Abstract
Ultrasound-guided needle biopsy of synovium is an increasingly performed procedure with a high diagnostic yield. In this review, we discuss the normal synovium, as well as the indications, technique, tissue handling and clinical applications of ultrasound-guided synovial biopsy.
INTRODUCTION
The synovium or synovial membrane is the soft tissue that lines the inner surface of freely moving joints, tendon sheaths and bursae. The normal synovium contains both an intima (20- to 40-μm thick) and a subintima. The intima is well innervated and richly vascularized with blood vessels and lymphatics. Under electronic microscopy, the intima comprises both Type A synoviocytes (which are macrophage-type cells) and Type B synoviocytes (which are fibroblast-type cells). Type A synoviocytes remove debris from the synovial fluid, while Type B synoviocytes produce specialized matrix components such as hyaluronan (hyaluronic acid), collagens and fibronectin, which diffuse out first into the intimal interstitial fluid and then into the synovial fluid. Synovial fluid is formed from fluid leaking through intimal capillaries. The products of the Type B synoviocytes, such as hyaluronic acid, help to make the synovial fluid more viscous and hence “trap” it within the joint. Synovial fluid is normally clear to yellow in colour and slightly less viscous than egg yolk so much so that the word synovium is probably derived from a combination of the Greek word “syn” (“with”) and the Latin word “ovum” (“egg”). Synovial fluid provides a non-adherent lubricant to facilitate joint movement and also provides nutrition for articular cartilage chondrocytes. The synovial membrane is pink, smooth and glistening. Synovial folds project into the joint, especially in those areas where some joint redundancy exists such as the suprapatellar or medial/lateral patellar recesses. Such synovial folds may be villous, lobulated or nodular in appearance.
Deep to the intima is the relatively acellular subintima (5-μm thick).1 This subintima, in contrast to normal serosa, lacks a basal membrane. The subintima is composed of either fatty, fibrofatty, fibrous or loose areolar tissue, while, less commonly, it may contain periosteal or fibrocartilaginous tissue.1 While the normal synovium has distinct intimal and subintimal layers, inflamed synovial tissue may lose this intimal/subintimal demarcation. In conditions such as rheumatoid arthritis, the intimal layer becomes markedly thickened with an increase in Type A synoviocytes (which are macrophagic, especially CD68-positive macrophages) over Type B synoviocytes (which are fibroblastic). At the same time, the subintima becomes hypervascularized and oedematous because of capillary engorgement and lymphatic congestion and heavily infiltrated with T and B lymphocytes, plasma cells and macrophages with a concurrent increase in cytokine, interleukin and tumour necrosis factor α production as well as increased osteoclastic activity. In chronic inflammatory conditions, the intima and subintima may partially or wholly undergo atrophy and this is reflected in some synovial biopsies which fail to reveal any synovial tissue.
As the synovium is the most biologically active tissue within synovial joints, it is the tissue of choice to target if one wishes to understand more about the underlying joint pathology. Biopsy of the synovium can be performed either as an open surgical procedure, via arthroscopy, via fluoroscopy or using an ultrasound-guided procedure.2,3 Ultrasound-guided synovial biopsy is an increasingly performed procedure with a high diagnostic yield.4–6 It offers many advantages over arthroscopically guided or fluoroscopically guided synovial biopsy.
Five reports of ultrasound-guided synovial biopsy have been published.7–11 Van Vugt et al7 provided the first description of ultrasound-guided synovial biopsy in 1997 reporting the use of an 18-G tru-cut needle and automated gun to biopsy the synovium in two patients with wrist arthritis under real-time ultrasound guidance. In 2004, Koski and Helle8 described a portal and forceps technique to biopsy synovial tissue in 37 patients with arthritis. This technique involved positioning a guidewire within the joint cavity under ultrasound guidance, dilatation of the tract to 6 Fr and then using a flexible or rigid biopsy forceps to obtain 5–8 synovial biopsies from each joint.8 Even the small joints of the hand were amenable to portal and forceps biopsy.8 Scire et al9 used a similar technique to obtain synovial tissue for histological grading and immunohistology in nine patients with rheumatoid arthritis. Kelly et al10 performed real-time ultrasound-guided tru-cut synovial biopsies (of the wrist, knee, small joints of the hand and elbow) on 57 patients with rheumatoid arthritis with the same aim. Marin et al11 reported (in French) another cohort of 83 synovial biopsies with biopsy being performed largely to confirm tumour or exclude infection. All, bar one, of these previous reports focused on the use of synovial biopsy to study inflammation in patients with rheumatoid arthritis. The one article by Marin et al11 that addressed ultrasound-guided synovial biopsy in more general terms was written primarily in French. This present review provides a comprehensive review of the background, indications, technique and limitations of synovial biopsy as a guide for the musculoskeletal and general radiologists. Whatever method of synovial biopsy is used, the aim is to obtain sufficient synovial tissue for analysis, using the least invasive method possible. The diagnostic yield and complication rate of the forceps and tru-cut needle methods seem comparable7–11 though clearly the forceps method is more cumbersome and probably time consuming as it requires the use of a guidewire, tract dilatation and forceps. As the forceps technique seems to confer no real advantage over the tru-cut method for routine clinical use, the latter is likely to become the method of choice for synovial biopsy.
INDICATIONS FOR SYNOVIAL BIOPSY
Synovial biopsy is undertaken to determine the cause and severity of synovial thickening or proliferation. There is no rationale to perform synovial biopsy in patients in whom the synovium is not thickened. Patients are most commonly referred for investigation of joint swelling or pain, and synovial thickening is encountered on either ultrasound or a prior imaging study such as MRI or CT. While ultrasound can readily depict synovial thickening, it does not have a high accuracy at determining the cause of the synovial thickening since considerable overlap can exist in the ultrasound appearances of synovial tumour, infection and inflammation.12 The superior tissue characterization of MRI, supplemented if necessary by radiographs or CT, usually enables synovial tumour to be reliably distinguished from infection or inflammation and often enables recognition of specific synovial tumour type.13,14 MR, however, cannot always reliably distinguish between infection and inflammation.13,15–17 Synovial biopsy is therefore generally undertaken to determine the cause of diffuse or localized synovial thickening of which there are three main causes. These are (i) synovial tumour, (ii) synovial infection and (iii) synovial inflammation.
The main synovial tumours are pigmented villonodular synovitis, focal nodular synovitis or synovial (osteo)chondromatosis. Less common synovial tumours are synovial haemangioma, diffuse articular lipomatosis (lipoma arborescens), synovial chondroma or fibroma (intracapsular and periarticular), synovial chondrosarcoma, synovial sarcoma, or lymphoma.18,19
Practically, any micro-organism can lead to joint infection. The main causative micro-organisms of joint infection in native joints are Staphylococcus aureus (which causes most acute bacterial joint infection in adults and children more than 2 years old including 80% of infections in patients with rheumatoid arthritis), Neisseria gonorrhoeae (which is a common source of joint infection in sexually active young adults), Streptococcal series, such as Streptoccocus viridans, Streptoccocus pneumonia and Group B Streptococci, as well as aerobic Gram-negative rods. Infections with Pseudomonas aeruginosa or Serratia species are usually related to intravenous drug abuse, while Aeromonas infections tend to be seen in patients with leukaemia.
In prosthetic joints, staphylococci remain the most common micro-organism, with S. aureus and coagulase-negative staphylococci each accounting for up to 25% of all prosthetic joint infections, followed by Gram negative micro-organisms (10%). A minority of prosthetic joint infections are caused by enterococci, streptococci and fungi. Of note, Proprionibacterium acnes has a particular predilection for prosthetic shoulder joint infection (up to 40% of cases).20
Usually, joint infection manifests as a joint effusion with mild to moderate synovial proliferation. In patients with significant joint fluid, joint aspiration alone is usually sufficient to make a diagnosis of joint infection. Synovial biopsy is performed therefore in that subset of patients with suspected joint infection though with little or no synovial fluid visible on ultrasound. Clearly, the aetiology, spectrum and progress of joint infection in these patients may not be the same as those patients with a more typical clinical manifestation of joint infection.
In patients with known rheumatoid arthritis and other systemic inflammatory arthropathies, colour or power Doppler ultrasound supplemented if necessary by contrast-enhanced ultrasound should suffice to determine the degree of synovial inflammation.21,22 Synovial biopsy in this group of patients is only performed currently in an experimental setting to either grade the degree of synovial inflammation histologically, quantify the number of synovial sublining macrophages (CDC8+) or undertake immunohistology, RNA extraction and gene expression profiling. In addition, synovial biopsy may occasionally be indicated to exclude superimposed infection or confirm the presence and type of crystal deposition.23 It should be noted that synovial biopsy cannot be used to diagnose a specific type of arthropathy such as rheumatoid arthritis or to distinguish between different types of arthropathy such as between rheumatoid or psoriatic arthropathy. Experience with synovial biopsy in the assessment of crystal arthropathy is, as yet, very limited.
TECHNIQUE
Preparation
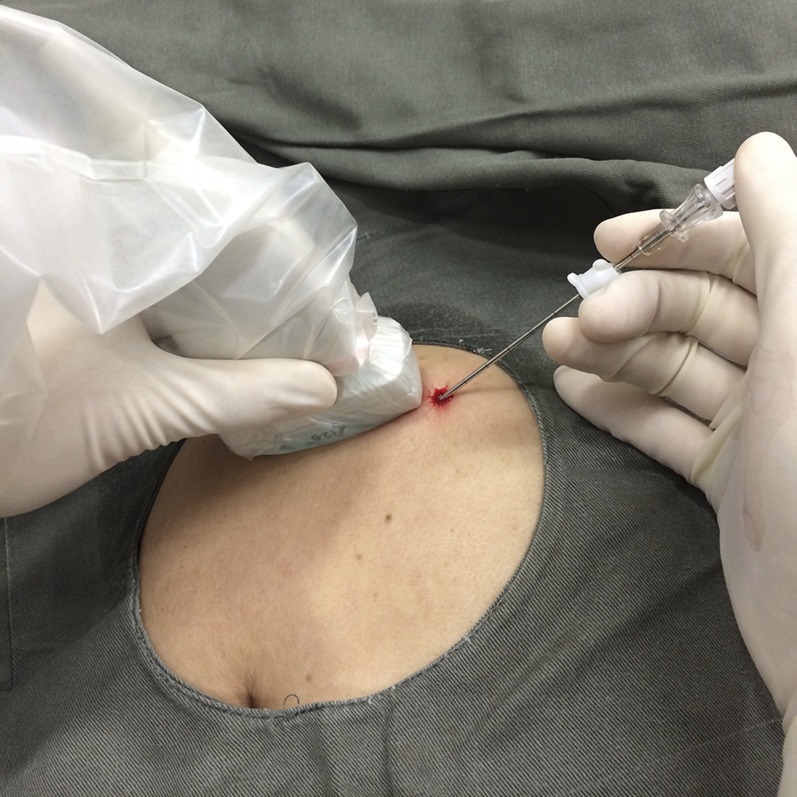
Tru-cut needle synovial biopsies are performed as an outpatient procedure under local anaesthesia. All standard pre-biopsy protocols are followed with regard to obtaining patient consent, and particularly in the hip joint, where surface tamponade cannot be easily applied, and ensuring a normal clotting profile. Before biopsy, the area of maximum synovial thickening is identified by ultrasound as this is usually the area chosen for biopsy. A 12- to 17-MHz linear array transducer is used for most joints except for the hip where a 9- to 5-MHz transducer is used. The skin of the selected entry site is then cleaned with antiseptic and dressed with a sterile surgical set (Figures 1–4). A sterile plastic cover is used for the ultrasound probe and sterile gel is applied for scanning. 1 ml of local anaesthetic (1% lignocaine) is injected initially to the skin. Then, under ultrasound guidance, about 2–5 ml is injected along the outer margin of the joint capsule. A 21-G intravenous needle is used for most joints, while a 20-G spinal needle is used for the hip joint. Ideally, no anaesthetic should be injected deep to the joint capsule or into the joint space if one is looking for infection in view of the potential bacteriocidal effect of lignocaine.24
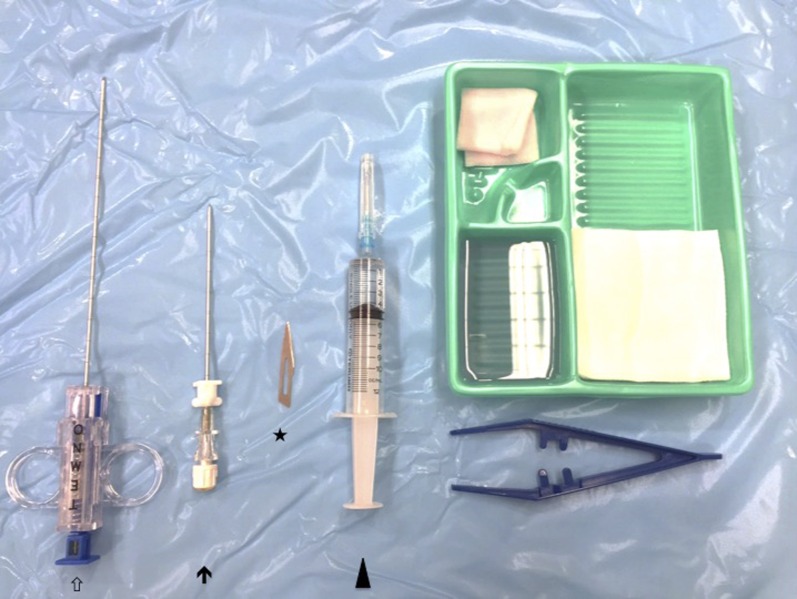
Figure 1.
Setup for ultrasound-guided synovial biopsy. Key components include a small (22 G) needle for local anaesthetic injection (arrow head), scalpel for skin incision (asterisk), coaxial needle (arrow) and tru-cut (e.g. Temno) biopsy needle (hollow arrow).
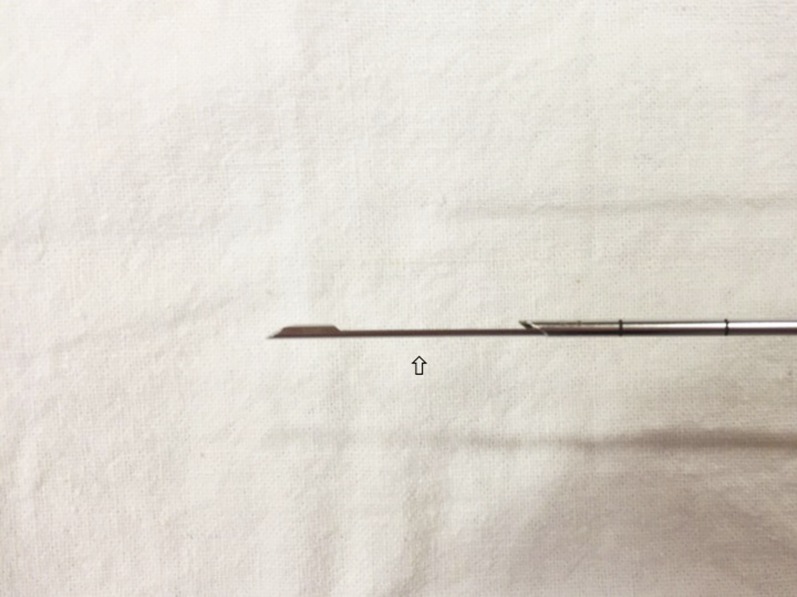
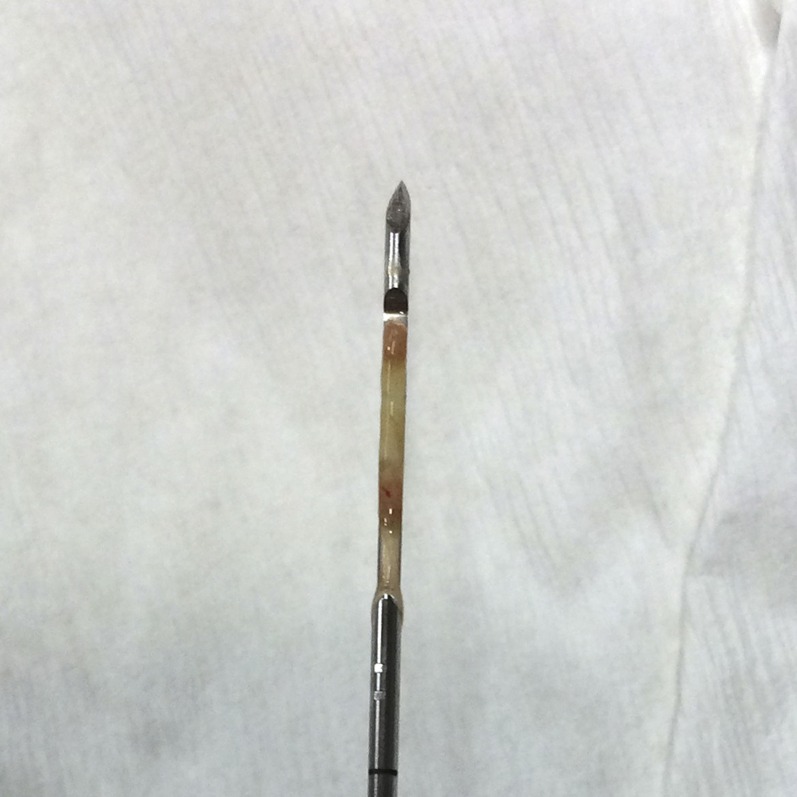
Figure 4.
Close up of the Temno needle when opened. The empty specimen slot (hollow arrow) is clearly seen.
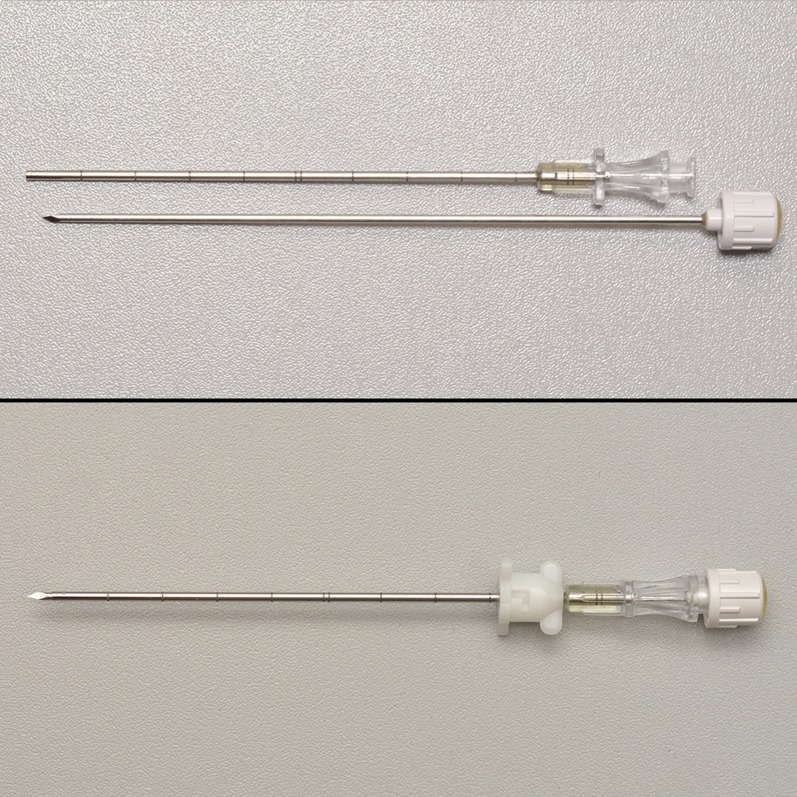
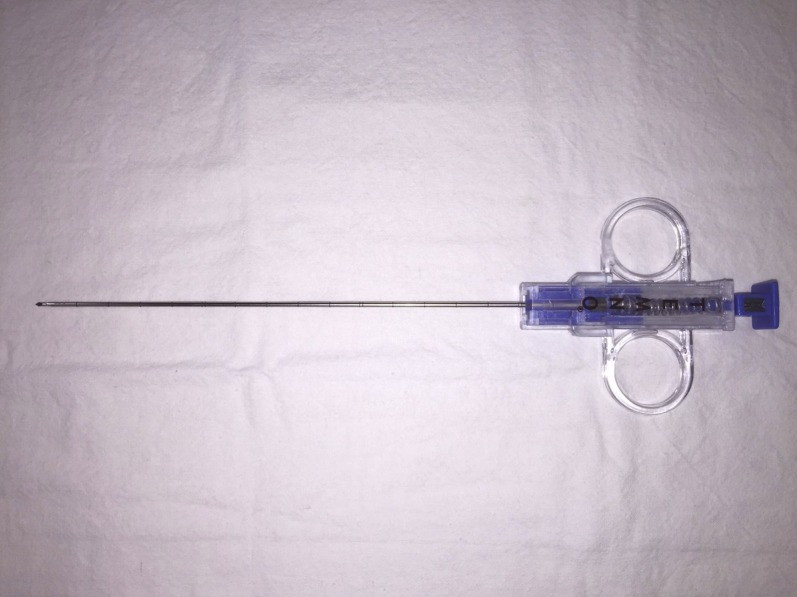
Figure 2.
Close up of coaxial needle which allows repeated biopsies via a single skin entry.
Figure 3.
A Temno needle can be used to perform tru-cut synovial biopsy.
Tru-cut biopsy
The technique of ultrasound-guided synovial tru-cut biopsy is very similar to that used for ultrasound-guided biopsy of extra-articular soft-tissue tumours albeit with a few specific modifications.4–6,25 For large- and medium-sized joints, a standard coaxial needle is used in conjunction with a standard tru-cut needle to facilitate obtaining multiple biopsy cores via a single skin entry (Figure 5). The coaxial needle size and ultrasound-guided needle path should be selected by the operator, after considering the ease of approach, the thickness of the synovium and the potential for neurovascular or other injury. Usually, a 15-G coaxial needle with a 16-G tru-cut needle would be suitable (Figure 6). For smaller joints such as the metacarpophalangeal joints or interphalangeal joints, a 16-G tru-cut needle may be used without a coaxial sheath.11 This is because it is difficult in superficial joints to gain purchase for a coaxial needle as the distance from skin to joint is so short. Alternatively, an assistant can ensure that the coaxial needle is maintained in the correct position just under the skin.
Figure 5.
Insertion of coaxial needle into the shoulder joint under ultrasound guidance. Following injection of local anaesthetic to the skin and pericapsular region (the latter under ultrasound guidance), a small stab-like skin incision is made with the scalpel prior to insertion of coaxial needle. A strict asepsis technique is used at all times.
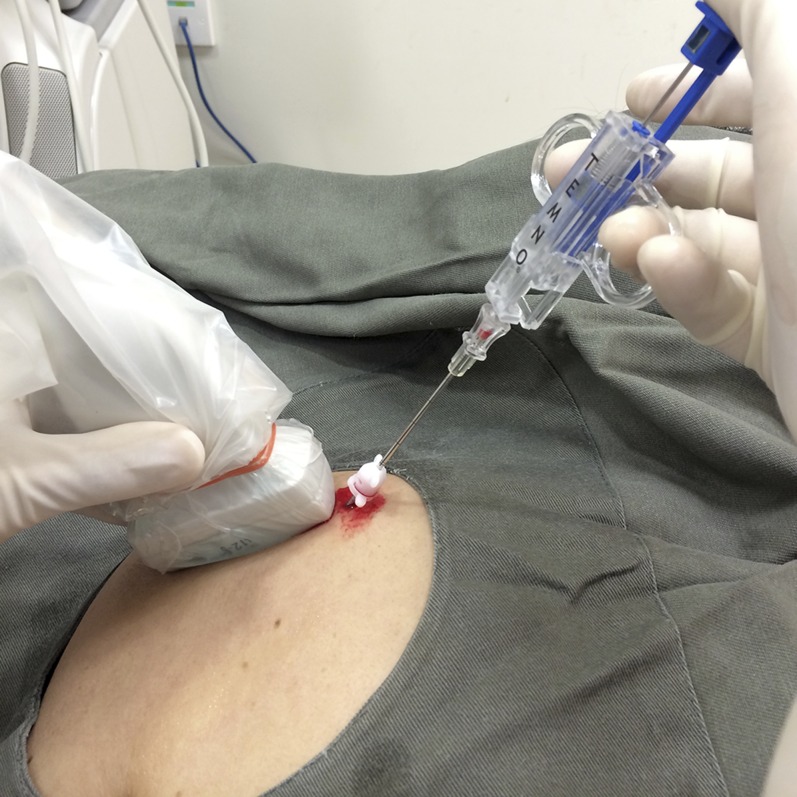
Figure 6.
Insertion of the tru-cut (e.g. Temno) needle into the coaxial needle (with inner trocar removed). Several tissue cores can then be obtained by reinserting the tru-cut needle into the joint via the coaxial needle. The coaxial needle is realigned, under ultrasound guidance, after each biopsy to allow subsequent biopsies to be obtained from different parts of the joint.
Generally, the synovial biopsy should be undertaken in that part of the joint where the synovium is thickest, provided safe percutaneous access route is possible. The coaxial needle tip is positioned, using real-time ultrasound guidance, just deep to the joint capsule and angled so that the biopsy needle path is along the line of the synovium about midway between the joint capsule and the joint cavity. Positioning the coaxial needle tip just deep to the capsule facilitates its redirection to biopsy different parts of the joints compared with a needle tip position embedded deep in the synovium. One should aim to align the biopsy needle along the synovium, preferably at regions with most frond-like proliferations and folds, to obtain good cores of tissue containing both the synovial intima and subintima, which is crucial for differentiating different types of synovial pathology. Repeated needle penetration into the joint cavity should be minimized, wherever possible, to help reduce the likelihood of haemarthrosis.
Number of specimen cores
Usually, at least three synovial biopsy cores are obtained which are sent for histology ± microbiology. The number of tissue cores obtained varies from centre to centre though clearly represents a balance between intended diagnostic yield, patient discomfort and time efficiency. One should aim to obtain the minimum number of cores that will provide sufficient synovial tissue to answer the question being addressed. Intuitively, it is better to obtain a few cores from different areas of the synovium than multiple cores from the same area. Marin et al11 obtained three cores per joint on average, while Kelly et al10 routinely obtained 12 tru-cut cores per joint. A larger number of synovial biopsies will be needed if immunohistological/RNA analysis or crystal analysis of the synovium is required. A smaller number of cores (3–6) is usually sufficient when synovial tumour is suspected.25 Should septic arthritis be suspected, any joint effusion if present should be first aspirated with the coaxial needle before the biopsy (Figures 7–11). Also, more tissue cores (such as 6–10 cores) may increase yield as more synovial tissue is available for culture. This is particularly so when biopsying synovium around prosthetic joints as there tends to be a high false-negative yield in this setting [JCM Sitt, 2015, personal communication].
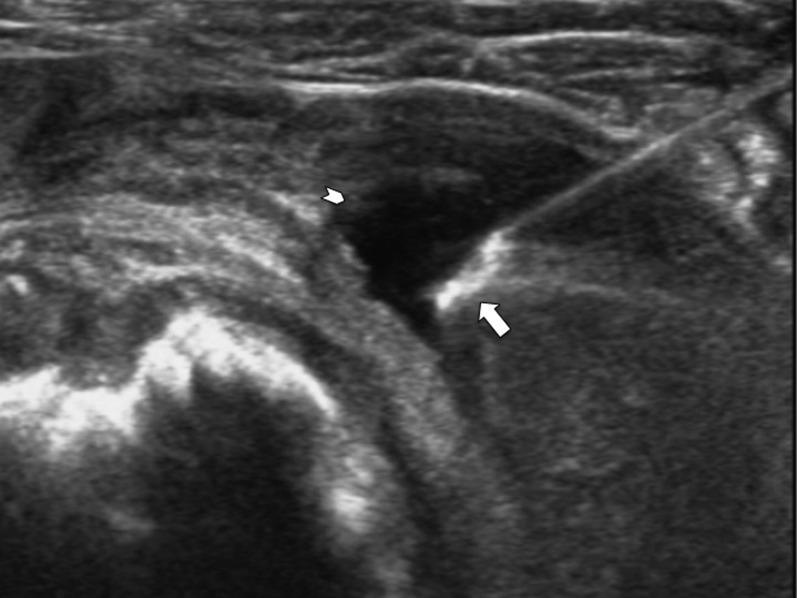
Figure 7.
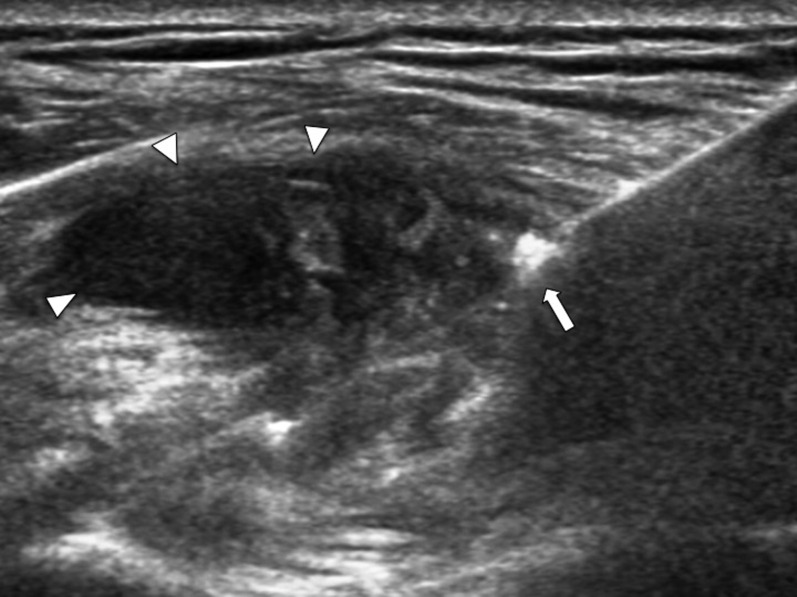
Transverse ultrasound of the shoulder joint showing a coaxial needle with its tip (arrow) within the joint effusion (arrowhead). The effusion was aspirated and sent for microbiology before tru-cut synovial biopsy. Culture of this specimen yielded tuberculosis.
Figure 11.
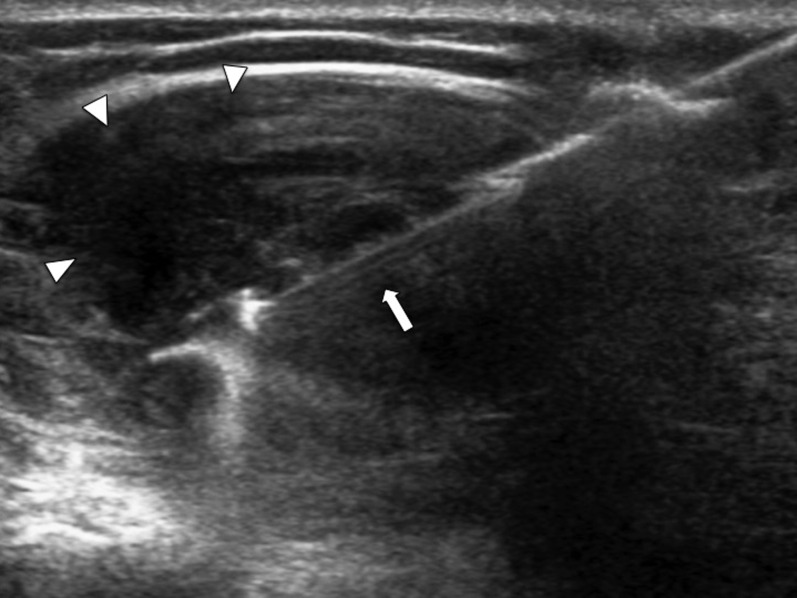
Transverse ultrasound of the knee joint showing the biopsy needle (arrow) during biopsy of thickened synovium in the lateral patellar recess (arrowheads).
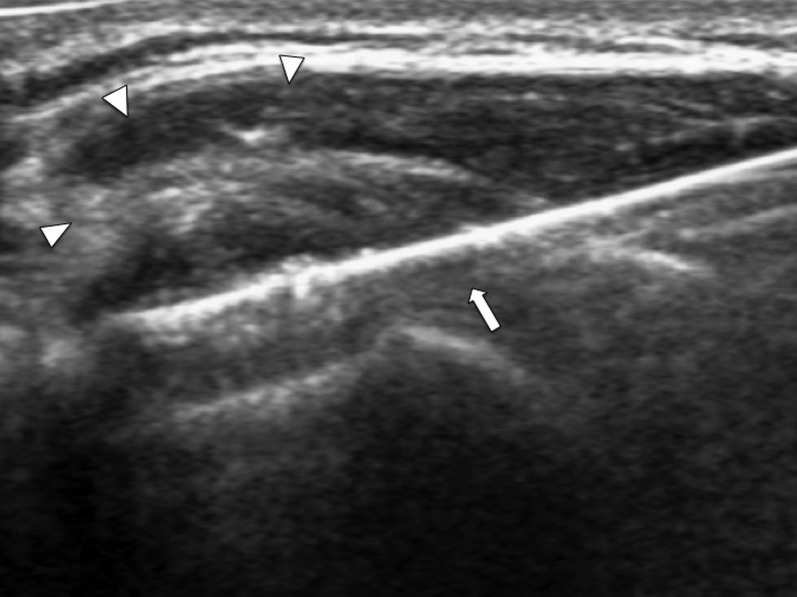
Figure 8.
Transverse ultrasound of the shoulder joint showing the echogenic tip of the coaxial needle (arrow) just piercing the thickened synovium (arrowheads). The tip of the coaxial system should be left at the outer margin of the thickened synovium to facilitate subsequent repositioning.
Figure 9.
Transverse ultrasound of the shoulder joint (same patient as Figure 8) showing the open specimen slot of the tru-cut biopsy needle (arrow) during biopsy of the thickened synovium (arrowheads).
Figure 10.
Core of synovial tissue within specimen slot of 16-G tru-cut needle. Histological and microbiological analysis revealed tuberculous infection.
Subsequent tissue handling
Any joint fluid aspirated before synovial biopsy should be sent for microbiological analysis (as fresh fluid specimen) and cytological analysis (in smear and 50% alcohol mixture) ± crystal detection (as fresh fluid specimen). Synovial biopsy specimens for histological analysis are immediately fixed in 4% paraformaldehyde solution for subsequent paraffin embedding. Specimens deemed for microbiological analysis are sent as fresh preparations in a sterile bottle. A small amount (∼1 ml) of sterile normal saline can be used to wash down the specimen from the trocar needle when necessary, but a large volume of saline should be avoided as this will potentially dilute the bacterial concentration of the specimen. Specimens for crystal analysis are sent as a fresh specimen without addition of saline or alcohol to avoid crystal dissolution.
POTENTIAL PITFALLS
Quantity insufficient for diagnosis
A minority of synovial biopsies (up to 10%) show no synovial tissue on histological examination despite apparent good needle positioning on image capture.10,11 The reason for this lack of synovial tissue may be technical though may also be related to relative synovial atrophy in patients with chronic arthritis as well as the patchy nature of synovial inflammation.3,10 We routinely try to acquire several cores from different areas of the joint through use of a coaxial system to minimize any such sampling error [JCM Sitt, 2015, personal communication].
Avoidance of antibiotics before biopsy for suspected septic arthritis
It is not completely clear whether the prior use of antibiotics reduces the likelihood of obtaining a positive yield in patients with joint infection. The type of joint (native or prosthetic) seems to have a greater influence on the likelihood of a positive yield than antibiotic therapy. For example, in our study, all patients with infected native joints yielded a positive growth on culture of synovial biopsy material, while only about 60% patients with infected prosthetic joints yielded a positive growth on culture of synovial biopsy material and this difference did not seem to be related to prior antibiotic therapy. Nevertheless, one should, whenever possible, aim to perform synovial biopsy early before starting antimicrobial treatment to minimize the possibility of a false-negative result from a partially treated infected joint.
Adverse effects and complications
Most tru-cut synovial biopsies are well-tolerated clinically. In the study by Kelly et al, only one in five patients reported mild post-procedural arthralgia, all of which resolved within 24 h with simple analgesics. Over two-thirds of patients expressed a willingness to undergo further biopsy if required.11 Synovial biopsies from small joints tend to be better tolerated than those of large joints, most likely owing to more efficient local anaesthesia.11
Synovial biopsy is considered a safe procedure that can be applied to all patients, even at the extremes of age. The relative risk of synovial biopsy is likely to be low for the knee, higher for the wrist, ankle and shoulder and highest for the elbow and hip reflecting a progressive increase in technical difficulty along with the potential for neurovascular injury. That said, the only adverse reactions reported to date are a vasovagal-type attack in a small number (1–2%) of patients at the time of biopsy and erysipelas in one patient which occurred 1 week following biopsy.8 No other significant immediate or long-term complications had been reported. Specifically, there have been no reports of joint infection, neurovascular injury or exacerbation of disease symptoms following synovial biopsy.
CONCLUSION
Ultrasound-guided synovial biopsy is a safe procedure that confers many advantages over arthroscopic synovectomy or fluoroscopic-guided biopsy. It is effective at confirming the presence of synovial tumour, excluding joint infection in patients with little or no joint effusion as well as providing tissue for histological grading of synovitis and immunohistology. We would encourage its more widespread use.
Contributor Information
Jacqueline C M Sitt, Email: jacquelinesitt@gmail.com.
James F Griffith, Email: griffith@cuhk.edu.hk.
Priscilla Wong, Email: priscillachwong@gmail.com.
REFERENCES
- 1.Smith MD. The normal synovium. Open Rheumatol J 2011; 5: 100–6. doi: 10.2174/1874312901105010100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerlag DM, Tak PP. How to perform and analyse synovial biopsies. Best Pract Res Clin Rheumatol 2009; 23: 221–32. doi: 10.1016/j.berh.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 3.Parlier-Cuau C, Hamze B, Champsaur P, Nizard R, Laredo JD. Percutaneous biopsy of the synovial membrane. Semin Musculoskelet Radiol 1997; 1: 189–96. doi: 10.1055/s-2008-1080138 [DOI] [PubMed] [Google Scholar]
- 4.Soudack M, Nachtigal A, Vladovski E, Brook O, Gaitini D. Sonographically guided percutaneous needle biopsy of soft tissue masses with histopathologic correlation. J Ultrasound Med 2006; 25: 1271–7. [DOI] [PubMed] [Google Scholar]
- 5.Torriani M, Etchebehere M, Amstalden E. Sonographically guided core needle biopsy of bone and soft tissue tumors. J Ultrasound Med 2002; 21: 275–81. [DOI] [PubMed] [Google Scholar]
- 6.Liu JC, Chiou HJ, Chen WM, Chou YH, Chen TH, Chen W, et al. Sonographically guided core needle biopsy of soft tissue neoplasms. J Clin Ultrasound 2004; 32: 294–8. doi: 10.1002/jcu.20038 [DOI] [PubMed] [Google Scholar]
- 7.Van Vugt RM, van Dalen A, Bijlsma JW. Ultrasound guided synovial biopsy of the wrist. Scand J Rheumatol 1997; 26: 212–14. doi: 10.3109/03009749709065683 [DOI] [PubMed] [Google Scholar]
- 8.Koski JM, Helle M. Ultrasound guided synovial biopsy using portal and forceps. Ann Rheum Dis 2005; 64: 926–9. doi: 10.1136/ard.2004.027409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scire CA, Epis O, Codullo V, Humby F, Morbini P, Manzo A, et al. Immunohistological assessment of the synovial tissue in small joints in rheumatoid arthritis: validation of a minimally invasive ultrasound-guided synovial biopsy procedure. Arthritis Res Ther 2007; 9: R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly S, Humby F, Filer A, Ng N, Di Cicco M, Hands RE, et al. Ultrasound-guided synovial biopsy: a safe, well-tolerated and reliable technique for obtaining high-quality synovial tissue from both large and small joints in early arthritis patients. Ann Rheum Dis 2015; 74: 611–17. doi: 10.1136/annrheumdis-2013-204603 [DOI] [PubMed] [Google Scholar]
- 11.Marin F, Lasbleiz J, Albert JD, Askri A, Werner-Leyval S, Duval H, et al. Synovial biopsy under US guidance: technical considerations and results. [In French.] J Radiol 2006; 87: 561–5. doi: 10.1016/S0221-0363(06)74038-0 [DOI] [PubMed] [Google Scholar]
- 12.Griffith JF. Diagnostic ultrasound: musculoskeletal. 1st edn. Philadelphia, PA: Amirsys, Elsevier; 2014. [Google Scholar]
- 13.Chung CB, Boucher R, Resnick D. MR imaging of synovial disorders of the knee. Semin Musculoskelet Radiol 2009; 13: 303–25. doi: 10.1055/s-0029-1242186 [DOI] [PubMed] [Google Scholar]
- 14.Frick MA, Wenger DE, Adkins M. MR imaging of synovial disorders of the knee: an update. Radiol Clin North Am 2007; 45: 1017–31, vii. doi: 10.1016/j.rcl.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 15.Narvaez JA, Narvaez J, Aguilera C, De Lama E, Portabella F. MR imaging of synovial tumors and tumor-like lesions. Eur Radiol 2001; 11: 2549–60. [DOI] [PubMed] [Google Scholar]
- 16.Wilson DJ. Soft tissue and joint infection. Eur Radiol 2004; 14(Suppl. 3): E64–71. doi: 10.1007/s00330-003-2048-8 [DOI] [PubMed] [Google Scholar]
- 17.Garner HW, Bestic JM. Benign synovial tumors and proliferative processes. Semin Musculoskelet Radiol 2013; 17: 177–8. doi: 10.1055/s-0033-1343095 [DOI] [PubMed] [Google Scholar]
- 18.Szendroi M, Deodhar A. Synovial neoformations and tumours. Baillieres Best Pract Res Clin Rheumatol 2000; 14: 363–83. [DOI] [PubMed] [Google Scholar]
- 19.Lee RK, Griffith JF, Ng AW, Ying Tam HK, Chan AW. Non-Hodgkin's lymphoma of the knee: a case report. Iran J Radiol 2015; 12: e7583. doi: 10.5812/iranjradiol.7583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peel TN, Buising KL, Choong PF. Diagnosis and management of prosthetic joint infection. Curr Opin Infect Dis 2012; 25: 670–6. doi: 10.1097/QCO.0b013e32835915db [DOI] [PubMed] [Google Scholar]
- 21.De Zordo T, Mlekusch SP, Feuchtner GM, Mur E, Schirmer M, Klauser AS. Value of contrast-enhanced ultrasound in rheumatoid arthritis. Eur J Radiol 2007; 64: 222–30. doi: 10.1016/j.ejrad.2007.07.011 [DOI] [PubMed] [Google Scholar]
- 22.Rezaei H, Torp-Pedersen S, af Klint E, Backheden M, Kisten Y, Györi N, et al. Diagnostic utility of musculoskeletal ultrasound in patients with suspected arthritis–a probabilistic approach. Arthritis Res Ther 2014; 16: 448. doi: 10.1186/s13075-014-0448-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerlag D, Tak PP. Synovial biopsy. Best Pract Res Clin Rheumatol 2005; 19: 387–400. doi: 10.1016/j.berh.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 24.Johnson SM, Saint John BE, Dine AP. Local anesthetics as antimicrobial agents: a review. Surg Infect (Larchmt) 2008; 9: 205–13. doi: 10.1089/sur.2007.036 [DOI] [PubMed] [Google Scholar]
- 25.Hung EH, Griffith JF, Ng AW, Lee RK, Lau DT, Leung JC. Ultrasound of musculoskeletal soft-tissue tumors superficial to the investing fascia. AJR Am J Roentgenol 2014; 202: W532–40. doi: 10.2214/AJR.13.11457 [DOI] [PubMed] [Google Scholar]