Abstract
Detecting focal abnormalities in MRI examinations of children with epilepsy can be a challenging task given the frequently subtle appearance of cortical dysplasia, mesial temporal sclerosis and similar lesions. In this report, we demonstrate the utility of double inversion recovery MRI in the detection of paediatric epileptogenic abnormalities, promoted primarily by increased lesion conspicuity due to complementary suppression of both cerebrospinal fluid and normal white matter signal.
INTRODUCTION
Identifying structural abnormalities that correspond to an epileptogenic focus is frequently a challenging task with the available MRI imaging techniques.1 Advances in spatial and contrast resolution have made possible the detection of subtle findings in patients with epilepsy, particularly focal cortical dysplasia (FCD) in paediatric patients. Given the cortical location of these lesions and the associated blurring of the underlying white matter, an MRI sequence that highlights cortical and subcortical pathology and increases the conspicuity of abnormal white matter is naturally a suitable candidate to be successfully employed in this clinical scenario.
Double inversion recovery (DIR) MRI has been proposed in central nervous system imaging, given its improved lesion-to-background contrast driven by simultaneous suppression of signal from both cerebrospinal fluid and normal white matter.2,3 The technique involves application of two inversion recovery pulses. The timing of the pulses is set so that the longitudinal magnetization from cerebrospinal fluid (CSF) and from white matter passes simultaneously through the null point. Image acquisition then proceeds with a standard fast spin echo sequence that samples the remaining magnetization, which is predominantly from grey matter.
DIR has been previously applied in the evaluation of multiple sclerosis and demonstrated increased sensitivity for depiction of cortical lesions, both at 1.5 T and at 3.0 T.4–9 Utilizing quantitative analysis of signal intensities, DIR has also proved beneficial in characterizing epileptogenic foci related to congenital and acquired neocortical pathology.10 In temporal lobe epilepsy, DIR has demonstrated superior sensitivity compared with T2 fluid-attenuated inversion-recovery and strong agreement with positron emission tomography for localization of epileptogenic focus.11,12 It has also proved valuable in the identification of cortical tubers in patients with tuberous sclerosis.13
The aim of this report is to illustrate the advantages and pitfalls of DIR imaging in patients with epilepsy, highlighting the increased conspicuity for detection of subtle congenital and acquired abnormalities.
CASE SERIES
Using data aggregation and search technology software for our institution's picture archiving and communication system/radiology information system, several illustrative cases of the diagnostic value and pitfalls of DIR were identified. This study was approved by the institutional review board.
IMAGE ACQUISITION
The DIR implementation currently employed at our institution consists of a coronal 3D acquisition of the whole head utilizing body transmit and local signal reception with dedicated 32-channel head coil. All imaging was performed on a 3.0-T clinical whole-body system (Siemens Tim Trio; Siemens Medical Solutions, Erlangen, Germany), with the DIR sequences acquired as a modification to a 3D T2 weighted acquisition (sampling perfection with application optimized contrasts using different flip angle evolution) permitting for flexibility in k-space sampling strategy, echo trains and flip angle evolution schemes, as well as two separate inversion times in a DIR operation block. Contrast optimization is achieved through simulation parameters provided inline, together with the spatially non-selective double-inversion preparation for suppression of both CSF and white matter. Sequence parameters for fat-suppressed coronal 3D DIR were prescribed as follows: TR 7500 ms; TE 327 ms; TI1 3000 ms; TI2 450 ms; excitations 1; voxel size 1-mm isotropic; turbo factor 256; BW 789 Hz/pixel; field of view 200 × 173 mm; parallel acceleration factor (iPAT) 2; acquisition time 7 min 24 s. Note that some of the cases presented in this essay were acquired using an eight-channel head coil and show a relatively lower spatial resolution when compared with our present technique.
NORMAL ANATOMY
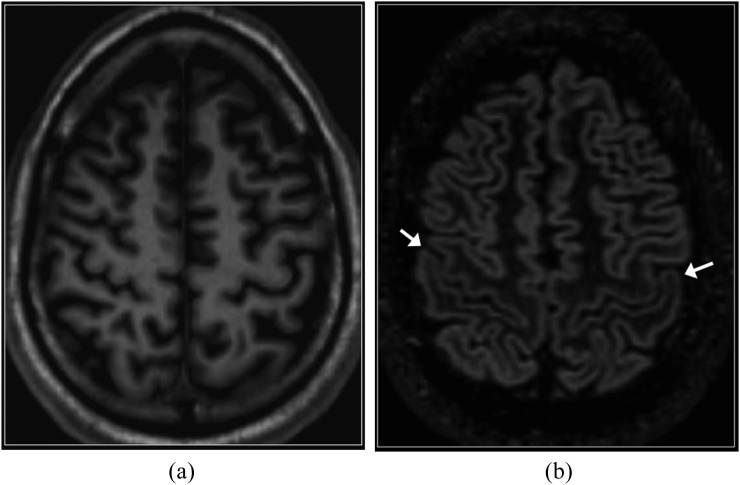
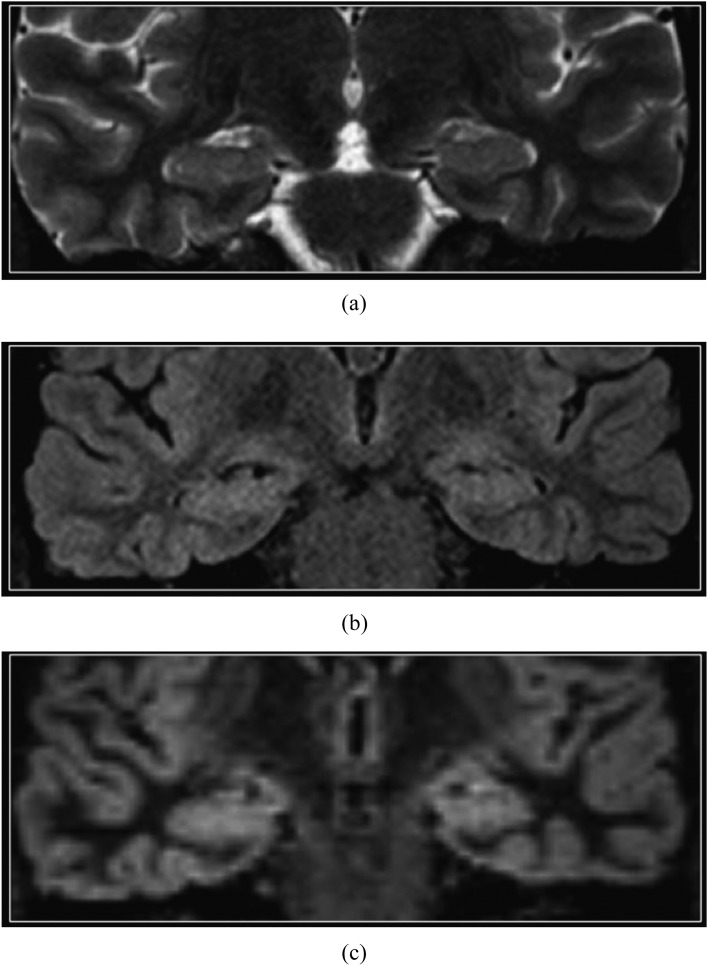
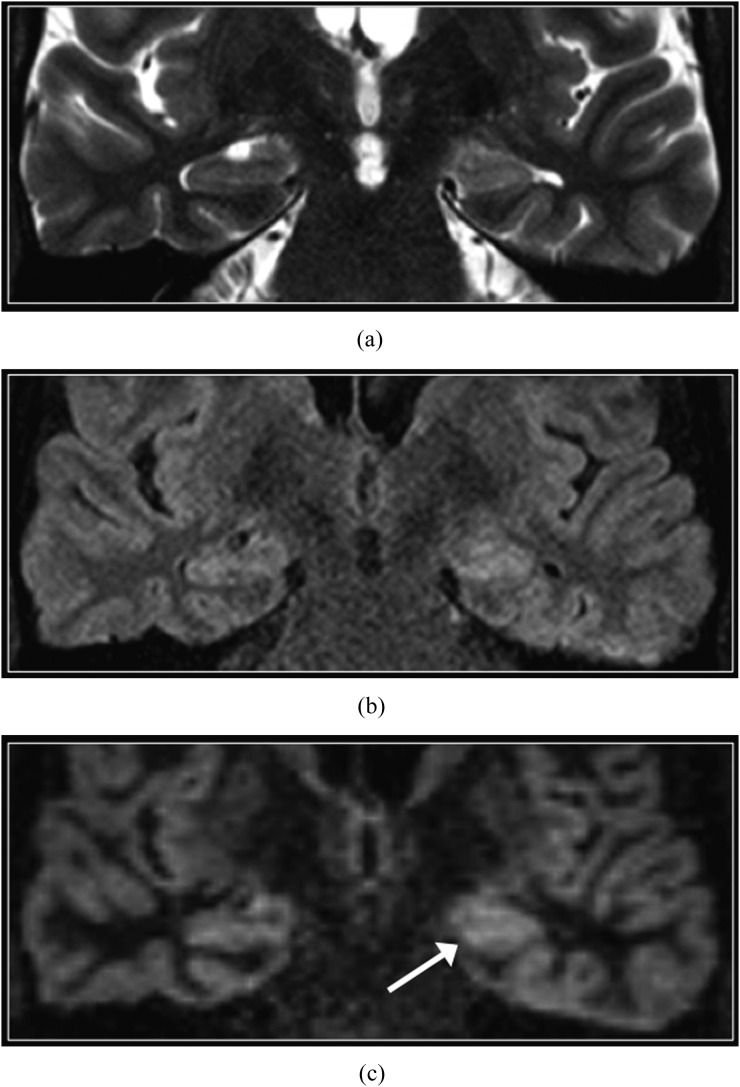
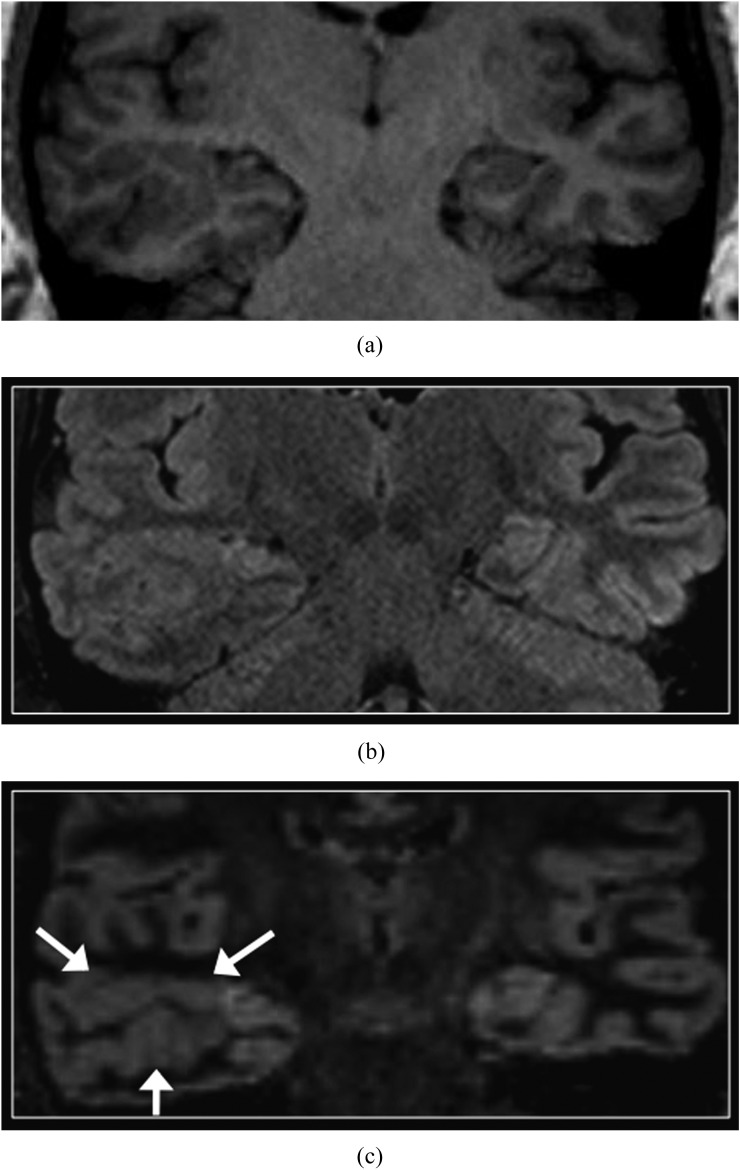
The normal perirolandic cortex appears thinner and less well-defined than the remainder of the neocortex on DIR imaging (Figure 1), owing to cytoarchitectural differences including increased myelination.14 In addition, the normal hippocampi and amygdala are slightly hyperintense than the supratentorial neocortex on DIR sequences and on other T2 weighted sequences (Figure 2).15
Figure 1.
Normal perirolandic cortex. Axial T1 weighted three-dimensional magnetization prepared rapid gradient echo (a) and reformatted double inversion recovery (DIR) (b) images. T1 weighted image shows very subtle high signal intensity in the perirolandic cortex with respect to the remainder of the cortex. On the DIR image, the perirolandic cortex appears relatively low in signal intensity (arrows).
Figure 2.
Normal hippocampus. Coronal T2 weighted (a), T2 fluid-attenuated inversion-recovery (b) and double inversion recovery (c) images. The normal hippocampi are slightly brighter than the normal supratentorial neocortex on T2 weighted sequences, owing to different cytoarchitecture.
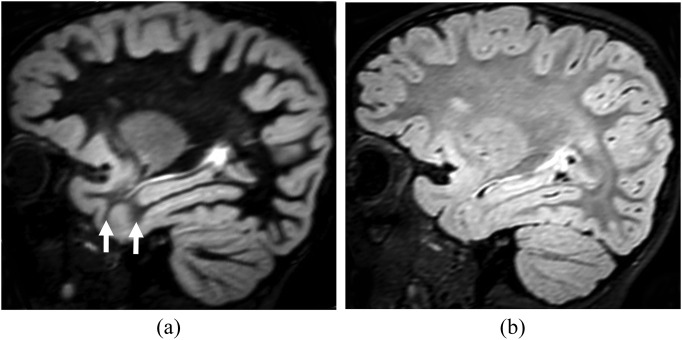
Normal areas of evolving myelination are conspicuous on DIR imaging, appearing as areas of brighter signal when compared with fully myelinated white matter. This is due to relatively diminished T2 shortening of the unmyelinated white matter (Figure 3). These can be distinguished from abnormal white matter by careful analysis of their distribution, for example, in the anterior temporal poles and juxtacortical white matter in children between 1 and 2 years of age.
Figure 3.
Incomplete myelination in a 20-month-old child. Sagittal reformatted double inversion recovery (DIR) (a) and sagittal T2 fluid-attenuated inversion-recovery (b) images show relatively increased signal intensity in the white matter of the anterior temporal lobes when compared with the frontal white matter. This difference, due to incomplete myelination, is most conspicuous on the DIR image (arrows). Despite the apparent blurring of the grey–white matter junction, this is a normal finding when symmetric.
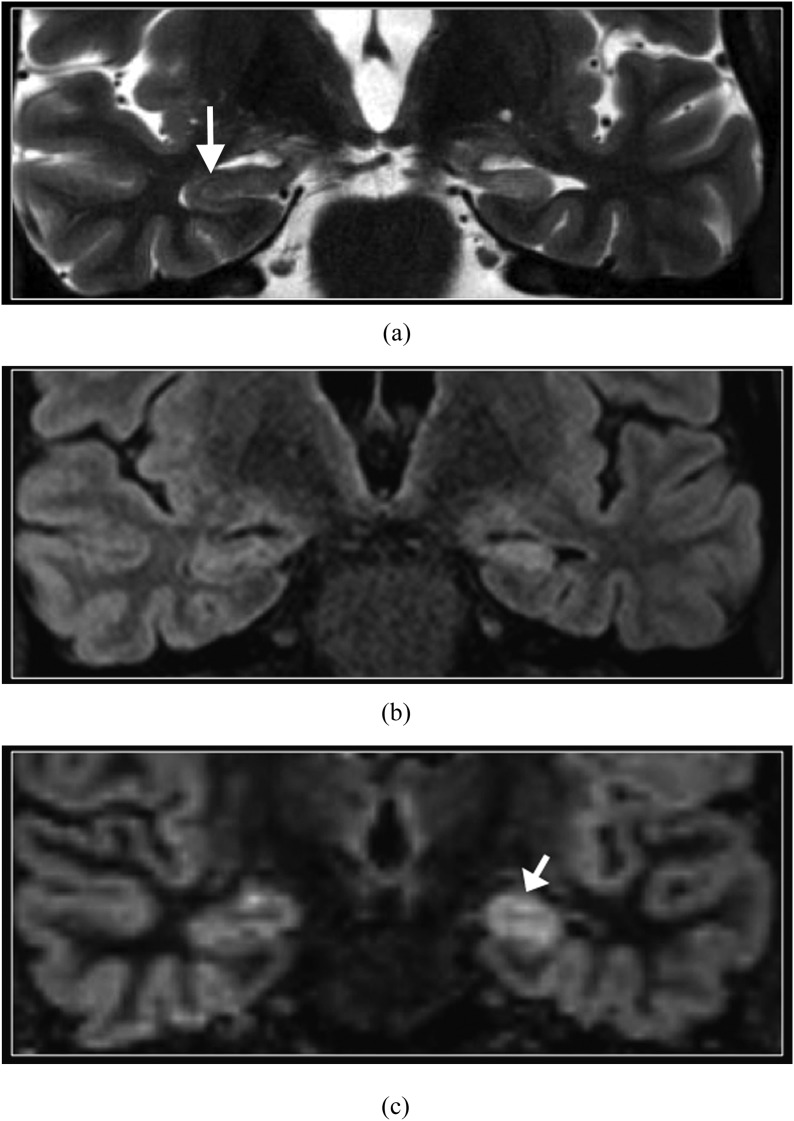
MESIAL TEMPORAL SCLEROSIS
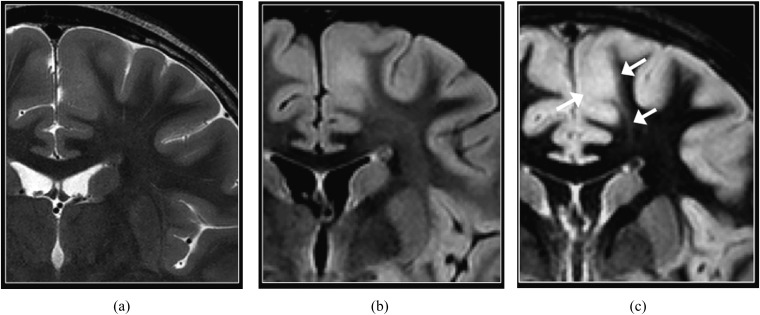
Although relatively less common in children than in adults suffering from refractory epilepsy,16 mesial temporal sclerosis nevertheless represents a diagnostic challenge on MRI when the findings of hippocampal atrophy, T2 hyperintense signal and disruption of internal architecture are subtle, such as in the early stage. DIR imaging is beneficial by demonstrating increased conspicuity of asymmetric signal intensity of the hippocampi (Figure 4). However, the internal architecture of the hippocampus is better depicted on T2 weighted imaging.
Figure 4.
Mesial temporal sclerosis. Coronal T2 weighted (a), T2 fluid-attenuated inversion-recovery (b) and double inversion recovery (DIR) (c) images. The left hippocampus is atrophic and hyperintense (arrow in c). Asymmetry of signal intensity of the hippocampi is most conspicuous on the DIR image. However, the normal internal architecture of the right hippocampus is better depicted on the T2 weighted image (arrow in a).
Recent seizures may cause swelling and T2 hyperintense signal in the affected hippocampus. In this setting, DIR imaging conspicuously demonstrates the asymmetric signal hyperintensity of the hippocampi (Figure 5).
Figure 5.
Ictal changes. Coronal T2 weighted (a), T2 fluid-attenuated inversion-recovery (b) and double inversion recovery (DIR) (c) images. The left hippocampus is swollen and hyperintense but retains its normal internal architecture on the T2 weighted image. Asymmetry of signal intensity of the hippocampi is most conspicuous on the DIR image (arrow).
FOCAL CORTICAL DYSPLASIA AND TUBEROUS SCLEROSIS
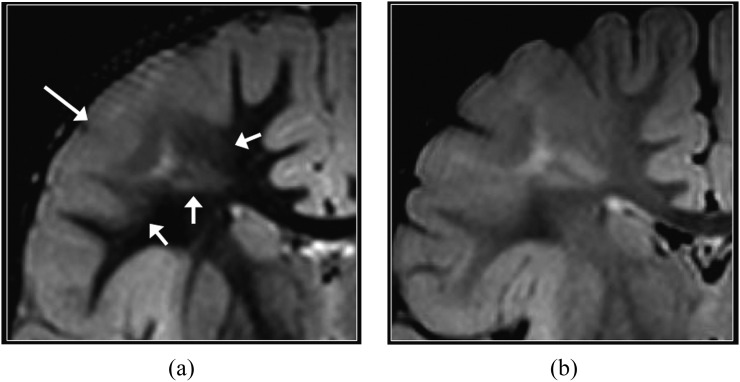
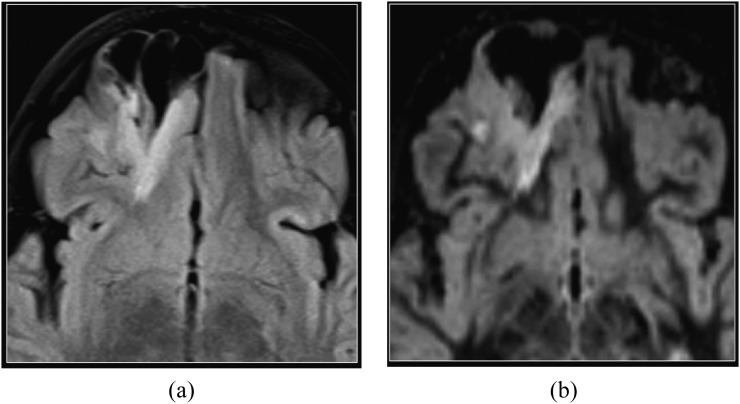
FCD, particularly the Type IIB, and cortical tubers are commonly associated with radial glial abnormality extending to the wall of the lateral ventricle (also known as the transmantle sign). DIR imaging is excellent in depicting the transmantle sign and the boundaries of the white matter signal abnormality (Figures 6 and 7). It also depicts subtle Type I FCDs that are sometimes located in the temporal pole and characterized by incomplete asymmetric myelination of the subcortical white matter.
Figure 6.
Focal cortical dysplasia (FCD). Coronal T2 weighted (a), T2 fluid-attenuated inversion-recovery (b) and double inversion recovery (DIR) (c) images. Blurring of the grey–white matter junction is present in the left paramedian superior frontal gyrus, consistent with FCD. The transmantle sign suggestive of FCD Type II is identified as curvilinear hyperintense signal extending from the grey–white matter junction to the superolateral margin of the lateral ventricle and is most conspicuous on the DIR image (arrows). FCD is found in almost 50% of children who undergo surgery for intractable epilepsy.17
Figure 7.
Tuberous sclerosis. Coronal double inversion recovery (DIR) (a) and T2 fluid-attenuated inversion-recovery (b) images show an area of thick cortex with blurring of the grey–white matter junction cortical tuber in the right frontal lobe, consistent with a cortical tuber in this patient with tuberous sclerosis (long arrow). The margins of the associated transmantle signal abnormality (short arrows) are more precisely delineated on the DIR image.
PERIVENTRICULAR NODULAR HETEROTOPIA
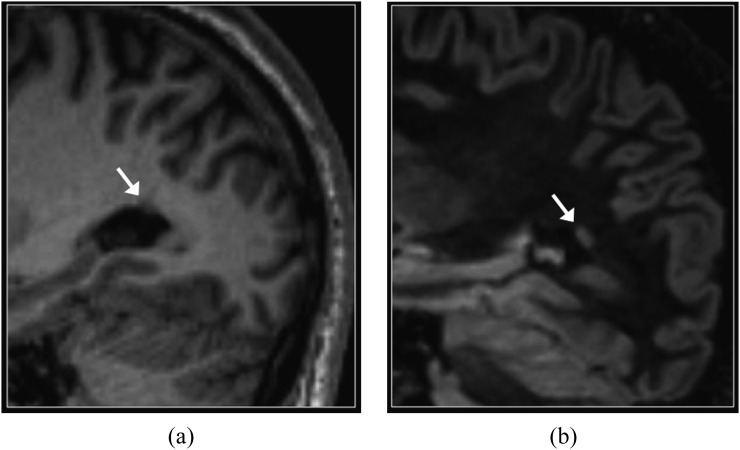
The suppression of normal white matter signal achieved with DIR imaging results in increased conspicuity of small foci of heterotopic grey matter (Figure 8). DIR may also help distinguish nodular heterotopia from small foci of gliosis, since the latter are typically brighter than normal grey matter.
Figure 8.
Periventricular nodular heterotopia. Sagittal T1 weighted three-dimensional magnetization prepared rapid gradient echo (a) and reformatted double inversion recovery (DIR) (b) images. A small focus of heterotopic grey matter (arrows) is more conspicuous on the DIR image. The signal intensity of heterotopia is similar to that of normal grey matter.
POLYMICROGYRIA
Not all malformations of cortical development present with abnormal signal intensity on DIR imaging. Polymicrogyria occurs in later stages of cortical development (late migration/early organization); therefore, the abnormal cortex is relatively less dysplastic and may not exhibit the high signal intensity typically seen in FCD on T2 weighted images. Volumetric T1 weighted sequences also demonstrate similar signal intensity between normal cortex and areas of polymicrogyria (Figure 9).
Figure 9.
Polymicrogyria. Coronal T1 weighted three-dimensional magnetization prepared rapid gradient echo (a), T2 fluid-attenuated inversion-recovery (b) and double inversion recovery (DIR) (c) images. An area of abnormal gyral pattern with irregular contours is present in the right temporal lobe (arrows), consistent with polymicrogyria. On all images, including DIR, there is no appreciable difference in the signal intensity of the normal and abnormal cortex. Note also mild atrophy of the ipsilateral hippocampus, without associated signal abnormality. Adjacent areas of subcortical heterotopia were also present (not shown).
NEUROGLIAL TUMOURS
DIR imaging provides high contrast between tumour and adjacent normal-appearing cortex (Figure 10). This facilitates the delineation of the extent of abnormal tissue prior to surgery for refractory epilepsy, particularly since neuroglial tumours may be associated with areas of cortical dysplasia that may contribute to epileptogenesis.18
Figure 10.
Dysembryoplastic neuroepithelial tumour. Axial T2 fluid-attenuated inversion-recovery (a) and reformatted axial double inversion recovery (DIR) (b) images of an intra-axial mass in the right frontal lobe with peripheral cystic components remodelling the inner table of the calvarium indicating slow growth. DIR provides precise delineation between tumour and adjacent normal-appearing cortex.
DISCUSSION
Detection of focal pathology in MRI of children with intractable epilepsy aids pre-surgical planning and intraoperative management. However, abnormalities such as FCD are often subtle, justifying the effort in developing MR sequences that provide optimal contrast between normal and abnormal tissue. For example, Kadom et al19 reported on the utility of T1 weighted magnetization transfer images to detect FCDs in infants and young children with incomplete myelination. Chan et al20 reported accurate diagnosis and localization of FCD utilizing high-resolution T2 weighted fast multiplaner inversion recovery images. Excellent contrast between grey and white matter can also be achieved with DIR, consisting of two inversion pulses applied to simultaneously suppress the signal from two tissues with different longitudinal relaxation times. By selectively suppressing CSF and normal white matter signals, grey matter-only images can be obtained. Recent developments allow DIR to be performed utilizing multichannel coils at higher magnetic fields, providing high-resolution isotropic MR images.
DIR imaging has a potential beneficial role in lesion detection and lateralization in patients with epilepsy. We illustrate the role of DIR imaging in epilepsy in such cases as malformations of cortical development, mesial temporal sclerosis and neoplasms. In evaluating such cases, it is important to remain aware that differences in cytoarchitecture cause inherently higher signal intensities of the hippocampi (and to a lesser extent of the insular cortices) with DIR. Distinguishing the boundaries of an infiltrative tumour or cortical dysplasia from adjacent normal cortex or white matter is facilitated by DIR. Furthermore, given the increased conspicuity for identification of cortical tubers, DIR can be employed to subjectively quantify the burden of disease in tuberous sclerosis. However, despite the aforementioned advantages of DIR imaging, its diagnostic value is limited in the incompletely myelinated brain, where suppression of the MR signal from normal white matter relies on its narrow range of T1 values. Optimizing the inversion time for suppression of the normal unmyelinated white matter at different stages of brain development is an area of ongoing attention. In temporal lobe epilepsy, DIR was the most sensitive sequence for detection of unilateral abnormal white matter signal in the anterior aspect of the affected temporal lobe.11 In the normal unmyelinated temporal lobes, the relatively higher signal intensity of the anterior white matter should always be mild and symmetric.
In summary, this clinical report highlights the applications of DIR in paediatric epilepsy and raises awareness of potential interpretative pitfalls. Further studies are required to establish the accuracy of DIR for detection of epileptogenic abnormalities and to quantify its value in the evaluation of paediatric patients with intractable epilepsy.
CONCLUSION
Given the increased conspicuity in the depiction of subtle abnormalities, we suggest acquisition of DIR imaging when imaging children with refractory epilepsy. Further studies are required to quantify the increased diagnostic accuracy provided by the DIR technique relative to conventional MRI sequences.
Contributor Information
Bruno P Soares, Email: brunopassebon@gmail.com, bruno.soares@emory.edu.
Samuel G Porter, Email: sgporter1@gmail.com.
Amit M Saindane, Email: asainda@emory.edu.
Seena Dehkharghani, Email: seena.dehkharghani@emory.edu.
Nilesh K Desai, Email: nilesh.k.desai@emory.edu.
REFERENCES
- 1.Vezina LG. MRI-negative epilepsy: protocols to optimize lesion detection. Epilepsia 2011; 52(Suppl. 4): 25–7. doi: 10.1111/j.1528-1167.2011.03147.x [DOI] [PubMed] [Google Scholar]
- 2.Redpath TW, Smith FW. Technical note: use of a double inversion recovery pulse sequence to image selectively grey or white brain matter. Br J Radiol 1994; 67: 1258–63. doi: 10.1259/0007-1285-67-804-1258 [DOI] [PubMed] [Google Scholar]
- 3.Turetschek K, Wunderbaldinger P, Bankier AA, Zontsich T, Graf O, Mallek R, et al. Double inversion recovery imaging of the brain: initial experience and comparison with fluid attenuated inversion recovery imaging. Magn Reson Imaging 1998; 16: 127–35. doi: 10.1016/S0730-725X(97)00254-3 [DOI] [PubMed] [Google Scholar]
- 4.Geurts JJ, Pouwels PJ, Uitdehaag BM, Polman CH, Barkhof F, Castelijns JA. Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology 2005; 236: 254–60. doi: 10.1148/radiol.2361040450 [DOI] [PubMed] [Google Scholar]
- 5.Calabrese M, De Stefano N, Atzori M, Bernardi V, Mattisi I, Barachino L, et al. Detection of cortical inflammatory lesions by double inversion recovery magnetic resonance imaging in patients with multiple sclerosis. Arch Neurol 2007; 64: 1416–22. doi: 10.1001/archneur.64.10.1416 [DOI] [PubMed] [Google Scholar]
- 6.Nelson F, Poonawalla AH, Hou P, Huang F, Wolinsky JS, Narayana PA. Improved identification of intracortical lesions in multiple sclerosis with phase-sensitive inversion recovery in combination with fast double inversion recovery MR imaging. AJNR Am J Neuroradiol 2007; 28: 1645–9. doi: 10.3174/ajnr.A0645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wattjes MP, Lutterbey GG, Gieseke J, Träber F, Klotz L, Schmidt S, et al. Double inversion recovery brain imaging at 3T: diagnostic value in the detection of multiple sclerosis lesions. AJNR Am J Neuroradiol 2007; 28: 54–9. doi: 10.3174/ajnr.A0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon B, Schmidt S, Lukas C, Gieseke J, Träber F, Knol DL, et al. Improved in vivo detection of cortical lesions in multiple sclerosis using double inversion recovery MR imaging at 3 Tesla. Eur Radiol 2010; 20: 1675–83. doi: 10.1007/s00330-009-1705-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vural G, Keklikoğlu HD, Temel Ş, Deniz O, Ercan K. Comparison of double inversion recovery and conventional magnetic resonance brain imaging in patients with multiple sclerosis and relations with disease disability. Neuroradiol J 2013; 26: 133–42. doi: 10.1177/197140091302600201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rugg-Gunn FJ, Boulby PA, Symms MR, Barker GJ, Duncan JS. Imaging the neocortex in epilepsy with double inversion recovery imaging. Neuroimage 2006; 31: 39–50. doi: 10.1016/j.neuroimage.2005.11.034 [DOI] [PubMed] [Google Scholar]
- 11.Morimoto E, Kanagaki M, Okada T, Yamamoto A, Mori N, Matsumoto R, et al. Anterior temporal lobe white matter abnormal signal (ATLAS) as an indicator of seizure focus laterality in temporal lobe epilepsy: comparison of double inversion recovery, FLAIR and T2W MR imaging. Eur Radiol 2013; 23: 3–11. doi: 10.1007/s00330-012-2565-4 [DOI] [PubMed] [Google Scholar]
- 12.Morimoto E, Okada T, Kanagaki M, Yamamoto A, Fushimi Y, Matsumoto R, et al. Evaluation of focus laterality in temporal lobe epilepsy: a quantitative study comparing double inversion-recovery MR imaging at 3T with FDG-PET. Epilepsia 2013; 54: 2174–83. doi: 10.1111/epi.12396 [DOI] [PubMed] [Google Scholar]
- 13.Cotton F, Rambaud L, Hermier M. Dual inversion recovery MRI helps identifying cortical tubers in tuberous sclerosis. Epilepsia 2006; 47: 1072–3. doi: 10.1111/j.1528-1167.2006.00529.x [DOI] [PubMed] [Google Scholar]
- 14.Karaarslan E, Arslan A. Perirolandic cortex of the normal brain: low signal intensity on turbo FLAIR MR images. Radiology 2003; 227: 538–41. doi: 10.1148/radiol.2272020311 [DOI] [PubMed] [Google Scholar]
- 15.Hirai T, Korogi Y, Yoshizumi K, Shigematsu Y, Sugahara T, Takahashi M. Limbic lobe of the human brain: evaluation with turbo fluid-attenuated inversion-recovery MR imaging. Radiology 2000; 215: 470–5. doi: 10.1148/radiology.215.2.r00ma06470 [DOI] [PubMed] [Google Scholar]
- 16.Harvey AS, Cross JH, Shinnar S, Mathern GW; ILAE Pediatric Epilepsy Surgery Survey Taskforce. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia 2008; 49: 146–55. doi: 10.1111/j.1528-1167.2007.01421.x [DOI] [PubMed] [Google Scholar]
- 17.Krsek P, Maton B, Korman B, Pacheco-Jacome E, Jayakar P, Dunoyer C, et al. Different features of histopathological subtypes of pediatric focal cortical dysplasia. Ann Neurol 2008; 63: 758–69. doi: 10.1002/ana.21398 [DOI] [PubMed] [Google Scholar]
- 18.Blümcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia 2011; 52: 158–74. doi: 10.1111/j.1528-1167.2010.02777.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadom N, Trofimova A, Vezina GL. Utility of magnetization transfer T1 imaging in children with seizures. AJNR Am J Neuroradiol 2013; 34: 895–8. doi: 10.3174/ajnr.A3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan S, Chin SS, Nordli DR, Goodman RR, DeLaPaz RL, Pedley TA. Prospective magnetic resonance imaging identification of focal cortical dysplasia, including the non-balloon cell subtype. Ann Neurol 1998; 44: 749–57. doi: 10.1002/ana.410440508 [DOI] [PubMed] [Google Scholar]