Abstract
Endovascular stroke treatment is a neurointerventional emergency where the main goal is the early recanalization of the occlusion within the critical time window, as safely as possible. Although the time window and rate of complications for endovascular stroke treatment differ with anterior and posterior circulation strokes, awareness of potential periprocedural complications is important, as they affect patient morbidity and mortality. Periprocedural complications are classified as haemorrhagic complications, procedure-/device-related, puncture site complications, and late-onset events including vascular stenosis. We present the digital subtraction angiography and CT imaging findings related to these complications in a study of 56 stroke patients, as they relate to previous findings in the literature.
INTRODUCTION
Endovascular stroke treatment is an emergency neurointerventional procedure being used with increasing frequency. Supported by recent trials, including multicentre randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands (MR CLEAN), the endovascular treatment for small core and anterior circulation proximal occlusion with emphasis on minimizing CT to recanalization times (ESCAPE) and the extending the time for thrombolysis in emergency neurological deficits—intra-arterial (EXTEND-IA), stent-assisted thrombectomy (SAT) affects stroke outcomes; stroke being the third leading cause of death in developed countries and the most common cause of serious, long-term disability.1–4 The aim of SAT in the anterior circulation is the recanalization of the occluded artery within the critical time window of 6 h for intra-arterial thrombolysis and of 8 h for SAT. In comparison, posterior circulation stroke can be treated up to 24 h after the onset.2 Compared with intra-arterial thrombolysis, SAT is preferred because of high recanalization rates; short procedure time, which restores cerebral blood flow following thrombectomy; and low incidence of haemorrhagic complications.1,2 In all of our patients with stroke, SAT was performed with Solitaire™ AB/FR stents (ev3; Covidien Vascular Therapies, Mansfield, MA).
Although SAT is performed as safely as possible, complications may occur during or after the procedure that can increase morbidity and mortality. These complications are generally classified as haemorrhagic, procedure-/device-related, vasospasm, ischaemic complications at another site and puncture site problems. Although the use of SAT has increased, haemorrhagic complications remain the most feared and the most frequent in the MR CLEAN trial (7.7%).2
We present the digital subtraction angiography (DSA; Allura Xper FD20; Philips Medical Systems, Best, Netherlands) and CT (SOMATOM® Sensation 16; Siemens Medical Solutions, Forchheim, Germany) imaging findings related to the periprocedural complications seen in 56 patients who received SAT between November 2012 and November 2014. Among these patients, thrombolysis in cerebral infarction grade 2b/3 was achieved in 45 of 56 patients, whereas in 4 of 56 patients, access or recanalization was not achieved.
The number of periprocedural complications is listed at Table 1.
Table 1.
The number of periprocedural complications
| Complications | Number |
|---|---|
| Haemorrhagic complications | |
| PH (1/2) and HI (1/2) | 8 |
| Symptomatic haemorrhage | 2 |
| Device-/procedure-related complications | |
| Stent detachment | 2 |
| Arterial dissection | 3 |
| Carotid-cavernous fistula | 1 |
| Vascular perforation | 1 |
| Arterial occlusions or ischemic complications in other locations | 2 |
| Vessel vasospasm | 2 |
| Reocclusion | 2 |
HI, haemorrhagic infarction; PH, parenchymal haematoma.
TECHNIQUE
In most of our cases, general anaesthesia was performed, which was the first line option in severe cases such as basilar and internal carotid artery (ICA) occlusions.
Simmons 2 catheter or vertebral catheters 100/125 cm (Stryker Neurovascular, Fremont, CA and Cook, Bloomington, IN) were used to engage the supra-aortic arteries; Terumo stiff guidewire (Terumo, Tokyo, Japan) was used to place the 6-Fr Envoy® (Cook) and 8-Fr balloon-guiding catheters (Balt, Montmorency, France) to the main arteries. Transend floppy microguidewire 300 cm (Stryker Neurovascular), Rebar® (Covidien, Irvine, CA) microcatheter and Solitaire (Covidien) stents were used for SAT referring to the artery diameter. The stent sizes used for middle cerebral artery (MCA) occlusions were 4–5 × 30 and 6 × 30 mm for basilar artery or ICA. After launching the guiding catheter to the parent artery, Transend microwire and Rebar microcatheter were used to reach the occlusion site. Having deployed the stent to the location of occlusion, we waited for 5 min before applying SAT with negative suction through the guiding catheter until the thrombectomy stent was driven out. A balloon-guiding catheter was preferred for ICA occlusions to reduce the thrombus migration in SAT. But in cases with distal small vessel occlusion after the large vessel recanalization, we used intra-arterial tissue plasminogen activator infusion.
HAEMORRHAGIC COMPLICATIONS
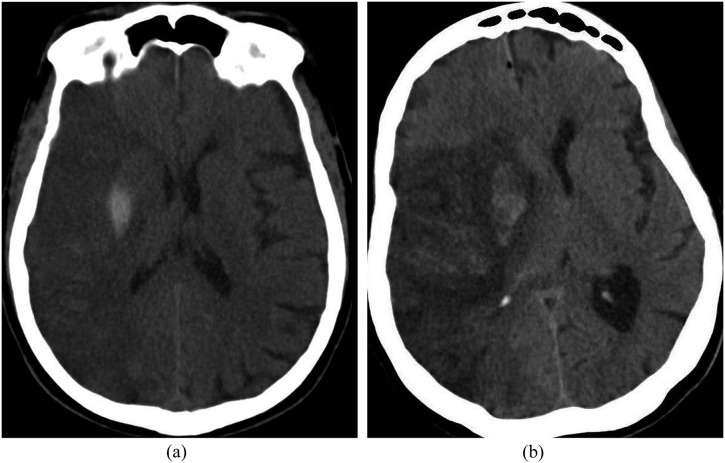
Haemorrhagic transformation is the most feared complication early after SAT. The European Cooperative Acute Stroke Study II classified intracranial haemorrhage with hemispheric stroke syndromes of the anterior circulation as haemorrhagic infarction (HI) or parenchymal haematoma (PH) according to the mass effect at the infarct area on CT. HI is defined as punctate (HI1) or more confluent (HI2) petechial haemorrhage with indistinct borders of the vascular territory (Figure 1a). The combined rate of HI1 and HI2 were reported as 0.4% (1 of 233 patients) in the MR CLEAN trial.2 PH has two subtypes with sharp borders related to the mass effect: less (PH1) or more (PH2) than 30% of the infarcted area (Figure 1b).5 PH1 was not seen in patients in the MR CLEAN trial, whereas the incidence of PH2 was 6%. Also, the term symptomatic haemorrhage refers to poor clinical outcome and was defined as an increase of four or more points in the National Institutes of Health Stroke Scale score compared with the pre-treatment score within 36 h of treatment in the Prolyse in Acute Cerebral Thromboembolism II study.5
Figure 1.
(a) Axial non-contrast CT image of a 74-year-old male with right middle cerebral artery (MCA) infarction that resulted in sulcal effacement and localized haemorrhage at the putamen. This was compatible with the European Cooperative Acute Stroke Study (ECASS) Type 2 haemorrhagic infarction. (b) Axial non-contrast CT scan showing a hypodense right middle cerebral artery territory infarction resulting in midline shift and compression of the lateral ventricles in a 77-year-old female. The ECASS parenchymal haemorrhage Type 1 including the right basal ganglia was observed on the second day after stroke, occupying <30% of the infarction.
PROCEDURE-/DEVICE-RELATED COMPLICATIONS
Stent detachment
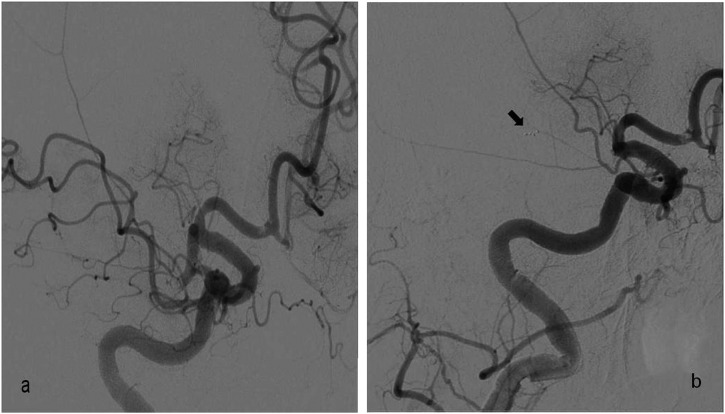
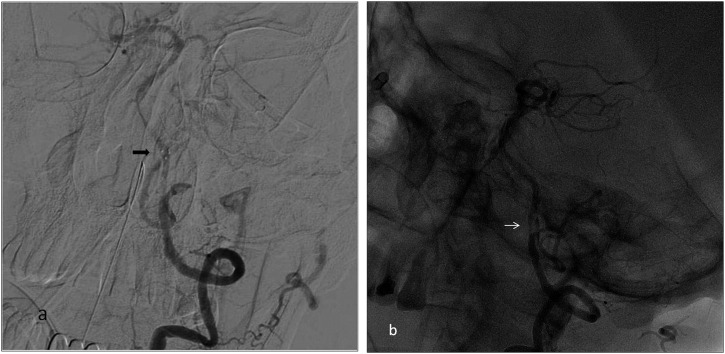
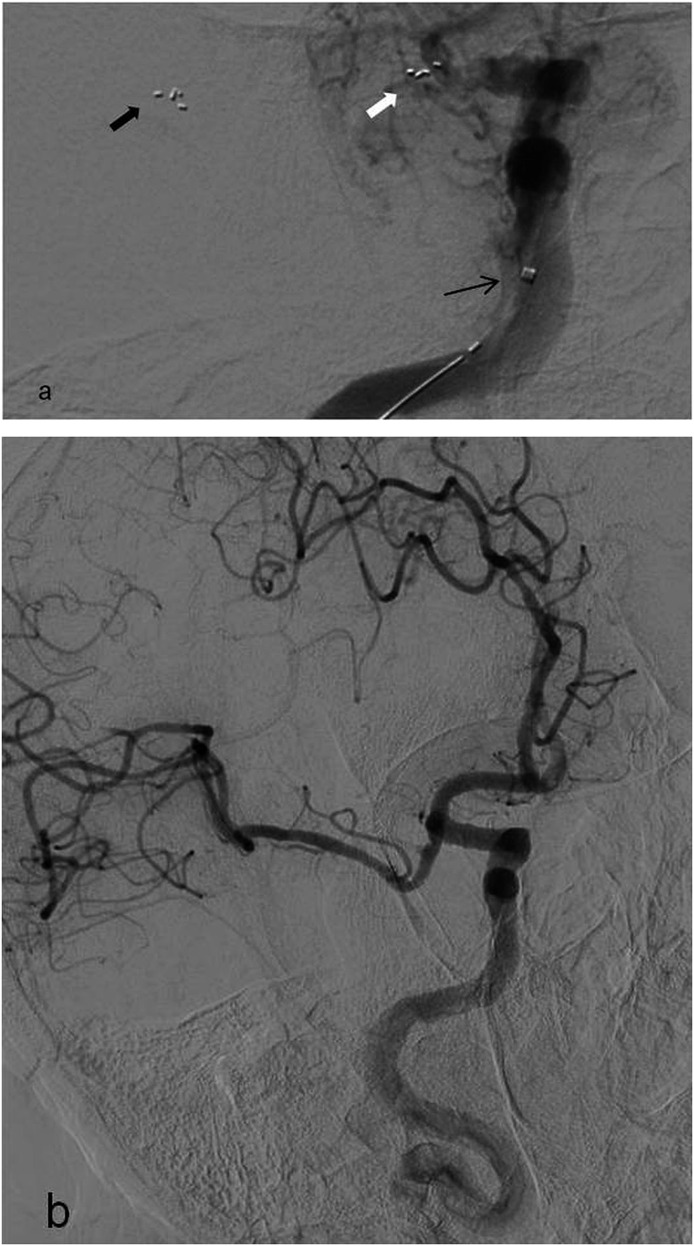
Inadvertent stent retriever detachment is a rarely defined complication of SAT with no percentage reported in recent trials. Potential causes are vascular pathologies such as arterial stenosis, tortuosity and wall calcification; increased number of stent passes during SAT; and stent structural features.6,7 Two stent detachments occurred in our study during the use of the Solitaire FR stent, which is a detachable stent. In the first case, stent detachment occurred at the dissected basilar artery with accompanying focal stenosis and, in the second case, at the MCA, which was assumed to be related to stent structural features (Figures 2 and 3). Two solutions are suggested in the literature to overcome this complication: leaving the stent in place and performing angioplasty, or removal of the detached stent and replacement with another thrombectomy stent within the treatment time window (Figure 4).6,7
Figure 2.
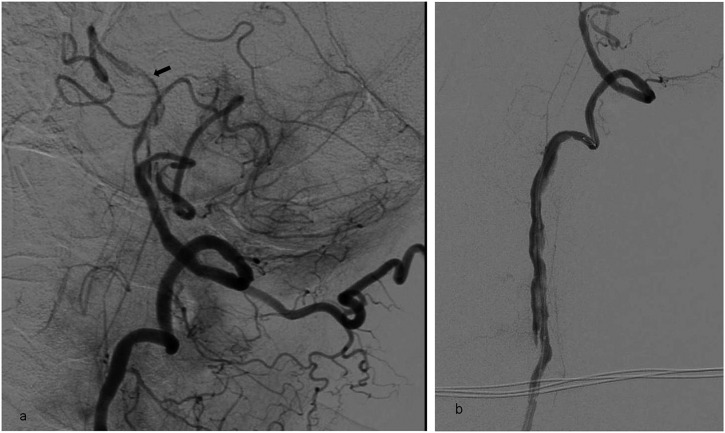
Digital subtraction angiography oblique image of the 77-year-old female; (a) right middle cerebral artery (MCA) M1 segment occlusion before stent-assisted thrombectomy (SAT). Note the small thrombus at the anterior cerebral artery A1 segment; (b) proximal progression of the MCA occlusion to the internal carotid artery tip in the same patient. Inadvertent stent detachment occurred during the second pass during SAT. (Arrow shows the distal markers of the detached stent.)
Figure 3.
Digital subtraction angiography (a) and unsubtracted (b) images showing detachment of the thrombectomy stent at the vertebrobasilar junction in a 63-year-old male with basilar dissection. (a) Arrow shows the distal marker of the detached stent. (b) At the basilar artery, there was a flap appearance and subintimal contrast filling (arrow) which was highly possible for the dissection. In this patient, during the procedure, dissection progressed downwards.
Figure 4.
Digital subtraction angiography (DSA) oblique image of a 74-year-old male patient; (a) inadvertent stent detachment at the right middle cerebral artery occlusion site. Another thrombectomy stent (white arrow) was deployed proximal to the detached stent (thick black arrow) to extract it. The thin black arrow indicates the deploy catheter of the second thrombectomy stent. DSA oblique image (b) reveals thrombolysis in cerebral infarction grade 2b recanalization of the occluded artery after the removal of the thrombectomy stent in the same patient.
Arterial dissection
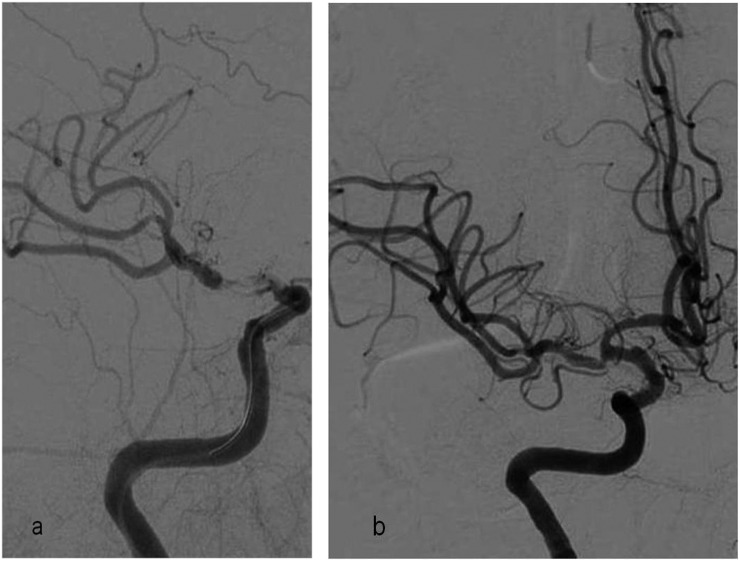
Iatrogenic dissection is a type of traumatic dissection that may occur during SAT. However, there are no known discussions in the literature of the relationship between the catheter or stent type and dissection.8 Akins et al9 reported an incidence of dissection of 3.5% with Solitaire AB/FR stents, and the MR CLEAN trial described procedure-related vessel dissections in four patients (1.7%).2 However, one of three dissections occurred because of movement of the inflated balloon-guiding catheter along the intimal surface of the vessel during SAT (Figure 5). The intimal injury that the thrombectomy devices cause on the vessel surface may be another reason for the dissection, which is worse in cases with pre-existing dissection (Figure 6). Another technical reason could be the stiff guidewire used to launch the guiding catheter in tortuous vessels (Figure 7).
Figure 5.
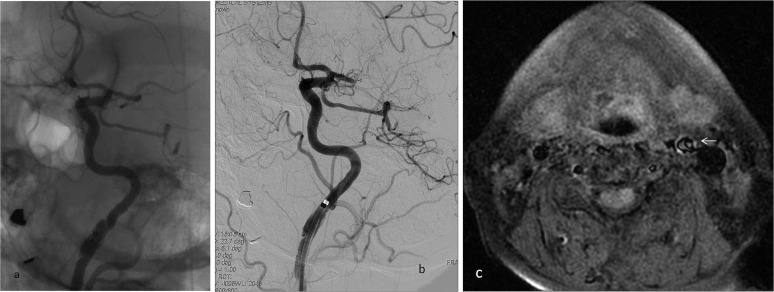
Unsubtracted (a) and digital subtraction angiography (b) images of a 65-year-old female with left middle cerebral artery stroke showing dissection at the left internal carotid artery (ICA) distal cervical segment because of friction from the 8-Fr balloon-guiding catheter on the intimal surface of the vessel that caused no flow disturbance in the parent vessel. T1 weighted axial fat saturated MRI (c) showing vessel stenosis because of dissection at the left ICA (arrow), which was noticed during the follow-up for the previous silent dissection. As dissection occurred initially during the endovascular intervention and stayed stable without progressing to occlusion, we did not further intervene.
Figure 6.
Digital subtraction angiography (DSA) image (a) of a 63-year-old male patient with basilar artery stroke showing basilar artery dissection (arrow) as a cause of stroke and partial recanalization of the basilar artery after the first pass of the thrombectomy stent. The DSA image (b) in the same patient showing extension of the dissection to the left vertebral artery after repeated interventional manoeuvres. A Wallstent® (Boston Scientific, Natick, MA) was placed to compress the dissection flap and restore flow to the vertebral artery; however, the patient died because of extensive brain stem infarction.
Figure 7.
Digital subtraction angiography image of a right middle cerebral artery (MCA) stroke in a 71-year-old female showing localized distal cervical internal carotid artery (ICA) dissection (arrow) which resulted from manoeuvres using the stiff hydrophilic guidewire tip while launching the guiding catheter to perform stent-assisted thrombectomy. Vasospasm was also seen in the right ICA.
Current treatment options are intravenous administration of heparin for 7 days, followed by Coumadin® (Bristol-Myers Squibb, New York, NY) for 3 months. If there is progressive flow disturbance in the dissected vessel, placing a stent at the dissected site is preferred.8
Carotid-cavernous fistula
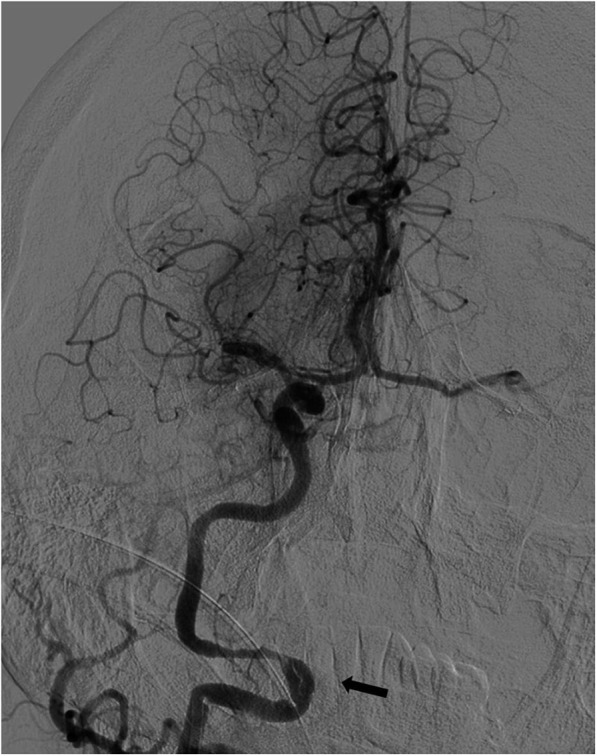
Carotid-cavernous fistulas (CCFs) are abnormal communications between the carotid arterial system and the cavernous sinus. CCFs are classified as direct or indirect and of high or low flow according to angiographic and haemodynamic features, respectively.10 CCF occurred during SAT in one of our patients who had both distal ICA and MCA occlusion (Figure 8). CCFs may spontaneously occlude during SAT or be treated according to the haemodynamic features, with coiling or detachable balloons.10
Figure 8.
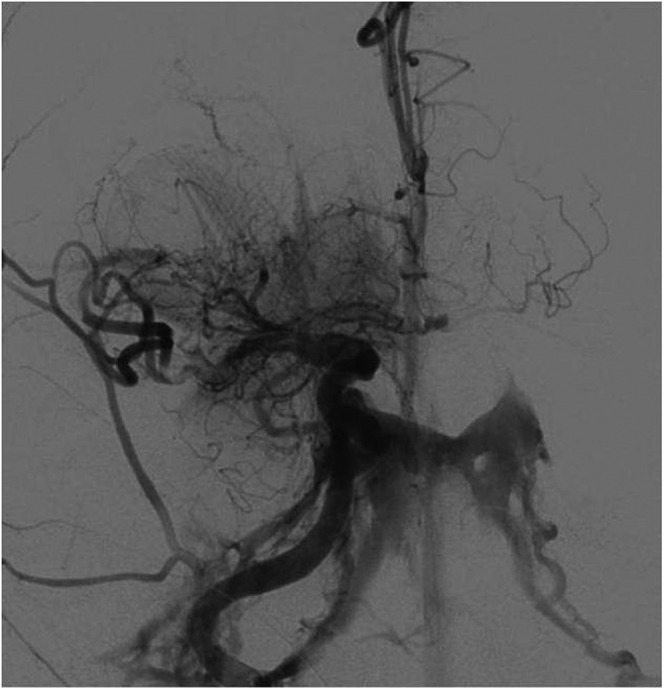
Digital subtraction angiography image of a 75-year-old female with right internal carotid artery stroke showing contrast filling of the cavernous sinus from a carotid-cavernous fistula which occurred during stent-assisted thrombectomy. There was partial recanalization of the middle cerebral artery after the first stent pass, and contrast blush of the lenticulostriate arteries was seen.
Vascular perforation
Vascular perforation is a serious complication of interventional procedures with an incidence of 1–9% and was described in two patients (0.9%) in the MR CLEAN trial. Vascular perforation is identified by extravasation of contrast material, which may be fatal.2,11 In cases of vascular perforation, withdrawal of the microcatheter is not recommended, as subarachnoid haemorrhage is potentially exacerbated by the pre-existing use of thrombolytics or heparin. Vascular perforation occurred in one of our cases while trying to gain access through the MCA occlusion with a microguidewire. As soon as bleeding was detected, we lowered the patient's blood pressure and, following runs, confirmed that bleeding had ceased. The contrast extravasation at the perforation site had excacerbated the haemorrhage appearance at the site of perforation on the CT scan (Figure 9). Although leaving the microcatheter in place and coiling or gluing with n-butyl cyanoacrylate injection are treatment options, lowering the blood pressure resolved the problem in our case.11
Figure 9.
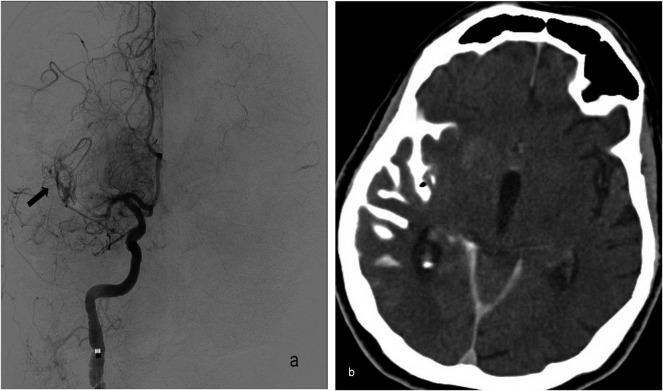
Digital subtraction angiography image (a) of a 77-year-old female patient with right middle cerebral artery (MCA) stroke showing perforation of the distal MCA branch from a microguidewire that caused localized contrast extravasation (arrow). This occurred while performing repetitive attempts to catheterize the right MCA occlusion when we were unable to access the hard plaque with a microcatheter to perform stent-assisted thrombectomy. Because recanalization was not achieved, the subarachnoid haemorrhage did not progress. Axial non-contrast control CT image (b) after the endovascular intervention demonstrating localized subarachnoid haemorrhage in the right temporal region extending to the Sylvian fissure.
Arterial occlusions or ischaemic complications in other locations
Dislodgement of occlusive plaque during intervention in another location is treated as a new embolus and is usually prevented by simultaneous aspiration from the guiding catheter during SAT. Embolization into new territories outside the target downstream territory of the occluded vessel was declared in 20 of the 233 patients (8.6%) in the MR CLEAN trial but Akins et al reported emboli to new vascular territory in 1 of 144 cases (0.7%).2,9 We experienced embolization of plaque in two patients in other vascular territories after the intervention during control runs and performed repeated SAT at these locations (Figure 10).
Figure 10.
Digital subtraction angiography (DSA) image (a) of bilateral middle cerebral artery occlusion in a 68-year-old male patient showing complete recanalization after the first stent-assisted thrombectomy. DSA image (b) of the same patient showing repeated embolization of the left anterior cerebral artery (ACA) (arrow) after total recanalization. He had a pre-diagnosis of chronic atrial fibrillation, and stroke occurred despite chronic Coumadin therapy. Control DSA image (c) showing catheterization of the reoccluded ACA that resulted in migration of the thrombus (arrow) and embolization to the distal ACA branches.
VASOSPASM
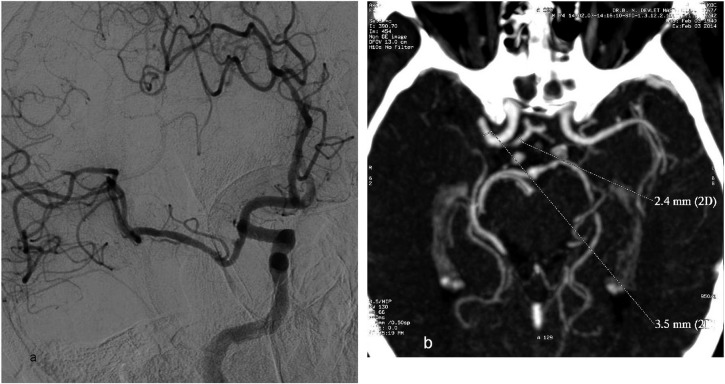
Vessel vasospasm, usually asymptomatic, may result from manipulations with the catheter and guidewires during intervention. Akins et al9 reported a 20% incidence of vasospasm without clinical sequelae. We experienced two cases of vasospasm with initial normal diameter on CT or DSA during the intervention as an uncommon complication of our neurointervention series (Figure 11). We added 15 ml of nimodipine to the saline flush during all interventions while controlling blood pressure, with successful outcomes in all patients without additional treatment for vasospasm.
Figure 11.
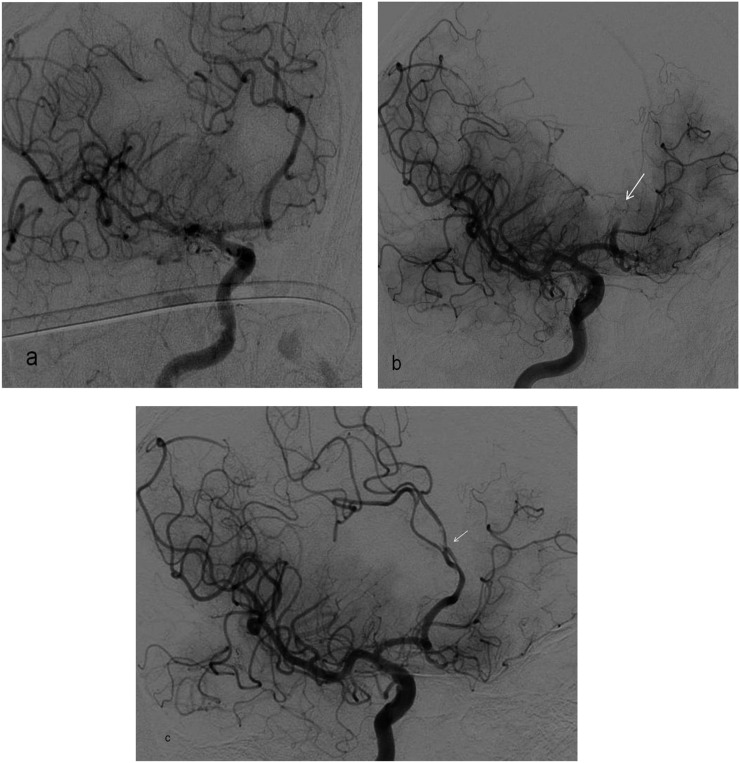
Vasospasm at the recanalized middle cerebral artery (MCA) M1 segment is seen on the oblique digital subtraction angiography image (a) of a 74-year-old male patient with right MCA stroke who underwent detached stent extraction after repeated stent-assisted thrombectomy (SAT). CT angiography maximum intensity projection image (b) showing the initial diameter of the MCA M1 segment (3.5 mm) and anterior cerebral artery A1 segment (2.4 mm) before SAT, which supports the presence of vasospasm after the intervention. 2D, two dimensions.
REOCCLUSION
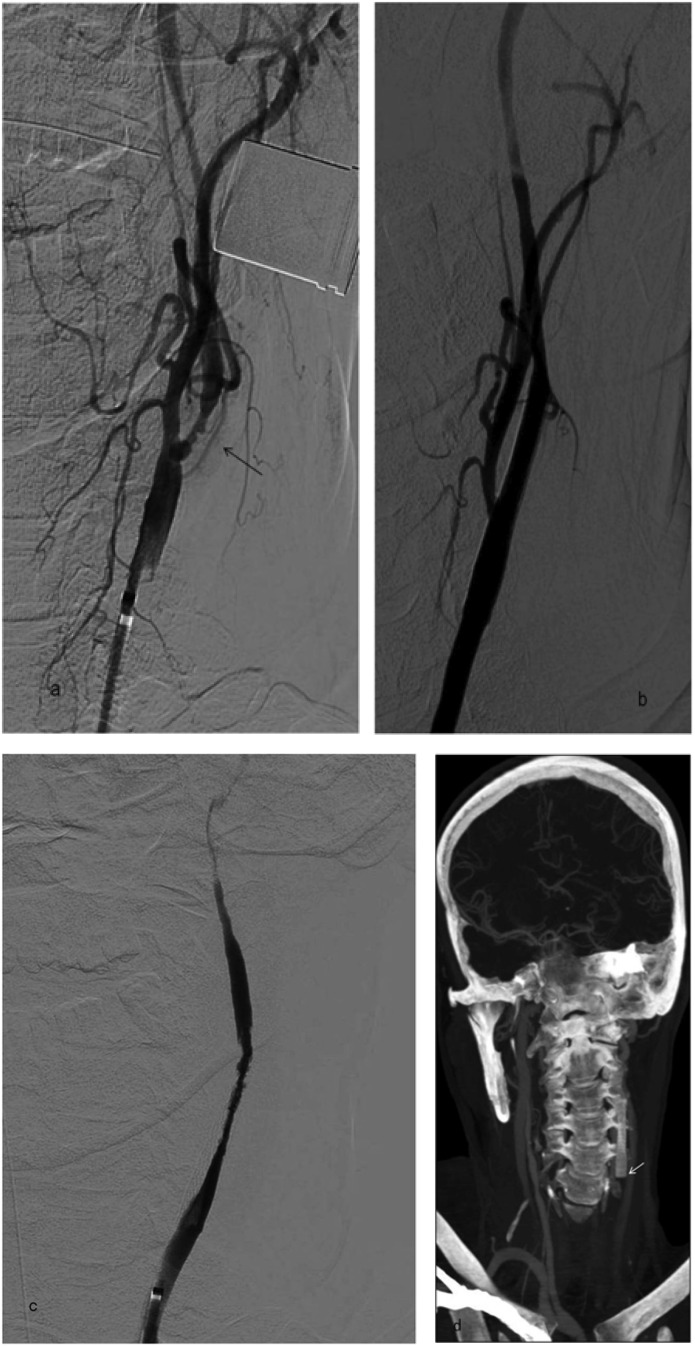
Reocclusion of the thrombectomy site is a rare complication in the literature with a frequency of 0.6% in one study,7 and an incidence of 6.4% during SAT in another study.12 Figures 12 and 13 show the CT and DSA images of two cases with reocclusion where the patients underwent repeated SAT. In the first case, left ICA stenosis with ulcerated plaques was observed at the thrombectomy site and a tapered stent was placed with double antiaggregant treatment. Occlusion of the stent occurred 2 h later, necessitating the second SAT (Figure 12). Although thrombolysis in cerebral infarction grade 3 was achieved in the second case, reocclusion occurred at the stenotic M1 segment of the MCA occlusion, which was observed on clinical follow-up (Figure 13).
Figure 12.
Digital subtraction angiography (DSA) image (a) of a 53-year-old female patient with left internal carotid artery (ICA) stroke showing proximal stenosis of the left internal carotid artery (ICA) that was revealed after the first stent pass during stent-assisted thrombectomy (SAT). The ulcerated plaques (arrow) caused ICA occlusion. As there was progressive reocclusion of the artery even after repeated thrombectomies, we assumed that it is the ruptured plaque that caused the emboli. In addition, no proof of embolism was found at echocardiography or atrial fibrillation at electrocardiography. DSA image (b) showing the tapered stent placed to the left common carotid artery (CCA)-ICA to recanalize the stenosis as slow flow was noticed at the left ICA on control runs. DSA image (c) showing occlusion of the delivered tapered stent 2 h after carotid artery stenting. Slow flow and reduced vascular diameter was seen. CT angiography maximum intensity projection image (d) showing progression of carotid stent occlusion to the distal CCA occlusion and the absence of contrast staining at the left CCA (arrow).
Figure 13.
Digital subtraction angiography oblique image (a) of a 66-year-old male patient with right middle cerebral artery (MCA) stroke showing MCA M1 segment occlusion and partial recanalization of the occlusion after stent deployment and the thrombus inside the stent. DSA oblique image (b) showing thrombolysis in cerebral infarction grade 3 recanalization after stent-assisted thrombectomy and stenosis of the proximal MCA M1 segment which had progressed to partial reocclusion during follow-up imaging.
CONCLUSION
We present the imaging findings related to complications seen during SAT in respect to the most recent trials. Recognition of complications and knowledge of the treatment options are important to design solutions within the critical time window in endovascular stroke treatment.
Contributor Information
Suha H Akpinar, Email: akpinarsuha@hotmail.com.
Guliz Yilmaz, Email: glz.yilmaz@hotmail.com.
REFERENCES
- 1.Darkhabani Z, Nguyen T, Lazzaro MA, Zaidat OO, Lynch JR, Fitzsimmons BF, et al. Complications of endovascular therapy for acute ischemic stroke and proposed management approach. Neurology 2012; 79 (13 Suppl. 1): S192–98. doi: 10.1212/WNL.0b013e31826958e3 [DOI] [PubMed] [Google Scholar]
- 2.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. ; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2014; 372: 11–20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. ; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–30. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 4.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. ; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–18. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 5.Trouillas P, von Kummer R. Classification and pathogenesis of cerebral hemorrhages after thrombolysis in ischemic stroke. Stroke 2006; 37: 556–61. doi: 10.1161/01.STR.0000196942.84707.71 [DOI] [PubMed] [Google Scholar]
- 6.Akpınar S, Yılmaz G. Spontaneous SolitaireTM AB thrombectomy stent detachment during stroke treatment. Cardiovasc Intervent Radiol 2015; 38: 475–8. doi: 10.1007/s00270-014-1022-y [DOI] [PubMed] [Google Scholar]
- 7.Gascou G, Lobotesis K, Machi P, Maldonado I, Vendrell JF, Riquelme C, et al. Stent retrievers in acute ischemic stroke: complications and failures during the perioperative period. AJNR Am J Neuroradiol 2014; 35: 734–40. doi: 10.3174/ajnr.A3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cloft HJ, Jensen EM, Kallmes DF, Dion JE. Arterial dissections complicating cerebral angiography and cerebrovascular interventions. AJNR Am J Neuroradiol 2000; 21: 541–5. [PMC free article] [PubMed] [Google Scholar]
- 9.Akins PT, Amar AP, Pakbaz RS, Fields JD; SWIFT Investigators. Complications of endovascular treatment for acute stroke in the SWIFT trial with solitaire and Merci devices. AJNR Am J Neuroradiol 2014; 35: 524–8. doi: 10.3174/ajnr.A3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korkmazer B, Kocak B, Tureci E, Islak C, Kocer N, Kizilkilic O. Endovascular treatment of carotid cavernous sinus fistula: a systematic review. World J Radiol 2013; 5: 143–55. doi: 10.4329/wjr.v5.i4.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen TN, Lanthier S, Roy D. Iatrogenic arterial perforation during acute stroke interventions. AJNR Am J Neuroradiol 2008; 29: 974–5. doi: 10.3174/ajnr.A0958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lefevre PH, Lainay C, Thouant P, Chavent A, Kazemi A, Ricolfi F. Solitaire FR as a first-line device in acute intracerebral occlusion: a single-centre retrospective analysis. J Neuroradiol 2014; 41: 80–6. doi: 10.1016/j.neurad.2013.10.002 [DOI] [PubMed] [Google Scholar]