 2 consecutive digestions in alpha-MEM containing 0.25% trypsin–0.1% EDTA and 50 μg/ml or 100 μg/ml collagenase P (Roche). The tissue was incubated at 37°C with shaking. The supernatant was discarded, and fresh digestion medium containing 100 μg/ml collagenase II (BioShop) and 0.25% trypsin–0.1% EDTA was added. The tissue was incubated at 37°C with vigorous shaking every 10 min for 60 min. The bone pieces and cells were collected by centrifugation (1,300 × g), washed with alpha-MEM, and plated in a 10-cm dish with alpha-MEM containing 10% FBS and 100 U/ml of penicillin-streptomycin. After 5 days, the bone cells migrated from the bone pieces and the medium was changed.
In vitro differentiation of primary osteoblasts and alizarin red staining for mineral deposition quantification were performed as described previously (20).
Calcein staining for mineral deposition analysis.
Primary osteoblasts were cultured in differentiation medium for 8 days. The cells were then fixed in 10% formalin–PBS buffer and stained with 0.25% calcein solution. After fixation, the cells were washed with calcein buffer (0.15 M NaCl–2% NaHCO3) and stained with Hoechst stain (catalog number H33258; Sigma-Aldrich). Images were captured by a EVOS FL cell imaging system at a ×40 magnification (Life Technologies).
Gene expression analysis.
Gene expression analysis was performed using a quantitative real time-PCR (qRT-PCR) system (model 7500; Applied Biosystems). Total RNA was extracted from the cells or limbs of embryonic day 14.5 mice with TRIzol reagent (catalog number 15596018; Ambion) and subjected to DNase I (catalog number M0303S; Applied Biosystems) treatment. The first-strand cDNA synthesis and qRT-PCR were performed using a high-capacity cDNA reverse transcription kit (Applied Biosystems) and SYBR green quantitative PCR master mix (Maxima; Fermentas), respectively. Relative gene expression analysis was performed as described previously (21). The primer sequences for Runx2, Sox9, Col10a1, Smpd3, Alpl, Col1a1, and Osx were the same as those published previously (5, 21). The primers F2 and R2 mentioned above were used to detect third loxP site expression in the Smpd3 gene.
Immunocytochemistry.
ATDC5 cells were cultured on glass chamber slides under differentiating conditions for 2 days and then subjected to treatment with either 0.1 μM PTHrP or 25 μM forskolin. After 48 h of treatment, cells were fixed in paraformaldehyde for 10 min on ice. Fixed cells were blocked with 5% BSA–TBST for 30 min and incubated with anti-SMPD3 antibody (Santa Cruz Biotechnology). Detection was by horseradish-peroxidase-conjugated secondary antibody from Abcam (Cambridge, MA).
Luciferase assay.
RCS cells were cotransfected with 500 ng of the Smpd3 reporter plasmids and 50 ng of the Renilla luciferase (R-Luc) plasmid using a MicroPorator system (pulse voltage, 1,100; pulse width, 20; pulse number, 1; Digital Bio, Seoul, South Korea). ATDC5 cells and HEK293 cells were cotransfected with 500 ng of the plasmid combinations indicated below together with 10 ng of the R-Luc plasmid (for ATDC5 cells, pulse voltage, 1,750; pulse width, 20; pulse number, 1; for HEK293 cells, pulse voltage, 1,300; pulse width, 10; pulse number, 3). Luciferase activity was measured at 48 h after transfection using a dual-luciferase reporter assay system (Promega, Madison, WI), as described before (20).
ChIP assay.
A chromatin immunoprecipitation (ChIP) sequencing (ChIP-seq) assay was performed as described previously (17). The ChIP assay was performed using an EZ chromatin immunoprecipitation kit (Millipore) following the manufacturer's protocol. An anti-SOX9 antibody (catalog number ab5535; Millipore) and control normal rabbit IgG antibody (catalog number sc-2027; Santa Cruz) were used for this assay. The following primers were used for PCR: 5′-AAGACATAGCCATACGTGAAAT-3′ and 5′-CCAGTGTTCCTGACCACAGAT-3′ amplified a 182-bp fragment for peak 1, while 5′-TCAACACCAAGGCAAGGAGA-3′ and 5′-TGGTCTGGCCTCCATTCACTG-3′ amplified a 228-bp fragment for peak 2.
Immunofluorescence.
Paraffin sections of humeri (7 μm) were subjected to antigen retrieval using 0.25 U/ml of chondroitinase ABC (Sigma-Aldrich) and blocked with 5% BSA in TBS–Triton X-100, followed by incubation with anti-type X collagen antibody from Abcam. Localization was detected with Alexa Fluor 488-conjugated AffiniPure donkey anti-rabbit immunoglobulin antibody from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). After washing 3 times with PBS, the nuclei were visualized with Hoechst stain (catalog number H33258; Sigma-Aldrich). Images were captured by use of the EVOS FL cell imaging system at a magnification of ×10 (Life Technologies).
TUNEL assay.
To analyze apoptosis, a terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay was performed using a TACS 2 TdT-DAB in situ apoptosis detection kit (Trevigen, Gaithersburg, MD) according to the manufacturer's instructions. Samples were counterstained with 1% methyl green, dehydrated, and mounted. TUNEL-positive cells were visualized (model DM200; Leica) with a 20× (numerical aperture, 0.40) objective and quantified.
Statistical analysis.
All results are shown as means ± standard deviations. Statistical analyses were performed by Student's t test or analysis of variance (Tukey's multiple-comparison test).
RESULTS
SOX9 regulates Smpd3 expression in chondrocytes.
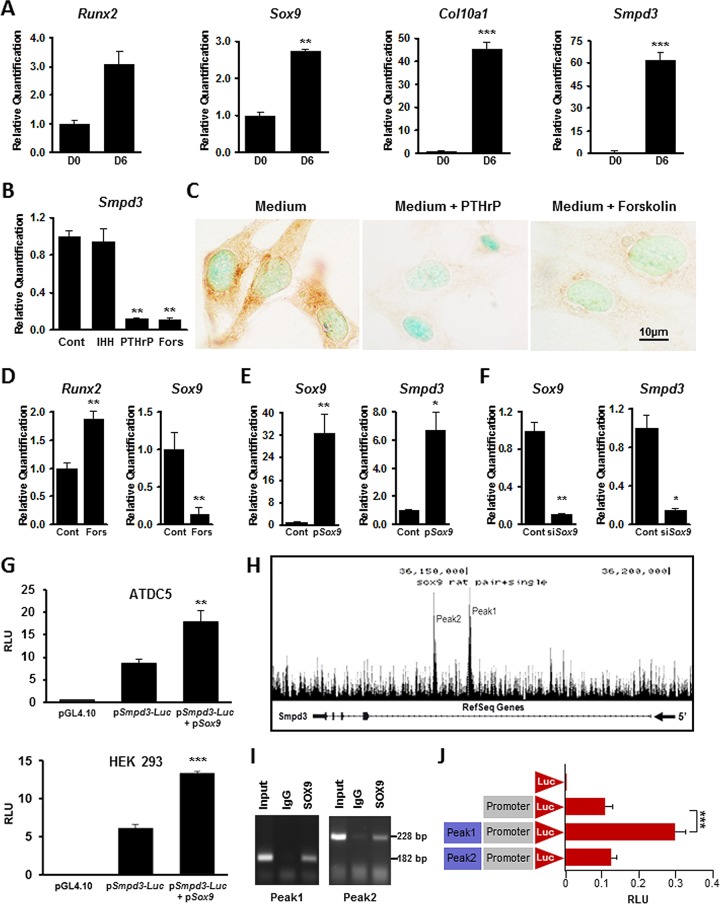
We used mouse ATDC5 cells to investigate the regulation of Smpd3 expression. These chondrogenic cells, when cultured under differentiating conditions for 6 days, showed upregulation of Runx2, Sox9, Col10a1, and Smpd3 expression (Fig. 1A). We next examined how Indian hedgehog (IHH) and parathyroid hormone-related peptide (PTHrP), two major signaling molecules involved in chondrogenesis and growth plate development, regulate the expression of Smpd3 (22). Treatment of differentiated ATDC5 cell cultures with IHH (5 μg/ml) for 1 h did not affect Smpd3 expression, while treatment with PTHrP (0.1 μM) or its surrogate, forskolin (25 μM), for 1 h markedly decreased Smpd3 expression (Fig. 1B). We confirmed these findings by immunocytochemistry using an anti-SMPD3 antibody; the SMPD3 protein was almost undetectable in differentiated ATDC5 cells after 48 h of treatment with PTHrP or forskolin (Fig. 1C).
FIG 1.
Regulation of Smpd3 gene expression in vitro. (A) qRT-PCR analysis showing a significant upregulation of Smpd3 and chondrogenic markers Runx2, Sox9, and Col10a1 in differentiated ATDC5 cells. D0, day 0; D6, day 6. (B) Gene expression analysis showing a significant decrease in Smpd3 expression in ATDC5 cells treated with either 0.1 μM PTHrP or 25 μM forskolin. There was no effect on Smpd3 expression in cells treated with 0.5 μg/ml of IHH. (C) Immunocytochemistry using anti-SMPD3 antibody on differentiated ATDC5 cells treated with 0.1 μM PTHrP or 25 μM forskolin, showing the downregulation of SMPD3 expression. (D) Gene expression analysis showing the upregulation of Runx2 expression but a significant downregulation of Sox9 expression in ATDC5 cells treated with 25 μM forskolin. (E) Transfection of ATDC5 cells with a Sox9 expression vector (pSox9) increased both Sox9 and Smpd3 expression. (F) Sox9 knockdown by siRNA (siSox9) significantly decreased both Sox9 and Smpd3 gene expression in chondrogenic ATDC5 cells. (G) Luciferase assay showing the basal activation of the Smpd3 promoter in both ATDC5 (top) and HEK293 (bottom) cells. Cotransfection experiments with pSox9 resulted in a significant upregulation of the Smpd3 promoter activity in these cell types. (H) ChIP-seq analysis showing the presence of two distinct peaks, peak 1 and peak 2, in intron 1 of the rat Smpd3 gene. (I) ChIP assay showing the presence of 182-bp and 228 bp fragments corresponding to the SOX9 binding regions in peak 1 and peak 2, respectively. (J) Luciferase assay showing an almost 3-fold increase of the mouse Smpd3 promoter activity in the presence of a sequence corresponding to peak 1 but not in the presence of a sequence corresponding to peak 2 in RCS cells. Cont, control; Fors, forskolin; RLU, relative light units. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Two transcription factors, RUNX2 and SOX9, play major roles in chondrogenesis (23). In a previous study, RUNX2 was shown to affect Smpd3 expression in C2C12 myoblasts (24). In order to identify the regulatory roles of these transcription factors in Smpd3 expression in ATDC5 cells, we examined how their expression is affected by PTHrP signaling. We found that while treatment with forskolin for 1 h increased Runx2 expression by almost 2-fold, there was almost a 5-fold decrease of Sox9 expression (Fig. 1D). This observation suggested that PTHrP-mediated suppression of Smpd3 expression in ATDC5 cells may involve SOX9. Indeed, we observed that the transient transfection of ATDC5 cells with a Sox9 expression vector (pSox9) increased Smpd3 expression by 48 h (Fig. 1E). On the other hand, when Sox9 was knocked down using siRNA in ATDC5 cells, Smpd3 expression was significantly decreased (Fig. 1F). This indicates that Sox9 is an important regulator of Smpd3 expression in chondrocytes.
We next examined whether SOX9 can upregulate Smpd3 proximal promoter activity. For this purpose, we generated a reporter construct (pSmpd3-Luc) by inserting a 1.9-kb Smpd3 promoter fragment (nucleotides −1 to −1929) upstream of the firefly luciferase gene. Transfection of this construct into ATDC5 cells resulted in a 10-fold increase of luciferase activity. This was further amplified by at least 2-fold when the reporter construct was cotransfected with a Sox9 expression vector (Fig. 1G, top). In a separate transfection experiment, we found that the proximal Smpd3 promoter is active in HEK293 cells. We also observed upregulation of the luciferase activity when these cells were cotransfected with the reporter construct and the Sox9 expression vector (Fig. 1G, bottom).
Recently, we performed ChIP-seq analyses using rat chondrosarcoma (RCS) cells and reported the genome-wide distribution of SOX9 binding sequences (17, 25). These analyses showed two prominent peaks (peaks 1 and 2), indicating two distinct SOX9 binding regions within intron 1 of the rat Smpd3 gene (Fig. 1H). In order to further validate these findings, we performed a ChIP assay using an anti-SOX9 antibody on RCS cell nuclear extracts and performed sequence-specific PCR covering these two regions. As shown in Fig. 1I, primers specific for both the suggested SOX9 binding regions in intron 1 of the Smpd3 gene resulted in amplicons of the expected sizes.
We then examined the ability of the suggested SOX9 binding regions to regulate the activity of the mouse Smpd3 promoter. For this purpose, the regions of intron 1 identified by the ChIP-Seq assay to be distinct peaks were PCR amplified from RCS cell DNA and cloned upstream of the Smpd3 promoter fragment in the luciferase reporter construct described above. Transfection experiments using RCS cells showed that the DNA fragment corresponding to peak 1 but not that corresponding to peak 2 resulted in an approximately 3-fold increase of Smpd3 promoter activity (Fig. 1J).
Chondrocyte differentiation and proliferation are not impaired in the developing fro/fro endochondral bones.
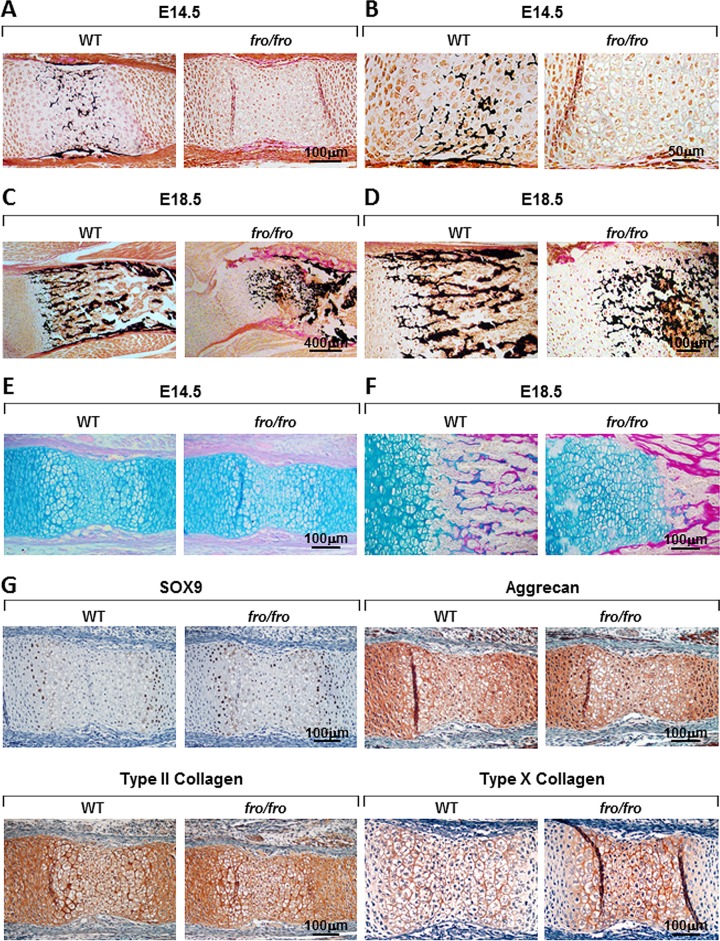
The bone and cartilage abnormalities in fro/fro mice appear during the early stages of skeletal development (5). We examined the progression of the growth plate cartilage abnormalities in the developing endochondral bones of fro/fro embryos by investigating their mineralization status, expression of chondrogenic markers, and proliferation of the chondrocytes. Von Kossa (VK) and van Gieson (VG) staining of humerus sections from embryonic day 14.5 (E14.5) fro/fro embryos showed a complete lack of minerals in the hypertrophic zones (Fig. 2A and B). Additionally, alcian blue (AB; which stains proteoglycans) and VG staining of demineralized bone sections showed comparable patterning of the cartilaginous anlagen in both wild-type (WT) and fro/fro embryos at this stage (Fig. 2E). We next performed the same analyses on the humerus sections from E18.5 WT and fro/fro embryos (Fig. 2C and D). Although the presence of mineralized extracellular matrix (ECM) is evident at this stage, AB-VG staining showed an expanded hypertrophic zone in fro/fro humerus sections (Fig. 2F).
FIG 2.
Cartilage phenotype of fro/fro mice. (A and C) VK-VG staining of the humerus sections from E14.5 (A) and E18.5 (C) WT and fro/fro embryo. fro/fro humeri show a delay in mineralization in the bone and cartilage at E14.5. (B and D) Magnified views of panels A and C, respectively. (E and F) AB-VG staining of the humerus sections from E14.5 (E) and E18.5 (F) WT and fro/fro embryo showing an abnormally expanded zone of hypertrophic chondrocytes in E18.5 fro/fro humeri. (G) Immunostaining of humerus sections from E14.5 WT and fro/fro embryos using anti-SOX9, antiaggrecan, and anti-type II collagen antibodies, showing that there is no noticeable difference in early chondrogenic differentiation markers in WT and fro/fro embryos. Note the increased accumulation of type X collagen in fro/fro humeri upon staining with an anti-type X collagen antibody.
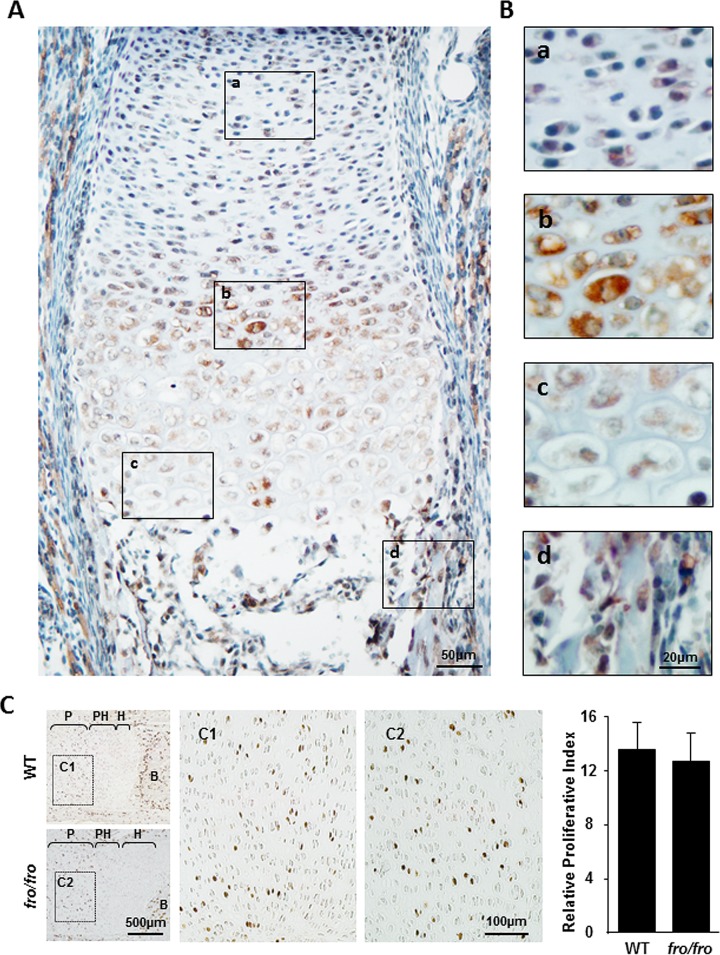
We compared the expression of various chondrogenic markers in the humerus sections of E14.5 WT and fro/fro embryos. Immunostaining of early chondrocyte markers, such as SOX9, aggrecan, and type II collagen, in the humeri of fro/fro mice indicated that chondrocyte differentiation is grossly unaffected in these mice compared to their WT counterparts. However, staining of type X collagen, a late marker of chondrocyte differentiation, was slightly more intense in the humeri of fro/fro embryos than in those of the WT embryos (Fig. 2G). We observed that SOX9 immunostaining was mostly localized in the nuclei of the prehypertrophic chondrocytes. Interestingly, in the growth plate, SMPD3 immunostaining was also most intense in this population of cells (Fig. 3A and B). In the developing bone, SMPD3 was detected in the osteoblasts both in the marrow space and in the bone collar. However, SMPD3 was not highly expressed in the proliferating zone of the growth plate, which suggests that this enzyme may not play a role in chondrocyte proliferation. We examined this possibility by labeling the proliferating chondrocytes of E16.5 embryos with BrdU. The numbers of BrdU-positive nuclei were comparable in WT and fro/fro humerus sections (Fig. 3C).
FIG 3.
Effects of Smpd3 expression on chondrocyte proliferation in fro/fro mice. (A and B) Immunostaining of a humerus section from an E15.5 WT embryo using an anti-SMPD3 antibody. High levels of SMPD3 expression are seen in the prehypertrophic region (b), while lower levels of expression are seen in the proliferating region (a) and the hypertrophic zone (c) of the growth plate. Strong Smpd3 expression was also detected in the osteoblasts (d). (B) Magnified views of the boxed areas in panel A. (C) Immunostaining of humerus sections from E16.5 WT and fro/fro embryos using an anti-BrdU antibody. P, proliferating zone; PH, prehypertrophic zone; H, hypertrophic zone; B, bone. (C1 and C2) Magnified views of the boxed areas in the proliferating zones of the humeri. (Right) Quantification of BrdU-positive nuclei shows that there is no significant difference in chondrocyte proliferation between WT and fro/fro mice.
Restoration of Smpd3 expression in chondrocytes partly corrects the skeletal abnormalities in fro/fro mice.
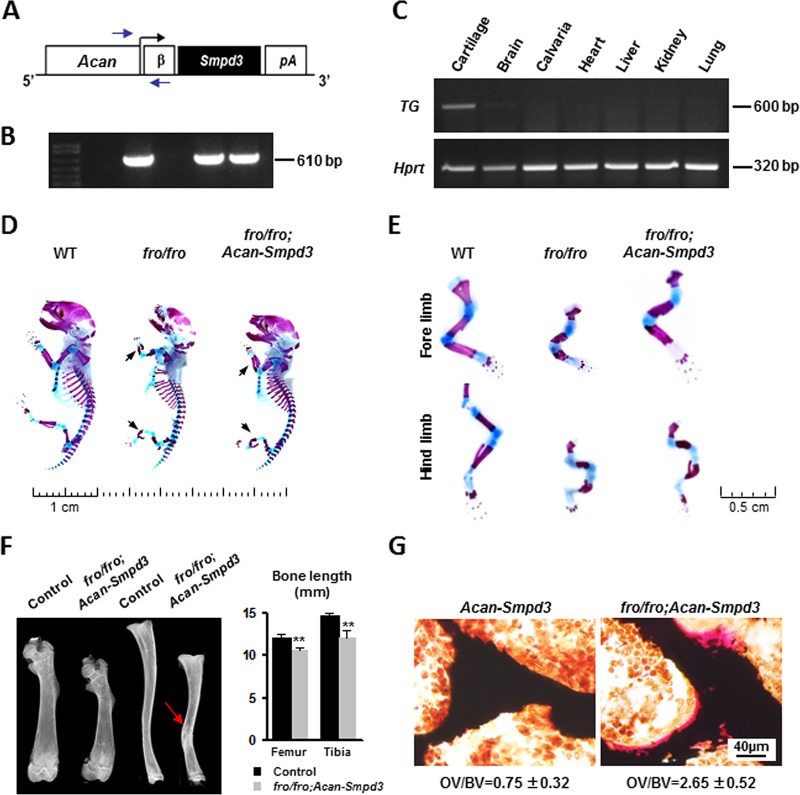
We expressed Smpd3 in chondrocytes under the control of a previously characterized Acan proximal promoter (Fig. 4A) (16). The presence of the Acan-Smpd3 transgene was verified by genotyping PCR using primers that annealed at the Acan promoter and the β-globin intron (Fig. 4B). The expression of Smpd3 in cartilage was confirmed by semiquantitative PCR using transgene-specific primers annealing within the SV40 polyadenylation signal sequence (Fig. 4C).
FIG 4.
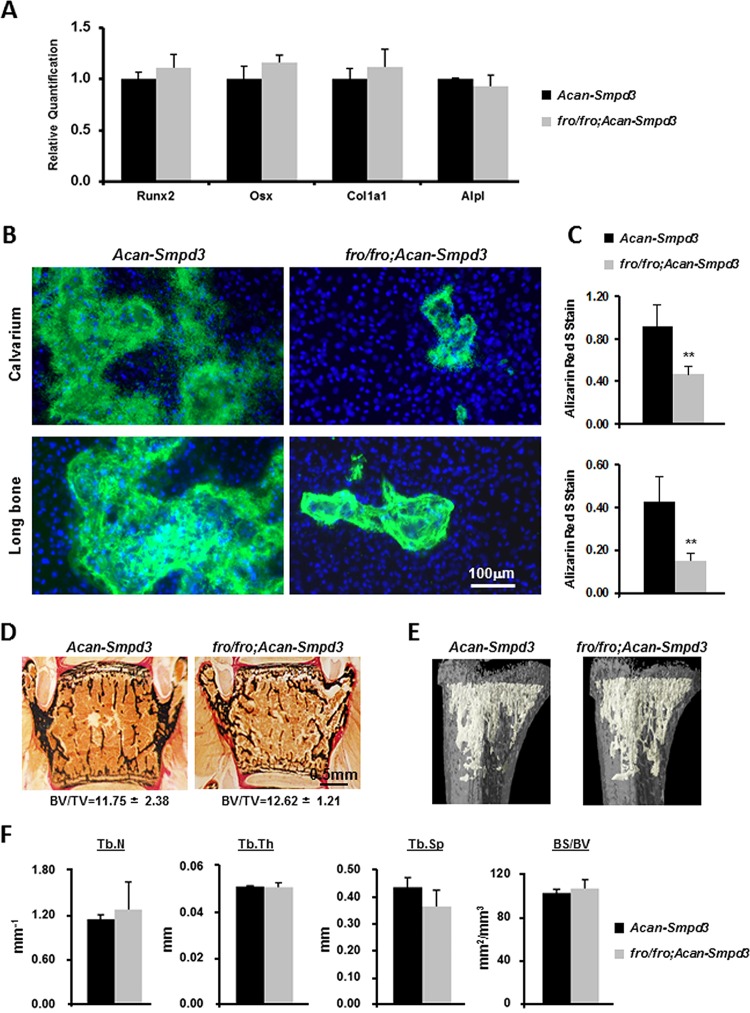
Generation and characterization of fro/fro; Acan-Smpd3 mice. (A) Schematic representation of the Acan-Smpd3 transgene construct. Arrows, positions of the primers used for genotyping; β, β-globin intron; pA, SV40 polyadenylation signal sequence. (B) A 610-bp amplicon was obtained from the PCR analysis of the transgenic DNA. (C) (Top) Semiquantitative RT-PCR analysis confirming the cartilage-specific expression of the transgene (TG). (Bottom) Hprt expression is shown as a loading control for cDNA. (D) Skeletal preparations of WT, fro/fro, and fro/fro; Acan-Smpd3 newborn pups stained with alizarin red and alcian blue show that in fro/fro; Acan-Smpd3 mice there is a partial correction of the skeletal deformities seen in fro/fro mice. Arrows, limb deformities. (E) Magnified view of the forelimbs and hind limbs of skeletal preparations of newborn WT, fro/fro, and fro/fro; Acan-Smpd3 pups. (F) X-ray analysis of the femur and tibia of 1-month-old mice showing that the long bones of fro/fro; Acan-Smpd3 mice are significantly shorter in length and slightly bent (arrow) compared to the control bones. **, P < 0.01. (G) Vertebral sections of 1-month-old mice stained with VK-VG showing a higher osteoid (unmineralized bone) volume over bone volume (OV/BV) in fro/fro; Acan-Smpd3 mice than the control mice.
We next crossed Acan-Smpd3 mice with +/fro mice to first generate +/fro; Acan-Smpd3 mice, which were then mated with +/fro mice to generate the compound fro/fro; Acan-Smpd3 mice in the F2 generations. Analysis of the skeletal phenotype by alcian blue (which stains the cartilage matrix) and alizarin red (which stains mineralized tissue) staining of newborn fro/fro; Acan-Smpd3 mice showed a partial correction of the skeletal phenotype seen in fro/fro mice (Fig. 4D and E). This was also confirmed by X-ray analysis of the femur and tibia of 1-month-old fro/fro; Acan-Smpd3 mice, in which the limbs were significantly shorter and slightly bent compared to those of the control mice (Fig. 4F). Furthermore, trabecular bones in the vertebrae of 1-month-old fro/fro; Acan-Smpd3 mice showed the presence of a significant amount of osteoid (unmineralized bone) compared to that seen in control mice (Fig. 4G). In order to validate this observation further, we isolated primary osteoblasts from the calvaria and long bones of both Acan-Smpd3 and fro/fro; Acan-Smpd3 mice. These cells were differentiated and examined for osteoblast marker genes Runx2, Osx (Sp7), Col1a1, and Alpl expression by qRT-PCR. There was no alteration of marker gene expression in fro/fro; Acan-Smpd3 osteoblasts (Fig. 5A). In agreement with the in vivo findings, we found that mineral accumulation in fro/fro; Acan-Smpd3 cultures was significantly lower than that in Acan-Smpd3 cultures (Fig. 5B and C).
FIG 5.
Osteoblast activity, histology, and micro-CT analyses of fro/fro; Acan-Smpd3 mice. (A) qRT-PCR analyses of osteoblast differentiation marker genes Runx2, Osx (Sp7), Col1a1, and Alpl in mouse primary osteoblasts isolated from the long bones of fro/fro; Acan-Smpd3 and control Acan-Smpd3 mice. Mouse primary osteoblasts were differentiated for 6 days. The expression of the marker genes was comparable between the two genotypes. (B) Calcein and Hoechst staining showing a reduction of mineral deposition in cultures of primary osteoblasts isolated from the calvaria and long bones of fro/fro; Acan-Smpd3 mice compared to that of control Acan-Smpd3 mice. (C) Spectrophotometric measurements of the deposited minerals after alizarin red staining of the mouse primary osteoblasts cultured under differentiating conditions. **, P < 0.01. (D) Vertebral sections of 1-month-old Acan-Smpd3 and fro/fro; Acan-Smpd3 mice stained by VK-VG. (E) Micro-CT images showing the morphology of the trabecular bones of Acan-Smpd3 and fro/fro; Acan-Smpd3 mice. (F) The trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular space (Tb.Sp), and bone surface over bone volume (BS/BV) were not significantly different between Acan-Smpd3 and fro/fro; Acan-Smpd3 mice.
We next performed histomorphometric analyses of the vertebral bone sections and found that the bone volume over tissue volume (BV/TV) in 1-month-old fro/fro; Acan-Smpd3 mice was comparable to that in the control mice (Fig. 5D). This observation was further confirmed by micro-CT analyses, as none of the trabecular bone parameters examined (trabecular number, trabecular thickness, trabecular space, and bone surface over bone volume) were affected (Fig. 5E and F).
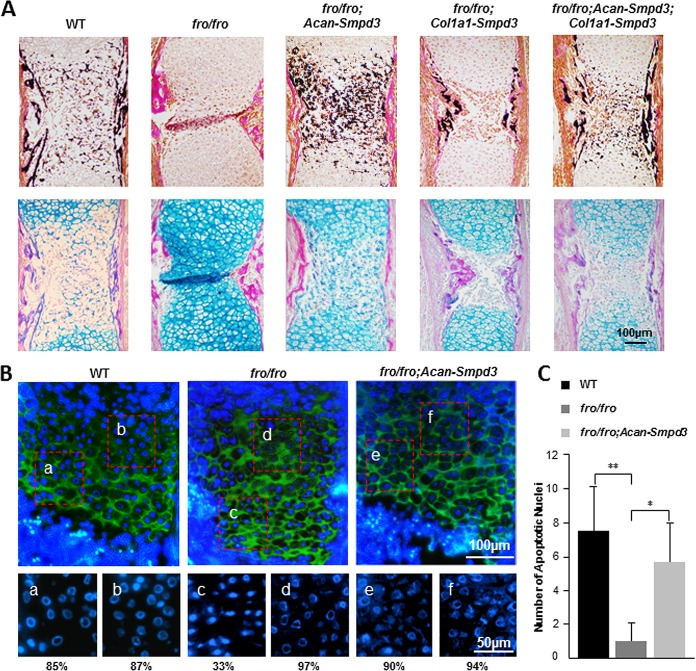
We also examined endochondral bone development in fro/fro; Acan-Smpd3 embryos. Histological analyses of E15.5 humeri of fro/fro; Acan-Smpd3 mice showed that the restoration of Smpd3 expression in the chondrocytes corrected the mineralization defects seen in the cartilaginous part of the midshaft region. However, the surrounding bone collar remained unmineralized. Earlier, we reported that the mineralization of the bone collar is normal in the humeri of fro/fro; Col1a1-Smpd3 mice, in which Smpd3 expression was restored in the osteoblasts only (5). In agreement with this finding, restoration of Smpd3 expression in both chondrocytes and osteoblasts in a complex model, fro/fro; Acan-Smpd3; Col1a1-Smpd3 mice, showed normal mineralization of both bone and cartilaginous tissues in the developing humeri (Fig. 6A).
FIG 6.
Analyses of the growth plate phenotype in fro/fro; Acan-Smpd3 mice. (A) VK-VG and AB-VG staining of the humerus sections from E15.5 WT, fro/fro, fro/fro; Acan-Smpd3, fro/fro; Col1a1-Smpd3, and fro/fro; Acan-Smpd3; Col1a1-Smpd3 embryos. The abnormal expansion of the hypertrophic zone in the midshaft region of the humeri seen in the fro/fro mice is not present in the fro/fro; Acan-Smpd3 mice. However, the bone collar remains unmineralized in these mice, which is corrected in the fro/fro; Col1a1-Smpd3 mice. All these abnormalities are corrected in the fro/fro; Acan-Smpd3; Col1a1-Smpd3 mice. (B) Immunofluorescence analysis of E16.5 WT, fro/fro, and fro/fro; Acan-Smpd3 humerus sections using an anti-type X collagen antibody. The nuclei were stained with Hoechst stain. Magnified views of the boxed areas in the upper panels are shown in the lower panels (Hoechst stain only). The numbers indicate the percentage of nuclei with ring-shaped/collapsed chromatin, which are considered to be in the early stage of the apoptotic cycle. (C) TUNEL-positive cells within an assigned area in the growth plates of E16.5 WT, fro/fro, and fro/fro; Acan-Smpd3 humerus samples were quantified (n = 4). Error bars represent standard deviations. *, P < 0.05; **, P < 0.01.
In order to examine the apoptotic properties of the hypertrophic chondrocytes, we first performed immunostaining of the humerus sections from E16.5 WT, fro/fro, and fro/fro; Acan-Smpd3 embryos using an anti-type X collagen antibody and counterstaining with Hoechst stain (Fig. 6B). This immunostaining identified the hypertrophic chondrocytes. We then counted the condensed ring-shaped and collapsed nuclei (considered to be at the early stage of the apoptotic cycle) in two different areas of the hypertrophic zone. Following this approach, we found that the nuclear DNA was mostly condensed, ring shaped, or collapsed throughout the hypertrophic zone in the WT embryos both in the early and in the late hypertrophic chondrocytes. However, in fro/fro embryos, although most of the early hypertrophic chondrocytes showed nuclear condensation comparable to that in the WT embryos, most of the late-stage hypertrophic chondrocytes did not show nuclear condensation. This anomaly was corrected in fro/fro; Acan-Smpd3 mice (Fig. 6B). Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay further confirmed these findings (Fig. 6C).
Generation of Smpd3flox/flox mice and conditional ablation of Smpd3 in bone and cartilage.
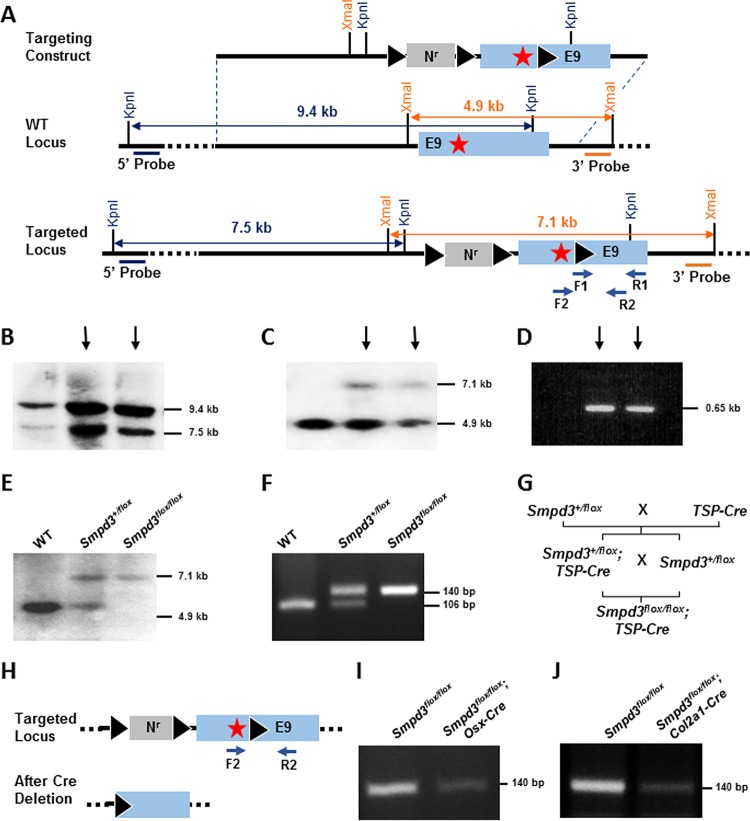
The fro mutation deletes part of intron 8 and part of the open reading frame (ORF) encoded by exon 9 of the Smpd3 gene (2). We decided to ablate the same region of the Smpd3 ORF using the Cre-loxP conditional gene-targeting approach. To achieve this, we generated a gene-targeting construct in which the part of the ORF in exon 9 was flanked by a floxed (flanked by two loxP sites) neomycin resistance cassette (Nr) within intron 8 and a third loxP site after the stop codon within exon 9 (Fig. 7A). For screening by Southern blotting, we used two radiolabeled (32P) DNA probes annealing at the 5′ and 3′ sequences external to the Smpd3 homologous sequence used in the targeting construct (Fig. 7A). Using the 5′ probe, we detected a 9.4-kb band for the WT locus and a 7.5-kb band for the targeted locus (Fig. 7B). Similarly, using the 3′ probe, we detected a 4.9-kb band denoting the WT locus and a 7.1 kb-band denoting the targeted locus (Fig. 7C). The ES cell DNAs were further characterized by PCR detecting the presence of the third loxP site (Fig. 7D). Finally, two independent Smpd3-targeted clones (indicated by the arrows in Fig. 7B to D) were karyotyped and selected for microinjection, and both resulted in chimeric offspring. Male chimeras were mated with female WT C57BL/6 mice, and germ line transmission of the conditional allele in the Smpd3+/flox and Smpd3flox/flox offspring was confirmed by both Southern blotting and PCR analysis (Fig. 7E and F).
FIG 7.
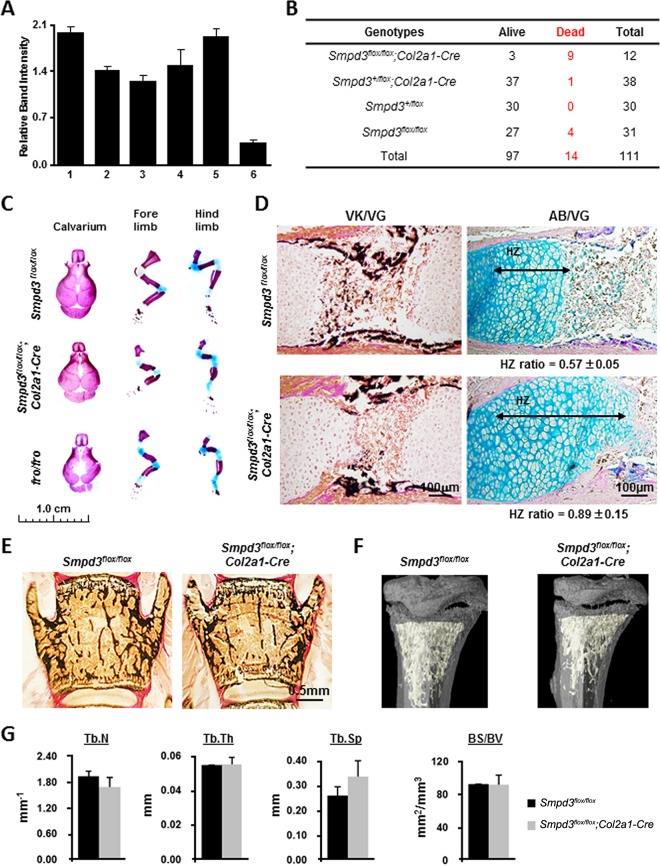
Tissue-specific deletion of Smpd3 gene. (A) Schematic representation of the targeting construct and the targeted Smpd3 locus. A neomycin resistance cassette (Nr) flanked by two loxP sites and a separate third loxP site were inserted into intron 8 and exon 9 downstream of the stop codon (star), respectively. 5′ and 3′ probes are indicated, and the primers (F1, R1, F2, and R2) used for PCR analyses are shown by arrows. KpnI and XmaI restriction sites were used for the Southern blot screening by the 5′ and 3′ probes, respectively. (B) ES cell clones with homologous recombination at the Smpd3 locus were identified by Southern blotting using the 5′ probe. The 9.4- and 7.5-kb bands represent the KpnI fragments generated from the WT and the targeted locus, respectively. (C) The screening of the targeted clones using the 3′ probe shows 4.9-kb and 7.1-kb bands denoting the XmaI fragments generated from the WT and the targeted locus, respectively. (D) PCR amplification of ES cell DNA using F1 and R1 primers showing amplification of a 0.65-kb fragment from the targeted clones containing the third loxP site. (B to D) Arrows indicate the clones used for microinjection. (E) Southern blot analysis of WT, heterozygote (Smpd3+/flox), and homozygote (Smpd3flox/flox) mice in the same litter using the 3′ probe. (F) Genotyping PCR using F2 and R2 primers to detect the targeted Smpd3 floxed allele (140 bp) and the WT allele (106 bp). (G) Breeding scheme showing the generation of Smpd3flox/flox; Osx-Cre and Smpd3flox/flox; Col2a1-Cre mice. TSP-Cre, tissue-specific Cre. (H) Schematic representation of the targeted locus before and after deletion of the floxed sequence in the presence of Cre recombinase. Arrows, the primers used for PCR analysis. (I and J) PCR to detect the deletion of the floxed Smpd3 sequence shown by a decrease of band intensity in the calvaria of Smpd3flox/flox; Osx-Cre mice and in the cartilage of Smpd3flox/flox; Col2a1-Cre mice.
We used two transgenic mouse models expressing Cre recombinase under the control of two tissue-specific promoters (TSP-Cre): Osx-Cre for both osteoblast- and late-stage chondrocyte-specific expression and Col2a1-Cre for chondrocyte-specific expression only (15). The TSP-Cre lines were bred with Smpd3+/flox mice to generate Smpd3flox/flox; Osx-Cre and Smpd3flox/flox; Col2a1-Cre mice (Fig. 7G). In the presence of a tissue-specific Cre recombinase, the floxed regions in the Smpd3 gene are deleted in a conditional manner. In order to detect this deletion event by PCR, we used a forward primer (F2) that annealed in exon 9 before the third loxP site and a reverse primer (R2) that annealed after the third loxP site within exon 9 of the Smpd3 gene (Fig. 7H). Our PCR analysis showed a marked decrease in the intensity of the amplicon in the DNA samples prepared from the calvaria of E15.5 Smpd3flox/flox; Osx-Cre embryos and the epiphyseal cartilage of Smpd3flox/flox; Col2a1-Cre embryos. DNAs from littermate Smpd3flox/flox calvarium and cartilage were used as controls for this analysis (Fig. 7I and J).
Smpd3flox/flox; Osx-Cre mice recapitulate the skeletal phenotype of fro/fro mice.
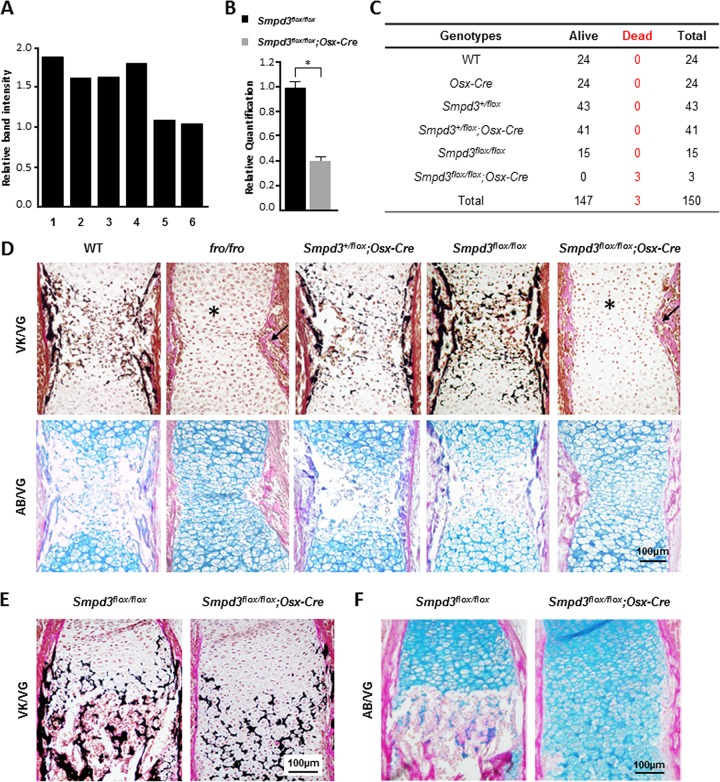
In order to examine the specificity of Cre-mediated deletion of Smpd3 in Smpd3flox/flox; Osx-Cre mice, we performed a PCR analysis using the primer pair F2 and R2 on DNAs prepared from skin, brain, stomach, liver, epiphyseal cartilage, and calvarium. No decrease of band intensity was detected in any of these tissues except in bone and cartilage (Fig. 8A). We next examined Smpd3 gene expression in the limbs (comprised of both bone and cartilaginous tissues) of E14.5 Smpd3flox/flox; Osx-Cre embryos by qRT-PCR. In agreement with the deletion PCR data, we observed a marked reduction in Smpd3 expression in the limb samples of Smpd3flox/flox; Osx-Cre mice (Fig. 8B).
FIG 8.
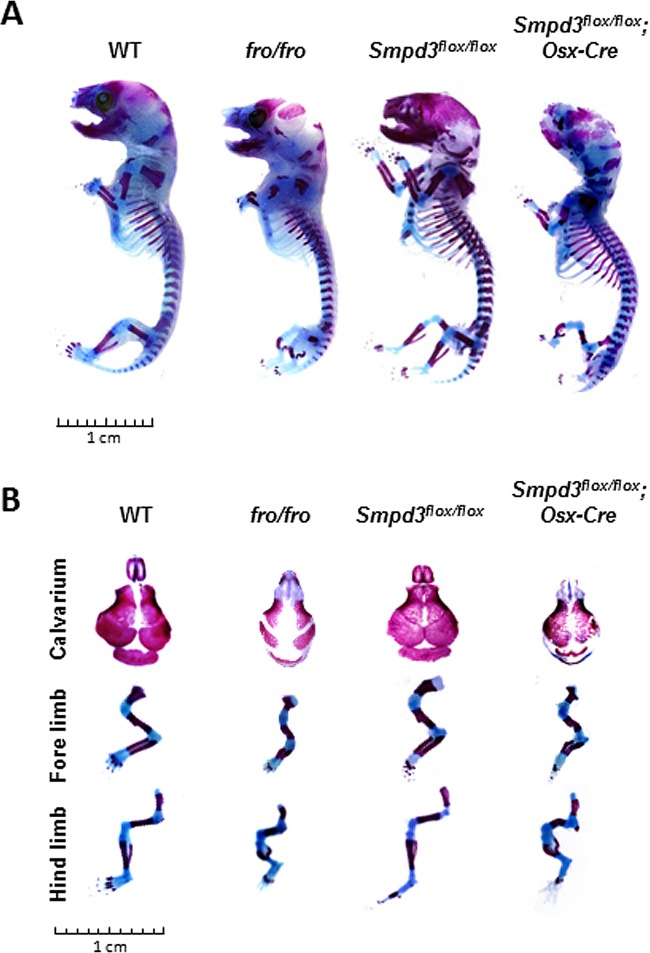
Deletion of Smpd3 in both osteoblasts and chondrocytes leads to abnormal bone and cartilage phenotypes in Smpd3flox/flox; Osx-Cre mice. (A) PCR analysis showing that the floxed Smpd3 sequence was deleted in the cartilage (epiphyseal cartilage of the limbs) and bone (calvaria) only. Bars: 1, skin; 2, brain; 3, stomach; 4, liver; 5, cartilage; 6, calvaria. (B) Gene expression analysis by qRT-PCR of E14.5 Smpd3flox/flox and Smpd3flox/flox; Osx-Cre whole long bones, showing that there is a significant reduction of Smpd3 expression in mice of the latter genotype. *, P < 0.05. (C) Smpd3+/flox; Osx-Cre mice were mated with Smpd3+/flox mice to generate Smpd3flox/flox; Osx-Cre mice. Records of the genotyping data for the pups obtained from these breedings show that all Smpd3flox/flox; Osx-Cre pups died perinatally. (D) Histological analyses of humeri from E15.5 WT, fro/fro, Smpd3+/flox; Osx-Cre, Smpd3flox/flox, and Smpd3flox/flox; Osx-Cre embryos. (Top) Paraffin sections stained with VK-VG showing a similar skeletal phenotype in the Smpd3flox/flox; Osx-Cre and fro/fro humeri. With both models, mice exhibit an abnormally expanded hypertrophic zone (*) and severely undermineralized cortical bone (arrow) (top). (Bottom) AB-VG staining of serial sections of the same samples showing an increased presence of hypertrophic chondrocytes in the midshaft of both Smpd3flox/flox; Osx-Cre and fro/fro humeri. The humeri of the controls, WT, Smpd3+/flox; Osx-Cre, and Smpd3flox/flox mice, were comparable. (E and F) Histological analyses of humeri from E18.5 Smpd3flox/flox and Smpd3flox/flox; Osx-Cre embryos. Paraffin sections stained with VK-VG (E) and AB-VG (F) showed the persistence of an abnormally expanded hypertrophic zone in the Smpd3flox/flox; Osx-Cre humeri.
As was the case with fro/fro mice, we also observed perinatal lethality of Smpd3flox/flox; Osx-Cre mice (Fig. 8C). On the other hand, all Smpd3flox/flox and Smpd3+/flox; Osx-Cre mice were viable and did not show any overt phenotypic abnormalities.
The examination of the humeri of E15.5 Smpd3flox/flox; Osx-Cre embryos showed the presence of an abnormally expanded hypertrophic zone and undermineralized cortical bones, which were also seen in fro/fro embryos (Fig. 8D). The humeri of the control mice, the Smpd3flox/flox and Smpd3+/flox; Osx-Cre mice, were comparable to those of the WT mice. This phenotype persisted even at later stages of development, as shown by histological analyses of the humeri of E18.5 Smpd3flox/flox; Osx-Cre embryos (Fig. 8E and F).
We next performed skeletal analyses of the carcasses of newborn Smpd3flox/flox; Osx-Cre pups and matching WT, fro/fro, and Smpd3flox/flox pups by staining them with alcian blue and alizarin red. Both Smpd3flox/flox; Osx-Cre and fro/fro pups showed a similar gross skeletal phenotype, hallmarked by severely bent limbs and a poorly mineralized skullcap (Fig. 9).
FIG 9.
Skeletal phenotypes of Smpd3flox/flox; Osx-Cre newborn pups. (A) Skeletal analyses were performed on WT, fro/fro, Smpd3flox/flox, and Smpd3flox/flox; Osx-Cre newborn pups. Alizarin red- and alcian blue-stained skeletal preparations show that both Smpd3flox/flox; Osx-Cre and fro/fro mice exhibit similar skeletal phenotypes. (B) Magnified view of skeletal preparations showing the calvaria' forelimbs, and hind limbs of newborn WT, fro/fro, Smpd3flox/flox, and Smpd3flox/flox; Osx-Cre pups.
Smpd3flox/flox; Col2a1-Cre mice partly recapitulate the skeletal phenotype of fro/fro mice.
We examined the deletion of the floxed sequence in the skin, heart, brain, calvarium, muscle, and epiphyseal cartilage of E15.5 Smpd3flox/flox; Col2a1-Cre embryos using the same PCR strategy described above; the band intensity was decreased in the DNA prepared from the epiphyseal cartilage only (Fig. 10A). Although we were unable to generate any viable Smpd3flox/flox; Osx-Cre pups, some Smpd3flox/flox; Col2a1-Cre pups survived past the perinatal stage and reached adulthood (Fig. 10B). We next stained the skeletal tissues of these and matching Smpd3flox/flox and fro/fro pups with alcian blue and alizarin red. Smpd3flox/flox; Col2a1-Cre pups showed limb deformities, albeit they were milder than those of fro/fro pups, while the limbs of Smpd3flox/flox pups were normal (Fig. 10C). The examination of the humeri of E15.5 Smpd3flox/flox; Col2a1-Cre embryos showed the presence of an abnormally expanded hypertrophic zone and undermineralized cartilage matrix in the midshaft region compared to that of control Smpd3flox/flox embryos. However, bone collars were normally mineralized in the Smpd3flox/flox; Col2a1-Cre developing endochondral bones (Fig. 10D). We also performed histology of the vertebrae and micro-CT analyses of the long bones of 7-week-old Smpd3flox/flox; Col2a1-Cre mice and their age- and gender-matched controls. There was no significant difference in any of the trabecular parameters (BV/TV, trabecular number, trabecular thickness, trabecular space, and bone surface over bone volume) between Smpd3flox/flox; Col2a1-Cre and control Smpd3flox/flox mice (Fig. 10E to G).
FIG 10.
Deletion of Smpd3 in chondrocytes leads to an abnormal cartilage phenotype in Smpd3flox/flox; Col2a1-Cre mice. (A) Quantification of PCR amplicon intensity showing that the floxed Smpd3 sequence was deleted only in the cartilage. Bars: 1, skin; 2, heart; 3, brain; 4, calvarium; 5, muscle; 6, cartilage. (B) Smpd3+/flox; Col2a1-Cre mice were mated with Smpd3flox/flox mice to generate Smpd3flox/flox; Col2a1-Cre mice. Records of the genotyping data for the pups obtained from these breedings show that some Smpd3flox/flox; Col2a1-Cre mice survive. (C) Skeletal preparations of newborn Smpd3flox/flox; Col2a1-Cre pups showing that these mice still exhibit the limb deformities seen in fro/fro mice. (D) (Left) Paraffin sections of humeri from E15.5 Smpd3flox/flox and Smpd3flox/flox; Col2a1-Cre embryos stained with VK-VG showed a delay in mineralization of the cartilage ECM in the latter genotype. (Right) AB-VG staining of serial sections of the same samples. Measurement of the ratio of the length of the hypertrophic zone over that of the growth plate (HZ ratio) confirms that the hypertrophic zone is elongated in the Smpd3flox/flox; Col2a1-Cre humeri. (E) Vertebral sections of 7-week-old Smpd3flox/flox; Col2a1-Cre and control mice stained with VK-VG showing comparable BV/TV values (14.96 ± 4.04 and 16.82 ± 6.97, respectively). (F) Micro-CT analyses of the tibiae of Smpd3flox/flox and Smpd3flox/flox; Col2a1-Cre mice. (G) The trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular space (Tb.Sp), and bone surface over bone volume (BS/BV) were not significantly different between Smpd3flox/flox and Smpd3flox/flox; Col2a1-Cre mice.
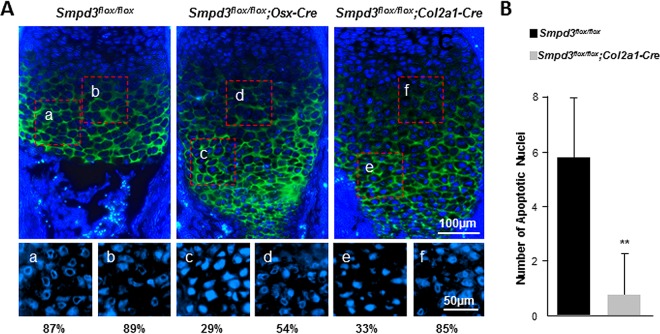
We next compared chondrocyte apoptosis in the developing growth plates of Smpd3flox/flox; Col2a1-Cre and Smpd3flox/flox; Osx-Cre embryos. The hypertrophic zone was identified by type X collagen immunostaining (Fig. 11A). As was the case with the fro/fro embryos, embryos of both conditional knockouts showed a decreased presence of late hypertrophic chondrocytes with condensed ring-shaped or collapsed nuclei (Fig. 11A). In agreement with this finding, there was also a reduction of late hypertrophic chondrocytes with TUNEL-positive nuclei at the chondro-osseous junctions of Smpd3flox/flox; Col2a1-Cre embryos (Fig. 11B). No TUNEL-positive nuclei were detected in the growth plates of Smpd3flox/flox; Osx-Cre mice.
FIG 11.
Analyses of chondrocyte apoptosis in Smpd3flox/flox; Osx-Cre and Smpd3flox/flox; Col2a1-Cre mice. (A) Immunofluorescence analysis of E15.5 Smpd3flox/flox, Smpd3flox/flox; Osx-Cre, and Smpd3flox/flox; Col2a1-Cre humerus sections using an anti-type X collagen antibody. The nuclei were stained with Hoechst stain. Magnified views of the boxed areas in the upper panels are shown in the lower panels (Hoechst stain only). The numbers indicate the percentage of nuclei with ring-shaped/collapsed chromatin, which are considered to be in the early stage of the apoptotic cycle. (B) TUNEL-positive cells within an assigned area in the growth plates of Smpd3flox/flox; Col2a1-Cre humerus samples were quantified (n = 4). No apoptotic nuclei were detected in Smpd3flox/flox; Osx-Cre humerus samples. Error bars represent standard deviations. **, P < 0.01.
DISCUSSION
ECM mineralization during skeletal development is a spatially and temporally regulated process. In the developing long bones of mice, mineralized tissues first appear at about embryonic day 14, when the ECM synthesized by the late hypertrophic chondrocytes starts accumulating hydroxyapatite minerals (4, 26). Concomitantly, osteoblast-derived ECM in the surrounding periosteum is also mineralized. SMPD3 has been shown to regulate the mineralization of both these ECMs. In agreement with the strong Smpd3 expression reported in both chondrocytes and osteoblasts, SMPD3 deficiency affects the initiation of mineralization in both cartilage and bone (4, 26).
It was shown earlier that bone morphogenetic protein (BMP) signaling and chondro-osteogenic transcription factor RUNX2 might be involved in the regulation of Smpd3 expression (24, 27). Furthermore, SMPD3 has been shown to act as a negative regulator of chondrocyte hypertrophy in the developing growth plates (24, 27). In support of the latter finding, we observed an increased deposition of type X collagen, a bona fide marker of the hypertrophic chondrocytes, in the expanded hypertrophic zone of cartilage in the developing fro/fro long bones.
In the growth plates, chondrocyte hypertrophy is tightly regulated by PTHrP, which is primarily expressed by the resting chondrocytes. PTHrP promotes chondrocyte proliferation but prevents their terminal differentiation (22). Considering the critical role of PTHrP in chondrocyte hypertrophy, we decided to examine the effects of this hormone and its surrogate, forskolin, on the regulation of Smpd3 expression in the ATDC5 chondrogenic cell line. When grown in a differentiation medium, these cells upregulated several chondrogenic differentiation markers along with Smpd3, which was markedly downregulated by both PTHrP and forskolin treatments. Interestingly, forskolin treatment also downregulated the expression of SOX9, a transcription factor essential for chondrogenesis. The latter finding prompted us to further investigate whether SOX9 regulates Smpd3 expression in chondrocytes.
We found that transfection of ATDC5 cells with a Sox9 expression vector upregulated endogenous Smpd3 expression. Transfection experiments using a reporter construct comprised of a 1.9-kb Smpd3 proximal promoter fragment driving the firefly luciferase gene also confirmed these data. However, we observed that, as was the case in ATDC5 chondrogenic cells, the Smpd3 proximal promoter was also active in nonchondrogenic HEK293 cells, even when they were not cotransfected with the Sox9 expression vector. This indicates that Smpd3 expression in the HEK293 cells is controlled by the endogenous transcriptional regulators.
Interestingly, although both ATDC5 and HEK293 cells demonstrated an upregulation of Smpd3 promoter-driven luciferase activity when cotransfected with the Sox9 expression vector, in silico DNA sequence analysis did not show any consensus SOX9 recognition site in the 2-kb Smpd3 promoter (see Fig. S1 in the supplemental material). Instead, we observed the presence of consensus SRY binding sites (AACAAA) at multiple regions in the analyzed sequence (28). This consensus SRY binding site has been shown to be recognized by SOX9, albeit with a lower affinity (29), which may explain why increased Sox9 expression in the cultured cells leads to a moderate induction of Smpd3 proximal promoter activity. However, it is also possible that SOX9 regulates the Smpd3 promoter via other transcriptional regulators. Recently, using ChIP-seq analyses, we have identified two possible SOX9 binding regions in intron 1 of the rat Smpd3 gene (17, 25). We show here that at least one of these regions may act as an enhancer of Smpd3 promoter activity in the chondrogenic cells. Taken together, our data suggest a complex regulation of Smpd3 expression in chondrocytes.
As shown by us previously, the abnormal accumulation of hypertrophic chondrocytes in the developing endochondral bones can be explained by impaired apoptosis of these cells (5). In agreement with this finding, we found that normal apoptosis of the late hypertrophic chondrocytes was impaired in both Smpd3flox/flox;Osx-Cre and Smpd3flox/flox; Col2a1-Cre embryos. On the other hand, this abnormality was corrected in fro/fro; Acan-Smpd3 mice. These observations suggest that local Smpd3 expression in late-stage chondrocytes is needed for their normal entry into the apoptotic cycle. The fact that these growth plate abnormalities are eventually corrected in fro/fro mice indicates that a secondary mechanism is in place to induce chondrocyte apoptosis in the absence of SMPD3. This inference is further supported by our observation that the apoptosis of early hypertrophic chondrocytes is not affected by SMPD3 deficiency.
Apart from reduced apoptosis, increased proliferation and differentiation of chondrocytes in the growth plate may also result in accumulation of hypertrophic chondrocytes in the developing long bones. However, we ruled out this possibility, as we did not observe any increase of chondrocyte proliferation in the developing limbs in fro/fro embryos. Also, the width of the proliferating and prehypertrophic zones appeared to be normal. Recently, it has been shown that hypertrophic chondrocytes may transdifferentiate to functional osteoblasts (30). Impairment of this pathway may contribute to the expansion of the hypertrophic zone. It is yet to be seen whether osteoblast differentiation from the chondrogenic lineage is affected in fro/fro mice.
In the current study, we generated a novel transgenic mouse model, Acan-Smpd3 mice, to restore Smpd3 expression in the developing cartilage. We decided to use this proximal Acan promoter fragment in our transgene because of the similar expression patterns of both Acan and Smpd3 during growth plate development (27, 31). In fro/fro; Acan-Smpd3 embryos, although growth plate cartilage mineralization was largely normalized, we did not observe a full correction of the skeletal deformities. This is most likely due to the absence of SMPD3 activity in the osteoblasts of these mice causing poor mineralization of both cortical and trabecular bones, thereby compromising their load-bearing capacities. This observation suggests that there may be an interdependency of SMPD3 functions in both cartilage and bone for proper skeletal development. In fact, this notion is supported by the fact that osteoblast-specific restoration of Smpd3 expression in fro/fro; Col1a1-Smpd3 embryos partly rescues the growth plate cartilage phenotype (5).
In order to further confirm our findings from the transgenic mouse rescue experiments, we performed conditional gene ablation studies. Here we report the generation and validation of a mouse model for the tissue-specific inactivation of Smpd3 in skeletal tissues. Our gene-targeting strategy aims to recapitulate the effects of the reported deletion mutation in the ORF of Smpd3 in fro/fro mice, which results in a nonfunctional protein (5).
In a conditional gene-targeting experiment, it is imperative that the genetic modifications introduced into the basic model (flox/flox mice) not result in any phenotypic alterations. Accordingly, all the phenotypic characteristics of Smpd3flox/flox mice were identical to those of age- and gender-matched WT mice. In our breeding experiments, we used Osx-Cre and Col2a1-Cre transgenic lines to either ablate Smpd3 in both late differentiation-stage chondrocytes and osteoblasts or just in chondrocytes, respectively. Although OSX was initially identified to be an osteoblast-specific transcription factor, more recent works have demonstrated that it is also expressed in the late-stage chondrocytes (14, 32, 33). The expression of OSX in the mineralizing cell types of the developing skeleton justifies the use of the Osx-Cre line in our breeding experiments that aimed to verify our original hypothesis that SMPD3 has a nonsystemic role in the skeletal tissues.
In agreement with our hypothesis, we observed a complete recapitulation of the fro/fro skeletal phenotype in the newborn Smpd3flox/flox; Osx-Cre mice, affecting both the intramembranous and endochondral bones. As was the case with fro/fro pups, none of the Smpd3flox/flox; Osx-Cre pups survived past the perinatal stage. We observed very similar histological features (e.g., abnormal mineralization of the periosteal bones and an expanded zone of hypertrophic chondrocytes surrounded by a poorly mineralized matrix in the growth plate) in both fro/fro and Smpd3flox/flox; Osx-Cre mice. On the other hand, we observed only an expanded zone of hypertrophic chondrocytes and a poorly mineralized cartilage ECM in the developing endochondral bones of Smpd3flox/flox;Col2a1-Cre mice. Taken together, these findings are complementary to those of our transgenic rescue experiments, confirming a cell-autonomous role for SMPD3 in osteoblasts and chondrocytes.
Until now, a parallel comparison of the relative contributions of osteoblast- and chondrocyte-derived SMPD3 was missing. We addressed this gap in knowledge to pave the way for further mechanical studies to understand the mode of action of SMPD3 in hard tissue mineralization. Our findings rule out the possibility of any major extraskeletal roles of SMPD3 on skeletal development. The transgenic experiments showed that local restoration of Smpd3 expression in both bone and cartilage in fro/fro; Acan-Smpd3; Col1a1-Smpd3 compound mutants resulted in a complete rescue of the skeletal phenotype. Additionally, as stated above, Smpd3flox/flox; Osx-Cre mice fully recapitulated the fro/fro phenotypes. These data do not support the conclusion drawn by Stoffel et al. stating that SMPD3 has a systemic/endocrine effect on skeletal development (10, 11). Additionally, Stoffel et al. suggested that there could be another mutation in the fro/fro mouse model resulting in osteogenesis and dentinogenesis imperfecta (10, 11). Our transgenic models, the fro/fro; Col1a1-Smpd3 and fro/fro; Acan-Smpd3 mouse models, disclaim this statement, as we were able to correct the phenotypic abnormalities seen in fro/fro mice simply by introducing the functional Smpd3 transgenes locally in the skeletal tissues.
Our histology and micro-CT analyses did not detect any change of bone volume in fro/fro; Acan-Smpd3 and Smpd3flox/flox; Col2a1-Cre mice when analyzed at 4 and 7 weeks, respectively. These findings were in agreement with those of Coleman et al., who reported that bone volume was not affected in fro/fro mice at 3 months of age (34).
Collectively, our data suggest a more important role for bone-derived SMPD3 in overall skeletal development and growth. However, it appears that Smpd3 expression in cartilage is not fully dispensable for normal skeletogenesis. The observation that skeletal ablation of Smpd3 results in perinatal lethality suggests a critical prosurvival role of the skeletal cells in whole-body metabolism. Indeed, bone has recently been suggested to be an endocrine organ regulating vital body functions, such as mineral homeostasis and energy metabolism (35, 36). Future work will reveal how the SMPD3 produced by the skeletal cells may affect some of these regulatory functions. Considering the pleiotropic roles of SMPD3 in both skeletal and nonskeletal tissues, the novel mouse models reported here will be useful for these future studies.
Supplementary Material
ACKNOWLEDGMENTS
We declare no conflict of interest.
We thank Mia Esser and Louise Marineau for animal husbandry, Marie-Helene Gaumond for technical support, Anujan Gunaratnam for generating a luciferase construct, and Veronique Lefebvre for providing the aggrecan promoter construct.
The core facility for skeletal phenotyping was supported by Le Réseau de Recherche en Santé Buccodentaire et Osseuse (RSBO). This work was supported by operating grants from the Canadian Institutes of Health Research (CIHR) Fund (number 123310 to M.M.). M.M. is an FRQS chercheur-boursier. P.M. is supported by the Shriners of North America. G.M. receives a studentship from the McGill University Health Center.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.01077-15.
REFERENCES
- 1.Shamseddine AA, Airola MV, Hannun YA. 2015. Roles and regulation of neutral sphingomyelinase-2 in cellular and pathological processes. Adv Biol Regul 57:24–41. doi: 10.1016/j.jbior.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubin I, Adams CP, Opsahl S, Septier D, Bishop CE, Auge N, Salvayre R, Negre-Salvayre A, Goldberg M, Guenet JL, Poirier C. 2005. A deletion in the gene encoding sphingomyelin phosphodiesterase 3 (Smpd3) results in osteogenesis and dentinogenesis imperfecta in the mouse. Nat Genet 37:803–805. doi: 10.1038/ng1603. [DOI] [PubMed] [Google Scholar]
- 3.Guenet JL, Stanescu R, Maroteaux P, Stanescu V. 1981. Fragilitas ossium: a new autosomal recessive mutation in the mouse. J Hered 72:440–441. [DOI] [PubMed] [Google Scholar]
- 4.Khavandgar Z, Murshed M. 2015. Sphingolipid metabolism and its role in the skeletal tissues. Cell Mol Life Sci 72:959–969. doi: 10.1007/s00018-014-1778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khavandgar Z, Poirier C, Clarke CJ, Li J, Wang N, McKee MD, Hannun YA, Murshed M. 2011. A cell-autonomous requirement for neutral sphingomyelinase 2 in bone mineralization. J Cell Biol 194:277–289. doi: 10.1083/jcb.201102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tani M, Hannun Y. 2007. Analysis of membrane topology of neutral sphingomyelinase 2. FEBS Lett 581:1323–1328. doi: 10.1016/j.febslet.2007.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannun YA, Obeid LM. 2008. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 8.Kolesnick R. 2002. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest 110:3–8. doi: 10.1172/JCI0216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchesini N, Luberto C, Hannun YA. 2003. Biochemical properties of mammalian neutral sphingomyelinase 2 and its role in sphingolipid metabolism. J Biol Chem 278:13775–13783. doi: 10.1074/jbc.M212262200. [DOI] [PubMed] [Google Scholar]
- 10.Stoffel W, Jenke B, Block B, Zumbansen M, Koebke J. 2005. Neutral sphingomyelinase 2 (smpd3) in the control of postnatal growth and development. Proc Natl Acad Sci U S A 102:4554–4559. doi: 10.1073/pnas.0406380102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoffel W, Jenke B, Holz B, Binczek E, Gunter RH, Knifka J, Koebke J, Niehoff A. 2007. Neutral sphingomyelinase (SMPD3) deficiency causes a novel form of chondrodysplasia and dwarfism that is rescued by Col2A1-driven smpd3 transgene expression. Am J Pathol 171:153–161. doi: 10.2353/ajpath.2007.061285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khavandgar Z, Alebrahim S, Eimar H, Tamimi F, McKee MD, Murshed M. 2013. Local regulation of tooth mineralization by sphingomyelin phosphodiesterase 3. J Dent Res 92:358–364. doi: 10.1177/0022034513478429. [DOI] [PubMed] [Google Scholar]
- 13.Kumar D, Lassar AB. 2009. The transcriptional activity of Sox9 in chondrocytes is regulated by RhoA signaling and actin polymerization. Mol Cell Biol 29:4262–4273. doi: 10.1128/MCB.01779-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodda SJ, McMahon AP. 2006. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133:3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 15.Ovchinnikov AD, Deng JM, Ogunrinu G, Behringer RR. 2000. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis 26:145–146. [PubMed] [Google Scholar]
- 16.Han Y, Lefebvre V. 2008. L-Sox5 and Sox6 drive expression of the aggrecan gene in cartilage by securing binding of Sox9 to a far-upstream enhancer. Mol Cell Biol 28:4999–5013. doi: 10.1128/MCB.00695-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh CD, Lu Y, Liang S, Mori-Akiyama Y, Chen D, de Crombrugghe B, Yasuda H. 2014. SOX9 regulates multiple genes in chondrocytes, including genes encoding ECM proteins, ECM modification enzymes, receptors, and transporters. PLoS One 9:e107577. doi: 10.1371/journal.pone.0107577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 19.Bakker AD, Klein-Nulend J. 2012. Osteoblast isolation from murine calvaria and long bones. Methods Mol Biol 816:19–29. doi: 10.1007/978-1-61779-415-5_2. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Khavandgar Z, Lin SH, Murshed M. 2011. Lithium chloride attenuates BMP-2 signaling and inhibits osteogenic differentiation through a novel WNT/GSK3-independent mechanism. Bone 48:321–331. doi: 10.1016/j.bone.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 21.Khavandgar Z, Roman H, Li J, Lee S, Vali H, Brinckmann J, Davis EC, Murshed M. 2014. Elastin haploinsufficiency impedes the progression of arterial calcification in MGP-deficient mice. J Bone Miner Res 29:327–337. doi: 10.1002/jbmr.2039. [DOI] [PubMed] [Google Scholar]
- 22.Kronenberg HM. 2006. PTHrP and skeletal development. Ann N Y Acad Sci 1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- 23.Zhou G, Zheng Q, Engin F, Munivez E, Chen Y, Sebald E, Krakow D, Lee B. 2006. Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc Natl Acad Sci U S A 103:19004–19009. doi: 10.1073/pnas.0605170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chae YM, Heo SH, Kim JY, Lee JM, Ryoo HM, Cho JY. 2008. Upregulation of Smpd3 via BMP2 stimulation and Runx2. BMB Rep 42:86–90. [DOI] [PubMed] [Google Scholar]
- 25.Oh CD, Maity SN, Lu JF, Zhang J, Liang S, Coustry F, de Crombrugghe B, Yasuda H. 2010. Identification of SOX9 interaction sites in the genome of chondrocytes. PLoS One 5:e10113. doi: 10.1371/journal.pone.0010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. 2008. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol 40:46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Kakoi H, Maeda S, Shinohara N, Matsuyama K, Imamura K, Kawamura I, Nagano S, Setoguchi T, Yokouchi M, Ishidou Y, Komiya S. 2014. Bone morphogenic protein (BMP) signaling up-regulates neutral sphingomyelinase 2 to suppress chondrocyte maturation via the Akt protein signaling pathway as a negative feedback mechanism. J Biol Chem 289:8135–8150. doi: 10.1074/jbc.M113.509331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tchenio T, Casella JF, Heidmann T. 2000. Members of the SRY family regulate the human LINE retrotransposons. Nucleic Acids Res 28:411–415. doi: 10.1093/nar/28.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mertin S, McDowall SG, Harley VR. 1999. The DNA-binding specificity of SOX9 and other SOX proteins. Nucleic Acids Res 27:1359–1364. doi: 10.1093/nar/27.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B. 2014. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet 10:e1004820. doi: 10.1371/journal.pgen.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Domowicz MS, Cortes M, Henry JG, Schwartz NB. 2009. Aggrecan modulation of growth plate morphogenesis. Dev Biol 329:242–257. doi: 10.1016/j.ydbio.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Shi Y, Regan J, Karuppaiah K, Ornitz DM, Long F. 2014. Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PLoS One 9:e85161. doi: 10.1371/journal.pone.0085161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh JH, Park SY, de Crombrugghe B, Kim JE. 2012. Chondrocyte-specific ablation of Osterix leads to impaired endochondral ossification. Biochem Biophys Res Commun 418:634–640. doi: 10.1016/j.bbrc.2012.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coleman RM, Aguilera L, Quinones L, Lukashova L, Poirier C, Boskey A. 2012. Comparison of bone tissue properties in mouse models with collagenous and non-collagenous genetic mutations using FTIRI. Bone 51:920–928. doi: 10.1016/j.bone.2012.08.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukumoto S. 2010. FGF23: phosphate metabolism and beyond. IBMS BoneKEy 7:268–278. doi: 10.1138/20100458. [DOI] [Google Scholar]
- 36.Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G. 2009. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.