Abstract
MRI-guided focused ultrasound surgery (MRgFUS) is a minimally invasive treatment guided by the most sophisticated imaging tool available in today's clinical practice. Both the imaging and therapeutic sides of the equipment are based on non-ionizing energy. This technique is a very promising option as potential treatment for several pathologies, including musculoskeletal (MSK) disorders. Apart from clinical applications, MRgFUS technology is the result of long, heavy and cumulative efforts exploring the effects of ultrasound on biological tissues and function, the generation of focused ultrasound and treatment monitoring by MRI. The aim of this article is to give an updated overview on a “new” interventional technique and on its applications for MSK and allied sciences.
MRI-GUIDED FOCUSED ULTRASOUND SURGERY
The use of MRI to guide interventional procedures would be desirable in a number of procedures thanks to its best tissue identification and characterization features. MRI provides a superior window on anatomy and can be considered a one-stop-source technique for pre-treatment and post-treatment evaluations as well as for treatment planning, with a complete spectrum of available imaging biomarkers;1 furthermore, MRI does not use ionizing radiation. The use of MRI as guide for procedures in musculoskeletal (MSK) diseases is particularly advantageous because of (a) the epidemiological impact and distribution of these disorders, from children to older adults; (b) specific features of MSK pathology, which is often benign, or chronic, for the most prevalent disorders; and (c) the need for multisession treatment or retreatment in several conditions. On the contrary, limitations are mainly related to costs and to compatible materials (for both safety and artefacts).
From an historical point of view, ultrasound in medicine was firstly employed for therapeutic purpose and later for diagnostics. After the first experimentations performed by the Curie brothers (and Joule) in the 19th century, Wood and Loomis2 reported their experience on the effects of ultrasound in 1927 and recognized how they could produce permanent alterations in biological systems. The most commonly known effect of the interaction between mechanical acoustic waves and biological system is thermal: absorption of acoustic energy in a tissue leads to heat. More recently, other effects on biological tissues (not related to the thermic effect) were demonstrated to be potentially useful in treating human pathology.3–5
In diagnostics, high-frequency and low-power ultrasound are used in order to avoid permanent effects on the body, whereas lower frequency and higher power ultrasound are used for therapeutic applications in order to obtain potential permanent effects. In more detail, observed effects seem to be reversible and/or of potential benefit for tissue biology at low power (∼100 mW cm−2), whereas ultrasound can produce instant tissue necrosis at very high power (∼1000 W cm−2).3–5 Based on frequency and power, ultrasound has been used for several applications in medicine, such as: high frequency (1–3 MHz), low power, separation technology—ultrasonic standing waves; low frequency (20–100 kHz), high/intermediate power, dentistry and surgery (scalpel, bone cutting), synthesis of microcapsules for drug delivery; high frequency (1–3 MHz) and intermediate/low power, physiotherapy, bone healing, destruction of blood clots (sonothrombolysis), transdermal drug delivery (sonophoresis), improved drug intake in cells (sonoporation), drug activation (sonodynamic therapy) and high-intensity focused ultrasound (HIFU).3–5
Bursts of focused ultrasound energy are three orders of magnitude more intense than diagnostic ultrasound. The use of therapeutic focused ultrasound surgery (FUS) dates back to 1940s, born with the intent to destroy the tissue in a focal spot inside the body. A major advantage of HIFU over other thermal ablation techniques was in its capability of enabling rapid heating of the target tissue volume without any percutaneous insertion of probes or seriously affecting tissue along the ultrasound propagation path.
MRI-GUIDED FOCUSED ULTRASOUND SURGERY, PRINCIPLES AND TECHNOLOGY
HIFU is currently considered as one of the most promising therapeutic applications of ultrasound, although additional knowledge is needed, with specific regard to the method of targeting the tissue to be destroyed and monitoring the target volume during the treatment.6–8
Therapeutic effects are obtained through both thermal and non-thermal interaction mechanisms. Ultrasound can be used to non-invasively produce different bioeffects via viscous heating, acoustic cavitation or their combination. At low intensities, acoustic streaming is likely to be significant, but at higher levels, heating and acoustic cavitation usually predominate; for pressures above a critical threshold, cavitation as the formation of vapour cavities (“bubbles” or “voids”) occurs.9 In order to manage these different effects, imaging methods are needed to map temperature changes and/or cavitation activity.6–8
HIFU was initially guided by ultrasound imaging; however, there were several limitations in identifying the target volume and in overviewing the treatment region by using ultrasound, as well as in monitoring the treated area during and after the procedure, especially in bone applications.10,11
MRI was proposed as potential imaging guide in 1991 at the University of Arizona.12 Experimental studies on animals were reported for the first time at the International Conference of Hyperthermic Oncology in 1992 and were subsequently published.13,14 The first application on human subjects was tested for breast fibroadenoma in 2001.15 The first commercially available system (ExAblate 2000; InSightec, Tirat-Carmel, Israel) was approved by European conformity (CE) in 2002 and by the US Food and Drug Administration (FDA) in 2004, mainly for the treatment of uterine fibroids.16 The acronyms FUS and MRgFUS (MRI-guided focused ultrasound surgery) were introduced to describe the most recent techniques associated with MRI guidance. MRgFUS is totally based on “clean” energy, both to treat and to guide. Radiologists approaching MRgFUS should become familiar with concepts such as acoustic field, acoustic absorption, attenuation, reflection, aberration and with spot/focal size, frequency, power, “sonication” time, and thermal/mechanical effects, cavitation etc. “Sonication” is the act of applying sound energy to agitate particles in a tissue. This can be featured with different parameters to obtain different results on tissues. Focused ultrasound are typically generated by a phased-array multielement transducer to produce a beam which enters the body from the skin surface, passes through different layers and gets to the target, delivering the majority of its energy there (Figure 1). Depending on the absorption rate and depth of the target, and the features of the tissues along the ultrasound propagation path (before the target), heating up to coagulative necrosis of the targeted tissue can be obtained. On the other hand, energy not delivered in the near field or in the target can still affect structures in the far field (beyond the target). The true deposition of energy within the target tissue depends not only on ultrasound parameters (e.g. acoustic intensity, exposure time etc.) but also on tissue features, structure and functional status.17–19 Intraoperative MRI allows dynamic control of energy deposition using real-time MRI thermometry1 (Figure 2). Through MR thermometry, it is possible to monitor the temperature in the target volume in near real time and calculate the thermal dose received by the treated region. Although several improvements have been introduced even for ultrasound guide,20 MRI shows a few advantages in terms of parameters of sonication (no need for fast sonication/cavitation), panoramic view of the field of treatment, excellent pre-/post-treatment evaluation and, as mentioned above, of temperature monitoring during the energy delivery.
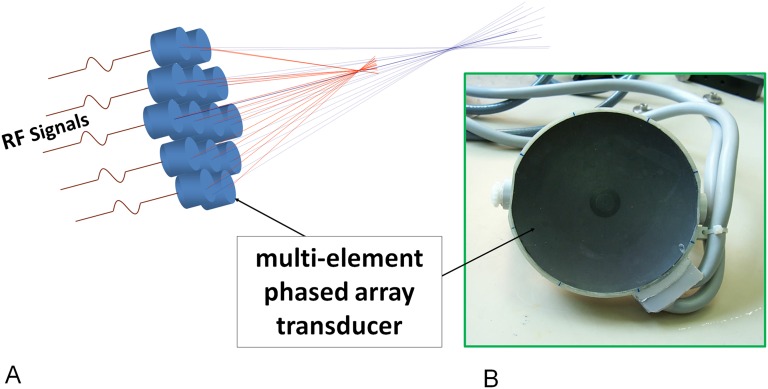
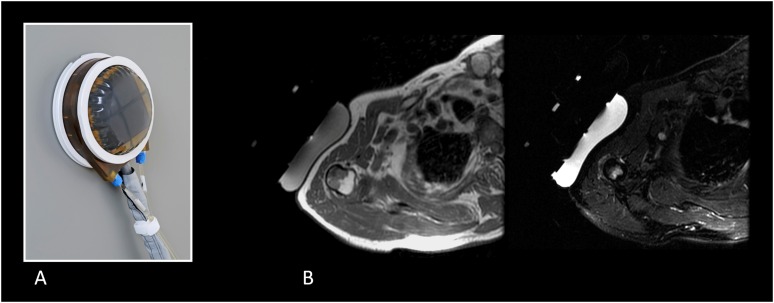
Figure 1.
Conventional multielement phased-array transducer for MRI-guided focused ultrasound surgery applications including bone (InSightec, Tirat Carmel, Israel). The transducer works to assure optimal and sharp focusing of the ultrasound beam, depending on the target. The focusing ensures that significant energy delivery is only at the desired focal point and is low at other locations, thus not impacting other tissues (a). The transducer may have a number of elements from piezoelectric materials that oscillate upon application of an alternating voltage resulting in the generation of ultrasound waves, with a matching layer on it (impedance matching) (b). RF, radiofrequency.
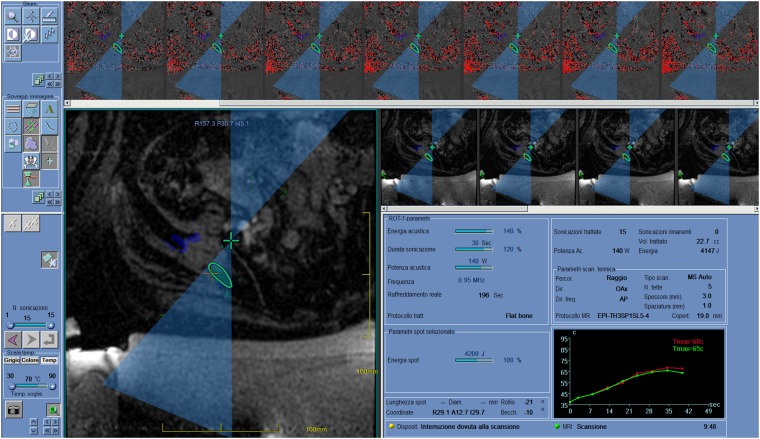
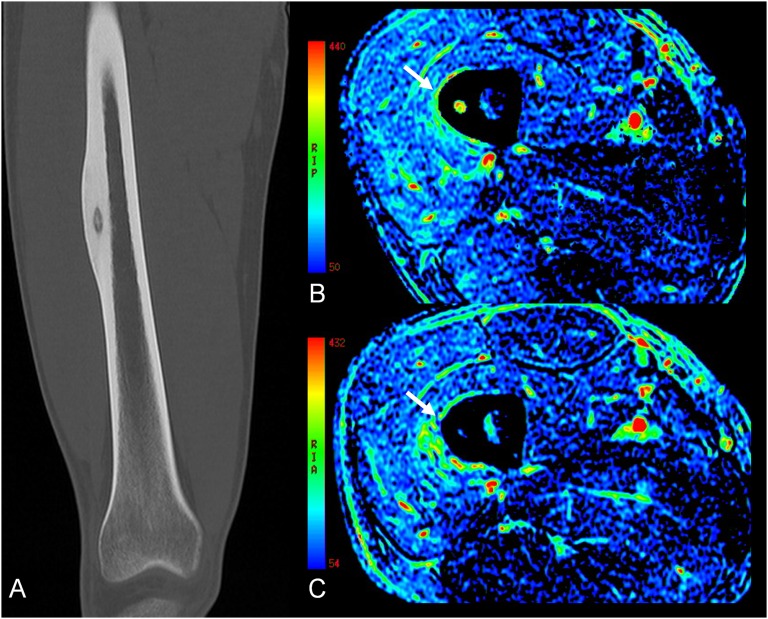
Figure 2.
Screenshot of a MRI-guided focused ultrasound surgery workstation after “sonication”, during the treatment of a painful bone metastasis from thyroid cancer in a 60-year-old female. The beam path is shown, with “near field” before the target (circle) and “far field” beyond. Typically, in bone applications, the focus (cross) is set beyond the cortical surface target to exploit the high absorption of the cortical bone. Areas/volumes covered and ablated by previous sonications are highlighted. The higher line shows thermal images during sonication. The graph at the bottom right shows the evolution of temperature during sonication, and this can be checked pixel by pixel.
In addition, prototypes of hybrid systems have also been designed to better address the needs for intraoperative evaluation of ultrasound effects, with combined ultrasound/MRI-guided HIFU machines integrating an ultrasound imaging array into a MRgFUS system, in order to simultaneously visualize thermal and mechanical effects (localized cavitation activity and temperature) via passive acoustic mapping and MR temperature imaging.21,22
Given the wide-ranging applicability of HIFU, numerous extracorporeal and intracorporeal (e.g. transrectal, transuretral, intravascular, interstitial etc.) devices have been designed to optimize application-specific treatment deliver. Today, tools tailored for different organs and clinical situations are available; brain, breast, prostate, abdominal organs and bone are approached by using different devices in order to achieve the best comfort and positioning of the patient as well as to obtain an effective FUS therapy.23
Currently, the first manufacturer in the MRgFUS field is InSightec with ExAblate series, followed by Philips Medical Systems (Eindhoven, Netherlands) with Sonoalleve. Advanced software interfaces have been developed and can be used in clinical practice in order to optimize the treatment plan and to improve the operator interaction and confidence with the system; furthermore, several additional tools currently contribute to enhance the safety and control of the procedure. In particular, for MSK applications, an accurate registration of pre-treatment CT and MR images can be obtained and can be useful in the treatment of bone lesions, especially in challenging sites to be treated or if accurate information on the extent, integrity and quality of the cortical bone are required.24
MRgFUS is currently employed in several fields (such as oncology, urology, gynaecology and MSK, and in other branches such as neurology and cardiovascular) at different levels, from pre-clinical to clinical activity, and with variable results in terms of safety and efficacy.1
MSK pathology is extremely common and involves the whole spectrum of population with several relationships to other diseases. Percutaneous treatments represent the modern surgical perspective, with very competitive effectiveness and associated cost drop, reduction of complication rate and rehabilitation time. However, the majority of these procedures still required an invasive approach, although less than traditional surgery, and they are still guided by ionizing radiations in most cases. MRgFUS offers several advantages in comparison with these techniques, since it is a radiation-free technique not requiring any surgical incision or invasive/mini-invasive approach; however, a large variety of technical, financial, clinical and practical challenges must be overcome to allow MRgFUS to break through into clinical practice and to make the transition from a research curiosity to a clinical standard of care.25,26
Mrgfus, LESSONS LEARNED FROM NON-MUSCULOSKELETAL APPLICATIONS
Clinical applications of HIFU were mainly focused on oncology; over the past 15 years, a growing number of trials have examined HIFU treatment of both benign and malignant tumours of the prostate, breast, liver, pancreas, kidney, uterus, thyroid, parathyroid, brain, connective tissue and bone, and in some of these emerging indications, HIFU potentially represents a serious alternative or adjunct to current standard treatments, including surgery, radiation therapy, chemotherapy immunotherapy and gene therapy.27–31
In addition, several applications have been tested in fields other than oncology, including MSK diseases and neurology (e.g. essential tremor, neuropathic pain etc.).32
The learning curve of MSK MRgFUS users should be addressed to (a) understand treatment effects and interaction between ultrasound and different tissues, (b) develop strategies to reach the target and the wanted effect and (c) build clinical, imaging and technical selection criteria for patients.
Not only bone but any tissue of the MSK system, as well as nerves and vessels, may be targeted within certain limits and purpose. The understanding of how organs with different content in connective tissue absorb ultrasound is critical, as well as the influence of tissue architecture on ultrasound beam distortion. The concepts of skin and near/far field “dose” (energy delivery) and strategies to reduce risks of burns or loss of energy within the path should be always taken into account. Correct shaving, preparation and positioning of the patient are essential. Bowel interposition, foreign material, large scars along the ultrasound beam path should be avoided32 (Figure 3). Changes in the treated tissue as well as in the surrounding background can significantly alter the ultrasound effect in a “lesion-to-lesion” interaction (occurring when the spatial separation of individual exposures is such that an existing lesion seems to affect the formation of a subsequent lesion).33 Cavitated areas may alter the correct propagation of ultrasound. Background propagation properties' dependence on temperature has been demonstrated through laboratory measurements of soft-tissue properties. For typical FUS applications, only the slow variations in tissue background parameters need to be accounted for when computing the outcome of a FUS sonication. The cumulative effect of slowly varying sound speed has been referred to in the literature as a thermal lens, or a thermoacoustic lens because of its beam-distorting properties.35,36
From the pioneers of FUS, such as William Fyer, to modern times, challenging organs were targeted, including moving organs such as the liver and those needed for trans-skull transmission.37,38 In more detail, new fascinating results have been presented for intracranial applications, demonstrating the possibility to produce focal intracranial thermal lesions or temporary opening of the blood–brain barrier, for treating movement disorders, and vascular, oncologic and psychiatric diseases,39 from thalamotomy to gene delivery.32 With specifically designed devices, MRgFUS can make the beam pass through the cortical bone without loosing power and energy and with no critical heating of superficial tissues and bone. Transcranial MR-guided focused ultrasound has been proposed for sonothrombolysis in the treatment of intracerebral haemorrhage and for acute stroke.40,41 Among other kinds of tissues, liquids and not gas, help in the transmission and should be considered as a “special tool” to make difficult cases easier; in this regard, for instance, some authors reported their experience in treating liver malignancies crossing the lung in the costophrenic angle by using intrapleural fluid infusion to create an acoustic window for MRgFUS near the liver dome.42 Portable high-intensity FUS devices were tested for several other applications in other tissues, including non-invasive venous ablation,43 reduction of subcutaneous adipose tissue and non-invasive body sculpting.44
An analysis of factors affecting clinical success of MRgFUS should be performed. The large experience on uterine fibroids suggests the influence of several factors on ablation [non-perfused volume (NPV)] and symptoms. NPV ratio, which is highly correlated with clinical success, is significantly higher in fibroids characterized by low signal intensity in contrast-enhanced T1 weighted fat-saturated MR images and lower in fibroids with septations, with subserosal component and in skin-distant fibroids.45 An adequate patient and strategy selection, combined with technical advances of the system, may lead to higher clinical success and low complication rate. This kind of experience suggests the need, in each field of MRgFUS application, of building clinical, imaging and technical selection criteria for patients' enrolment. In this scenario, well-designed and comprehensive as well as large studies are required on MRgFUS. Luo et al46 recently faced the problem and reported on for extracorporeal ultrasound-guided high-intensity focused ultrasound (USgHIFU), on the basis of 75 controlled trials including 833 cases of benign and 4559 cases of malignant diseases.
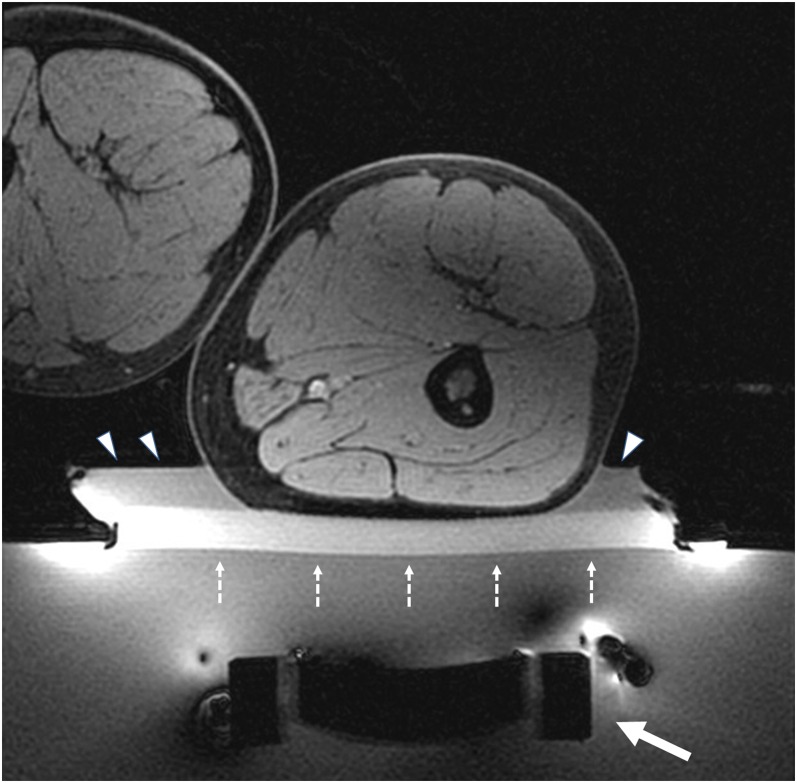
Figure 3.
Axial view of a patient lying prone on the MRI-guided focused ultrasound surgery table. The white arrow shows the transducer (Figure 1), which can move in any direction to get the most favourable acoustic window to the target. The perfect preparation of media and positioning device (e.g. gel pad, water bag etc.) in the near field are mandatory, in order not to disturb the beam to the target, and to avoid or minimize adverse effects (e.g. skin burns). In this case, the patient is on a gel pad (broken arrows) with degassed water (arrowheads).
FOCUSED ULTRASOUND, MRI AND MUSCULOSKELETAL TISSUES: PROS AND CONS
MRgFUS has been approved for the treatment of painful bone metastases in 2007 by CE and in 2012 by FDA, respectively.47–50 CE mark was later achieved for pain palliation, facet joint syndrome and benign bone tumours. In MRgFUS treatment, tissues with the maximum acoustic absorption capacity are the most desirable targets; in the MSK system, these are the cortical bone and high-density fibrous tissues.
Therefore, sonication power used for bone applications is typically low, making the treatment even more safe for surrounding tissues. Furthermore, spot size can be large since the focus can be placed beyond the cortical bone to exploit the heating distribution along the cortical surface (fewer sonications needed). However, surrounding tissues, including tendons, ligaments, vessels and nerves are heated by the heated bone, and the bone may also produce partial reflection in wanted or unwanted directions and this should be taken into consideration; with commercially available systems, cortical and trabecular bone is difficult to be crossed.
Superficial structures are at risk of skin burns; deeper structures are burdened by the different layers to be crossed from the beam. The focus can shift away from the desired point because of tissue inhomogeneity. The correction of distortions induced on the ultrasound beam during its propagation through defocusing obstacles is challenging and several improvements occurred in sonication parameters.51
Few layers of homogeneous tissues in terms of density, architecture and orientation are desirable for the optimal beam path to get the desired effect and to avoid dispersion of energy or dangerous deviation.52 The reflection and absorbing properties of the near field and target tissue should be considered (calcifications, scars etc.), as well as the distance of critical structures from heated tissues (skin, nerves, which are plunged in connective tissue). Nonetheless, the far field should be considered in light of the expected delivery of energy on the target: with bone as target the far field is protected by the low-energy sonication to be used and by the high absorption/reflection of bone; with a soft-tissue target, a higher energy sonication must be used and the beam will impact the far field still with a significant energy load. For instance, the effects of fascia lata on HIFU-induced lesions were demonstrated through comparison with and without fascia lata in bovine thigh muscle tissue; fascia lata was confirmed to contribute in increasing tissue necrosis, temperature elevation and echogenicity in ultrasound images.53
On the other side, the most important advancements in MRI-guiding HIFU (MRgHIFU) were on intraprocedural monitoring of treatment effects, therefore on MR thermometry.54,55 The goal of MRI-guided thermal therapy is to better control the treatment outcome by using real-time temperature mapping. In this regard, the temperature needs to be accurately measured during the treatment, but also to be related to thermal tissue damage.
Non-invasive temperature monitoring is feasible with MRI; several temperature-sensitive MR parameters are involved, such as T1 and T2 relaxation times, proton resonance frequency (PRF), diffusion coefficient, proton density, magnetization transfer and temperature-sensitive contrast agents. The most affirmed method to drive HIFU was based on PRF shift method. The temperature sensitivity of PRF was first observed by Hindman56 in 1966, and implemented for spectroscopy; later it was adapted for MRI-temperature monitoring by Ishihara et al57 and De Poorter et al.58 Two techniques have been developed from temperature imaging based on the PRF shift: spectroscopic imaging and phase-mapping method. MRI-derived temperature maps can be realized by using gradient-recalled echo imaging sequences; more in detail, phase changes (resulting from temperature-dependent changes in resonance frequency) are measured. PRF thermal coefficient is basically tissue type independent (except for adipose tissue); even when tissues have been coagulated, only a small influence has been observed. Indeed, this independence of the PRF shift from tissue type is true only for aqueous tissues. In water, the relationship between PRF and temperature is mainly based on changes in the hydrogen bonds, absent in adipose tissue; therefore, susceptibility effects are almost completely responsible for the temperature dependence of PRF in fat. The resulting sensitivity on temperature of fat is very small, making thermometry in fatty tissue difficult. The described difference in thermometry in aqueous and fatty tissues represents a challenging issue for temperature measurements by using PRF, since water and fat coexist in many biological tissues. PRF-thermometry is limited to water-based tissues and temperature is not measured in the bone and marrow, as well as in the adipose tissue. Because information on temperature within the bone is not provided by the existing techniques of MR thermometry, these are monitored by measuring temperature changes in the surrounding soft tissues.56–58
Efforts were directed to improve the accuracy of volumetric MR thermometry and in finding reliable methods to correlate the temperature in high-lipid tissues and in the bone. A model-based correction procedure for PRF shift thermometry errors caused by heat-induced magnetic susceptibility changes during HIFU ablation in tissues containing fat (breast fatty samples) was proposed by Baron et al.59 A non-parametric temperature controller with non-linear negative reaction for multipoint rapid MRgFUS was proposed by Petrusca et al.60 Experimental methods for improved spatial control of thermal lesions in MRgHIFU ablation were also proposed.61 Available and accurate methods for retrospective reconstruction of the temperature maps useful in research settings were investigated.62,63 A recent study by Ramsay et al64 proposes to monitor temperature changes in the cortical bone using a short echo-time gradient echo sequence. The feasibility of using T2 mapping to monitor the temperature change in subcutaneous adipose tissue layers was studied by Baron et al.59,65 Temperature monitoring and hyperthermic injury detection in fatty tissue was also tested by using X-ray CT during HIFU thermal treatment.66 Diakite et al67 recently presented a three-dimensional segmented echoplanar imaging pulse sequence implementation that simultaneously provides the proton resonance frequency shift temperature of aqueous tissue and the longitudinal relaxation time (T1) of fat during thermal ablation.
Another study evaluated the accuracy and precision of two non-invasive thermal diffusivity estimation methods,68 and MR acoustic radiation force imaging, in order to propose an elegant adjunct to MRgFUS for treatment planning and optimization, permitting in situ assessment of the focusing and targeting quality.69 Even real-time methods are being developed for motion-compensated MRI thermometry in MRgHIFU treatment of abdominal organs, which may be considered in the treatment of the chest wall.70
The development of reliable and accurate MRI methods was also devoted to post-treatment imaging. A recent study by Fite et al71 compared in mice the correlation between histological findings and common MRI protocols in the assessment of the extent of thermal damage. This research found a good correlation between non-enhancement area on contrast enhanced T1 weighted imaging immediately after ablation and the region of tissue receiving a thermal dose. Moreover, although both tumour T2 and apparent diffusion coefficient values changed from pre-ablation values, contrast enhanced T1 weighted images appeared to be more sensitive to changes in tissue viability following HIFU ablation.71
Other imaging options may help to better understand and investigate the connectivity of nerves and other functional structures (e.g. diffusion-tensor MRI).71
MRgFus TARGET AND APPLICATIONS IN MUSCULOSKELETAL PATHOLOGY
Oncology
Both benign and malignant, primary and secondary, bone tumours are the target of imaging-guided interventions. However, owing to the curative intent, and to good surgical and medical outcome of today's clinical practice, imaging-guided procedures are not considered as first-line treatment options for primary malignant bone or soft-tissue tumours. These are more advocated for benign tumours or for palliation in primary and overall secondary malignant bone tumours.73
Fire and ice: thermoablation is definitely the most realized effect of interventional imaging-guided procedures in the treatment of benign and malignant MSK tumoral lesions. Several techniques are used to determine this effect such as continuous radiofrequency, laser, microwaves and cryoablation. Different techniques have been associated with different imaging methods, with advantages and disadvantages, with a certain comfort of the patient; of course, each technique has shown a certain effectiveness in several clinical settings. Different combinations influence the choice, development and optimization of treatment management in different clinical scenarios and disorders (lesion, patient and aim features, site etc.). However, techniques such as radiofrequency ablation are usually still CT imaging guided and require at least a percutaneous approach.74
A recent article reviewed the clinical applications of FUS and provided a comprehensive overview of HIFU employment on bone tumours, describing background, results of clinical studies and suggesting future directions.75 MRgFUS has been first proposed with a potential in pain palliation of bone metastases, for its good results in patients non-responding to radiotherapy while applying acoustic energy on the bone surface resulting in bone cortex heating, and indirectly ablating the adjacent periosteum—the most innervated component of mature bone tissue, and tumour tissue, and a major source of pain.11,76 The bone is a common site for metastasis. The primary cancers that most frequently metastasize to bone are breast and prostate cancers (post-mortem incidence of bone metastases: breast 73%, prostate 68%, thyroid 42%, lung 36%, renal 35%), and these are among the most incident and curable worldwide. The treatment of painful bone metastases is of primary importance in terms of both the quality of life of the patient and economy, and this should be considered in light of the increasing survival of patients with cancers. Bone metastases are the most common cause of cancer-related pain.77 The development of strategies to improve the quality of life in patients with bone metastasis is fundamental and represents a major clinical challenge. In this scenario, MRgFUS can work with the aim to produce fast and long-lasting pain relief while providing several advantages: no ionizing radiation, lack of cumulative dose, several lesions might be treated per session, treatment might be repeated as many times as needed, fast pain relief, contraindications limited to those of the imaging guide (MRI) and general fitness to anaesthesiological choices.78–80
Among several experience, preliminary prospective cohort studies on using MRgFUS for painful bone metastases (in patients for whom other treatments were either ineffective or not feasible) were published between 2007 and 2009, with satisfying effects in terms of pain palliation. The first multicentre study on this topic was published by Liberman et al,81 including previous pioneering works from Catane et al82 and Gianfelice et al.83 The study by Liberman et al81 comprised 31 patients (32 bone lesions), and lead to CE mark of conformity of MRgFUS for the role of palliation in painful bone metastases. A 3-month follow-up was available for 25 patients. The average visual analogue scale (VAS) score decreased from 5.9 to 1.8 at the 3-month follow-up with 18 (72%) patients experiencing a significant reduction in pain and 9 (36%) with the VAS score dropped to 0; 52% of patients reported substantial pain relief within 3 days, 24% had no response and one patient (4%) experienced worsened pain levels. A reduction in opioid usage was registered in 67% of patients. No major complications were reported.81 Napoli et al10 presented a single-centre experience with 18 patients and found increased bone density with restoration of cortical borders in 5 of the 18 patients treated with MRgFUS (27.7%) (the overall pain response rate was 89% with complete pain relief in 72% of patients). According to MD Anderson criteria, complete and partial responses to treatment were observed in two (11.1%) and four (22.2%) cases, respectively, and the NPV values ranged between 20% and 93%, with mean NPV values substantially stable after the treatment.10 This represented the first concrete experience which actual extended the potential role of MRgFUS even to the hypothesis of local tumour control (Figures 2 and 4). Hurwitz et al recently published the results of the first Phase III trial supporting FDA approval in the USA, giving a new substantial contribution to the evidence of safety and efficacy of MRgFUS in the palliation of painful bone metastasis. In this study, 147 patients affected by painful bone metastases were randomly assigned to MRgFUS sonication or placebo group (3 : 1). The primary end point was represented by an improvement in self-reported pain score without pain medication increase during the 3 months after treatment (at least two-point decrease in the Numerical Rating Scale for pain score and equivalent daily dose intake of morphine not increasing by >25% when compared with the baseline dose). Response rate was 64.3% in the MRgFUS group and 20.0% in the placebo group (p < 0.001). MRgFUS was superior to placebo at 3 months [worst Numerical Rating Scale for pain score and the brief pain inventory-quality of life (p < 0.001)]. The most frequent adverse event was sonication pain, occurring in 32.1% of patients with MRgFUS. Pathological fractures were reported in two patients, third-degree skin burn and neuropathy were reported in one patient each; overall, 60.3% of the adverse effects were resolved within the same day of the treatment. This study demonstrated that MRgFUS is a non-invasive and safe treatment in painful bone metastases for patients in whom standard treatments have failed.11 Recently, an article published by an international consensus held at the Focused Ultrasound Therapy Second European Symposium in Rome (2013), Italy, focused on the point about current treatment goals, current indications, technical considerations, future directions including research priorities, and economic and logistical considerations.84 A systematic literature review of image-guided FUS and bone metastases was performed, scanning between 1980 and June 2014. An overview of all clinical studies and abstract results on image-guided FUS for painful bone metastases was presented. Preliminary clinical studies concluded that MRgFUS offers a potentially safe and effective non-invasive treatment option for radiation refractory metastatic bone pain, with >70% of patients experiencing pain reduction after MRgFUS treatment. The biological mechanism of pain relief induced by FUS treatment has not been completely elucidated, although it is generally assumed, as previously mentioned, that periosteal denervation induced by cortical heating plays a major role with duration of pain relief of at least 3 months. More aggressive ablations may lead to local tumour control and improve pain relief but may also generate more adverse events. In patients with advanced disease and limited survival (6 months), local tumour control should not be pursued since the potential additional risks of more aggressive treatment strategies do not outweigh the benefits for these patients.84
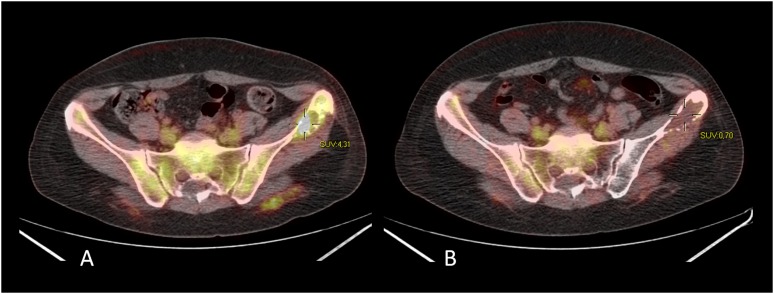
Figure 4.
Bone metastasis from breast cancer before (a) and 3 months after (b) MRI-guided focused ultrasound surgery at fluorine-18 fludeoxyglucose positron emission tomography/CT imaging. SUV, standardized uptake value.
Important benefits of FUS compared with radiotherapy are (a) the absence of ionizing radiation, (b) the ability to induce pain relief within 3 days of treatment, whereas radiotherapy may have a delay in response of up to 4 weeks, (c) the higher response rate, longer response duration if compared with re-irradiation, (d) fewer side effects, (e) no need to interrupt chemotherapy for FUS, (f) generally only a single session is needed, although repeat exposures are possible. Disadvantages of FUS include (a) the common need for anaesthesia and (b) for patients to be able to undergo MRI for MRgFUS, and (c) potential positioning problems, to get full access and window to the target area (possible solutions with MRgFUS conformal bone system or USgFUS). Moreover, FUS is currently not suitable for skull metastases or spinal metastases (except for the posterior elements, in safe conditions e.g. below the level of the conus medullaris). Spinal metastases cannot be faced at the moment because of concerns about thermal damage to the spinal cord, and for technical and accessibility challenges.85 Since approximately one-third of patients with painful bone metastases have lesions in the thoracic or lumbar spine, efforts on significant engineering development are being directed to accomplish this goal. It has been anticipated that in the future, it will be technically possible to treat metastases in the entire vertebral column.85
Consensus has been reached that FUS is an acceptable secondary treatment option for patients who have painful bone metastases in a non-spinal site, for whom radiotherapy has not been effective. FUS can be considered as a primary palliative treatment option in patients for whom radiotherapy may be contraindicated (e.g. prior radiation) or has been refused. Future directions and research purpose include the local tumour control and the role as primary treatment, the potential integration of FUS in a multimodality setting treatment of spinal metastasis, and the design of registry and randomized clinical trials. Selection criteria and understanding of specific biological response of different cancers to FUS thermoablation should be also investigated. Currently, a randomized controlled trial of MRgFUS vs radiotherapy for the primary palliative treatment of metastatic bone pain is open to accrual in Europe (trial registry no. NCT01091883).
Today, palliative treatment of bone metastases is still the only application in oncology and musculoskeletal pathology for which FUS has received approval in the USA.
Traditionally, MRgHIFU treatments consist of multiple single focal point sonications, referred to as point-by-point ablation. In 2011, a different MRgFUS system with volumetric ablation capabilities was CE marked for the treatment of painful bone metastases. 11 patients underwent 13 treatments for 12 bone metastases. No major adverse events were observed during or after the procedure. At 3 days after the ablation, significant decrease in pain scores was observed in 6 of 11 (55%) patients. At 1-month follow-up (available for nine patients), a significant decrease in pain scores was observed with six of nine patients obtaining pain response (overall response rate 67%; 95% confidence interval 35–88%).86 A bone-dedicated transducer (conformal bone system) has been developed and is currently approved by CE mark. This is a mobile multielement (1000 elements) transducer with no mechanical but electronic steering which is directly applied to the skin surface to be used as acoustic window to access the bone lesion. The frequency is fixed to 0.55 MHz, and the transducer is provided with a water-permeable membrane to provide acoustic coupling and integrated built-in skin cooling system. The experience in using this kind of transducer is still limited87 (Figure 5).
Figure 5.
Conformal bone system. A transducer dedicated to bone applications was developed and is currently European Conformity (CE) approved. (a) This is a mobile multielement (1000-elements, 0.55-MHz) transducer with electronic steering which can be directly applied to the skin surface to be used as acoustic window to access the bone lesion and to improve the comfort of the patient. In (b) the transducer is applied for the treatment of a metastasis to the humerus from prostate cancer, with the patient lying supine; T2 weighted images with fat saturation show a slight oedema of the surrounding soft tissue after treatment.
Experience in the treatment of primary malignant bone tumours comes from Chinese researchers and authors and is limited to USgHIFU. The first FUS bone treatment was performed in China, for a tibial osteosarcoma in December 1997. Several articles presented series of patients treated mainly for limb salvage or with FUS with palliative intent, in combination or not with chemotherapy.88–91 In 2010, Chen et al92 reported long-term follow-up results of a non-randomized clinical trial of USgFUS for the treatment of 80 primary malignant tumours (60 Stage IIb and 20 Stage IIIb patients according to Enneking staging). 62 patients affected by osteosarcoma, 1 with periosteal osteosarcoma and 3 with Ewing's sarcoma underwent FUS combined with chemotherapy. The remaining 14 patients with chondrosarcoma, malignant giant-cell tumour of the bone, sarcoma of the periosteum or with unknown histology received FUS alone. Complete ablation of the tumour was observed at follow-up images, whereas ablation covering >50% of the tumour tissue was seen in the remaining 11 patients. The overall 5-year survival rate was 51%, with 64% and 16% for patients with Stage IIb and III disease, respectively. Only 5 out of the 69 patients who underwent complete ablation had local cancer recurrence during the follow-up period (5–87 months). All patients experienced mild pain and, among other adverse events, 28% were major complications; in this group, 11 patients required surgery and 8 presented severe peripheral nerve damage. In these last cases, the distance between the damaged nerves and the tumour margin was <10 mm, suggesting that 10 mm is a reasonable safety margin to avoid nerve damage in FUS treatments. Although several articles were published about this topic, there is only limited evidence to consider the technique suitable for this application. Much stronger evidence and trials might allow HIFU to enter a limb-salvage treatment approach or to be considered for neoadjuvant–adjuvant therapy in primary bone tumours. An editorial by Konski commented on the study by Li et al;90 HIFU may provide another treatment option for patients with primary bone tumours who are not surgical candidates or who refuse surgery, but these data need to be confirmed. A reply by Bielack et al93 suggested that experimental approaches should not be considered “safe” unless they are proven to produce equivalent local control rates, and we think that this opinion should be shared and supported.
Italian researchers were mainly involved in testing MRgFUS for the treatment of benign bone tumour, especially osteoid osteoma, with excellent results in terms of efficacy and safety;94,95 in particular, one recent prospective multicentre study95 including 30 consecutive patients with non-spinal osteoid osteoma reported a complete clinical success rate of 90% without adverse events.94,95 Anecdotal experiences have been reported for other epiphyseal benign bone lesions (e.g. periosteal chondroma). However, this topic will be specifically addressed by other authors in this special issue (Figure 6).
Figure 6.
Osteoid osteoma of the femur [CT image, (a)] treated by MRI-guided focused ultrasound surgery, with perfusion imaging before (b) and after (c) the treatment showing the nidus blowing out (arrows).
Standardization of validated assessment instruments facilitates comparisons of clinical studies and should be pursued. Taking into account overall response inclusive of complete and partial responses as a baseline for comparisons, the responder group varied within 92–100%, 85–87% and 64–87% for primary benign, primary malignant and metastatic tumours, respectively. In treatments with a curative aim, the recurrence rate was 0–14%, and in palliative treatments, the pain progression was 0–13%. A comprehensive review of results demonstrates the efficacy of FUS for both palliative and curative purposes in the treatment of bone tumours. Major complications were reported in the ranges of 0%, 0–28% and 0–4% for primary benign, malignant and metastatic tumours, respectively.75
Degenerative diseases
Osteoarthritis (OA) is a common, disabling and costly disease. The predominant symptom is pain. Effective non-invasive treatment approaches are missing. The vast majority of joint replacement surgery procedures are performed because of pain. The need for new therapeutic options is even enhanced by the ageing of the population, responsible for the increasing symptomatic population affected by OA. The management of OA is still a matter of debate.96,97 OA disease is heterogeneous and characterized by failure of the synovial joint organ. The determinants of pain in OA are not well understood but are believed to involve multiple interactive pathways, and inflammatory mediators contributing to sensitize nociceptors. An important component of the biological contribution to pain comes from the multitude of tissues containing nociceptive fibres within the joint; the subchondral bone, periosteum, periarticular ligaments, periarticular muscle and joint capsule including its inner synovial lining are all richly innervated and are the likely source of nociception in OA.98 The cartilage is in the “spotlight” in the field of OA; nonetheless, it is important to note that this disease of the whole joint concurrently affects other tissues that do contain nociceptors. A recent study suggested that areas of denuded cartilage are related to symptoms. Other bone-related causes of pain include periostitis associated with osteophyte formation, subchondral microfractures, bone attrition and bone angina due to decreased blood flow and elevated intraosseous pressure, resulting in bone marrow lesions at imaging. Synovial causes of pain also include irritation of sensory nerve endings within the synovium from osteophytes and synovial inflammation. The National Institute for Health and Care Excellence suggests to consider referral for joint surgery for people with OA who experience joint symptoms (pain, stiffness and reduced function) with substantial impact on their quality of life and refractory to non-surgical treatment.97
MRgFUS has been recently applied for the treatment of pain related to facet joint syndrome and to knee OA by two different research groups. Their preliminary experiences have been described in two pilot studies, demonstrating the safety of the procedure and the potential efficacy while achieving satisfying results in terms of pain relief. The treatments were performed by using both “conventional” and “conformal” bone systems.99,100
Low back pain is a leading cause of activity limitation and work absence throughout the world, imposing a high economic burden on individuals, families, industry and governments.101 Facet joint pain represents a consistent part of low back pain, and its treatment and management are subjects of great controversy. Several methodological limitations have led to poor evidence and non-widely approved conclusions for current treatment options. Facet joints have a rich innervation arising from the medial and lateral branches of the dorsal rami with dual innervation from the medial branches arising from the posterior rami. In most cases, the treatment strategy is still unsatisfactory. MRgFUS received CE mark for facet joint syndrome in June 2013, after a preliminary experience, with great potential, of Weeks et al99 who published in 2012 the first reliable clinical application of MRgFUS for OA, specifically for facet joint syndrome (Figure 7). In a Phase I observational pilot study, the authors enrolled 18 patients with a positive response to facet joint interventions. MRgFUS was performed at the levels of pain according to symptomatology, previous invasive treatment and MRI grading of facet joint OA, and evaluated pain, function and quality of life. At 6 and 12 months, they found a reduction in both average and worst numerical rating scale pain scores of about 60.2%/51.2%, with of 45.9% in Oswestry disability questionnaire score and 61.9% reduction in the brief pain inventory interference score. According to EuroQol, they observed an improvement in the health state score based on UK coefficients of +0.379 (0.317–0.696). No major adverse events were observed. They demonstrated, in such a difficult clinical entity, that MRgFUS is safe, free of complications, effective and well tolerated, after a rigorous diagnosis including diagnostic blocks, which are the best approach to select patients for treatment, since no physical examination findings are pathognomonic for diagnosis.99 Summary of pre-clinical phase for facet joint treatment was published by Harnof et al.102
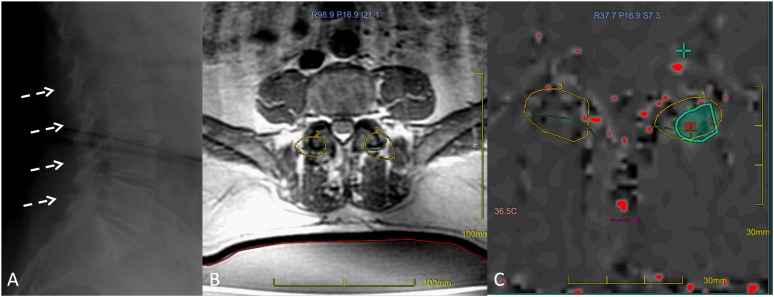
Figure 7.
MRI-guided focused ultrasound surgery (MRgFUS) treatment for facet joint syndrome. Osteoarthritis with facet joint involvement is shown in (a) (broken arrows). A screenshot of the MRgFUS procedure shows facet joints targeted on the MR planning image (b), and a thermal map image (c) caught during sonication highlights the thermal effect reached on the target (ablation/denervation) (courtesy of Dr Mattia Squarcia, Hospital Clinic-Centre Mèdic Alomar Barcelona, Spain).
The risk of mobility disability attributable to knee OA alone, affecting general well-being and performance at work, is greater than that due to any other medical condition in people aged 65 years and over. A study by Izumi et al100 opened the application of MRgFUS to other important joints, such as the knee. Although there is still not a marking of conformity or approval about the use of FUS in knee OA, this study showed the safety and potential efficacy of MRgFUS in eight patients with medial knee pain, eligible for total knee arthroplasty. The pain intensity during walking was assessed by VAS before and after treatment, and pressure pain thresholds were also evaluated in the sonication area and in control sites 1 month after treatment. Six patients (75%) showed immediate pain alleviation after treatment, and four of them demonstrated long-lasting effect at 6-month follow-up (mean VAS reduction; 72.6%), with pressure pain thresholds significantly increased after treatment in responders. No adverse side effects or complications were observed.100
The above mentioned studies open to a new field, with potentially very huge impact, both in terms of new options in treating pain from the most prevalent joint disorders, OA, as well as in new insights into joint function pathophysiology.
Miscellaneous and soft-tissue lesions
Very few articles propose HIFU for MSK soft-tissue ablation. All major studies use USgHIFU. An experience by Wang et al103 showed the potential of using HIFU in treating extra-abdominal desmoid tumours. Ten patients with pathologically proven extra-abdominal desmoid tumours were submitted to USgHIFU ablation. Curative aim was possible in two patients with new solitary tumours, whereas the procedure was performed with palliative aim in eight patients with multiple, recurrent tumours. The mean size of the largest tumour was 9.2 cm (range 5.9–12.8 cm). Large areas of coagulation necrosis were obtained in all patients. 25 treated tumours significantly shrank in volume (>50%) during a mean follow-up of 30 months (range 8–55 months); in 2 patients with solitary tumours, complete tumour necrosis was observed. HIFU was repeated in two patients for growing residual tumours. No major complications were observed.
In a series of patients with solid malignancies, Orgera et al104 evaluated the feasibility, local tumour response and clinical results of USgHIFU. Among these, soft-tissue metastases from colorectal carcinoma and muscle metastasis from lung, and one abdominal liposarcoma were presented. All bone and soft-tissue were described palliated in symptoms, with complete response to positron emission tomography (PET)/CT, multidetector CT or MRI; the liposarcoma was almost completely ablated at MRI.104
Local control of primary synovial spindle cell sarcoma of the chest wall was attempted by Hu et al.105 After four cycles of chemotherapy, local recurrence of the sarcoma was detected. Subsequent extended resection confirmed synovial sarcoma. After five cycles of a new chemotherapy option, the sarcoma relapsed again. The patient subsequently received five courses of HIFU; this was described to completely ablate the sarcoma without complications.105
Other experiences also involving MRgFUS in the treatment of aggressive fibromatosis, haemangiomas (with particular features), as well as other soft-tissue masses have been presented in abstracts and conferences as anecdotal and must be confirmed by more extensive research.106
From pre-clinical evidence to practice
A number of studies investigated the effects of ultrasound on biological tissues. The best known method of HIFU is thermal ablation, but interest in non-thermal, mechanical destruction is increasing. The advantages of mechanical ablation are that thermal protein denaturation remains limited, and less damage is created to the surrounding tissue by thermal diffusion.107 During HIFU applications, tissue necrosis may be obtained by heating, or the tissue can be emulsified by cavitation; as recently reported, the tissue can also be emulsified by using repetitive millisecond boiling (caused by shock wave heating).108 During treatment, ultrasound reflections from distal media interfaces can shift prescribed treatment locations. By targeting the focus “behind” the bone, the same result can be achieved with a single sonication only. MRgFUS by both energy deposition methods can be used to produce controlled well-localized damage to soft tissue in close proximity to the bone, with minimal collateral damage.109 Comparing the effect of normal incidence reflections from air, acrylic (modelling bone) and rubber on treatment location, temperature elevation and heating patterns by performing ultrasound exposures, results demonstrated a shift in treatment location towards the distal interface when targeted closer than 2 cm from the interface, especially for acrylic: ultrasound wave reflections from a distal air interface had less effect than the acrylic interface (modelling bone) on the heating pattern and focal location.110
“Paleoresearch” of interactions between MSK tissues and HIFU were published.111–113
Thermal effects due to high ultrasound absorption in bone pose an ongoing safety issue regarding the heating of the soft tissue adjacent to the bone surface. Mathematical models have been developed to predict the temperature rise at bone/soft-tissue interface.114 The comprehension of effects on bony structure and function after HIFU is of crucial importance. Several experiences on bone remodelling processes induced by thermo-related coagulative necrosis were also reported. Bucknor et al115 investigated hyperacute (<1 h) changes following MRgFUS at MR and CT in relation to sonication number and energy in a swine bone model; increasing the sonications' number from four to six caused a significant increase in the depth of the intramedullary hypoenhanced zone (from 2.9 to 6.5 mm; p < 0.001) within similar volumes. No significant difference was observed between low- and high-energy ablations. CT images did not reveal structural abnormalities. This study concluded that the number of sonications can be used to increase the treatment depth within the target in the bone. T2 weighted and contrast-enhanced MR, and not CT, can show the hyperacute structural changes of the bone and surrounding tissues.115
Recently, Bucknor et al also provided additional information by studying bone remodelling in the swine femur after MRgHIFU ablation with MRI, CT, Na(18)F-PET and histopathological examination, as a function of sonication energy. Eight pigs were evaluated before and 3 and 6 weeks after MRgHIFU with 3-T MRI and 64-section CT [4 pigs were also evaluated using (18)f-sodium fluoride-PET (Na(18)F-PET) and histopathological examination]. Ablation sizes at MRI 3 and 6 weeks after MRgFUS were similar between proximal (low-energy) and distal (high-energy) lesions, although distal ablation lesions demonstrated evidence of subperiosteal new bone formation at CT, with a subtle focus of new ossification at 3 weeks and a larger focus of ossification at 6 weeks. New bone formation was associated with increased uptake at Na(18)F-PET in three of four animals undergoing PET; this was confirmed at histopathological examination in all four animals. MRgHIFU ablation of bone may result in progressive remodelling, with both subcortical necrosis and subperiosteal new bone formation. This may be related to the use of high energies. MRI, CT and PET are suitable non-invasive techniques to monitor bone remodelling after MRgHIFU ablation.116
Another interesting study was conducted by Herman et al117 in 2013. This author investigated biomechanical properties of the bone treated by MRgHIFU in four minipigs. The treatment was performed in six consecutive right normal ribs, whereas the corresponding left ribs were used as controls. A reduction in bone biomechanical properties was observed 6 weeks after treatment in the treated ribs, with a mean ± standard deviation yield load ratio and maximum ratios of 0.69 ± 0.11 and 0.71 ± 0.13, respectively (both p = 0.031). Some recovery trend was observed at 12 weeks after treatment. Significant reduction in mean osteon size was reported at histological analysis at 2 weeks after treatment in control vs treated bones, respectively (p = 0.005). An approximate 30% reduction in mechanical strength was observed in the treated bones at 6 weeks post-treatment, with a reversible trend at 12 weeks post-treatment (reduced from 30% to 25–20%).117
Safety guidelines are required for treating tissue masses on or near the bone by using MRgFUS,118 since the presence of bone would directionally change the spatial distribution of acoustic pressure, and thermal and cavitation effects for oblique incidence of HIFU.119 In addition, HIFU may be generated as pulsed or continuous depending on the device and medical purpose. HIFU treatments on muscle tissue induce severe changes in gene expression; however, continuous or pulsed HIFU cause the upregulation of the same genes, meaning that the tissue reaction is not influenced by the type of damage.120 Effects of ultrasound were studied also on other tissues and components of the MSK system, such as muscles, tendons, ligaments, and synovial, meniscal and discal tissues, as well as on nerves and vessels.121–124
HIFU beam was tested on bovine tendons ex vivo, highlighting the potential of HIFU as a non-invasive treatment option for chronic tendinosis.125 Foldes et al126 proposed to study the feasibility of MRgFUS to non-invasively perform synovectomy in rabbit knees, with partial synovectomy resulting in five animals. Studies evaluated probes as an alternative technology for meniscal debridement in the bovine knee,127 and for collagen shrinkage in intervertebral disc.128,129 A number of studies reported the therapeutic potential of low-intensity pulsed ultrasound in the induction of bone repair,130 as well as in avoiding the development of disuse muscle atrophy partly via activation of satellite cells.131
The effects of focused and unfocused ultrasound on nerves, vessels and adipocytes also deserve special consideration both for therapeutic implications and for safety issues.131 Studies on functional effects and of the potential disruptive effects of FUS on nerves date back to the mid-20th century. FUS of the whole nerve produces differential conduction blocking of mammalian nerve fibres. The smallest (C) fibres are more sensitive, whereas the largest (A-alpha) are less vulnerable. Complete reversible blocking can be reached through graded doses of ultrasound.133,134 Single pulses of FUS have been observed to significantly modify neuronal excitability in vitro. Pulsed FUS can stimulate the receptor and conductive nerve structures of humans and animals as well as the neurons of the central nervous system of invertebrates.135 Colucci et al136 more recently studied the application of FUS on sciatic nerves in bullfrogs. The nerve action potential was shown to decrease in the experiments and correlated with temperature elevation measured in the nerve. The action potential recovered either completely, partially or not at all, depending on the parameters of the ultrasound exposure. These results indicate that a thermal mechanism of FUS can be used to block nerve conduction, either temporarily or permanently.136 Foley et al137 investigated the effects of various exposures (intensity, duration) of HIFU on sciatic nerve conduction in rats, with the aim to identify HIFU exposures that produce biological effects ranging from partial to complete conduction block, indicating potential use of HIFU as an alternative to current clinical methods of inducing nerve conduction block.137 HIFU was demonstrated to attenuate neural responses of sciatic nerves isolated from normal or neuropathic rats, with diabetic nerves being less suppressed by HIFU and more vulnerable to permanent damage.138 Neurolysis, blocks, neuromodulation are possible applications of FUS.139,140
Among studies on vascular applications, Ishikawa et al141 demonstrated how, in rats, the response of the artery to HIFU varied with intensity. Vascular contraction without tissue degeneration occurred at low intensity; by increasing the intensity, the tissue degeneration was detectable in histology and the vascular diameter was reduced, and finally, at high intensity, the blood flow was occluded. Although these phenomena appeared to be mainly due to thermal effects, mechanical effects might have some role, particularly on vascular contraction. HIFU was also investigated for sympathetic denervation.141
A study was aimed at detecting changes in the cell membrane of Sarcoma 180 (S180) cells induced by FUS and to probe the underlying mechanism. The results revealed that the instant cell damage effects induced by ultrasound might be related to the improved membrane lipid peroxidation levels post-treatment. The physicochemical properties of S180 cell membrane were changed by FUS. The findings also implied an exposure time-dependent pattern and suggested that the lipid peroxidation produced by acoustic cavitation might play important roles in these actions.142 The histological changes, including the antitumour immunological response, after HIFU treatment were examined in soft-tissue sarcoma. HIFU, even when administered as a single shot, induces apoptosis of tumour cells and intratumoral infiltration of macrophages and lymphocytes.143
Non-surgical opportunities
Thermal therapy can be performed in two regimes. The first one is high-temperature ablation, with temperatures in the range of 50–80 °C (or higher) applied for a short time interval to rapidly induce coagulation necrosis through processes such as protein denaturation. The second is low-temperature ablation, with temperatures in the range of 43–45 °C applied for a longer time interval (tens of minutes) to directly induce cancer cells' death or to make them more sensitive to cytotoxic agents and/or radiation.144–153
Staruch et al154 studied control strategies and drug delivery with temperature-sensitive liposome hyperthermia in bone, generated with MRgFUS. Significant increases in doxorubicin concentration occurred in heated vs unheated marrow (8.2-fold) and muscle (16.8-fold). Enhancement occurred for 0- and 10-mm offsets, suggesting that localized drug delivery in bone is possible with both hyperthermia and thermal ablation. Thus, this demonstrated that MRgFUS can achieve localized hyperthermia in bone for image-guided drug delivery in the bone with temperature-sensitive drug carriers.154
Potential mechanism inducing apoptosis in sonodynamic therapy and FUS were investigated in S180 cells in vitro;155 in particular, how pulsed HIFU-mediated nanoparticle delivery in murine muscle.156
Current commercially available MRgFUS devices are marketed for their thermal ablation applications. In the future, lower energy treatments may play a significant role in mediating targeted drug and gene delivery for cancer treatment.27
SAFETY
HIFU treatments are usually carried out in a single session, often as a day case procedure, with the patient either fully conscious, lightly sedated or under locoregional or general anaesthesia. Errors in the preparation of patient and in positioning should be accurately ruled out. Small scars can be avoided by placing appropriate sticks and with a good planning of the procedure.
In the Phase III trial for treatment of skeletal metastasis, the most common treatment-related adverse events were sonication pain (32.1% of patients) for insufficient anaesthesia, followed by pathological fractures (two patients) for insufficient anaesthesia, severe skin burn and neuropathy (one patient each). Around 60% of the adverse effects resolved on the same day of the procedure.11
In the study of treatment of primary malignant bone tumours with USgHIFU by Chen et al, all patients experienced mild pain, and among other adverse events, 28% were major complications where 11 of these patients required surgery and 8 presented severe peripheral nerve damage.92,157
Overall, studies of benign tumours reported 0–66% minor and 0% major complications. Patients treated for primary malignant tumours presented higher complication ranges: 45–100% minor and 0–28% major complications. Finally, patients with metastatic tumours presented complication ranges similar to the first group: 0–51% minor and 0–4% major complications. The most frequently observed complications were mild skin burn (also depending on operator skills), usually resolving by 1–2 weeks after FUS, and sonication pain during treatment (also depending on anaesthesia choices). Major problems were in large part due to lack of guidelines to protect normal structures (e.g. there were eight serious nerve injuries, since treatment guidelines did not limit proximity to nerves). Other primary malignant studies implemented a 1-cm tumour margin limit and major complications were limited to 8%. However, MRgFUS produced only 4% (4/112) major complications in patients with bone metastases. Overall, the data provide strong evidence that FUS is safe for treatment of bone metastases and primary benign lesions, whereas other applications are still under investigation.75 Adverse events of extracorporeal USgHIFU therapy were also reported.158,159
One last issue deserves special consideration: the use of contrast agents before HIFU treatment. According to a recent study performed by Hijnen et al,160 MRgHIFU treatment seems not induce the dissociation of gadolinium (Gd)-diethylene-triamine-penta-acetate. In small-tissue volumes, no significant effect on the long-term in vivo Gd retention was found. However, care must be taken with the use of proton resonance frequency shift-based MR thermometry for HIFU guidance in combination with Gd, since the susceptibility artefact induced by Gd can severely influence treatment outcome.160
CLOSING REMARKS AND CONCLUSIONS
MRgFUS is an emerging technology which enhances the potential efficacy of HIFU/FUS by influencing the accuracy of treatment planning and monitoring (by using MRI). MRgFUS is a completely non-invasive technique. The physician can correctly localize tumours or lesions, optimally deliver acoustic energy (sonicate), monitor energy deposition in real time and accurately control the deposited thermal dose.
The basic knowledge of running evidence in pre-clinical setting helps to introduce physicians to this technology and to enlighten MRgFUS users to new potential treatment strategies and applications.
MRgFUS was approved and is gaining consensus for bone metastases, other bone tumours and other applications including degenerative disease. The core of the current program for research and innovation of the European Union, “Horizon 2020”, is “active and healthy ageing”. This includes the importance of quality of life and the need for successful mini-invasive therapy in order to postpone any major and invasive intervention which may require long rehabilitation times, leading to potential morbidity, mortality or needing revision. The “palliative” role of any potential treatment approach in pain relief while restoring better quality of life will be central in any of the possible future scenarios of ageing. MRgFUS has been demonstrated to be safe and effective in treating pain caused by several medical conditions, and also to have more deep potential in tumour ablation.
Although MRgFUS is still at the starting point, and several limitations must be taken into consideration, it seems to be a candidate to hold a prominent position in the next clinical generation of therapeutic applications.
Contributor Information
Alberto Bazzocchi, Email: abazzo@inwind.it.
Alessandro Napoli, Email: alessandro.napoli@uniroma1.it.
Beatrice Sacconi, Email: beatrice.sacconi@fastwebnet.it.
Giuseppe Battista, Email: giuseppe.battista@unisi.it.
Giuseppe Guglielmi, Email: g.guglielmi@unifg.it.
Carlo Catalano, Email: carlo.catalano@uniroma1.it.
Ugo Albisinni, Email: ugo.albisinni@ior.it.
REFERENCES
- 1.Napoli A, Anzidei M, Ciolina F, Marotta E, Cavallo Marincola B, Brachetti G, et al. MR-guided high-intensity focused ultrasound: current status of an emerging technology. Cardiovasc Intervent Radiol 2013; 36: 1190–203. doi: 10.1007/s00270-013-0592-4 [DOI] [PubMed] [Google Scholar]
- 2.Richards WT, Loomis AL. The chemical effects of high frequency sound waves I. A preliminary survey. J Am Chem Soc 1927; 49: 3086–100. [Google Scholar]
- 3.ter Haar G. Therapeutic applications of ultrasound. Prog Biophys Mol Biol 2007; 93: 111–29. doi: 10.1016/j.pbiomolbio.2006.07.005 [DOI] [PubMed] [Google Scholar]
- 4.Mason TJ. Therapeutic ultrasound an overview. Ultrason Sonochem 2011; 18: 847–52. doi: 10.1016/j.ultsonch.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 5.Miller DL, Smith NB, Bailey MR, Czarnota GJ, Hynynen K, Makin IR, et al. Overview of therapeutic ultrasound applications and safety considerations. J Ultrasound Med 2012; 31: 623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy JE, Ter Haar GR, Cranston D. High intensity focused ultrasound: surgery of the future? Br J Radiol 2003; 76: 590–9. doi: 10.1259/bjr/17150274 [DOI] [PubMed] [Google Scholar]
- 7.Haar GT, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia 2007; 23: 89–104. [DOI] [PubMed] [Google Scholar]
- 8.Haar GT, Coussios C. High intensity focused ultrasound: past, present and future. Int J Hyperthermia 2007; 23: 85–7. [DOI] [PubMed] [Google Scholar]
- 9.Yang X, Roy RA, Holt RG. Bubble dynamics and size distributions during focused ultrasound insonation. J Acoust Soc Am 2004; 116: 3423–31. doi: 10.1121/1.1823251 [DOI] [PubMed] [Google Scholar]
- 10.Napoli A, Anzidei M, Marincola BC, Brachetti G, Ciolina F, Cartocci G, et al. Primary pain palliation and local tumor control in bone metastases treated with magnetic resonance-guided focused ultrasound. Invest Radiol 2013; 48: 351–8. doi: 10.1097/RLI.0b013e318285bbab [DOI] [PubMed] [Google Scholar]
- 11.Hurwitz MD, Ghanouni P, Kanaev SV, Iozeffi D, Gianfelice D, Fennessy FM, et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J Natl Cancer Inst 2014; 106: pii: dju082. doi: 10.1093/jnci/dju082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cline HE, Schenck JF, Hynynen K, Watkins RD, Souza SP, Jolesz FA. MR-guided focused ultrasound surgery. J Comput Assist Tomogr 1992; 16: 956–65. doi: 10.1097/00004728-199211000-00024 [DOI] [PubMed] [Google Scholar]
- 13.Cline HE, Schenck JF, Watkins RD, Hynynen K, Jolesz FA. Magnetic resonance-guided thermal surgery. Magn Reson Med 1993; 30: 98–106. doi: 10.1002/mrm.1910300115 [DOI] [PubMed] [Google Scholar]
- 14.Hynynen K, Darkazanli A, Unger E, Schenck JF. MRI-guided noninvasive ultrasound surgery. Med Phys 1993; 20: 107–15. doi: 10.1118/1.597093 [DOI] [PubMed] [Google Scholar]
- 15.Hynynen K, Pomeroy O, Smith DN, Huber PE, McDannold NJ, Kettenbach J, et al. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology 2001; 219: 176–85. doi: 10.1148/radiology.219.1.r01ap02176 [DOI] [PubMed] [Google Scholar]
- 16.Hindley J, Gedroyc WM, Regan L, Stewart E, Tempany C, Hynyen K, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. AJR Am J Roentgenol 2004; 183: 1713–19. doi: 10.2214/ajr.183.6.01831713 [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Bai J, Li F, Du Y, Wen S, Hu K, et al. Study of a “biological focal region” of high-intensity focused ultrasound. Ultrasound Med Biol 2003; 29: 749–54. doi: 10.1016/S0301-5629(02)00785-8 [DOI] [PubMed] [Google Scholar]
- 18.Hill CR, Rivens I, Vaughan MG, ter Haar GR. Lesion development in focused ultrasound surgery: a general model. Ultrasound Med Biol 1994; 20: 259–69. doi: 10.1016/0301-5629(94)90066-3 [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Li J, Gong X, Zhang D. Nonlinear absorption in biological tissue for high intensity focused ultrasound. Ultrasonics 2006; 44(Suppl. 1): e27–30. doi: 10.1016/j.ultras.2006.06.035 [DOI] [PubMed] [Google Scholar]
- 20.Choi JW, Lee JY, Hwang EJ, Hwang I, Woo S, Lee CJ, et al. Portable high-intensity focused ultrasound system with 3D electronic steering, real-time cavitation monitoring, and 3D image reconstruction algorithms: a preclinical study in pigs. Ultrasonography 2014; 33: 191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arvanitis CD, McDannold N. Integrated ultrasound and magnetic resonance imaging for simultaneous temperature and cavitation monitoring during focused ultrasound therapies. Med Phys 2013; 40: 112901. doi: 10.1118/1.4823793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherwood V, Civale J. Development of a hybrid magnetic resonance and ultrasound imaging system. Biomed Res Int 2014; 2014: 914347. doi: 10.1155/2014/914347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yiallouras C, Damianou C. Review of MRI positioning devices for guiding focused ultrasound systems. Int J Med Robot 2015; 11: 247–55. [DOI] [PubMed] [Google Scholar]
- 24.Noorda YH, Bartels LW, Huisman M, Nijenhuis RJ, van den Bosch MA, Pluim JP. Registration of CT to pre-treatment MRI for planning of MR-HIFU ablation treatment of painful bone metastases. Phys Med Biol 2014; 59: 4167–79. doi: 10.1088/0031-9155/59/15/4167 [DOI] [PubMed] [Google Scholar]
- 25.Schlesinger D, Benedict S, Diederich C, Gedroyc W, Klibanov A, Larner J. MR-guided focused ultrasound surgery, present and future. Med Phys 2013; 40: 080901. doi: 10.1118/1.4811136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellis S, Rieke V, Kohi M, Westphalen AC. Clinical applications for magnetic resonance guided high intensity focused ultrasound (MRgHIFU): present and future. J Med Imaging Radiat Oncol 2013; 57: 391–9. doi: 10.1111/1754-9485.12085 [DOI] [PubMed] [Google Scholar]
- 27.Maloney E, Hwang JH. Emerging HIFU applications in cancer therapy. Int J Hyperthermia 2015: 31: 302–9. [DOI] [PubMed] [Google Scholar]
- 28.Cavallo Marincola B, Pediconi F, Anzidei M, Miglio E, Di Mare L, Telesca M, et al. High-intensity focused ultrasound in breast pathology: non-invasive treatment of benign and malignant lesions. Expert Rev Med Devices 2015; 12: 191–9. doi: 10.1586/17434440.2015.986096 [DOI] [PubMed] [Google Scholar]
- 29.Anzidei M, Marincola BC, Bezzi M, Brachetti G, Nudo F, Cortesi E, et al. Magnetic resonance-guided high-intensity focused ultrasound treatment of locally advanced pancreatic adenocarcinoma: preliminary experience for pain palliation and local tumor control. Invest Radiol 2014; 49: 759–65. doi: 10.1097/RLI.0000000000000080 [DOI] [PubMed] [Google Scholar]
- 30.Aubry JF, Pauly KB, Moonen C, Haar GT, Ries M, Salomir R, et al. The road to clinical use of high-intensity focused ultrasound for liver cancer: technical and clinical consensus. J Ther Ultrasound 2013; 1: 13. doi: 10.1186/2050-5736-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Napoli A, Anzidei M, De Nunzio C, Cartocci G, Panebianco V, De Dominicis C, et al. Real-time magnetic resonance-guided high-intensity focused ultrasound focal therapy for localised prostate cancer: preliminary experience. Eur Urol 2013; 63: 395–8. doi: 10.1016/j.eururo.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 32.Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med 2013; 369: 640–8. doi: 10.1056/NEJMoa1300962 [DOI] [PubMed] [Google Scholar]
- 33.Froling V, Kroncke TJ, Schreiter NF, Scheurig-Muenkler C, Collettini F, Hamm B, et al. Technical eligibility for treatment of magnetic resonance-guided focused ultrasound surgery. Cardiovasc Intervent Radiol 2014; 37: 445–50. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, ter Haar G, Hill CR. Influence of ablated tissue on the formation of high-intensity focused ultrasound lesions. Ultrasound Med Biol 1997; 23: 921–31. doi: 10.1016/S0301-5629(97)00016-1 [DOI] [PubMed] [Google Scholar]
- 35.Hallaj IM, Cleveland RO, Hynynen K. Simulations of the thermo-acoustic lens effect during focused ultrasound surgery. J Acoust Soc Am 2001; 109: 2245–53. doi: 10.1121/1.1360239 [DOI] [PubMed] [Google Scholar]
- 36.Miller NR, Bograchev KM, Bamber JC. Ultrasonic temperature imaging for guiding focused ultrasound surgery: effect of angle between imaging beam and therapy beam. Ultrasound Med Biol 2005; 31: 401–13. doi: 10.1016/j.ultrasmedbio.2004.11.014 [DOI] [PubMed] [Google Scholar]
- 37.Fry FJ. Transkull transmission of an intense focused ultrasonic beam. Ultrasound Med Biol 1977; 3: 179–84. doi: 10.1016/0301-5629(77)90069-2 [DOI] [PubMed] [Google Scholar]
- 38.Tobias J, Hynynen K, Roemer R, Guthkelch AN, Fleischer AS, Shively J. An ultrasound window to perform scanned, focused ultrasound hyperthermia treatments of brain tumors. Med Phys 1987; 14: 228–34. doi: 10.1118/1.596074 [DOI] [PubMed] [Google Scholar]
- 39.Lipsman N, Mainprize TG, Schwartz ML, Hynynen K, Lozano AM. Intracranial applications of magnetic resonance-guided focused ultrasound. Neurotherapeutics 2014; 11: 593–605. doi: 10.1007/s13311-014-0281-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monteith SJ, Kassell NF, Goren O, Harnof S. Transcranial MR-guided focused ultrasound sonothrombolysis in the treatment of intracerebral hemorrhage. Neurosurg Focus 2013; 34: E14. doi: 10.3171/2013.2.FOCUS1313 [DOI] [PubMed] [Google Scholar]
- 41.Holscher T, Ahadi G, Fisher D, Zadicario E, Voie A. MR-guided focused ultrasound for acute stroke: a rabbit model. Stroke 2013; 44(6 Suppl. 1): S58–60. [DOI] [PubMed] [Google Scholar]
- 42.Wijlemans JW, de Greef M, Schubert G, Moonen CT, van den Bosch MA, Ries M. Intrapleural fluid infusion for MR-guided high-intensity focused ultrasound ablation in the liver dome. Acad Radiol 2014; 21: 1597–602. doi: 10.1016/j.acra.2014.06.015 [DOI] [PubMed] [Google Scholar]
- 43.Henderson PW, Lewis GK, Shaikh N, Sohn A, Weinstein AL, Olbricht WL, et al. A portable high-intensity focused ultrasound device for noninvasive venous ablation. J Vasc Surg 2010; 51: 707–11. doi: 10.1016/j.jvs.2009.10.049 [DOI] [PubMed] [Google Scholar]
- 44.Robinson DM, Kaminer MS, Baumann L, Burns AJ, Brauer JA, Jewell M, et al. High-intensity focused ultrasound for the reduction of subcutaneous adipose tissue using multiple treatment techniques. Dermatol Surg 2014; 40: 641–51. [DOI] [PubMed] [Google Scholar]
- 45.Mindjuk I, Trumm CG, Herzog P, Stahl R, Matzko M. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol 2015; 25: 1317–28. doi: 10.1007/s00330-014-3538-6 [DOI] [PubMed] [Google Scholar]
- 46.Luo J, Ren X, Yu T. Efficacy of extracorporeal ultrasound-guided high intensity focused ultrasound: an evaluation based on controlled trials in China. Int J Radiat Biol 2015: 95: 480–5. doi: 10.3109/09553002.2015.1021962 [DOI] [PubMed] [Google Scholar]
- 47.Jolesz FA, McDannold N. Current status and future potential of MRI-guided focused ultrasound surgery. J Magn Reson Imaging 2008; 27: 391–9. doi: 10.1002/jmri.21261 [DOI] [PubMed] [Google Scholar]
- 48.Jolesz FA. MRI-guided focused ultrasound surgery. Annu Rev Med 2009; 60: 417–30. doi: 10.1146/annurev.med.60.041707.170303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hynynen K. MRI-guided focused ultrasound treatments. Ultrasonics 2010; 50: 221–9. doi: 10.1016/j.ultras.2009.08.015 [DOI] [PubMed] [Google Scholar]
- 50.Hynynen K. MRIgHIFU: a tool for image-guided therapeutics. J Magn Reson Imaging 2011; 34: 482–93. doi: 10.1002/jmri.22649 [DOI] [PubMed] [Google Scholar]
- 51.Li D, Shen G, Bai J, Chen Y. Focus shift and phase correction in soft tissues during focused ultrasound surgery. IEEE Trans Biomed Eng 2011; 58: 1621–8. doi: 10.1109/TBME.2011.2106210 [DOI] [PubMed] [Google Scholar]
- 52.Ritchie R, Collin J, Coussios C, Leslie T. Attenuation and de-focusing during high-intensity focused ultrasound therapy through peri-nephric fat. Ultrasound Med Biol 2013; 39: 1785–93. doi: 10.1016/j.ultrasmedbio.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 53.Kun G, Wan M. Effects of fascia lata on HIFU lesioning in vitro. Ultrasound Med Biol 2004; 30: 991–8. doi: 10.1016/j.ultrasmedbio.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 54.Cline HE, Hynynen K, Hardy CJ, Watkins RD, Schenck JF, Jolesz FA. MR temperature mapping of focused ultrasound surgery. Magn Reson Med 1994; 31: 628–36. doi: 10.1002/mrm.1910310608 [DOI] [PubMed] [Google Scholar]
- 55.Hardy CJ, Cline HE, Watkins RD. One-dimensional NMR thermal mapping of focused ultrasound surgery. J Comput Assist Tomogr 1994; 18: 476–83. doi: 10.1097/00004728-199405000-00024 [DOI] [PubMed] [Google Scholar]
- 56.Hindman JC. Proton resonance shift of water in the gas and liquid states. J Chem Phys 1996; 44: 4582. [Google Scholar]
- 57.Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Y, Kuroda K, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med 1995; 34: 814–23. doi: 10.1002/mrm.1910340606 [DOI] [PubMed] [Google Scholar]
- 58.De Poorter J, De Wagter C, De Deene Y, Thomsen C, Stahlberg F, Achten E. Noninvasive MRI thermometry with the proton resonance frequency (PRF) method: in vivo results in human muscle. Magn Reson Med 1995; 33: 74–81. doi: 10.1002/mrm.1910330111 [DOI] [PubMed] [Google Scholar]
- 59.Baron P, Deckers R, de Greef M, Merckel LG, Bakker CJ, Bouwman JG, et al. Correction of proton resonance frequency shift MR-thermometry errors caused by heat-induced magnetic susceptibility changes during high intensity focused ultrasound ablations in tissues containing fat. Magn Reson Med 2014; 72: 1580–9. doi: 10.1002/mrm.25063 [DOI] [PubMed] [Google Scholar]
- 60.Petrusca L, Auboiroux V, Goget T, Viallon M, Muller A, Gross P, et al. A nonparametric temperature controller with nonlinear negative reaction for multi-point rapid MR-guided HIFU ablation. IEEE Trans Med Imaging 2014; 33: 1324–37. doi: 10.1109/TMI.2014.2310704 [DOI] [PubMed] [Google Scholar]
- 61.Viallon M, Petrusca L, Auboiroux V, Goget T, Baboi L, Becker CD, et al. Experimental methods for improved spatial control of thermal lesions in magnetic resonance-guided focused ultrasound ablation. Ultrasound Med Biol 2013; 39: 1580–95. doi: 10.1016/j.ultrasmedbio.2013.03.018 [DOI] [PubMed] [Google Scholar]
- 62.Todd N, Vyas U, de Bever J, Payne A, Parker DL. Reconstruction of fully three-dimensional high spatial and temporal resolution MR temperature maps for retrospective applications. Magn Reson Med 2012; 67: 724–30. doi: 10.1002/mrm.23055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Todd N, Prakash J, Odeen H, de Bever J, Payne A, Yalavarthy P, et al. Toward real-time availability of 3D temperature maps created with temporally constrained reconstruction. Magn Reson Med 2014; 71: 1394–404. doi: 10.1002/mrm.24783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramsay E, Mougenot C, Kazem M, Laetsch TW, Chopra R. Temperature-dependent MR signals in cortical bone: Potential for monitoring temperature changes during high-intensity focused ultrasound treatment in bone. Magn Reson Med 2015; 74: 1095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baron P, Ries M, Deckers R, de Greef M, Tanttu J, Kohler M, et al. In vivo T2-based MR thermometry in adipose tissue layers for high-intensity focused ultrasound near-field monitoring. Magn Reson Med 2014; 72: 1057–64. doi: 10.1002/mrm.25025 [DOI] [PubMed] [Google Scholar]
- 66.Weiss N, Sosna J, Goldberg SN, Azhari H. Non-invasive temperature monitoring and hyperthermic injury onset detection using X-ray CT during HIFU thermal treatment in ex vivo fatty tissue. Int J Hyperthermia 2014; 30: 119–25. doi: 10.3109/02656736.2014.883466 [DOI] [PubMed] [Google Scholar]
- 67.Diakite M, Odeen H, Todd N, Payne A, Parker DL. Toward real-time temperature monitoring in fat and aqueous tissue during magnetic resonance-guided high-intensity focused ultrasound using a three-dimensional proton resonance frequency T1 method. Magn Reson Med 2014; 72: 178–87. doi: 10.1002/mrm.24900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dillon CR, Payne A, Christensen DA, Roemer RB. The accuracy and precision of two non-invasive, magnetic resonance-guided focused ultrasound-based thermal diffusivity estimation methods. Int J Hyperthermia 2014; 30: 362–71. doi: 10.3109/02656736.2014.945497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Auboiroux V, Viallon M, Roland J, Hyacinthe JN, Petrusca L, Morel DR, et al. ARFI-prepared MRgHIFU in liver: simultaneous mapping of ARFI-displacement and temperature elevation, using a fast GRE-EPI sequence. Magn Reson Med 2012; 68: 932–46. doi: 10.1002/mrm.23309 [DOI] [PubMed] [Google Scholar]
- 70.Celicanin Z, Auboiroux V, Bieri O, Petrusca L, Santini F, Viallon M, et al. Real-time method for motion-compensated MR thermometry and MRgHIFU treatment in abdominal organs. Magn Reson Med 2014; 72: 1087–95. doi: 10.1002/mrm.25017 [DOI] [PubMed] [Google Scholar]
- 71.Fite BZ, Wong A, Liu Y, Mahakian LM, Tam SM, Aina O, et al. Magnetic resonance imaging assessment of effective ablated volume following high intensity focused ultrasound. PLoS One 2015; 10: e0120037. doi: 10.1371/journal.pone.0120037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wintermark M, Huss DS, Shah BB, Tustison N, Druzgal TJ, Kassell N, et al. Thalamic connectivity in patients with essential tremor treated with MR imaging-guided focused ultrasound: in vivo fiber tracking by using diffusion-tensor MR imaging. Radiology 2014; 272: 202–9. doi: 10.1148/radiol.14132112 [DOI] [PubMed] [Google Scholar]
- 73.Masciocchi C, Conchiglia A, Gregori LM, Arrigoni F, Zugaro L, Barile A. Critical role of HIFU in musculoskeletal interventions. Radiol Med 2014; 119: 470–5. doi: 10.1007/s11547-014-0414-z [DOI] [PubMed] [Google Scholar]
- 74.Botsa E, Mylona S, Koutsogiannis I, Koundouraki A, Thanos L. CT image guided thermal ablation techniques for palliation of painful bone metastases. Ann Palliat Med 2014; 3: 47–53. doi: 10.3978/j.issn.2224-5820.2014.04.02 [DOI] [PubMed] [Google Scholar]
- 75.Rodrigues DB, Stauffer PR, Vrba D, Hurwitz MD. Focused ultrasound for treatment of bone tumours. Int J Hyperthermia 2015: 1–12. [DOI] [PubMed] [Google Scholar]
- 76.Napoli A, Anzidei M, Marincola BC, Brachetti G, Noce V, Boni F, et al. MR imaging-guided focused ultrasound for treatment of bone metastasis. Radiographics 2013; 33: 1555–68. doi: 10.1148/rg.336125162 [DOI] [PubMed] [Google Scholar]
- 77.Carter JA, Ji X, Botteman MF. Clinical, economic and humanistic burdens of skeletal-related events associated with bone metastases. Expert Rev Pharmacoecon Outcomes Res 2013; 13: 483–96. doi: 10.1586/14737167.2013.820959 [DOI] [PubMed] [Google Scholar]
- 78.Suva LJ, Washam C, Nicholas RW, Griffin RJ. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol 2011; 7: 208–18. doi: 10.1038/nrendo.2010.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hechmati G, Cure S, Gouepo A, Hoefeler H, Lorusso V, Luftner D, et al. Cost of skeletal-related events in European patients with solid tumours and bone metastases: data from a prospective multinational observational study. J Med Econ 2013; 16: 691–700. doi: 10.3111/13696998.2013.779921 [DOI] [PubMed] [Google Scholar]
- 80.Rades D, Schild SE, Abrahm JL. Treatment of painful bone metastases. Nat Rev Clin Oncol 2010; 7: 220–9. doi: 10.1038/nrclinonc.2010.17 [DOI] [PubMed] [Google Scholar]
- 81.Liberman B, Gianfelice D, Inbar Y, Beck A, Rabin T, Shabshin N, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann Surg Oncol 2009; 16: 140–6. doi: 10.1245/s10434-008-0011-2 [DOI] [PubMed] [Google Scholar]
- 82.Catane R, Beck A, Inbar Y, Rabin T, Shabshin N, Hengst S, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases–preliminary clinical experience. Ann Oncol 2007; 18: 163–7. doi: 10.1093/annonc/mdl335 [DOI] [PubMed] [Google Scholar]
- 83.Gianfelice D, Gupta C, Kucharczyk W, Bret P, Havill D, Clemons M. Palliative treatment of painful bone metastases with MR imaging–guided focused ultrasound. Radiology 2008; 249: 355–63. doi: 10.1148/radiol.2491071523 [DOI] [PubMed] [Google Scholar]
- 84.Huisman M, Ter Haar G, Napoli A, Hananel A, Ghanouni P, Lovey G, et al. International consensus on use of focused ultrasound for painful bone metastases: Current status and future directions. Int J Hyperthermia 2015: 31: 251–9. [DOI] [PubMed] [Google Scholar]
- 85.Mavrogenis AF, Angelini A, Vottis C, Pala E, Calabro T, Papagelopoulos PJ, et al. Modern palliative treatments for metastatic bone disease: awareness of merits, demerits and guidance. Clin J Pain 2015. Epub ahead of print. doi: 10.1097/AJP.0000000000000255 [DOI] [PubMed] [Google Scholar]
- 86.Huisman M, Lam MK, Bartels LW, Nijenhuis RJ, Moonen CT, Knuttel FM, et al. Feasibility of volumetric MRI-guided high intensity focused ultrasound (MR-HIFU) for painful bone metastases. J Ther Ultrasound 2014; 2: 16. doi: 10.1186/2050-5736-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joo B, Park MS, Lee SH, Choi HJ, Lim ST, Rha SY, et al. Pain palliation in patients with bone metastases using magnetic resonance-guided focused ultrasound with conformal bone system: a preliminary report. Yonsei Med J 2015; 56: 503–9. doi: 10.3349/ymj.2015.56.2.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, et al. Extracorporeal focused ultrasound surgery for treatment of human solid carcinomas: early Chinese clinical experience. Ultrasound Med Biol 2004; 30: 245–60. doi: 10.1016/j.ultrasmedbio.2003.10.010 [DOI] [PubMed] [Google Scholar]
- 89.Li C, Wu P, Zhang L, Fan W, Huang J, Zhang F. Osteosarcoma: limb salvaging treatment by ultrasonographically guided high-intensity focused ultrasound. Cancer Biol Ther 2009; 8: 1102–8. doi: 10.4161/cbt.8.12.8556 [DOI] [PubMed] [Google Scholar]
- 90.Li C, Zhang W, Fan W, Huang J, Zhang F, Wu P. Noninvasive treatment of malignant bone tumors using high-intensity focused ultrasound. Cancer 2010; 116: 3934–42. doi: 10.1002/cncr.25192 [DOI] [PubMed] [Google Scholar]
- 91.Wang Y, Wang W, Tang J. Primary malignant tumours of the bony pelvis: US-guided high intensity focused ultrasound ablation. Int J Hyperthermia 2013; 29: 683–7. doi: 10.3109/02656736.2013.840806 [DOI] [PubMed] [Google Scholar]
- 92.Chen W, Zhu H, Zhang L, Li K, Su H, Jin C, et al. Primary bone malignancy: effective treatment with high-intensity focused ultrasound ablation. Radiology 2010; 255: 967–78. doi: 10.1148/radiol.10090374 [DOI] [PubMed] [Google Scholar]
- 93.Bielack SS, Marina N, Bernstein M. High-intensity focused ultrasound (HIFU) is not indicated for treatment of primary bone sarcomas. Cancer 2011; 117: 2822; author reply 22–3. doi: 10.1002/cncr.25881 [DOI] [PubMed] [Google Scholar]
- 94.Napoli A, Mastantuono M, Cavallo Marincola B, Anzidei M, Zaccagna F, Moreschini O, et al. Osteoid osteoma: MR-guided focused ultrasound for entirely noninvasive treatment. Radiology 2013; 267: 514–21. doi: 10.1148/radiol.13120873 [DOI] [PubMed] [Google Scholar]
- 95.Geiger D, Napoli A, Conchiglia A, Gregori LM, Arrigoni F, Bazzocchi A, et al. MR-guided focused ultrasound (MRgFUS) ablation for the treatment of nonspinal osteoid osteoma: a prospective multicenter evaluation. J Bone Joint Surg Am 2014; 96: 743–51. doi: 10.2106/JBJS.M.00903 [DOI] [PubMed] [Google Scholar]
- 96.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage 2013; 21: 1145–53. doi: 10.1016/j.joca.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.National Clinical Guideline Centre (UK). Osteoarthritis: care and management in adults. London, UK: National Institute for Health and Care Excellence; 2014. [PubMed] [Google Scholar]
- 98.Hunter DJ, Guermazi A, Roemer F, Zhang Y, Neogi T. Structural correlates of pain in joints with osteoarthritis. Osteoarthritis Cartilage 2013; 21: 1170–8. doi: 10.1016/j.joca.2013.05.017 [DOI] [PubMed] [Google Scholar]
- 99.Weeks EM, Platt MW, Gedroyc W. MRI-guided focused ultrasound (MRgFUS) to treat facet joint osteoarthritis low back pain–case series of an innovative new technique. Eur Radiol 2012; 22: 2822–35. doi: 10.1007/s00330-012-2628-6 [DOI] [PubMed] [Google Scholar]
- 100.Izumi M, Ikeuchi M, Kawasaki M, Ushida T, Morio K, Namba H, et al. MR-guided focused ultrasound for the novel and innovative management of osteoarthritic knee pain. BMC Musculoskelet Disord 2013; 14: 267. doi: 10.1186/1471-2474-14-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Palazzo C, Ravaud JF, Papelard A, Ravaud P, Poiraudeau S. The burden of musculoskeletal conditions. PLoS One 2014; 9: e90633. doi: 10.1371/journal.pone.0090633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harnof S, Zibly Z, Shay L, Dogadkin O, Hanannel A, Inbar Y, et al. Magnetic resonance-guided focused ultrasound treatment of facet joint pain: summary of preclinical phase. J Ther Ultrasound 2014; 2: 9. doi: 10.1186/2050-5736-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y, Wang W, Tang J. Ultrasound-guided high intensity focused ultrasound treatment for extra-abdominal desmoid tumours: preliminary results. Int J Hyperthermia 2011; 27: 648–53. doi: 10.3109/02656736.2011.597047 [DOI] [PubMed] [Google Scholar]
- 104.Orgera G, Monfardini L, Della Vigna P, Zhang L, Bonomo G, Arnone P, et al. High-intensity focused ultrasound (HIFU) in patients with solid malignancies: evaluation of feasibility, local tumour response and clinical results. Radiol Med 2011; 116: 734–48. doi: 10.1007/s11547-011-0634-4 [DOI] [PubMed] [Google Scholar]
- 105.Hu X, Cai H, Zhou M, He H, Tian W, Hu Y, et al. New clinical application of high-intensity focused ultrasound: local control of synovial sarcoma. World J Surg Oncol 2013; 11: 265. doi: 10.1186/1477-7819-11-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fu SZ, Wang B, Huang HP, Huang LL. Clinical study on hemangiomas treatment with high-intensity focused ultrasound (60 cases). [In Chinese.] Zhonghua Zheng Xing Wai Ke Za Zhi 2012; 28: 252–5. [PubMed] [Google Scholar]
- 107.Hoogenboom M, Eikelenboom D, den Brok MH, Heerschap A, Futterer JJ, Adema GJ. Mechanical high-intensity focused ultrasound destruction of soft tissue: working mechanisms and physiologic effects. Ultrasound Med Biol 2015; 41: 1500–17. doi: 10.1016/j.ultrasmedbio.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 108.Khokhlova TD, Canney MS, Khokhlova VA, Sapozhnikov OA, Crum LA, Bailey MR. Controlled tissue emulsification produced by high intensity focused ultrasound shock waves and millisecond boiling. J Acoust Soc Am 2011; 130: 3498–510. doi: 10.1121/1.3626152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kopelman D, Inbar Y, Hanannel A, Pfeffer RM, Dogadkin O, Freundlich D, et al. Magnetic resonance guided focused ultrasound surgery. Ablation of soft tissue at bone-muscle interface in a porcine model. Eur J Clin Invest 2008; 38: 268–75. doi: 10.1111/j.1365-2362.2008.01931.x [DOI] [PubMed] [Google Scholar]
- 110.Hipp E, Partanen A, Karczmar GS, Fan X. Safety limitations of MR-HIFU treatment near interfaces: a phantom validation. J Appl Clin Med Phys 2012; 13: 3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lele PP, Parker KJ. Temperature distributions in tissues during local hyperthermia by stationary or steered beams of unfocused or focused ultrasound. Br J Cancer Suppl 1982; 5: 108–21. [PMC free article] [PubMed] [Google Scholar]
- 112.Hynynen K, DeYoung D. Temperature elevation at muscle-bone interface during scanned, focused ultrasound hyperthermia. Int J Hyperthermia 1988; 4: 267–79. doi: 10.3109/02656738809051103 [DOI] [PubMed] [Google Scholar]
- 113.O'Neill TP, Winkler AJ, Wu J. Ultrasound heating in a tissue-bone phantom. Ultrasound Med Biol 1994; 20: 579–88. doi: 10.1016/0301-5629(94)90094-9 [DOI] [PubMed] [Google Scholar]
- 114.Myers MR. Transient temperature rise due to ultrasound absorption at a bone/soft-tissue interface. J Acoust Soc Am 2004; 115: 2887–91. doi: 10.1121/1.1707091 [DOI] [PubMed] [Google Scholar]
- 115.Bucknor MD, Rieke V, Do L, Majumdar S, Link TM, Saeed M. MRI-guided high-intensity focused ultrasound ablation of bone: evaluation of acute findings with MR and CT imaging in a swine model. J Magn Reson Imaging 2014; 40: 1174–80. doi: 10.1002/jmri.24451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bucknor MD, Rieke V, Seo Y, Horvai AE, Hawkins RA, Majumdar S, et al. Bone remodeling after MR imaging-guided high-intensity focused ultrasound ablation: evaluation with MR imaging, CT, Na(18)F-PET, and histopathologic examination in a swine model. Radiology 2015; 274: 387–94. doi: 10.1148/radiol.14132605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Herman A, Avivi E, Brosh T, Schwartz I, Liberman B. Biomechanical properties of bone treated by magnetic resonance-guided focused ultrasound—an in vivo porcine model study. Bone 2013; 57: 92–7. doi: 10.1016/j.bone.2013.06.032 [DOI] [PubMed] [Google Scholar]
- 118.Smith NB, Temkin JM, Shapiro F, Hynynen K. Thermal effects of focused ultrasound energy on bone tissue. Ultrasound Med Biol 2001; 27: 1427–33. doi: 10.1016/S0301-5629(01)00454-9 [DOI] [PubMed] [Google Scholar]
- 119.Zhang S, Li C, Yin H, Wang S, Wan M. Surface vibration and nearby cavitation of an ex vivo bovine femur exposed to high intensity focused ultrasound. J Acoust Soc Am 2013; 134: 1656–62. doi: 10.1121/1.4812891 [DOI] [PubMed] [Google Scholar]
- 120.Hundt W, Yuh EL, Steinbach S, Bednarski MD, Guccione S. Comparison of continuous vs. pulsed focused ultrasound in treated muscle tissue as evaluated by magnetic resonance imaging, histological analysis, and microarray analysis. Eur Radiol 2008; 18: 993–1004. doi: 10.1007/s00330-007-0848-y [DOI] [PubMed] [Google Scholar]
- 121.Solomon SB, Nicol TL, Chan DY, Fjield T, Fried N, Kavoussi LR. Histologic evolution of high-intensity focused ultrasound in rabbit muscle. Invest Radiol 2003; 38: 293–301. [DOI] [PubMed] [Google Scholar]
- 122.Hundt W, Yuh EL, Steinbach S, Bednarski MD, Guccione S. Mechanic effect of pulsed focused ultrasound in tumor and muscle tissue evaluated by MRI, histology, and microarray analysis. Eur J Radiol 2010; 76: 279–87. doi: 10.1016/j.ejrad.2009.05.044 [DOI] [PubMed] [Google Scholar]
- 123.Zhang J, Mougenot C, Partanen A, Muthupillai R, Hor PH. Volumetric MRI-guided high-intensity focused ultrasound for noninvasive, in vivo determination of tissue thermal conductivity: initial experience in a pig model. J Magn Reson Imaging 2013; 37: 950–7. doi: 10.1002/jmri.23878 [DOI] [PubMed] [Google Scholar]
- 124.Vasquez B, Navarrete J, Farfan E, Cantin M. Effect of pulsed and continuous therapeutic ultrasound on healthy skeletal muscle in rats. Int J Clin Exp Pathol 2014; 7: 779–83. [PMC free article] [PubMed] [Google Scholar]
- 125.Muratore R, Akabas T, Muratore IB. High-intensity focused ultrasound ablation of ex vivo bovine achilles tendon. Ultrasound Med Biol 2008; 34: 2043–50. doi: 10.1016/j.ultrasmedbio.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 126.Foldes K, Hynynen K, Shortkroff S, Winalski CS, Collucci V, Koskinen SK, et al. Magnetic resonance imaging-guided focused ultrasound synovectomy. Scand J Rheumatol 1999; 28: 233–7. [DOI] [PubMed] [Google Scholar]
- 127.O'Daly BJ, Morris E, Gavin GP, O'Keane C, O'Byrne JM, McGuinness GB. High power, low frequency ultrasound: meniscal tissue interaction and ablation characteristics. Ultrasound Med Biol 2011; 37: 556–67. [DOI] [PubMed] [Google Scholar]
- 128.Forslund C, Persson J, Stromqvist B, Lidgren L, McCarthy ID. Effects of high-intensity focused ultrasound on the intervertebral disc: a potential therapy for disc herniations. J Clin Ultrasound 2006; 34: 330–8. doi: 10.1002/jcu.20242 [DOI] [PubMed] [Google Scholar]
- 129.Persson J, Stromqvist B, Zanoli G, McCarthy I, Lidgren L. Ultrasound nucleolysis: an in vitro study. Ultrasound Med Biol 2002; 28: 1189–97. doi: 10.1016/S0301-5629(02)00566-5 [DOI] [PubMed] [Google Scholar]
- 130.Jung YJ, Kim R, Ham HJ, Park SI, Lee MY, Kim J, et al. Focused low-intensity pulsed ultrasound enhances bone regeneration in rat calvarial bone defect through enhancement of cell proliferation. Ultrasound Med Biol 2015; 41: 999–1007. doi: 10.1016/j.ultrasmedbio.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 131.Poliachik SL, Khokhlova TD, Wang YN, Simon JC, Bailey MR. Pulsed focused ultrasound treatment of muscle mitigates paralysis-induced bone loss in the adjacent bone: a study in a mouse model. Ultrasound Med Biol 2014; 40: 2113–24. doi: 10.1016/j.ultrasmedbio.2014.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kyriakou Z, Corral-Baques MI, Amat A, Coussios CC. HIFU-induced cavitation and heating in ex vivo porcine subcutaneous fat. Ultrasound Med Biol 2011; 37: 568–79. doi: 10.1016/j.ultrasmedbio.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 133.Young RR, Henneman E. Functional effects of focused ultrasound on mammalian nerves. Science 1961; 134: 1521–2. doi: 10.1126/science.134.3489.1521 [DOI] [PubMed] [Google Scholar]
- 134.Ballantine HT, Jr, Hueter TF, Nauta WJ, Sosa DM. Focal destruction of nervous tissue by focused ultrasound: biophysical factors influencing its application. J Exp Med 1956; 104: 337–60. doi: 10.1084/jem.104.3.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mihran RT, Barnes FS, Wachtel H. Transient modification of nerve excitability in vitro by single ultrasound pulses. Biomed Sci Instrum 1990; 26: 235–46. [PubMed] [Google Scholar]
- 136.Colucci V, Strichartz G, Jolesz F, Vykhodtseva N, Hynynen K. Focused ultrasound effects on nerve action potential in vitro. Ultrasound Med Biol 2009; 35: 1737–47. doi: 10.1016/j.ultrasmedbio.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Foley JL, Little JW, Vaezy S. Effects of high-intensity focused ultrasound on nerve conduction. Muscle Nerve 2008; 37: 241–50. doi: 10.1002/mus.20932 [DOI] [PubMed] [Google Scholar]
- 138.Lee YF, Lin CC, Cheng JS, Chen GS. High-intensity focused ultrasound attenuates neural responses of sciatic nerves isolated from normal or neuropathic rats. Ultrasound Med Biol 2015; 41: 132–42. doi: 10.1016/j.ultrasmedbio.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 139.Juan EJ, Gonzalez R, Albors G, Ward MP, Irazoqui P. Vagus nerve modulation using focused pulsed ultrasound: potential applications and preliminary observations in a rat. Int J Imaging Syst Technol 2014; 24: 67–71. doi: 10.1002/ima.22080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gulati A, Loh J, Gutta NB, Ezell PC, Monette S, Erinjeri JP, et al. Novel use of noninvasive high-intensity focused ultrasonography for intercostal nerve neurolysis in a swine model. Reg Anesth Pain Med 2014; 39: 26–30. doi: 10.1097/AAP.0000000000000028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ishikawa T, Okai T, Sasaki K, Umemura S, Fujiwara R, Kushima M, et al. Functional and histological changes in rat femoral arteries by HIFU exposure. Ultrasound Med Biol 2003; 29: 1471–7. doi: 10.1016/S0301-5629(03)00951-7 [DOI] [PubMed] [Google Scholar]
- 142.Li T, Hao Q, Wang X, Liu Q. The effect of focused ultrasound on the physicochemical properties of Sarcoma 180 cell membrane. [in Chinese.] Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2009; 26: 941–6. [PubMed] [Google Scholar]
- 143.Chida S, Okada K, Suzuki N, Komori C, Shimada Y. Infiltration by macrophages and lymphocytes in transplantable mouse sarcoma after irradiation with high-intensity focused ultrasound. Anticancer Res 2009; 29: 3877–82. [PubMed] [Google Scholar]
- 144.Weber-Adrian D, Thevenot E, O'Reilly MA, Oakden W, Akens MK, Ellens N, et al. Gene delivery to the spinal cord using MRI-guided focused ultrasound. Gene Ther 2015; 22: 568–77. doi: 10.1038/gt.2015.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hijnen N, Langereis S, Grull H. Magnetic resonance guided high-intensity focused ultrasound for image-guided temperature-induced drug delivery. Adv Drug Deliv Rev 2014; 72: 65–81. doi: 10.1016/j.addr.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 146.Ranjan A, Jacobs GC, Woods DL, Negussie AH, Partanen A, Yarmolenko PS, et al. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J Control Release 2012; 158: 487–94. doi: 10.1016/j.jconrel.2011.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Okita K, Sugiyama K, Takagi S, Matsumto Y. Microbubble behavior in an ultrasound field for high intensity focused ultrasound therapy enhancement. J Acoust Soc Am 2013; 134: 1576–85. doi: 10.1121/1.4812880 [DOI] [PubMed] [Google Scholar]
- 148.Lorenzato C, Cernicanu A, Meyre ME, Germain M, Pottier A, Levy L, et al. MRI contrast variation of thermosensitive magnetoliposomes triggered by focused ultrasound: a tool for image-guided local drug delivery. Contrast Media Mol Imaging 2013; 8: 185–92. doi: 10.1002/cmmi.1515 [DOI] [PubMed] [Google Scholar]
- 149.Choi SY, Kim YS, Seo YJ, Yang J, Choi KS. Gas-filled phospholipid nanoparticles conjugated with gadolinium play a role as a potential theragnostics for MR-guided HIFU ablation. PLoS One 2012; 7: e34333. doi: 10.1371/journal.pone.0034333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Park J, Zhang Y, Vykhodtseva N, Jolesz FA, McDannold NJ. The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound. J Control Release 2012; 162: 134–42. doi: 10.1016/j.jconrel.2012.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.de Smet M, Hijnen NM, Langereis S, Elevelt A, Heijman E, Dubois L, et al. Magnetic resonance guided high-intensity focused ultrasound mediated hyperthermia improves the intratumoral distribution of temperature-sensitive liposomal doxorubicin. Invest Radiol 2013; 48: 395–405. doi: 10.1097/RLI.0b013e3182806940 [DOI] [PubMed] [Google Scholar]
- 152.Carlisle R, Choi J, Bazan-Peregrino M, Laga R, Subr V, Kostka L, et al. Enhanced tumor uptake and penetration of virotherapy using polymer stealthing and focused ultrasound. J Natl Cancer Inst 2013; 105: 1701–10. doi: 10.1093/jnci/djt305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.O'Neill BE, Karmonik C, Sassaroli E, Li KC. Estimation of thermal dose from MR thermometry during application of nonablative pulsed high intensity focused ultrasound. J Magn Reson Imaging 2012; 35: 1169–78. doi: 10.1002/jmri.23526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Staruch R, Chopra R, Hynynen K. Hyperthermia in bone generated with MR imaging-controlled focused ultrasound: control strategies and drug delivery. Radiology 2012; 263: 117–27. doi: 10.1148/radiol.11111189 [DOI] [PubMed] [Google Scholar]
- 155.Tang W, Liu Q, Wang X, Wang P, Zhang J, Cao B. Potential mechanism in sonodynamic therapy and focused ultrasound induced apoptosis in sarcoma 180 cells in vitro. Ultrasonics 2009; 49: 786–93. doi: 10.1016/j.ultras.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 156.O'Neill BE, Vo H, Angstadt M, Li KP, Quinn T, Frenkel V. Pulsed high intensity focused ultrasound mediated nanoparticle delivery: mechanisms and efficacy in murine muscle. Ultrasound Med Biol 2009; 35: 416–24. doi: 10.1016/j.ultrasmedbio.2008.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 2003; 14: S199–202. doi: 10.1097/01.RVI.0000094584.83406.3e [DOI] [PubMed] [Google Scholar]
- 158.Yu T, Luo J. Adverse events of extracorporeal ultrasound-guided high intensity focused ultrasound therapy. PLoS One 2011; 6: e26110. doi: 10.1371/journal.pone.0026110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Grassi DG, Gavier B, Trucco J, Bana MT. Acute pancreatitis after high-intensity focused ultrasonography for body sculpting. Ann Intern Med 2014; 160: 71. doi: 10.7326/L14-5000-3 [DOI] [PubMed] [Google Scholar]
- 160.Hijnen NM, Elevelt A, Grull H. Stability and trapping of magnetic resonance imaging contrast agents during high-intensity focused ultrasound ablation therapy. Invest Radiol 2013; 48: 517–24. doi: 10.1097/RLI.0b013e31829aae98 [DOI] [PubMed] [Google Scholar]