Abstract
Low back pain and neuralgia due to spinal pathology are very common symptoms debilitating numerous patients with peak prevalence at ages between 45 and 60 years. Intervertebral discs and facet joints act as pain sources in the vast majority of the cases. Diagnosis is based on the combination of clinical examination and imaging studies. Therapeutic armamentarium for low back pain and neuralgia due to intervertebral discs and/or facet joints includes conservative therapy, injections, percutaneous therapeutic techniques and surgical options. Percutaneous, therapeutic techniques are imaging-guided, minimally invasive treatments which can be performed as outpatient procedures. In cases of facet joint syndrome, they include, apart from injections, neurolysis with radiofrequency/cryoablation, MR-guided high-intensity focused ultrasound and percutaneous fixation techniques. In case of discogenic pain, apart from infiltrations, therapeutic techniques can be classified in to two main categories: decompression (mechanical, thermal, chemical) techniques and biomaterials implantation/disc cell therapies. Strict sterility measures are a prerequisite and should include extensive local sterility and antibiotic prophylaxis. This article will report clinical and imaging findings for each pathology type and the association with treatment decision. In addition, we will describe in detail all possible treatment techniques for low back pain and neuralgia, and we will report recently published results of these techniques summarizing the data concerning safety and effectiveness as well as the level of evidence. Finally, we will try to provide a rational approach for the therapy of low back pain and neuralgia by means of minimally invasive imaging-guided percutaneous techniques.
INTRODUCTION
There is a controversy concerning the original substrate of low back pain and neuralgia because of spinal pathology. There are evolutionary theories suggesting that the vertical support structure works but not as well as the horizontal structure of a quadruped which denotes a “walking bridge”.1 On the other hand, more recent studies blame spinal stress, lack of exercise and poor posture for spinal symptoms because of degeneration.2 The fact is that degeneration in the spine is quite common with resultant low back pain, with or without lower extremity pain. Spinal pathology pain is governed by 54–80% lifetime prevalence and 15–45% annual prevalence.3 Low back pain and neuralgia constitute a major portion of pain syndromes with significant social and economic costs and are the most common disability causes in ages <45 years.4,5 Intervertebral discs and facet joints act as pain sources in the vast majority of the cases.5–7
Despite the high prevalence of low back pain and neuralgia, it is often difficult to reach a definite diagnosis which on most cases is based upon a combination of clinical examination and imaging studies.8–10 Imaging studies include non-invasive modalities such as X-rays, CT and MRI or minimally invasive techniques such as myelography, percutaneous discography and diagnostic infiltrations.5
Proposed therapies for low back pain and neuralgia due to intervertebral discs and/or facet joints include conservative therapy, injections (interlaminar, caudal or transforaminal and intra-articular), percutaneous (minimally invasive) techniques and surgical treatments.10–17 In case of facet joint syndrome, therapeutic armamentarium includes apart from conservative therapies and injections, neurolysis with radiofrequency (RF)/cryoablation, MR-guided high-intensity focused ultrasound (HIFU) and percutaneous fixation techniques.16–22 In case of discogenic pain, alternatives to conservative therapy and injections include percutaneous minimally invasive therapeutic techniques and surgical options. The former can be classified in to two main categories: decompression (mechanical, thermal, chemical) techniques and biomaterials implantation/disc cell therapies.12,16,17,23,24
Strict sterility measures and informed consent are a prerequisite. Surgical skin preparation is necessary, and the operative field should be draped with sterile sheets. Prophylactic antibiosis should be administered in disc decompression techniques, facet joint denervation and fixation techniques. Absolute contraindications include a patient unwilling to consent to the procedure and local or systemic infection. Haemorrhagic diathesis should be corrected, and anticoagulant therapy should be interrupted according to international guidelines.16,17
Supplies for spinal injections include needles of various gauges (18–25 G), lengths (88–150 mm) and tip shapes (Crawford or Tuochy). Anesthetics used include short- or long-acting agents (e.g. lidocaine 1–2%, bupivacaine 0.25–0.5% etc.) depending on the operator's preference. Steroid preparations include particulate or non-particulate agents (e.g. methylprednisolone suspension, triamcinolone, betamethasone injectable suspension, cortivasol etc.). As far as steroids are concerned only preservative-free agents should be used in order to avoid arachnoiditis in case of inadvertent extension in the subarachnoid space. Concerning contrast medium for the verification of correct needle placement, only agents approved for myelography should be used in order to avoid complications in case of inadvertent intrathecal injection. In our department, we perform all our spinal injections with 22 G diameter, 90- to 120-mm length tuochy spinal needle; in our practice, we perform a maximum of four injection sessions per year with a maximum of two injections per session.
This article will report clinical and imaging findings for each pathology type and the association to treatment decision. In addition, we will describe in details all possible treatment techniques for low back pain and neuralgia (due to facet joint and intervertebral disc pathology), and we will report recently published results on these techniques summarizing the data concerning safety and effectiveness as well as the level of evidence. Finally, we will try to provide a rational approach for the therapy of low back pain and neuralgia (due to facet joint and intervertebral disc pathology) by means of minimally invasive imaging-guided percutaneous techniques.
FACET JOINTS
Facet joints are innervated by the medial branches of the dorsal rami; in lumbar facet joints, there are free and encapsulated nerve endings as well as nerves containing substance P and calcitonin gene-related peptide.7,25,26 The symmetry and correct orientation of facet joints seem to be fundamental for correct disc function and its protection from abnormal stress, mainly during twisting. Lumbar facet joints constitute a common source of pain accounting for 27–40% of low back pain.27 Degeneration affecting facet joints results in remodeling as an effort of surface increase.28 Imaging findings suggestive of facet joint syndrome include joint space narrowing, intra-articular vacuum phenomenon or fluid, osteophytes (usually at the upper articular surface of the lower vertebra), synovial cyst formation and hypertrophy of flaval ligaments.5,29 Patients complain of low back pain radiating to the thigh, iliac crest and rarely to the groin; pain is exacerbated during pressure, hyperextension, torsion and lateral bending or is worse when waking up from bed or trying to stand after prolonged sitting.5,7
Percutaneous techniques for symptomatic facet joint syndrome include injections, neurolysis with RF/cryoablation, MR-guided HIFU and percutaneous fixation techniques.16–22 The initial approach in symptomatic patients with facet joint syndrome should always be standard conservative therapy [non-steroidal anti-inflammatory drugs (NSAIDs), analgesics, physical therapies and/or bracing]. This course can be combined to intra-articular or median branch injections. Alternatively, percutaneous injections can be performed as an intermediate step between conservative therapy, percutaneous techniques (denervation or fixation) and surgical options.
Percutaneous injections
Facet joint injections in the lumbar spine are indicated for diagnostic purposes (in order to differentiate symptomatic from asymptomatic facet joint alterations) or for pain reduction and mobility improvement in cases of painful facet joint syndrome, synovial cysts or spondylolysis.5,7,16,17,30 In the vast majority of the reported studies, the injected mixture contains a long-acting corticosteroid (either soluble or non-soluble) with local anesthetic.7,16,17 Efficacy of corticosteroids is based upon their anti-inflammatory and antioedematous effect, their immunosuppressive action and the inhibition of neural transmission within the C fibers.7,31 Concerning painful facet joint syndrome, intra-articular injection of steroids is effective with a slight superiority over intramuscular route of administration.32
Special attention must be given to a patient's medical record; infiltrations with corticosteroids might worsen diabetes in a patient, changing the disease from non-insulin to insulin dependent whilst in patients with HIV under antiretroviral therapy rarely Cushing symptoms emerge after steroid infiltration.32,33 Alternative injected solutions described in the literature for facet joints include local anaesthetic alone, normal saline or dextrose, ozone or hyaluronic acid.7,30,34–37 However, it must be noted that all these injected substances have not been studied as extensively as the mixture of corticosteroid with local anaesthetic.
Imaging guidance for facet joint injections increases technical and clinical efficacy and at the same time decreases potential complications rate.16,17,30 Concerning facet joint injections, imaging modalities used for guidance include fluoroscopy, ultrasound, CT and MRI.16,17,30,38,39 Owing to the difficulty in image interpretation by operators unfamiliar with the complex spinal sonography, navigation and reconstruction systems are used to generate and fuse sonographic images with the collected CT data sets facilitating multiplanar imaging of ultrasound-guided spine injections.38 The ideal target point is the inferior portion of the joint where the posterior joint recess lies (slightly inferolateral to the inferior articular process) (Figure 1). Facet joint injections are performed as outpatient procedures; patients should be accompanied home and avoid driving for the first hours since local anaesthetic could result in delayed reflexes. According to recent systematic reviews,7 there is good evidence for diagnostic facet joint infiltrations with 75–100% pain relief as the criterion standard and fair evidence for therapeutic infiltrations with 50–74% pain relief.
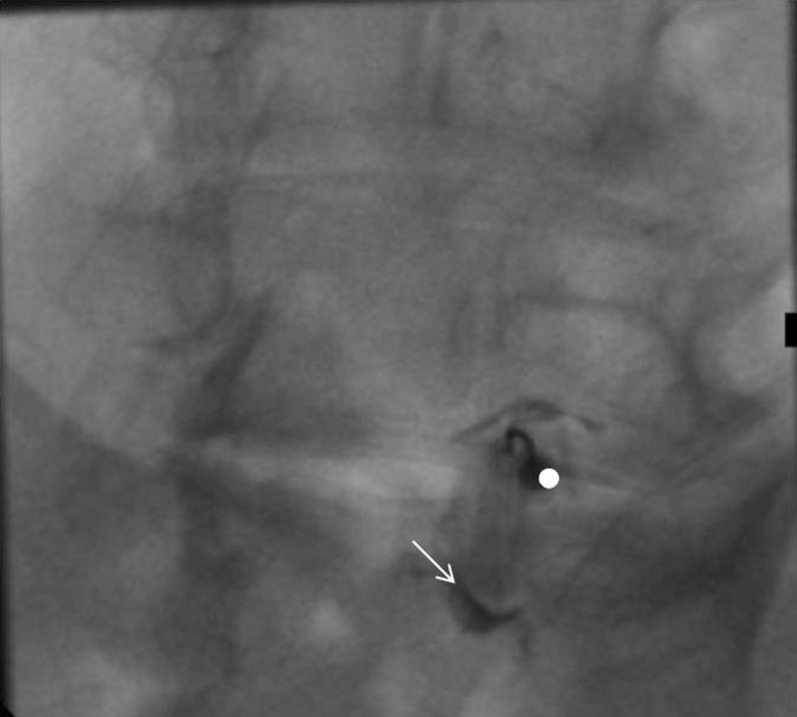
Figure 1.
Oblique (“Scottie dog”) fluoroscopy view during facet joint infiltration: needle (white dot) is within the L5–S1 left facet joint—contrast medium (white arrow) injection verifies correct intra-articular needle position.
Medial branch infiltrations are performed by injecting corticosteroid mixed to local anesthetic right next to the medial branch of the dorsal ramus. Ideal target point is where the superior articular process connects to the base of the transverse process. Medial branch injections are performed not only for pain reduction but for diagnostic purposes as well in order to evaluate which patient could benefit from facet joint denervation by means of RF.
Facet joint denervation
Each facet joint in the lumbar spine is innervated by nerve fibers from medial branch nerves at two levels; for example, the L4–L5 facet joint is innervated by the L3 medial branch superiorly and the L4 medial branch inferiorly.40 The ideal candidate for facet joint denervation is the patient who has experienced significant pain relief (even transient) from intra-articular or medial branch infiltrations. Medial branch of the dorsal ramus courses at the junction of the superior articular process with the transverse process (Figure 2). The nerve fibers at that level are destroyed by heat (RF ablation) or cold (cryoablation application). By destroying these fibers, there is a break in the communication link that signals pain from the spine to the brain. Owing to the dual nerve supply of a given facet joint, RF electrodes or cryoprobes are placed in two subsequent levels. Patients may experience significant pain reduction for a mean of 1–2 years, with pain relief success rate of 55–85% at 1 year.16,17,41–43
Figure 2.
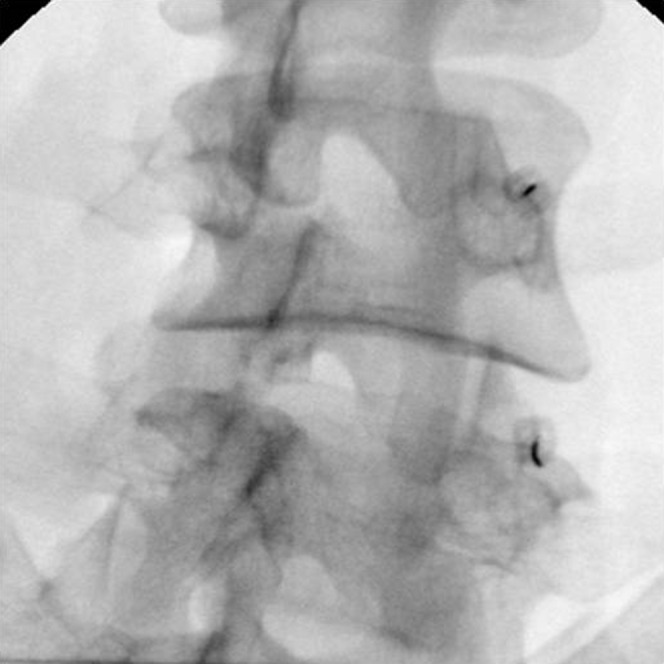
Oblique (“Scottie dog”) fluoroscopy view during facet joint denervation: radiofrequency electrodes have been placed at the junction of the superior articular process with the transverse process (in two subsequent levels) where medial branch of the dorsal ramus courses.
Facet joint denervation is indicated in symptomatic patients who have exhausted or are unresponsive to conservative therapies (NSAIDs and analgesics, physical therapies) and are responsive to diagnostic blocks (with local anaesthetic) performed on the medial branches supplying the painful facet joints. Such a block is considered positive when there is definite improvement of low back pain over 50% for at least 3 h.43
Facet joint denervation seems to be more effective than placebo in pain control and functional improvement, and there is evidence Level 1 concerning the practice of this technique.44 When compared with percutaneous steroid infiltrations, both techniques are governed by favorable short- and midterm results in terms of pain relief and function improvement with a slight superiority of denervation; however, there are no data and evidence for cost-effectiveness.44,45
An alternative, totally non-invasive approach of facet joint denervation is MR-guided HIFU during which the target point is the entire facet surface where nerve endings terminate to innervate the joint.19 Advantages of this technique include the lack of radiation and the total non-invasive character which eliminates the risk of bleeding and infection; on the other hand, the long duration of the technique, the limited availability and high cost along with the limited evidence for practice constitute significant disadvantages.19
Percutaneous fixation techniques
Recently, novice percutaneous approaches have been proposed for posterior facet joint fixation aiming to obtain near equivalent immediate stabilization of the lumbar spine.46 These techniques are performed under local anesthesia and/or mild sedation. Owing to their percutaneous nature, they do not need deep dissection preserving thus muscle anatomy and the adjacent facet joint.47 Combined imaging guidance by means of C-arm and CT or fluoroscopy with cone beam CT enhances correct fixation placement and avoids soft-tissue or vascular damages by means of augmenting proper choice of the instrument's correct size, length and the orientation of the fixation.48
The most commonly used technique for posterior percutaneous facet joint fixation is the transarticular screw fixation; alternatively, intra-articular implants have been proposed.49 Fixation techniques could be considered the last resort of percutaneous approach.
INTERVERTEBRAL DISCS
Intervertebral disc herniation is associated with a rupture of the annulus fibrosus and subsequent release of nucleus pulposus. Pathophysiology of discogenic pain includes mechanical pressure effect, inflammatory reaction and neovascularization.6 Mechanical effect can be either due to direct pressure from the herniated disc or as an indirect effect resulting in ischaemia caused by pressure upon afferent arterioles and venous stasis. Inflammatory reaction is a cell-mediated autoimmune response associated with production of phospholipase A2, prostaglandin, leukotriene and matrix metalloproteinase.
Therapeutic armamentarium for low back pain and neuralgia due to intervertebral discs includes conservative therapy, injections (interlaminar, caudal or transforaminal), percutaneous therapeutic techniques and surgical options.6,12,14–18 Percutaneous therapies for discogenic pain, apart from injections, include techniques which can be classified in to two main categories: decompression (mechanical, thermal, chemical) techniques and biomaterials implantation/disc cell therapies6,12,14–18,23,24 (Table 1). The initial approach in symptomatic patients with intervertebral disc herniation should always be a minimum of 4–6 weeks standard conservative therapy course with NSAIDs, analgesics, physical therapies and/or bracing. This course can be combined to epidural injections. Alternatively, percutaneous injections can be performed as an intermediate step between conservative therapy, percutaneous decompression techniques and surgical options.
Table 1.
Percutaneous, imaging-guided intervertebral disc techniques
| Technique | Method | Definition |
|---|---|---|
| Mechanical decompression | Different vendors in the market propose different devices | All these devices are percutaneously placed inside the nucleus pulposus of the intervertebral disc, and during function, they remove a part of the nucleus, decreasing thus the intradiscal pressure in order to allow the herniated fragment to implode inwards |
| Mechanical high rotation per minute device with spiral tips | ||
| Mechanical high rotation per minute device with metallic laminae | Herniotome extracts hernia or portion of the hernia in order to decrease pressure upon the nerve root | |
| Water-driven suction-cutting probe | ||
| Pneumatically driven, suction-cutting probe | ||
| Herniotome | ||
| Thermal decompression | Percutaneous laser decompression | Laser energy vaporizes a part of the nucleus pulposus |
| Intradiscal electrothermal therapy | Flexible thermal resistive coil (electrode or catheter) coagulates the disc tissue with radiant heat | |
| Intervertebral disc nucleoplasty | Bipolar radiofrequency energy causes molecular dissociation and dissolves nuclear material | |
| Pulsed RF | Pulsed RF uses a high-frequency electric field administered in controlled pulses in order to disrupt the nerve cells carrying the pain signal | |
| Chemical decompression | DiscoGel® (gelified ethanol) | Dehydration of nucleus pulposus |
| Ozon therapy | Chemical breakdown of the nucleus pulposus | |
| Biomaterial implantation | Hydrogel | Aim in intervertebral disc regeneration |
| Cellular therapies | Platelet-rich plasma, stem cell therapy |
RF, radiofrequency.
Percutaneous decompression techniques
Percutaneous decompression techniques are based on the Hijikata theory (1975) which stated that “Reduction of intradiscal pressure reduced the irritation of the nerve root and the pain receptors in the annulus and peridiscal area” (Table 2).6 Based on the principle that the intervertebral disc is a closed hydraulic space, a small volume change inside the nucleus pulposus results in significant pressure change with subsequent inwards imploding of the herniated fragment.12
Table 2.
Review of the literature
| Condition studied | Technique studied | Study | Patient number | Conclusions |
|---|---|---|---|---|
| Facet joint syndrome | Injections | Lakemeier et al45 | 56 | Favourable short- and midterm results in terms of pain relief and function improvement |
| Ribeiro et al32 | 60 | Slight superiority of the intra-articular injection of steroids over intramuscular injection | ||
| Fotiadou et al50 | 55 | Long-term pain improvement was achieved in 79% | ||
| Denervation | Masala et al22 | 92 | Medial branch RF neurotomy has a well-established effectiveness in pain and quality of life improvement | |
| Birkenmaier et al43 | 46 | Medial branch cryodenervation is a safe and effective treatment for lumbar facet joint pain | ||
| Fixation | Marcia et al20 | 38 | VAS pain reduction—ODI improvement | |
| Manfre49 | 8 | Fast and safe technique when facet posterior fixation is needed | ||
| Amoretti et al48 | 107 | Clinical results were classified as excellent in 92 (86%) and good in 15 (14%) patients | ||
| Intervertebral disc herniation | Injections | Gossner51 | 231 | Safe technique when non-particulate steroids are used |
| Fotiadou et al50 | 31 | Long-term pain improvement was achieved in 83% | ||
| Decompression techniques | Erginousakis et al52 | 62 | When compared with conservative therapy, PDD shows improved amelioration of symptoms at 12- and 24-month follow-up | |
| Muto et al53 | 2200 | 75% success rate at 18 months (intradiscal ozone) | ||
| Léglise et al54 | 25 | VNS for lumbar pain and radicular pain decreased in 42% and 50% of patients, respectively, after the use of DiscoGel® | ||
| Fukui and Rohof55 | 15 | The mean pain severity score (NRS) improved from 7.27 ± 0.58 pre-treatment to 2.5 ± 0.94 at the 6-month follow-up (pulsed RF) | ||
| Gerszten et al56 | 90 | Patients treated with coblation had significantly reduced pain and better quality of life scores than those treated using repeated injections |
NRS, numeric rating scale; ODI, Oswestry Disability Index; PDD, percutaneous disc decompression; RF, radiofrequency; VAS, visual analogue scale; VNS, visual numeric scale.
Ideal candidate is an adult (capable of providing informed consent) suffering from symptomatic contained, small- to medium-sized intervertebral disc herniation occupying less than one-third or half of the canal diameter at MRI.6,12,16,17,53 Ideally, there should be failure of 4–6 weeks conservative course therapy usually combined with at least one session of steroid infiltration. Whenever low back pain and leg pain coexist, neuralgia should be of higher intensity. Finally, symptoms should be consistent with the segmental level where herniation is illustrated at MRI (for example, an L4–L5 right foraminal herniation is expected to produce right L4 root neuralgia).6,12,16,17,53
Absolute contraindications for percutaneous techniques making the case a surgical emergency include sphincter dysfunction, extreme sciatica and progressive neurologic deficit.6,12,16,17,53 Other contraindications include an asymptomatic herniation, a sequestered disc fragment (which does not belong any more to a closed hydraulic system), local or systemic infection, spondylolisthesis and patient refusal to provide informed consent.6,12,16,17,53 Haemorrhagic diathesis should be corrected, and anticoagulant therapy should be interrupted according to international guidelines prior to percutaneous disc decompression techniques.
Under imaging guidance and percutaneous posterolateral approach, a trocar is inserted in the intervertebral disc. The trocar's final position should be at the middle between the two vertebral end-plates, towards the anterior third of the disc and on the midline. Concerning intervertebral disc approach, imaging modalities used for guidance include fluoroscopy, CT and MRI.6,12,16,17,57 Once in the desired position, through the trocar, any decompression device available in the market can be inserted in the disc (Figure 3).
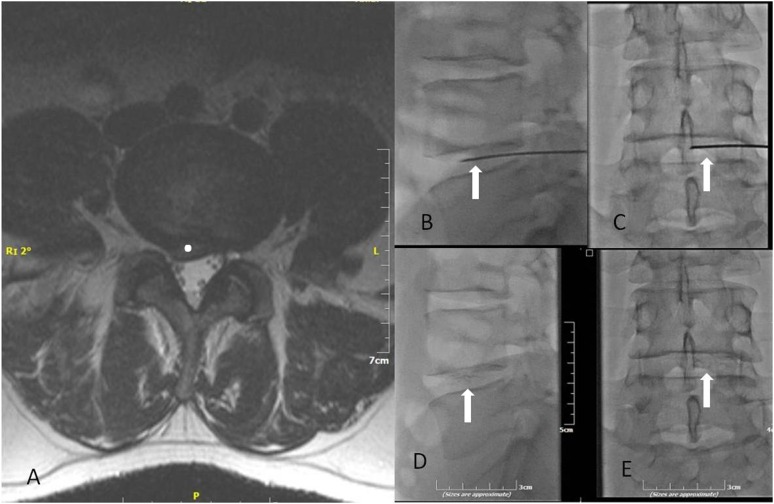
Figure 3.
(a) T2 weighted axial sequence illustration of a posterolateral L4–L5 intervertebral disc herniation (white dot) with pressure effect upon dural sac. (b, c) The same patient. Correct final trocar positioning inside the intervertebral disc—trocar (white arrows) should be located in the anterior third of the disc in the lateral fluoroscopy view (b) and towards the midline in anteroposterior projection (c). (d, e) The same patient. Lateral fluoroscopy (d) and anteroposterior projection (e) illustrating dispersion of DiscoGel® (white arrows) inside the L4–L5 intervertebral disc.
Decompression devices can be classified as mechanical, thermal and chemical. Mechanical decompression devices include either a pneumatically or water-driven suction-cutting probe or a high rotation per minute device with spiral tips or metallic laminae which extract a portion of nucleus pulposus.6,12,16,17 Alternatively, a probe inserted through a curved cannula with lateral window can be used to extract hernia or portion of the hernia in order to decrease pressure upon the nerve root.58 Thermal decompression devices include laser fibers, RF electrodes (for continuous or pulsed RF energy application), and nucleoplasty based on coblation technology.6,12,16,17,55–57,59 Thermal techniques modify intradiscal cytokines associated with disc degeneration, destroy nociceptors in the periphery of the annulus and fuse collagen annular fibers with resultant shrinkage at the disc's periphery.12 Chemical decompression techniques include intradiscal injection of ozone or of a gellified ethyl alcohol.6,16,17,53,54,60,61 Ozone is an unstable irritating gas leading to breakdown of the nucleus pulposus, inflammation reduction and reduction of nerve root oedema. Gellified ethyl alcohol consists of 96% pure ethyl alcohol mixed with tungsten (radio-opaque element); intradiscal injection results in local necrosis of the nucleus pulposus. Injection of gellified ethyl alcohol is advised to be performed under continuous fluoroscopy.6,16,17
Concerning percutaneous decompression techniques (of any kind) for the treatment of intervertebral disc herniation, significant pain reduction is achieved in 75–80% of patients with long-term stable effects.6,12,16,17 Comparison of mechanical decompression and conservative therapy favor the former with pain reduction effect being more significant and longer lasting in the patients treated with percutaneous technique.52 Complications are rarely encountered; the most fearsome complication is infection with spondylodiscitis being encountered in 0.24% of patients and 0.091% of disc level of the cases.6 Less frequently encountered complications include iatrogenic complications such as puncture of dura or nerve root and haemorrhage.6 Despite the fact that these techniques have been utilized for many years, the current evidence for percutaneous decompression techniques is limited to fair mainly because of a lack of literature (both randomized and observational).62–64
Biomaterial implantation/disc cell therapies
There is a continuous interest increase upon cellular therapies aiming in regeneration of nucleus pulposus in a degenerated intervertebral disc. Requirements of such therapies include cells which produce proteoglycan-rich matrix in a challenging natural environment, as a degenerated intervertebral disc is with low cellular density of the intervertebral disc and low quality of nutritional pathway (vertebral end plate changes).65,66 Cellular sources (autologous or allogenic) for such therapies include nucleus pulposus cells, chondrocytes and mesenchymal stem cells derived from the bone marrow or adipose tissue.66 Several agents have been intradiscally injected in animal studies with promising results including platelet-rich plasma, growth differentiation factor 5, osteogenic protein 1 and bone morphogenetic protein 2 and 17.63 Preliminary results in a restricted number of clinical studies report no safety issues; however, long-term efficacy is still to be proven.66 The quest for stem cell therapies aims in prolonged survival of these cells in the discal environment.
Biomaterials (in the form of hydrogel stick implants or any other form available) are percutaneously placed in the centre (nucleus pulposus) of a degenerated disc aiming to restore normal biomechanical function. Theoretically, these biomaterials are designed to increase disc height and at the same time retain their shape under pressure. Concerning biomaterial implantation inside the degenerated disc, long-term efficacy as well as safety and potential implant migration are still to be proven.67 The quest for replacement of nucleus pulposus aims in biocompatible materials with load-bearing and anchoring capacities.
EPIDURAL INJECTIONS
The dural membrane which surrounds the spinal cord and nerve roots along with the osseous and ligamentous parts which form the vertebral canal is the actual anatomic boundary of the epidural space. The spinal canal is bordered by the spinous process, two vertebral laminae, two vertebral pedicles, two transverse processes and posterior vertebral wall. Inside the spinal canal, the epidural space lies anterior to the flaval ligaments and contains fat, areolar tissue, lymphatics, blood vessels, nerve roots and the thecal sac (Figure 4). The thecal sac divides the epidural space into the anterior compartment (bordered anteriorly by the vertebral body and intervertebral disc, and posteriorly by the longitudinal ligament) and the posterior compartment (bordered posteriorly by the flaval ligaments and vertebral laminae). The neural foramen is the lateral margin of the epidural space whilst the sacral hiatus is its inferior margin. A sleeve of dura and arachnoid matter covers the exiting nerves until the intervertebral foramen where it thins to become the epineurium. The margins of neural foramen include the facet joint (posterior margin), the vertebral pedicles (superior and inferior margin) and the vertebral body/intervertebral disc (anterior margin). In the lumbar spine, nerve and vessels exit the foramen in its anterior and cephalad part.
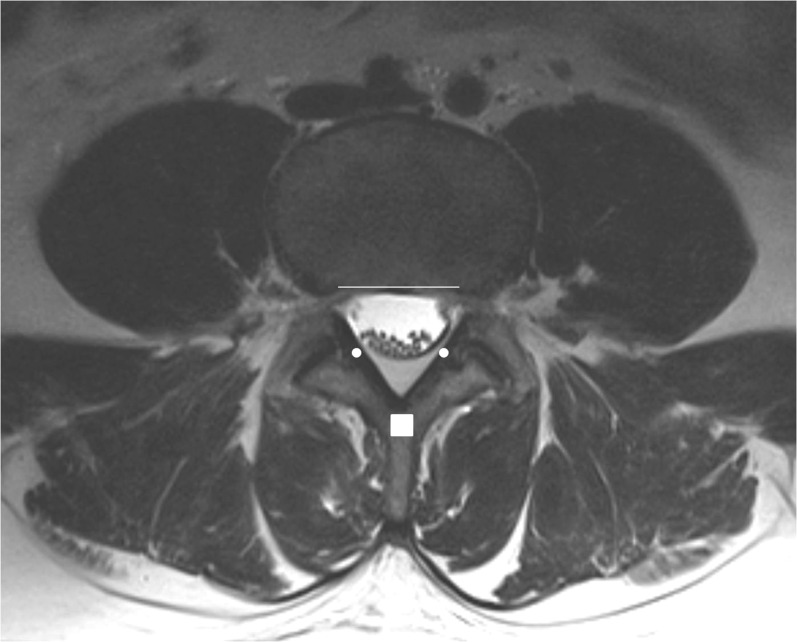
Figure 4.
Epidural space is a circumference bounded by the spinous process (white rectangle), flaval ligaments (white dots) and the posterior vertebral wall (white line). Inside the circumference lies the thecal sac which divides the epidural space into the anterior and posterior compartments.
Interlaminar epidural infiltrations involve the insertion of a needle between the laminae of successive vertebral bodies (Figure 5). Transforaminal epidural injections involve the insertion of a needle in the lateral and lower part of the intervertebral foramen. Caudal infiltrations involve the insertion of a needle inside the epidural space through the sacral hiatus. The choice of what approach to use should be made by the treating physician.68
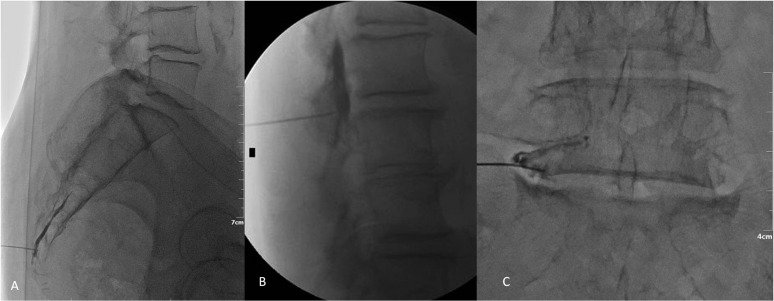
Figure 5.
(a) Lateral fluoroscopy view during epidural infiltration through the sacrococcygeal hiatus (caudal infiltration). Contrast medium injection verifies correct needle location and desired dispersion inside the epidural space. (b) Different patient. Lateral fluoroscopy view during epidural infiltration with an interlaminar approach. Contrast medium injection verifies correct needle location inside the epidural space and outside the dural sac as well as desired dispersion inside the epidural space. (c) Different patient. anteroposterior fluoroscopy view during epidural infiltration with a transforaminal approach. Needle should be located at the lateral and lower margin of the foramen. Contrast medium injection verifies correct needle location (intraforaminal, extravascular).
Indications for epidural injections include radiculopathy/radiculitis (degenerative, infectious or traumatic), spinal stenosis and spondylosis with axial pain. Epidural injections are performed as outpatient procedures. Depending on the type of local anaesthetic used, the patient should be monitored in a recovery room for 15–60 min; the patient should be accompanied home and should avoid driving for the first hours since the local anaesthetic could result in delayed reflexes.
In symptomatic patients who are not surgical candidates, epidural injections can be combined with conservative therapy aiming to provide pain reduction and mobility improvement. Transforaminal approach can be performed for radicular pain diagnosis in cases of confusing imaging; in patients complaining of low back pain, transforaminal infiltrations have no diagnostic value because the injectate will affect not only the spinal nerve but also the dura, posterior longitudinal ligament, intervertebral disc and facet joint. Interlaminar or caudal infiltrations have no diagnostic efficacy. Concerning therapeutic purposes, transforaminal approach can be reserved for patients with unilateral symptoms or for patients who have had spinal surgery at the level to be treated. Interlaminar injections should be avoided at the level of prior laminectomy because of the increased chance of dural puncture. Especially in the cervical spine, dural puncture can lead to persistent cerebrospinal fluid leakage and headache. Caudal injections through the sacrococcygeal hiatus seem to be the safest approach to the epidural space with an extremely low risk for dural puncture (the thecal sac ends in the S1 or S2 level in most patients) and for negligible bleeding risk. Caudal approach requires a larger volume of injectate in order to deliver steroid to the pathological site. In case of post-operative spine with tight stenosis, transforaminal and caudal injections seem to have better results than the interlaminar approach. Alternatively, in these patients, operators prefer a combined interlaminar and caudal approach.
According to recently published Consensus Opinions from Multidisciplinary Working Group and National Organizations, all epidural injections in the lumbar spine should be performed under imaging guidance and a test dose of contrast medium.68 Concerning epidural injections, imaging modalities used for guidance include fluoroscopy, CT and MRI.16,17,50,51,69–71 Ultrasound guidance with navigation systems can be used for periradicular (i.e. transforaminal) injections.38 Blind epidural interlaminar injections without any imaging guidance result in incorrect needle placement in >25% of the cases.72
Recent systematic reviews support that there is good evidence for epidural injections in cases of radiculitis due to intervertebral disc herniation and fair evidence in cases of radiculitis due to spinal stenosis; in all cases, there is evidence illustrating superiority of steroids mixed to local anaesthetic rather than local anaesthetic alone.13–15,69–71,73
Epidural injections are rarely associated with minor complications and side effects, such as dural puncture, pain exacerbation, vasovagal reaction and headache; all these side effects and complications do not involve any permanent impairment.68 However, rarely there are reported cases of catastrophic spinal cord injuries post epidural infiltrations with steroids and local anaesthetic; the number of these reported cases suggests that the risk is not negligible.68,74 Possible mechanisms for such injury include unintended intra-arterial injection of particulate steroids or vessel penetration resulting in perforation, pseudoaneurysm or vasospasm.68,74 The most possible injury mechanism is the unintended intra-arterial injection of particulate steroids which could act as embolization particles.68,74 Proper imaging guidance and use of contrast medium for verification of correct needle placement minimizes the risk of such catastrophic injury.68,74 In addition, one can use non-particulate steroids in the initial injection and extension tubing can be used to minimize the risk of needle being dislodged.68
Contributor Information
Dimitrios K Filippiadis, Email: dfilippiadis@yahoo.gr.
Alexis Kelekis, Email: akelekis@med.uoa.gr.
REFERENCES
- 1.Krogman WM. The scars of human evolution. Scientific American 1951; 185: 54–7. [Google Scholar]
- 2.Delaney PM, Hubka MJ. The diagnostic utility of McKenzie clinical assessment for lower back pain. J Manipulative Physiol Ther 1999; 22: 628–30. doi: 10.1016/S0161-4754(99)70024-2 [DOI] [PubMed] [Google Scholar]
- 3.Abdi S, Datta S, Trescot AM, Schultz DM, Adlaka R, Atluri SL, et al. Epidural steroids in the management of chronic spinal pain: a systematic review. Pain Physician 2007; 10: 185–212. [PubMed] [Google Scholar]
- 4.Bhangle SD, Sapru S, Panush RS. Back pain made simple: an approach based on principles and evidence. Cleve Clin J Med 2009; 76: 393–9. doi: 10.3949/ccjm.76a.08099 [DOI] [PubMed] [Google Scholar]
- 5.Kelekis A, Filippiadis DK. Percutaneous therapy versus surgery in chronic back pain: how important is imaging in decision-making. Imaging Med 2013; 5: 187–96. doi: 10.2217/iim.13.15 [DOI] [Google Scholar]
- 6.Kelekis AD, Filippiadis DK, Martin JB, Brountzos E. Standards of practice: quality assurance guidelines for percutaneous treatments of intervertebral discs. Cardiovasc Intervent Radiol 2010; 33: 909–13. doi: 10.1007/s00270-010-9952-5 [DOI] [PubMed] [Google Scholar]
- 7.Falco FJ, Manchikanti L, Datta S, Sehgal N, Geffert S, Onyewu O, et al. An update of the systematic assessment of the diagnostic accuracy of lumbar facet joint nerve blocks. Pain Physician 2012; 15: E869–907. [PubMed] [Google Scholar]
- 8.Manchikanti L, Boswell MV, Singh V, Benyamin RM, Fellows B, Abdi S, et al. ; ASIPP-IPM. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician 2009; 12: 699–802. [PubMed] [Google Scholar]
- 9.Manchikanti L, Falco FJ, Boswell MV, Hirsch JA. Facts, fallacies, and politics of comparative effectiveness research: Part 1. Basic considerations. Pain Physician 2010; 13: E23–54. [PubMed] [Google Scholar]
- 10.Manchikanti L, Falco FJ, Boswell MV, Hirsch JA. Facts, fallacies, and politics of comparative effectiveness research: Part 2. Implications for interventional pain management. Pain Physician 2010; 13: E55–79. [PubMed] [Google Scholar]
- 11.Brox JI, Nygaard ØP, Holm I, Keller A, Ingebrigsten T, Reikerås O. Four-year follow-up of surgical versus non-surgical therapy for chronic low back pain. Ann Rheum Dis 2010; 69: 1643–8. doi: 10.1136/ard.2009.108902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buy X, Gangi A. Percutaneous treatment of intervertebral disc herniation. Semin Intervent Radiol 2010; 27: 148–59. doi: 10.1055/s-0030-1253513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benyamin RM, Manchikanti L, Parr AT, Diwan S, Singh V, Falco FJ, et al. The effectiveness of lumbar interlaminar epidural injections in managing chronic low back and lower extremity pain. Pain Physician 2012; 15: E363–404. [PubMed] [Google Scholar]
- 14.Manchikanti L, Buenaventura RM, Manchikanti KN, Ruan X, Gupta S, Smith HS, et al. Effectiveness of therapeutic lumbar transforaminal epidural steroid injections in managing lumbar spinal pain. Pain Physician 2012; 15: E199–245. [PubMed] [Google Scholar]
- 15.Parr AT, Manchikanti L, Hameed H, Conn A, Manchikanti KN, Benyamin RM, et al. Caudal epidural injections in the management of chronic low back pain: a systematic appraisal of the literature. Pain Physician 2012; 15: E159–98. [PubMed] [Google Scholar]
- 16.Kelekis AD, Somon T, Yilmaz H, Bize P, Brountzos EN, Lovblad K, et al. Interventional spine procedures. Eur J Radiol 2005; 55: 362–83. doi: 10.1016/j.ejrad.2005.03.024 [DOI] [PubMed] [Google Scholar]
- 17.Santiago FR, Kelekis A, Alvarez LG, Filippiadis DK. Interventional procedures of the spine. Semin Musculoskelet Radiol 2014; 18: 309–17. doi: 10.1055/s-0034-1375572 [DOI] [PubMed] [Google Scholar]
- 18.Jasper JF. Radiofrequency cannula with active tip radio-opaque marker: image analysis for facet, gray ramus, and dorsal root ganglion techniques. Pain Physician 2008; 11: 863–75. [PubMed] [Google Scholar]
- 19.Harnof S, Zibly Z, Shay L, Dogadkin O, Hanannel A, Inbar Y, et al. Magnetic resonance-guided focused ultrasound treatment of facet joint pain: summary of preclinical phase. J Ther Ultrasound 2014; 2: 9. doi: 10.1186/2050-5736-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcia S, Saba L, Anselmetti GC, Marini S, Piras E, Marras M, et al. Effectiveness of percutaneous screws for treatment of degenerative lumbar low back pain. Cardiovasc Intervent Radiol 2014; 37: 1329–35. doi: 10.1007/s00270-013-0786-9 [DOI] [PubMed] [Google Scholar]
- 21.Marcia S, Masala S, Marini S, Piras E, Marras M, Mallarini G, et al. Osteoarthritis of the zygapophysial joints: efficacy of percutaneous radiofrequency neurotomy in the treatment of lumbar facet joint syndrome. Clin Exp Rheumatol 2012; 30: 314. [PubMed] [Google Scholar]
- 22.Masala S, Nano G, Mammucari M, Marcia S, Simonetti G. Medial branch neurotomy in low back pain. Neuroradiology 2012; 54: 737–44. doi: 10.1007/s00234-011-0968-6 [DOI] [PubMed] [Google Scholar]
- 23.Kelekis A, Filippiadis DK. Percutaneous treatment of cervical and lumbar herniated disc. Eur J Radiol 2015; 84: 771–6. doi: 10.1016/j.ejrad.2014.02.023 [DOI] [PubMed] [Google Scholar]
- 24.Fukui S, Nitta K, Iwashita N, Tomie H, Nosaka S, Rohof O. Intradiscal pulsed radiofrequency for chronic lumbar discogenic low back pain: a one year prospective outcome study using discoblock for diagnosis. Pain Physician 2013; 16: E435–42. [PubMed] [Google Scholar]
- 25.Kim JS, Kroin JS, Buvanendran A, Li X, van Wijnen AJ, Tuman KJ, et al. Characterization of a new animal model for evaluation and treatment of back pain due to lumbar facet joint osteoarthritis. Arthritis Rheum 2011; 63: 2966–73. doi: 10.1002/art.30487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyagi M, Ohtori S, Ishikawa T, Aoki Y, Ozawa T, Doya H, et al. Up-regulation of TNFalpha in DRG satellite cells following lumbar facet joint injury in rats. Eur Spine J 2006; 15: 953–8. doi: 10.1007/s00586-005-1031-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Data S, Lee M, Falco FJ, Bryce DA, Hayek SM. Systematic assessment of diagnostic accuracy and therapeutic utility of lumbar facet joint interventions. Pain Physician 2009; 12: 437–60. [PubMed] [Google Scholar]
- 28.Fujiwara A, Lim TH, An HS, Tanaka N, Jeon CH, Andersson GB, et al. The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine (Phila Pa 1976) 2000; 25: 3036–44. doi: 10.1097/00007632-200012010-00011 [DOI] [PubMed] [Google Scholar]
- 29.Gallucci M, Limbucci N, Paonessa A, Splendiani A. Degenerative disease of the spine. Neuroimag Clin N Am 2007; 17: 87–103. doi: 10.1016/j.nic.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 30.Filippiadis DK, Mazioti A, Velonakis G, Kelekis A. Image guided percutaneous facet joint infiltrations: review of the knowledge for diagnostic and therapeutic approach. J Med Diagn Meth 2013; 2: 4. [Google Scholar]
- 31.Johansson A, Hao J, Sjölund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand 1990; 34: 335–8. doi: 10.1111/j.1399-6576.1990.tb03097.x [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro LH, Furtado RN, Konai MS, Andreo AB, Rosenfeld A, Natour J. Effect of facet joint injection versus systemic steroids in low back pain: a randomized controlled trial. Spine (Phila Pa 1976) 2013; 38: 1995–2002. doi: 10.1097/BRS.0b013e3182a76df1 [DOI] [PubMed] [Google Scholar]
- 33.Herold MA, Günthard HF. Cushing syndrome after steroid-infiltration in two HIV-patients with antiretroviral therapy. [In German.] Praxis (Bern 1994) 2010; 99: 863–5. doi: 10.1024/1661-8157/a000190 [DOI] [PubMed] [Google Scholar]
- 34.Colen S, Haverkamp D, Mulier M, van den Bekerom MP. Hyaluronic acid for the treatment of osteoarthritis in all joints except the knee: what is the current evidence? BioDrugs 2012; 26: 101–12. doi: 10.2165/11630830-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 35.DePalma MJ, Ketchum JM, Queler ED, Trussell BS. Prospective pilot study of painful lumbar facet joint arthropathy after intra-articular injection of hylan G-F 20. PM R 2009; 1: 908–15. doi: 10.1016/j.pmrj.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 36.Eippert F, Finsterbusch J, Bingel U, Büchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science 2009; 326: 404. doi: 10.1126/science.1180142 [DOI] [PubMed] [Google Scholar]
- 37.Bonetti M, Fontana A, Martinelli F, Andreula C. Oxygen-ozone therapy for degenerative spine disease in the elderly: a prospective study. Acta Neurochir Suppl 2011; 108: 137–42. doi: 10.1007/978-3-211-99370-5_21 [DOI] [PubMed] [Google Scholar]
- 38.Galiano K, Obwegeser AA, Bale R, Harlander C, Schatzer R, Schocke M, et al. Ultrasound-guided and CT-navigation-assisted periradicular and facet joint injections in the lumbar and cervical spine: a new teaching tool to recognize the sonoanatomic pattern. Reg Anesth Pain Med 2007; 32: 254–7. [DOI] [PubMed] [Google Scholar]
- 39.Galiano K, Obwegeser AA, Walch C, Schatzer R, Ploner F, Gruber H. Ultrasound-guided versus computed tomography-controlled facet joint injections in the lumbar spine: a prospective randomized clinical trial. Reg Anesth Pain Med 2007; 32: 317–22. [DOI] [PubMed] [Google Scholar]
- 40.Bogduk N, Wilson AS, Tynan W. The human lumbar dorsal rami. J Anat 1982; 134: 383–97. [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen SP, Raja SN. Pathogenesis, diagnosis, and treatment of lumbar zygapophysial (facet) joint pain. Anesthesiology 2007; 106: 591–614. doi: 10.1097/00000542-200703000-00024 [DOI] [PubMed] [Google Scholar]
- 42.Boswell MV, Colson JD, Sehgal N, Dunbar EE, Epter R. A systematic review of therapeutic facet joint interventions in chronic spinal pain. Pain Physician 2007; 10: 229–53. [PubMed] [Google Scholar]
- 43.Birkenmaier C, Veihelmann A, Trouillier H, Hausdorf J, Devens C, Wegener B, et al. Percutaneous cryodenervation of lumbar facet joints: a prospective clinical trial. Int Orthop 2007; 31: 525–30. doi: 10.1007/s00264-006-0208-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poetscher AW, Gentil AF, Lenza M, Ferretti M. Radiofrequency denervation for facet joint low back pain: a systematic review. Spine (Phila Pa 1976) 2014; 39: E842–9. doi: 10.1097/BRS.0000000000000337 [DOI] [PubMed] [Google Scholar]
- 45.Lakemeier S, Lind M, Schultz W, Fuchs-Winkelmann S, Timmesfeld N, Foelsch C, et al. A comparison of intraarticular lumbar facet joint steroid injections and lumbar facet joint radiofrequency denervation in the treatment of low back pain: a randomized, controlled, double-blind trial. Anesth Analg 2013; 117: 228–35. doi: 10.1213/ANE.0b013e3182910c4d [DOI] [PubMed] [Google Scholar]
- 46.Su BW, Cha TD, Kim PD, Lee J, April EW, Weidenbaum M, et al. An anatomic and radiographic study of lumbar facets relevant to percutaneous transfacet fixation. Spine (Phila Pa 1976) 2009; 34: E384–90. doi: 10.1097/BRS.0b013e3181a39665 [DOI] [PubMed] [Google Scholar]
- 47.Milchteim C, Yu WD, Ho A, O'Brien JR. Anatomical parameters of subaxial percutaneous transfacet screw fixation based on the analysis of 50 computed tomography scans: clinical article. J Neurosurg Spine 2012; 16: 573–8. doi: 10.3171/2012.3.SPINE11449 [DOI] [PubMed] [Google Scholar]
- 48.Amoretti N, Amoretti ME, Hovorka I, Hauger O, Boileau P, Huwart L. Percutaneous facet screw fixation of lumbar spine with CT and fluoroscopic guidance: a feasibility study. Radiology 2013; 268: 548–55. doi: 10.1148/radiol.13120907 [DOI] [PubMed] [Google Scholar]
- 49.Manfré L. CT-Guided transfacet pedicle screw fixation in facet joint syndrome: a novel approach. Interv Neuroradiol 2014; 20: 614–20. doi: 10.15274/INR-2014-10031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fotiadou A, Wojcik A, Shaju A. Management of low back pain with facet joint injections and nerve root blocks under computed tomography guidance. A prospective study. Skeletal Radiol 2012; 41: 1081–5. doi: 10.1007/s00256-011-1353-6 [DOI] [PubMed] [Google Scholar]
- 51.Gossner J. Safety of CT-guided lumbar nerve root infiltrations. analysis of a two-year period. Interv Neuroradiol 2014; 20: 533–7. doi: 10.15274/INR-2014-10082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Erginousakis D, Filippiadis DK, Malagari A, Kostakos A, Brountzos E, Kelekis NL, et al. Comparative prospective randomized study comparing conservative treatment and percutaneous disk decompression for treatment of intervertebral disk herniation. Radiology 2011; 260: 487–93. doi: 10.1148/radiol.11101094 [DOI] [PubMed] [Google Scholar]
- 53.Muto M, Andreula C, Leonardi M. Treatment of herniated lumbar disc by intradiscal and intraforaminal oxygen-ozone (O2-O3) injection. J Neuroradiol 2004; 31: 183–9. [DOI] [PubMed] [Google Scholar]
- 54.Léglise A, Lombard J, Moufid A. DiscoGel(®) in patients with discal lumbosciatica. Retrospective results in 25 consecutive patients. Orthop Traumatol Surg Res 2015; 101: 623–6. doi: 10.1016/j.otsr.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 55.Fukui S, Rohof O. Results of pulsed radiofrequency technique with two laterally placed electrodes in the annulus in patients with chronic lumbar discogenic pain. J Anesth 2012; 26: 606–9. doi: 10.1007/s00540-012-1385-7 [DOI] [PubMed] [Google Scholar]
- 56.Gerszten PC, Smuck M, Rathmell JP, Simopoulos TT, Bhagia SM, Mocek CK, et al. ; SPINE Study Group. Plasma disc decompression compared with fluoroscopy-guided transforaminal epidural steroid injections for symptomatic contained lumbar disc herniation: a prospective, randomized, controlled trial. J Neurosurg Spine 2010; 12: 357–71. doi: 10.3171/2009.10.SPINE09208 [DOI] [PubMed] [Google Scholar]
- 57.Streitparth F, Walter T, Wonneberger U, Schnackenburg B, Philipp CM, Collettini F, et al. MR guidance and thermometry of percutaneous laser disc decompression in open MRI: an ex vivo study. Cardiovasc Intervent Radiol 2014; 37: 777–83. doi: 10.1007/s00270-013-0734-8 [DOI] [PubMed] [Google Scholar]
- 58.Amoretti N, Huwart L, Marcy PY, Foti P, Hauger O, Boileau P. CT- and fluoroscopy-guided percutaneous discectomy for lumbar radiculopathy related to disc herniation: a comparative prospective study comparing lateral to medial herniated discs. Skeletal Radiol 2013; 42: 49–53. doi: 10.1007/s00256-012-1422-5 [DOI] [PubMed] [Google Scholar]
- 59.Eichen PM, Achilles N, Konig V, Mosges R, Hellmich M, Himpe B, et al. Nucleoplasty, a minimally invasive procedure for disc decompression: a systematic review and meta-analysis of published clinical studies. Pain Physician 2014; 17: E149–73. [PubMed] [Google Scholar]
- 60.Crockett MT, Moynagh M, Long N, Kilcoyne A, Dicker P, Synnott K, et al. Ozone-augmented percutaneous discectomy: a novel treatment option for refractory discogenic sciatica. Clin Radiol 2014; 69: 1280–6. doi: 10.1016/j.crad.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 61.Theron J, Cuellar H, Sola T, Guimaraens L, Casasco A, Courtheoux P. Percutaneous treatment of cervical disc using gelified ethanol. AJNR Am J Neuroradiol 2010; 31: 1454–6. doi: 10.3174/ajnr.A1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manchikanti L, Singh V, Falco FJ, Calodney AK, Onyewu O, Helm S, 2nd, et al. An updated review of automated percutaneous mechanical lumbar discectomy for the contained herniated lumbar disc. Pain Physician 2013; 16(Suppl. 2): SE151–84. [PubMed] [Google Scholar]
- 63.Manchikanti L, Falco FJ, Benyamin RM, Caraway DL, Deer TR, Singh V, et al. An update of the systematic assessment of mechanical lumbar disc decompression with nucleoplasty. Pain Physician 2013; 16(Suppl. 2): SE25–54. [PubMed] [Google Scholar]
- 64.Manchikanti L, Singh V, Calodney AK, Helm S, 2nd, Deer TR, Benyamin RM, et al. Percutaneous lumbar mechanical disc decompression utilizing Dekompressor®: an update of current evidence. Pain Physician 2013; 16(Suppl. 2): SE1–24. [PubMed] [Google Scholar]
- 65.Bae WC, Masuda K. Emerging technologies for molecular therapy for intervertebral disk degeneration. Orthop Clin North Am 2011; 42: 585–601. doi: 10.1016/j.ocl.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kregar Velikonja N, Urban J, Fröhlich M, Neidlinger-Wilke C, Kletsas D, Potocar U, et al. Cell sources for nucleus pulposus regeneration. Eur Spine J 2014; 23(Suppl. 3): S364–74. doi: 10.1007/s00586-013-3106-9 [DOI] [PubMed] [Google Scholar]
- 67.Durdag E, Ayden O, Albayrak S, Atci IB, Armagan E. Fragmentation to epidural space: first documented complication of Gelstix(TM.). Turk Neurosurg 2014; 24: 602–5. doi: 10.5137/1019-5149.JTN.9328-13.1 [DOI] [PubMed] [Google Scholar]
- 68.Rathmell JP, Benzon HT, Dreyfuss P, Huntoon M, Wallace M, Baker R, et al. Safeguards to prevent neurologic complications after epidural steroid injections: consensus opinions from a multidisciplinary working group and national organizations. Anesthesiology 2015; 122: 974–84. doi: 10.1097/ALN.0000000000000614 [DOI] [PubMed] [Google Scholar]
- 69.Bui J, Bogduk N. A systematic review of the effectiveness of CT-guided, lumbar transforaminal injection of steroids. Pain Med 2013; 14: 1860–5. doi: 10.1111/pme.12243 [DOI] [PubMed] [Google Scholar]
- 70.Manchikanti L, Singh V, Cash KA, Pampati V, Damron KS, Boswell MV. Effect of fluoroscopically guided caudal epidural steroid or local anesthetic injections in the treatment of lumbar disc herniation and radiculitis: a randomized, controlled, double blind trial with a two-year follow-up. Pain Physician 2012; 15: 273–86. [PubMed] [Google Scholar]
- 71.Wewalka M, Abdelrahimsai A, Wiesinger GF, Uher EM. CT-guided transforaminal epidural injections with local anesthetic, steroid, and tramadol for the treatment of persistent lumbar radicular pain. Pain Physician 2012; 15: 153–9. [PubMed] [Google Scholar]
- 72.Renfrew DL, Moore TE, Kathol MH, el-Khoury GY, Lemke JH, Walker CW. Correct placement of epidural steroid injections: fluoroscopic guidance and contrast administration. AJNR Am J Neuroradiol 1991; 12: 1003–7. [PMC free article] [PubMed] [Google Scholar]
- 73.MacVicar J, King W, Landers MH, Bogduk N. The effectiveness of lumbar transforaminal injection of steroids: a comprehensive review with systematic analysis of the published data. Pain Med 2013; 14: 14–28. doi: 10.1111/j.1526-4637.2012.01508.x [DOI] [PubMed] [Google Scholar]
- 74.Wybier M, Gaudart S, Petrover D, Houdart E, Laredo JD. Paraplegia complicating selective steroid injections of the lumbar spine. Report of five cases and review of the literature. Eur Radiol 2010; 20: 181–9. doi: 10.1007/s00330-009-1539-7 [DOI] [PubMed] [Google Scholar]