Abstract
Objective:
The purpose of this study was to investigate the relationship between Ki-67 proliferation indexes and apparent diffusion coefficient (ADC) values of low-grade and atypical/anaplastic (high-grade) meningiomas.
Methods:
Pre-operative diffusion-weighted imaging and histopathological evaluation of 44 patients with meningiomas were performed retrospectively. Regions of interest (ROIs) were manually drawn on the ADC images. In total six ROI measurements were taken in three consecutive slices, and the average of the mean ADC value was used. The relationship between the ADC and Ki-67 values was investigated, and the ADC values of the low-grade and high-grade meningiomas were compared.
Results:
31 (70%) patients had low-grade the meningiomas. 10 (23%) patients had atypical and 3 (7%) had anaplastic meningiomas. ADC values of the low-grade and high-grade meningiomas were 0.81 ± 0.12 × 10−3 and 0.66 ± 0.08 × 10−3 mm2 s−1, respectively. Ki-67 proliferation indexes were 2.19% ± 1.14% for low-grade and 11.20% ± 9.80% for high-grade meningiomas. A statistically significant negative correlation between Ki-67 proliferation index and ADC values of the low-grade and high-grade meningiomas was detected (r2 = 0.326, p < 0.001). High-grade meningiomas had lower ADC values than that of low-grade meningiomas. There was statistically significant difference between the ADC values of the low-grade and high-grade meningiomas (p < 0.001).
Conclusion:
Our data provide an inverse correlation between the ADC and Ki-67 proliferation index values of meningiomas. ADC values can be used for histopathological characterization of the meningiomas and pre-surgical planning.
Advances in knowledge:
The purpose of this study was to investigate the relationship between Ki-67 proliferation indexes and ADC values of low-grade and atypical/anaplastic (high-grade) meningiomas. In addition, we compared the ADC and Ki-67 proliferative index values of the low-grade and atypical/anaplastic (high-grade) meningiomas. We concluded that there was an inverse correlation between the ADC and Ki-67 proliferation index values in meningiomas, and we have found statistically significant difference between the ADC values of the low-grade and high-grade meningiomas. ADC values can be used for histopathological characterization of the meningiomas and pre-surgical planning.
INTRODUCTION
Meningiomas are generally slow-growing, intracranial neoplasms, which thought to be arisen from the arachnoid “cap” cells located at the outer layer of the arachnoid mater and the arachnoid villi.1,2 Meningiomas are the most common benign, extra-axial intracranial tumours accounting for about 35% of all tumours.3 They are more common in older population and females.3 Meningiomas were divided into three grades according to the World Health Organization (WHO) 2007 classification. They were low grade (grade I), atypical (grade II) and anaplastic (grade III).1 Atypical meningiomas consist of 4.7–7% and anaplastic meningiomas consist of 1–2.8% of all meningiomas.1 Low-grade meningiomas are slow growing and consist of 80–90% of all meningiomas.1,2
The recurrence is the main problem and increases patient morbidity and mortality. For that reason, pre-operative evaluation and characterization of meningiomas would be very important before resection, for an appropriate surgical and treatment planning. Atypical and anaplastic meningiomas are more prone to recurrence.4 After surgery, atypical and anaplastic meningiomas recur in about 40% and 50–80% within 5 years, respectively.5 On the other hand, recurrence rate of benign meningiomas can be up to 25%.1 Compared with the WHO 2000 classification the WHO 2007 classification1 mentioned that the presence of brain invasion in histologically benign variants and this group's prognosis should be considered as grade II.6 The recurrence cannot be explained with only the grades of the tumours. The tumours have a tendency to recur, which depends on the grade, brain infiltration, proliferative activity and extent of resection. Immunohistochemistry, using antibodies that are reactive against various proliferating cellular antigens, is one of the ways of determining proliferative activity in tumours.7 The Ki-67 monoclonal antibody (MIB-1) is an important marker of cellular proliferation, which has been widely used in the routine histological evaluation of different tumours.8 Ki-67/MIB-1 monoclonal antibody, which is reactive against the nuclear antigen Ki-67, expressed during cell cycle (G1, S, G2 and M) but absent in G0.7 The Ki-67 MIB-1-positive tumour cells are often correlated with the clinical course and prognosis of several types of human neoplasms. An elevated Ki-67 proliferation index is associated with an increased recurrence rate and has prognostic value in meningiomas.2,5,7
On MRI, meningiomas present typically as well-circumscribed mass, with strong enhancement after gadolinium administration, with a peritumoral rim and a dural tail. Atypical imaging features such as heterogeneous enhancement, marked perilesional oedema, irregular cerebral surface, cystic appearance, intratumoral haemorrhage and parenchymal invasion can be detected but are not specific or reliable diagnostic features for the differentiation of the atypical and malignant meningiomas from benign lesions.9 Diffusion-weighted imaging (DWI) is an imaging technique which shows abnormalities of tissue structure by detecting changes in water mobility, which is not possible with other imaging techniques.10 Diffusion-weighted (DW) MRI and apparent diffusion coefficient (ADC) measurements can be useful in determining tumour cell density and nucleus/cytoplasm ratio, which are related to malign potential of the tumours.5,10,11
The purpose of this study was to investigate the relationship between Ki-67 proliferation indexes and ADC values of low-grade and atypical/anaplastic (high-grade) meningiomas. In addition, we compared the ADC and Ki-67 proliferative index values of the low-grade and atypical/anaplastic (high-grade) meningiomas.
METHODS AND MATERIALS
Subjects
The study included all patients who had the diagnosis of meningioma, had histopathological evaluation with Ki-67 proliferation indexes and had undergone pre-operative DWI in Medipol University Hospital between November 2012 and August 2014. None of the patients had undergone any other therapy before the surgery. The local institutional review board approved this retrospective study.
The MRI examinations of 44 patients, who had the inclusion criteria, were retrospectively evaluated. The mean age (±standard deviation) was 53.63 ± 14.45 years with an age range of 28–80 years, and the female/male ratio was 29/15.
Imaging techniques
All MRI examinations were performed on a 3-T clinical scanner (Philips Achieva TX, Philips Healthcare, Best, Netherlands). MRI protocol included: axial and sagittal T1 weighted (T1W) spin echo (SE) sequence with repetition time (TR)/echo time (TE) = 400/10 ms, slice thickness = 5 mm, interslice gap = 1 mm and field of view (FOV) = 24 cm; an axial T2 weighted (T2W) turbo spin echo sequence with TR/TE = 3000/80 ms, slice thickness = 5 mm, interslice gap = 1 mm and FOV = 24 cm; and sagittal three-dimensional fluid-attenuated inversion-recovery sequences with TR/TE = 4500/270 ms and inversion time (TI) = 1650. After the administration of 0.1-mmol kg−1 gadolinium, contrast-enhanced axial and coronal T1W SE (TR/TE = 400/10 ms) images were obtained.
DWI was performed with a single-shot SE echoplanar imaging sequence with three gradient directions in the axial plan. The following parameters were used: TR/TE = 3190/90 ms, slice thickness = 5 mm, interslice gap = 1 mm, FOV = 24 cm and b-values = 0 and 1000 s mm−2. ADC maps were automatically generated. We recalled all the results of all the patient examinations in order to re-evaluate on the workstation which was provided by the vendor.
Image evaluation
A radiologist who was blinded to the cases and unaware of the histopathological diagnosis randomly reviewed conventional and DW MR images. Regions of interest (ROIs) were manually drawn on the ADC images. The ADC measurements were taken on the workstation which was provided by the vendor. ROIs were drawn in the solid parts of the lesions and cystic–necrotic, and haemorrhagic and calcified parts were avoided. Those areas were identified on the T2W and contrast-enhanced T1W images (Figures 1 and 2). Totally six ROI measurements were taken in three consecutive slices, and the average of the mean ADC values was used. ROI areas ranged between 0.2 and 1 cm2. According to the size and morphology of the meningioma when three slices were not available, the ADC values of three ROIs in two slices were calculated. The mean ADC value of the normal white matter was calculated by the same method in the centrum semiovale. The mean ADC values of the peritumoral oedemas were calculated by the same method.
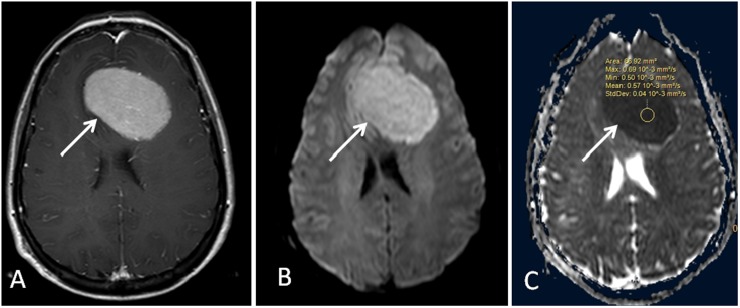
Figure 1.
Low-grade meningioma and meningothelial subtype (arrows). (a) Contrast-enhanced coronal T1 weighted image shows enhancing extra-axial meningioma in the right temporo-occipital region. (b) Diffusion-weighted MR image. (c). Apparent diffusion coefficient map with region of interest measurement. Max, maximum; Min, minimum; StdDev, standard deviation.
Figure 2.
High-grade meningioma (arrows). (a) Contrast-enhanced coronal T1 weighted image shows enhancing extra-axial meningioma in the frontal region. (b) Diffusion-weighted MR image. (c). Apparent diffusion coefficient map with region of interest measurement. Max, maximum; Min, minimum; StdDev, standard deviation.
Pathology
The pathological diagnoses of the meningiomas were based on the WHO 2007 classification system for brain tumours.1 Tumour proliferation indexes were reported as the percentage of tumour cell nuclei labelled with the Ki-67 (clone MIB-1) monoclonal antibody in formalin-fixed paraffin tissue sections. For each case, areas with the highest number of positive-staining tumour nuclei were selected for counting. The low-grade meningiomas were classified into subgroups according to the WHO classification. For further evaluation, we grouped the meningiomas as low grade (grade I) and high grade (grade II: atypical and grade III: anaplastic).
Statistical analysis
Comparison of the Ki-67 proliferation index values was made between low-grade and high-grade meningiomas, using an independent unpaired Student's t-test. Correlation coefficients were also calculated between the mean ADC and Ki-67 proliferation index values for all meningiomas using linear regression. Correlation analysis was performed using the Pearson's product–moment correlation with statistical software. A value of p < 0.05 was considered statistically significant.
RESULTS
Totally 44 meningiomas were evaluated retrospectively. 31 (70%) patients had low-grade meningiomas. 10 (23%) patients had atypical meningiomas and 3 (7%) patients had anaplastic meningiomas (Table 1). The low-grade meningiomas consisted of 14 (45%) meningothelial, 10 (32%) transitional, 4 (13%) secretory, 2 (7%) fibroblastic and 1 (3%) angiomatous (Table 2). Oedema was detected in 17 meningiomas (39%). The mean ADC value (1.54 ± 0.15 × 10−3 mm2 s−1) of the oedema was significantly higher than that of the normal white matter. There was no statistically difference between the ADC values of the oedema of the low-grade and high-grade meningeal tumours (p > 0.05).
Table 1.
Characteristics of low-grade (LG) and high-grade (HG) meningiomas
| Findings | LG | HG | p-value |
|---|---|---|---|
| Patient number | 31 | 13 | |
| ADC (×10−3; mm2 s−1) | 0.81 ± 0.12 | 0.66 ± 0.08 | <0.001 |
| Ki-67 (%) | 2.19 ± 1.14 | 11.20 ± 9.80 | 0.007 |
| ADCwm (×10−3; mm2 s−1) | 0.73 ± 0.03 | 0.72 ± 0.027 | >0.05 |
| ADCm/ADCwm | 1.12 ± 0.18 | 0.91 ± 0.12 | 0.002 |
ADC, apparent diffusion coefficient; ADCm/ADCwm, the ratios of the ADC values of the meningiomas to the white matter; ADCwm, the ADC value of the white matter; Ki-67, Ki-67 proliferation index.
A statistically significant negative correlation between Ki-67 proliferation index and ADC values of the LG and HG meningiomas was detected (r2 = 0.326, p < 0.001).
Table 2.
Apparent diffusion coefficient (ADC) and Ki-67 proliferation index (Ki-67) values of the subtypes of the low-grade (LG) meningiomas
| LG | Patient number | ADC (×10−3; mm2 s−1) | Ki-67 (%) |
|---|---|---|---|
| Meningothelial | 14 | 0.79 ± 0.11 | 2.64 ± 1.28 |
| Transitional | 10 | 0.77 ± 0.87 | 2.00 ± 0.09 |
| Secretory | 4 | 1.00 ± 0.10 | 1.50 ± 1.00 |
| Fibroblastic | 2 | 0.80 ± 0.12 | 1.50 ± 0.12 |
| Angiomatous | 1 | 0.87 | 2.00 |
ADC values of the low-grade and high-grade meningiomas were 0.81 ± 0.12 × 10−3 and 0.66 ± 0.08 × 10−3 mm2 s−1, respectively. Ki-67 proliferation indexes were 2.19 ± 1.14% for low-grade meningiomas and 11.20 ± 9.80% for high-grade meningiomas. A statistically significant negative correlation between Ki-67 proliferation index and ADC values of the low-grade and high-grade meningiomas was detected (r2 = 0.326, p < 0.001). The mean ADC of the white matter (WM) for low-grade and high-grade meningiomas were 0.73 ± 0.03 × 10−3 and 0.72 ± 0.027 × 10−3 mm2 s−1, respectively. The ratios of the ADC values of the meningiomas to the white matter (ADCm/ADCwm) were calculated. They were 1.12 ± 0.18 for low-grade meningiomas and 0.91 ± 0.12 for high-grade meningiomas. A statistically significant negative correlation between Ki-67 proliferation index and ADCm/ADCwm values of the low-grade and high-grade meningiomas was also detected (r2 = 0.297, p = 0.001) (Table 1).
In low-grade meningiomas, 24 lesions (78%) had an elevated ADC value compared with that of the normal white matter and low Ki-67 proliferation index (Figure 1). On the other hand, the majority (n = 11, 84%) of the high-grade meningiomas showed high Ki-67 proliferation index and a low ADC value compared with that of the normal white matter (Figure 2). There was statistically significant difference between the Ki-67 proliferation indexes of the low-grade and high-grade meningiomas (p = 0.007). There were some exceptions in low-grade and high-grade groups. Two of the meningothelial meningiomas with 4% Ki-67 proliferation index showed lower ADC values than that of the normal white matter (0.68 × 10−3 mm2 s−1). One of these meningiomas showed progression in 1-year follow-up MRI. One high-grade meningioma had a low Ki-67 proliferation index (1%), with a high ADC value than that of the white matter (0.75 × 10−3 mm2 s−1). Unfortunately, we did not know the clinical course of this case.
High-grade meningiomas had a lower ADC values than that of low-grade meningiomas. There was a statistically significant difference between the ADC values of the low-grade and high grade meningiomas (p < 0.001). A statistically significant difference between the ADCm/ADCwm values of the low-grade and high-grade meningiomas was also detected (p = 0.002). ADC values of the subtypes of the low-grade meningiomas were 0.79 ± 0.11 × 10−3 mm2 s−1 for meningothelial, 0.77 ± 0.87 × 10−3 mm2 s−1 for transitional, 1.00 ± 0.10 × 10−3 mm2 s−1 for secretory and 0.80 ± 0.12 × 10−3 mm2 s−1 for fibroblastic. One angiomatous meningioma had an ADC value of 0.87 ×10−3 mm2 s−1 (Table 2). For the subgroups of the low-grade meningiomas, Ki-67 proliferation indexes were 2.64 ± 1.28% for meningothelial, 2.00 ± 0.09% for transitional, 1.50 ± 1.00% for secretory, 1.50 ± 0.12% for fibroblastic and 2.00% for angiomatous meningiomas (Table 2). There was no statistically significant difference between the ADC values of the meningothelial (0.79 ± 0.11 × 10−3 mm2 s−1) and transitional meningiomas (0.77 ± 0.87 × 10−3 mm2 s−1) (p = 0.664). Statistically significant differences were detected between the mean ADC values of the meningothelial (0.79 ± 0.11 × 10−3 mm2 s−1) and secretory meningiomas (1.00 ± 0.10 × 10−3 mm2 s−1) (p = 0.016) and between the mean ADC values of the transitional (0.77 ± 0.87 × 10−3 mm2 s−1) and secretory meningiomas (1.00 ± 0.10 × 10−3 mm2 s−1) (p = 0.017).
DISCUSSION
Meningiomas are one of the most common intracranial tumours. Although they are slow growing and most of them are low-grade, the recurrence is the main problem that determines the prognosis. Although tumour grade and subtype are strong prognostic factors in meningiomas, the recurrence is still unpredictable. The Ki-67 proliferation index is an important tool in addition to routine histological evaluation. The Ki-67 proliferation index correlates with increased risk of recurrence and appears as an important prognostic factor in meningiomas.5,12,13
The therapeutic approach can change according to the histopathological properties of the meningiomas. Determining the histopathological grades, types and proliferative activity of the meningiomas will help in pre-surgical planning, in predicting the prognosis and in the additional use of other therapies such as radiotherapy.14–17 MRI is an important tool for the diagnosis, but conventional MRI techniques are limited in differentiating the low-grade and high-grade (atypical and anaplastic) meningiomas.
DW MRI has been used for the diagnosis and follow-up of the brain tumours. Water motion diffusivity is greater in the extracellular space than in the intracellular space. The cellularity and the nucleus/cytoplasm ratio of the tumour will determine the restriction of the water molecules.10 Malignant lesions are densely cellular neoplasms, which have a greater restriction of water diffusion than less cellular tumours; this results in decreased ADC values.10 The use of ADC values in determining the histopathological grades of the various intracranial tumours was investigated.18,19
We found that there was a statistically significant inverse correlation between ADC and Ki-67 proliferation index values in meningiomas (r2 = 0.326, p < 0.001). Two previous studies16,17 have investigated the relationship of ADC values and Ki-67 proliferation index in meningiomas. Tang et al17 found statistically significant inverse correlation between ADC and Ki-67 proliferation values, which was consistent with our results. Ginat et al16 detected an inverse relationship between ADC and Ki-67 proliferation index values in high-grade meningiomas, which was not statistically significant. They included only the high-grades meningiomas in their study, and the average Ki-67 proliferation index for atypical and anaplastic meningiomas were not significantly different.
Tang et al17 had described patterns of relationships between the ADC and Ki-67 proliferation index values. Combination of high ADC values and low Ki-67 proliferation index values of low-grade meningiomas was the common pattern. In our study, 24 (78%) of the low-grade meningiomas showed an elevated ADC value compared with that of the normal white matter and low Ki-67 proliferation index, as generally expected according to their histopathological properties. On the other hand, two (6%) of the meningothelial meningiomas with 4% Ki-67 proliferation index showed lower ADC values than that of the normal white matter (0.68 × 10−3 mm2 s−1). One of these meningiomas showed progression on 1-year follow-up MRI. The clinical course of the other menangioma was unknown. These findings were consistent with the second described pattern.17 In the high-grade meningiomas, most of them had low ADC and high Ki-67 proliferation index values (84%). This pattern reflects the high cellularity and active proliferation activity2,5,7 and high risk of recurrence.5,12,13 In our study group, we had no data that were consistent with the pattern of low ADC and low Ki-67 proliferation index values in aggressive meningiomas. One high-grade meningioma had a low Ki-67 proliferation index (1%), with a high ADC value than that of the white matter (0.75 × 10−3 mm2 s−1). Unfortunately, the clinical course of this case was unknown. The lack of patients' follow-up was the limitation of our study. The future prospective studies with larger patient groups could be useful for detecting the correlation of these patterns with the clinical course and long-term imaging findings.
In the literature, there were studies that have investigated the use of DWI in determining the histopathological grades of the meningiomas.9,11,14,15,18–23 The results were controversial. In our study, in addition, we compared the ADC values of the meningiomas. We have found statistically significant difference in ADC and ADCm/ADCwm values between the low-grade and high-grade meningiomas (p < 0.05). Some of these studies14,15,18,22 investigating the effectiveness of the DWI in differentiating the subtypes of benign meningiomas had different findings. Hakyemez et al14 had found significant difference between the angiomatous and the meningotelial and transitional meningiomas. These findings were not consistent with that of other studies.15,18,22 In our study, the low-grade meningiomas consisted of 14 meningothelial, 10 transitional, 4 secretory, 2 fibroblastic and 1 angiomatous. Because of the small number of the subtypes, we compared the only ADC values of meningothelial, transitional and secretory meningiomas. There was no statistically significant difference between the ADC values of the meningothelial and transitional meningiomas (p = 0.664). Statistically significant differences were detected between the mean ADC values of the meningothelial and secretory meningiomas (p = 0.016) and between the mean ADC values of the transitional and secretory meningiomas (p = 0.017). We found that secretory meningiomas had the highest ADC values than the other subtypes, which was consistent with the previous studies.14,20 The ADC values of peritumoral oedema were significantly higher than that of the normal white matter. In this study, no significant difference was found in the ADC values of the peritumoural oedema of the low-grade and high-grade meningiomas as that of the previous studies.9,14,22
CONCLUSION
In our study, we concluded that there was an inverse correlation between the ADC and Ki-67 proliferation index values in meningiomas, and we have found statistically significant difference in ADC values between the low-grade and high-grade meningiomas. ADC values can be used for histopathological characterization of the meningiomas and pre-surgical planning.
Contributor Information
Ozdil Baskan, Email: ozdil.baskan@yahoo.com.
Gokalp Silav, Email: ihsahmet@yahoo.com.
Fatih Han Bolukbasi, Email: xstyx22@hotmail.com.
Ozlem Canoz, Email: ozigrwin@yahoo.com.
Serdar Geyik, Email: alyayin@yahoo.com.
Ilhan Elmaci, Email: fykmlm@outlook.com.
REFERENCES
- 1.Perry A, Louis DN, Scheithauer BW, Budka H, Von Deimling A. Meningiomas. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, eds. WHO classification of tumours of the central nervous system. Lyon: IARC Press; 2007. pp. 164–72. [Google Scholar]
- 2.Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol 2010; 99: 379–91. doi: 10.1007/s11060-010-0342-2 [DOI] [PubMed] [Google Scholar]
- 3.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumours diagnosed in the United States in 2005–2009. Neuro Oncol 2012; 14(Suppl. 5): v1–49. doi: 10.1093/neuonc/nos218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durand A, Labrousse F, Jouvet A, Bauchet L, Kalamaridès M, Menei P, et al. WHO grade II and III meningiomas: a study of prognostic factors. J Neurooncol 2009; 95: 367–75. doi: 10.1007/s11060-009-9934-0 [DOI] [PubMed] [Google Scholar]
- 5.Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol 2006; 5: 1045–54. doi: 10.1016/S1474-4422(06)70625-1 [DOI] [PubMed] [Google Scholar]
- 6.Wang DJ, Xie Q, Gong Y, Mao Y, Wang Y, Cheng HX, et al. Histopathological classification and location of consecutively operated meningiomas at a single institution in China from 2001 to 2010. Chin Med J (Engl) 2013; 126: 488–93. [PubMed] [Google Scholar]
- 7.Abry E, Thomassen IØ, Salvesen ØO, Torp SH. The significance of Ki-67/MIB-1 labeling index in human meningiomas: a literature study. Pathol Res Pract 2010; 206: 810–15. doi: 10.1016/j.prp.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 8.Chiloiro S, Bianchi A, Doglietto F, de Waure C, Giampietro A, Fusco A, et al. Radically resected pituitary adenomas: prognostic role of Ki 67 labeling index in a monocentric retrospective series and literature review. Pituitary 2014; 17: 267–76. doi: 10.1007/s11102-013-0500-6 [DOI] [PubMed] [Google Scholar]
- 9.Nagar VA, Ye JR, Ng WH, Chan YH, Hui F, Lee CK, et al. Diffusion-weighted MR imaging: diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am J Neuroradiol 2008; 29: 1147–52. doi: 10.3174/ajnr.A0996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmainda KM. Diffusion-weighted MRI as a biomarker for treatment response in glioma. CNS Oncol 2012; 1: 169–80. doi: 10.2217/cns.12.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe Y, Yamasaki F, Kajiwara Y, Takayasu T, Nosaka R, Akiyama Y, et al. Preoperative histological grading of meningiomas using apparent diffusion coefficient at 3T MRI. Eur J Radiol 2013; 82: 658–63. doi: 10.1016/j.ejrad.2012.11.037 [DOI] [PubMed] [Google Scholar]
- 12.Torp SH, Lindboe CF, Grønberg BH, Lydersen S, Sundstrøm S. Prognostic significance of Ki-67/MIB-1 proliferation index in meningiomas. Clin Neuropathol 2005; 24: 170–4. [PubMed] [Google Scholar]
- 13.Roser F, Samii M, Ostertag H, Bellinzona M. The Ki-67 proliferation antigen in meningiomas. Experience in 600 cases. Acta Neurochir (Wien) 2004; 146: 37–44. doi: 10.1007/s00701-003-0173-4 [DOI] [PubMed] [Google Scholar]
- 14.Hakyemez B, Yildirim N, Gokalp G, Erdogan C, Parlak M. The contribution of diffusion-weighted MR imaging to distinguishing typical from atypical meningiomas. Neuroradiology 2006; 48: 513–20. doi: 10.1007/s00234-006-0094-z [DOI] [PubMed] [Google Scholar]
- 15.Santelli L, Ramondo G, Della Puppa A, Ermani M, Scienza R, d'Avella D, et al. Diffusion-weighted imaging does not predict histological grading in meningiomas. Acta Neurochir (Wien) 2010; 152: 1315–19. doi: 10.1007/s00701-010-0657-y [DOI] [PubMed] [Google Scholar]
- 16.Ginat DT, Mangla R, Yeaney G, Wang HZ. Correlation of diffusion and perfusion MRI with Ki-67 in highgrade meningiomas. AJR Am J Roentgenol 2010; 195: 1391–5. doi: 10.2214/AJR.10.4531 [DOI] [PubMed] [Google Scholar]
- 17.Tang Y, Dundamadappa SK, Thangasamy S, Flood T, Moser R, Smith T, et al. Correlation of apparent diffusion coefficient with Ki-67 proliferation index in grading meningioma. AJR Am J Roentgenol 2014; 202: 1303–8. doi: 10.2214/AJR.13.11637 [DOI] [PubMed] [Google Scholar]
- 18.Kono K, Inoue Y, Nakayama K, Shakudo M, Morino M, Ohata K, et al. The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol 2001; 22: 1081–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Yamasaki F, Kurisu K, Satoh K, Arita K, Sugiyama K, Ohtaki M, et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology 2005; 3: 985–91. doi: 10.1148/radiol.2353031338 [DOI] [PubMed] [Google Scholar]
- 20.Filippi CG, Edgar MA, Ulug AM, Prowda JC, Heier LA, Zimmerman RD. Appearance of meningiomas on diffusion-weighted images: correlating diffusion constants with histopathologic findings. AJNR Am J Neuroradiol 2001; 22: 65–72. [PMC free article] [PubMed] [Google Scholar]
- 21.Pavlisa G, Rados M, Pazanin L, Padovan RS, Ozretic D, Pavlisa G. Characteristics of typical and atypical meningiomas on ADC maps with respect to schwannomas. Clin Imaging 2008; 32: 22–7. doi: 10.1016/j.clinimag.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 22.Sanverdi SE, Ozgen B, Oguz KK, Mut M, Dolgun A, Soylemezoglu F, et al. Is diffusion-weighted imaging useful in grading and differentiating histopathological subtypes of meningiomas? Eur J Radiol 2012; 81: 2389–95. doi: 10.1016/j.ejrad.2011.06.031 [DOI] [PubMed] [Google Scholar]
- 23.Toh CH, Castillo M, Wong AM, Wei KC, Wong HF, Ng SH, et al. Differentiation between classic and atypical meningiomas with use of diffusion tensor imaging. AJNR Am J Neuroradiol 2008; 29: 1630–5. doi: 10.3174/ajnr.A1170 [DOI] [PMC free article] [PubMed] [Google Scholar]