Abstract
In this comprehensive review, we discuss the main interventions performed in the foot and ankle for Achilles tendinopathy, Morton's neuromas and Plantar fasciitis as well as techniques for intra-articular and peritendinous injections. We present the different imaging techniques and injectable agents that can be used in clinical practice, trying to help the reader decide the most appropriate way of managing the patient with a problem in the ankle and foot.
ULTRASOUND-GUIDED INJECTIONS AROUND TENDONS
Tendinopathy and tenosynovitis are very common in the foot and ankle. They can be associated with mechanic factors, chronic repetitive stress or overuse injury, age-related degeneration or can be secondary to inflammatory arthritis.1,2 Tendinopathy can be also associated to previous surgery, especially for ankle fractures, resulting to tendon impingement by internal fixation devices.2
The imaging investigation of a tendon lesion in the ankle and foot includes MRI and ultrasound.2–4 MRI is used for diagnostic purposes but is generally less accurate than ultrasound, as ultrasound has better spatial resolution.2,3 Ultrasound is the imaging test of choice to diagnose the presence of acute or chronic tendinopathy, to assess its activity and to guide intervention around tendons.3,4 Normal tendons appear as hyperechoic bands consisting of fine intrasubstance hyper- and hypoechoic fibrils. Tendons display anisotropy, and therefore, it is necessary to always keep the transducer perpendicular to the tendon to maximize tendon echogenicity and avoid misinterpretation.3,4 The tendon sheath cannot be discriminated when not distended with fluid, and there is no detectable vascularity in colour Doppler imaging.3,4
The ultrasound imaging features of acute tendinopathy of the ankle and foot tendons include fusiform hypoechoic thickening of the affected tendon with loss of normal fascicular echostructure.3,4 There can be hypoechoic synovial thickening surrounding the tendon and areas of microfissuring and mucinous degeneration into the tendon substance (Figure 1). Evaluation using Doppler imaging may reveal the presence of neovascularity in and around the tendon, a feature associated with active inflammation.3,4 At a more chronic stage, there can be thinning of the tendon although its continuity can be maintained. Tenosynovitis is characterized by distension of the synovial tendon sheath by fluid, which may or may not be accompanied by the abnormal appearance of the tendon fibres.3,4
Figure 1.
(a) Axial ultrasound image of the tibialis posterior (TP) and flexor digitorum longus (FDL) tendons at the level of the medial malleolus and (b) longitudinal ultrasound image of TP distally to the medial malleolus of a 32-year-old patient with inflammatory arthritis. There is effusion in the tendon sheath of both tendons together with an intratendinous hypoechoic area in the FDL tendon, corresponding to an area of mucinous degeneration. FDL is also swollen and larger than TP, which is the reverse of normal.
Besides establishing the diagnosis of tendinopathy or tenosynovitis, ultrasound can also be used to guide the injection of steroid and local anaesthetic into the tendon sheath.5–7 Patient positioning depends on the anatomic location, and a linear high-frequency transducer (7–18 MHz) is used to identify the pathological tendon and guide the needle. If possible, a hockey stick transducer is used, as it allows more space to manipulate the needle. Doppler imaging prior to the injection should be used to identify surrounding vascular structures and to detect areas of increased neovascularity as target areas.5–7 The optimal needle entry point and the course of needle must be assessed prior to skin preparation. The injection is performed under sterile conditions using a 23-G needle, and the injection can be carried out with the needle oriented axially or longitudinally to then probe under direct ultrasound guidance.5–7 If there is a significant synovial sheath effusion, this can be aspirated prior to injecting. Care is taken to deliver the steroid in the synovial sheath around the tendon and not into the tendon, as intratendinous injections are associated with collagen breakdown and risk of rupture.8 In cases of difficulty in discriminating the synovial sheath from the tendon (when not distended with fluid), a preliminary injection of local anaesthetic may be useful to distend the cavity before injecting the steroid. Careful consideration and discussion with the patient should be made before steroid injection around a weight-bearing tendon (especially the posterior tibial tendon), as the resulting relief of pain can lead to overuse by the patient, which can worsen anatomic lesions and predispose to rupture. In these cases, the patient may benefit from temporary immobilization in a boot. Biomechanical assessment is also important to prevent recurrence of tendinopathy.
Peritendinous hyaluronic acid injections are also commercially available for application in impingement tendinopathies and post-operative peritendinous adhesions.9,10 However, there is only scarce evidence, and therefore the use of viscosupplementation around tendons should be limited before further investigation on safety and efficacy is undertaken. Surgical treatment of tendinopathy is indicated after failure of conservative measures or in recalcitrant cases.11
INJECTABLE SUBSTANCES AND TECHNIQUES
Steroid injections into soft tissues, bursae, tendon sheaths and joints are very commonly used in clinical practice for their anti-inflammatory properties.12 In most institutions, the two corticosteroids used most routinely are triamcinolone acetonide and methylprednisolone acetate. These are generally mixed with a local anaesthetic, either lidocaine 1% or a long-lasting anaesthetic such as bupivacaine or ropivacaine 0.25%. Several potential side effects of corticosteroids are known.12–16 These include local side effects, including skin atrophy, skin depigmentation and fat necrosis. Methylprednisolone is less prone to causing skin atrophy than triamcinolone and, therefore, is preferred when injecting superficial lesions. Moreover, intratendinous steroid injections increase direct tendon damage and in animal studies increase your risk of rupture,12–16 as they have been shown to suppress tenocyte activity and collagen synthesis and reduce tendon cell viability.13–15 There are also several systemic side effects, such as facial flushing, menstrual irregularity in females and vivid dreams for several days afterwards.16 Steroids should also be used with caution in patients with diabetes and in patients using the antismoking drug Zyban, as they can increase the risk of fits.17 For the above reasons, the use of steroids for tendinopathy is not recommended by the British National Formulary for drugs and the British Society of Rheumatology, and they are banned substances by the World Anti-Doping Agency in competition.
Autologous blood treatment arose over 40 years ago in the equine world.18 Some of the initial studies were performed on rabbit tendons showing an increase in collagen Type 1 and increased maturity of repair tissue.19 The use of autologous blood and dry needling in patellar tendinosis was published in 2007 by James et al.20 Platelet-rich plasma was introduced to reduce the after effect of the autologous blood, which can clot in the syringe and cause a lot of soreness because of the other factors within the blood which are irritants. A new product was therefore developed by centrifuging the blood and producing both protein-rich and protein-poor plasma. The protein-rich plasma appears to increase the amount of growth factors and enhances the amount of collagen Type 1 to aid increased healing.21–24 This, however, made the procedure more complex, and a need for sterility became important as the blood was being handled before being reinjected into the patient. The autologous blood or platelet rich plasma (PRP) treatment has been used extensively in conjunction with a dry needling technique.25–27 Therefore, we do not know whether it is the dry needling that has the beneficial effect or whether the autologous blood or PRP is the main benefactor.25–27 In view of the excitement in the use of autologous blood therapy, especially in the sporting world, the National Institute of Clinical Excellence (NICE, UK) has issued a statement which includes information on actions that should be taken by the performing clinicians.28 In this statement, NICE pointed out that it is safe, but the evidence on efficacy remains inadequate. Therefore, they advise that this procedure should only be used with special arrangements for clinical governance, consent and audit or research, making sure that the patients are made aware of the above issues and the results are audited and reviewed.28
Sodium hyaluronate has been shown to regulate angiogenesis in healing rabbit Achilles tendons.29 It may also have lubricating properties promoting tendon gliding. As yet, there are no trials using it in the treatment of human Achilles tendinosis, but it does appear promising in the elbow.30
Tenocyte transplant is a new method of treatment in tendinosis.31 The aim is that by using skin-derived tenocytes that have been cultured in vitro, cells are placed at the site of tendinosis to help produce Type 1 and Type 2 collagen where needed. This procedure started in the equine world, has now been translated into the human population and has been used in patellar tendinopathy with some success.31
ULTRASOUND-GUIDED INTERVENTION FOR THE ACHILLES TENDON
The Achilles tendon is the largest weight-bearing tendon in the body, and it is formed of a spiral of fibres with contribution from both gastrocnemius muscles and the soleus muscle. Tendinosis is one of the commonest pathologies of the Achilles tendon.32 It has a multifactorial aetiology including the role of genetics, other disease processes and tendon injury. There are a number of theories for the development of tendinosis, but it is likely to be due to failed healing because of these factors.32,33
High-resolution ultrasound is the imaging test of choice for assessing Achilles tendinosis, showing tendon thickening and neovascularization, with vessels usually inserting in the tendon through the ventral side of it from the Kager's fat4 (Figure 2). Using ultrasound, a number of interventional procedures can be accurately performed in order to treat Achilles tendinosis.4,7,34 The ultrasound-guided techniques can be combined and enhanced by physical therapy such as eccentric loading35,36 (Table 1). Eccentric loading exercises have been shown to be beneficial unless the Achilles tendinosis is of the insertional type.35,36 The main problems are that these are very painful and therefore subjects do not perform these exercises diligently.35,36
Figure 2.
Longitudinal (a) and axial (b) images of a tendinopathic Achilles tendon showing hypoechoic thickening of the tendon and increased Doppler signal because of neovascularization.
Table 1.
Treatments for chronic tendinopathy (adapted from Riley33 with permission from Macmillan Publishers Ltd)
| Treatment | Putative target or mode of action |
|---|---|
| Rest or modification of activity | Removal of precipitating factors and prevention of reinjury |
| Orthotics | Alters biomechanics to prevent provocation |
| Cryotherapy | Reduction of acute inflammation and decrease in cell metabolism |
| Non-steroidal anti-inflammatory drugs | Reduction of inflammation through inhibition of prostaglandin synthesis; this could be bad for healing |
| Eccentric loading exercise | Thought to promote restoration of normal tissue structure, possibly through an effect on cell activity and matrix remodelling from stretching the muscle–tendon unit |
| Laser treatment | Possible analgesic effects by suppressing prostaglandin E2 production and unspecified (unknown) effects on cell activity |
| Ultrasound (therapeutic) | Thermal effects on tissue, stimulation of cell activity and increased blood flow |
| Corticosteroid injection | Reduction of inflammation and other unknown effects (generally inhibitory of protein synthesis) |
| Glyceryl trinitrate patch | Vasodilatation-promoting healing |
| Actovegin | Unknown (suggested to promote glucose uptake and other effects on tendon cell metabolism that promote repair and resolution) |
| Sclerosant injection | Blocks tendon blood flow (targets neovascularization and associated nerve in growth) |
| Autologous blood | Contains growth factors (e.g. transforming growth factor β and platelet-derived growth factor) that promote matrix synthesis and tissue repair |
| Platelet-rich plasma | Contains concentrated growth factors (e.g. transforming growth factor β and platelet-derived growth factor) that promote matrix synthesis and tissue repair |
| Dry needling | Microtrauma and bleeding bringing inflammatory response |
| High-volume saline stripping | Strips blood vessels from tendon to decrease neovessels and neural tissue |
| Hyperosmolar dextrose (prolotherapy) | Healing by local inflammatory response |
| Extracorporeal shockwave treatment | Mechanical stimulation. Anti-inflammatory by effect on nitric oxide production. Useful in calcific tendinosis |
| Hyaluronic acid | Regulation of angiogenesis and lubricant |
| Surgical debridement | Strips blood vessels to decrease new vessels |
| Tenocyte transplant | Production of Types 1 and 2 collagen |
Steroids have been used in the past to treat Achilles tendinosis. However, there is solid evidence against steroid injections around Achilles tendon, as they do not have a long-term effect, and there is a significant risk of tendon damage or rupture.12–16 The other risk is that of fat atrophy both superficially and in the Kager's fat pad depending on the site of injection.
Glyceryl trinitrate has been shown to be of benefit in non-insertional Achilles tendinosis as compared with placebo at 6 months and 3 years.37,38 The main side effect is headache, and it is not licensed in the UK for this use.39
Extracorporeal shockwave treatment has been investigated in the Achilles tendon. Under ultrasound guidance, a device is used to pass acoustic shock waves through the skin towards the tendon. The mechanism of action includes overstimulation of a painful area causing decreased pain signals to the brain stem, so called “hyperstimulation analgesia”; regeneration causing the tenocytes to produce more collagen; and breaking of tendon calcification.40 Evidence shows only minor improvement in function, pain and alignment score.40–45 The NICE has issued a statement on extracorporeal shockwave therapy in Achilles tendinopathy concluding that it is safe, but there is no consensus opinion regarding efficacy. Therefore, they advise that this procedure should only be used with special arrangements for clinical governance, consent and audit or research.44,45
Saline stripping whether in small or large volumes is used by some groups and has been used with good effect. The process of using saline stripping along the Achilles tendon is to strip off the blood vessels which also bring nerve fibres from the anterior aspect of the tendon. By stripping these vessels, it has been noticed that there is a reduction in pain and a correlation between the number of vessels and the amount of pain is evident46,47 (Figure 3). The technique includes injections into the pre-Achilles fascia using 10 ml of 0.5% bupivacaine, 25 mg hydrocortisone acetate and up to 40 ml of normal saline under ultrasound guidance. Published evidence suggests short-term improvement with pain and function scores and a significant improvement in the Victorian Institute of Sport Assessment–Achilles tendon (VISA-A) score at 30 weeks and at 12 months post injection.46,47
Figure 3.
A transverse ultrasound image showing a needle anterior to the Achilles tendon while performing saline stripping of the tendon.
Chronic inflammation of the paratenon may result in adhesions between the paratenon and the Achilles tendon, leading to paratenonitis. Brisement, also known as paratenon stripping, includes the injection of saline solution into the tendon/peritenon interspace to break up adhesions. Under ultrasound guidance, a needle is placed deep to the paratenon in short axis views and a solution of normal saline and 1% lidocaine is gently injected. Brisement injections have long been used, although there is lack of data in the literature to specifically evaluate this technique.34
Some institutions are using sclerosants such as polidocanol inside or close to the vessels in conjunction with eccentric loading exercises with promising results.48,49 The sclerosant treatment can be repeated, but it is not clear how often this treatment should be performed. Electrocoagulation is a method of closing new vessels. There has only been one pilot study to date in Achilles tendinosis, suggesting that this therapy destroyed the accompanying nerves rather than the vessels.50
Prolotherapy using very concentrated dextrose or neat alcohol is thought to cause a local inflammatory response to promote healing.51,52 Intratendinous injection of hyperosmolar dextrose has recently been studied in the Achilles tendon, under ultrasound guidance. 33 patients had 6 weekly injections until symptoms resolved. There was no placebo control. There was an improvement in visual analog scale scores at follow-up at a mean of 1 year by telephone interview.51,52
Regarding PRP and autologous blood therapies, despite the initial enthusiasm on the technique from the sporting world, there are a number of recent met-analyses which have questioned the role of such therapies in Achilles tendinosis.25–27
We are therefore left with a conundrum as to which is the most effective treatment for Achilles tendinosis. There is also lack of good quality long-term blinded studies to assess each technique individually, as mixing techniques and injectable agents have led to confusion. It is interesting to note that a recent article in the BMJ suggests eccentric loading with low-level laser treatment or extracorporeal shockwave treatment was the most effective.32 Eccentric loading exercise is often not performed well by the patient because of pain. Interventional techniques should only be performed after several months of eccentric loading exercise has been performed.
OTHER SOFT-TISSUE INJECTIONS IN THE ANKLE AND FOOT
Ultrasound can be used to evaluate soft-tissue lesions and palpable lumps at the ankle and foot. These include benign and malignant tumours related to tendons or bones, pseudotumors and ganglia.53,54 Ganglia represent by far the most common mass of the ankle. Ultrasound can be used to identify and characterize the mass and also provide guidance to aspirate a ganglion or to biopsy a soft-tissue lesion.4–6 Ultrasound reveals the characteristic cystic ultrasound appearance of ganglia, identifies the presence of vascular structures or cutaneous nerves in the proximity of the ganglion and allows for the needle to be placed accurately and safely into the ganglion to drain and decompress it.55,56 Aspiration may be followed by injection of steroid to treat the ganglion. The procedure is well tolerated and has minimal complications. However, there is a higher risk of failure than surgical treatment, and following multiple ganglion aspirations, surgical treatment is indicated.57 Ultrasound examination can assist in surgical treatment, by identifying the origin of the ganglion.
In addition, ultrasound has proven useful in localizing and guiding removal of foreign bodies.58
IMAGE-GUIDED JOINT INJECTIONS
Ultrasound and fluoroscopy are used to guide injections in the foot and ankle. The most commonly injected joints are the ankle, posterior subtalar and mid-tarsal joints.
The ankle joint is accessed from the dorsal aspect avoiding the dorsalis pedis artery which lies medially between the tibialis anterior and extensor digitorum tendons. It is easier to avoid the tendons if using ultrasound guidance. The probe can be placed in a longitudinal or transverse plane to guide the needle.5 An anterior–medial or anterolateral approach can be used59 (Figures 4 and 5).
Figure 4.
Longitudinal ultrasound image of the ankle showing a needle approaching the joint by an anterior approach.
Figure 5.
Transverse ultrasound image of the ankle showing a needle approaching the joint by a lateral approach.
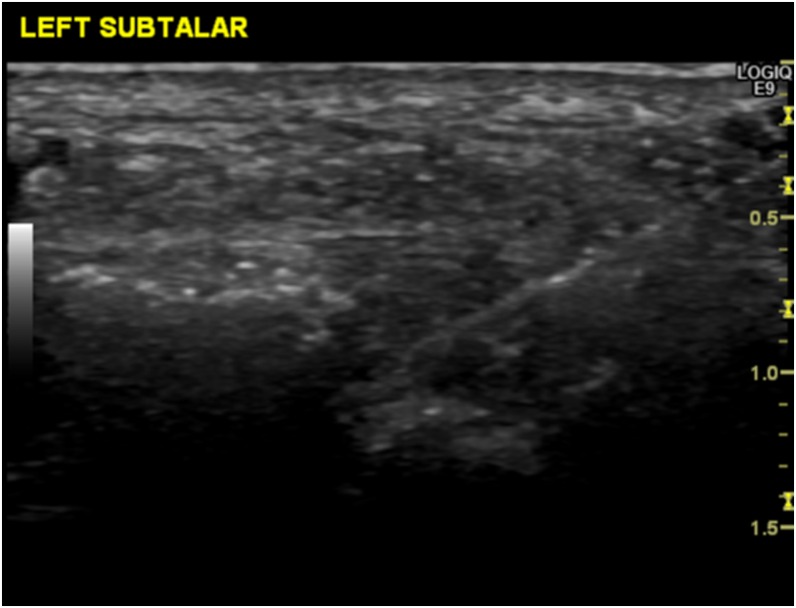
Access to the posterior subtalar joint is either via a medial or lateral approach. This is technically more demanding with ultrasound, as it is deep to the tibialis posterior or peroneal tendons, respectively (Figure 6). Some operators may therefore find fluoroscopic guidance easier, but the tendons and arteries need to be palpated and the needle placement checked with iodinated contrast prior to injecting steroid or other substances such as hyaluronic acid (Figure 7). Image guidance is superior to freehand injections, especially when the joint is arthritic.60 The midtarsal joints are easily assessed from the dorsum of the foot by both fluoroscopy and ultrasound.
Figure 6.
Longitudinal ultrasound image of the posterior subtalar joint showing a needle approaching the joint.
Figure 7.
Fluoroscopically guided injection into the posterior subtalar joint.
MORTON'S NEUROMA
Morton's neuroma (MN) is a painful condition of the foot caused by the fusiform enlargement of a digital branch of the medial or lateral plantar nerve.61–63 It is caused by nerve fibre degeneration and excessive intraneural and juxtaneural reparative fibrosis. It is therefore not a true nerve tumour but rather represents a perineural fibroma as the result of impingement and microdamage to the common digital nerve, caused by several factors, including excessive weight-bearing stress, inflammatory bursitis, ischaemia, entrapment of the metatarsal nerve and compression by the metatarsal ligament. The principal mechanism leading to metatarsal impingement is collapse of the plantar arch, which leads to fore foot rotation whilst walking. Therefore, during the walking movement, pressure is placed on the medial side of the great toe creating hallux valgus and in turn metatarsal impingement. MNs are usually multiple, with a predilection for the third common digital nerve and commonly affect middle-aged females and athletes.61–63
MN presents as burning pain or paraesthesia at the forefoot radiating to the toes. Symptoms are aggravated by walking in tight high-heeled shoes and may be relieved by removing shoes and massaging.61–63 To help provide an accurate clinical assessment, a number of provocative tests can be performed. Although there is no single pathognomonic test, the thumb index finger test and the Mulder's click are the most sensitive and specific, resulting, respectively, in pain and a palpable click at the site of the suspected MN.64–66
Imaging investigations include ultrasound and MRI. Ultrasound is an accurate and cost-effective way to confirm the diagnosis of MN in cases of equivocal clinical findings. As proved by several studies, ultrasound has a sensitivity ranging between 85% and 100% for detecting a web space abnormality.66,67 Compared with MR, ultrasound has a similar or slightly higher sensitivity,68–70 particularly for detecting MNs smaller than 5 mm.70 Therefore, MRI is reserved for equivocal or atypical cases to reveal a low-signal-intensity mass in T1 and T2 weighted images and to distinguish MN from other soft-tissue masses with high signal intensity in T2 images, including bursae, schwannomas or other neoplasms. The ultrasound examination technique includes scanning both in the coronal plane (axial to the metatarsal heads) and the sagittal plane (parallel to the metatarsal heads).4,5 The ultrasound appearance of a MN includes a non-specific hypoechoic mass parallel to the metatarsal heads4,5 (Figure 8). The ultrasound findings may mimic the appearance other hypoechoic soft-tissue masses in the interdigital space, including fibroma, epidermoid, ganglion, mass-like mucoid degeneration and giant cell tumour of the tendon sheath.64,71 Identification of the presumed plantar digital nerve in continuity with the mass in the sagittal plane improves diagnostic confidence67 as well as the “Gingko leaf sign”, an appearance encountered in the coronal plane defined as the biconcave shape of the mass caused from compression by adjacent structures (Figure 9). Intermetatarsal adventitial bursitis may mimic a MN or coexist with it.71,72 Differentiation form bursitis and correct assessment of the size of the neuroma can be achieved by pressing with the thump while scanning on the web space, causing the bursa to collapse (Figure 8). Most MNs range in size from 3 to 10 mm,64 and the finding of an interdigital mass >20 mm in length should raise suspicion of an abnormality other than a neuroma.67
Figure 8.
Ultrasound images of the third intermetatarsal space in the sagittal plane (longitudinal to the metatarsal shaft) before and during the application of pressure on the skin of the dorsal side of the metatarsal space. The application of pressure causes the intermetatarsal bursa to collapse and allows for the differentiation between Morton's neuroma and bursitis and for the correct assessment of the size of the neuroma. The hypoechoic lesion shown by the arrows on image (a) corresponds to a Morton's neuroma with coexisting bursitis, whereas the smaller lesion also shown by arrows on image (b) corresponds to the Morton's neuroma after the collapse of the bursa.
Figure 9.
Ultrasound image of the third intermetatarsal space in the coronal plane (axial to metatarsal shaft) showing a hypoechoic mass between the metatarsal heads. The mass exhibits the characteristic appearance of the “ginkgo leaf sign” (arrows). The larger portion of the mass (thin arrows) corresponds to the gingko leaf and the thin portion connecting the mass to the nerve corresponds to the stalk of the leaf (thick arrows).
Clinical correlation of the ultrasound findings is essential for the diagnosis but also for guiding treatment, as asymptomatic sonographic thickening of the intermetatarsal nerves is found in up to 54% of cases.72 Provocative manoeuvres can be performed during ultrasound scanning to increase diagnostic confidence, such as squeezing the metatarsal heads or pressing with the index finger on the plantar side on the web space while scanning.64
The conservative treatment of MN includes modification of footwear and orthosis, as well as localized injections of local anaesthetics, steroids or alcohol into the affected web space. The primary treatment is to support the plantar arch with orthotics, which should be tried before any injection techniques are considered. In cases of injections for symptomatic therapy, ultrasound can be used to deliver the injectate under image guidance in the MN to increase accuracy and efficacy.67 It has been proved that, compared with blind techniques, ultrasound guidance can result in better short- and long-term pain relief for corticosteroid injections, can reduce the need for additional procedures in a series of alcohol injections, can reduce the surgical referral rate and can add efficacy to a single injection.73,74
Ultrasound-guided local anaesthetic injections can be used as a diagnostic tool to temporarily block the nerve and confirm the diagnosis; however, the effects last short and do not improve the results of surgery.75 Local injection of steroids either alone or in combination with a local anaesthetic agent are more widely used in the clinical practice. There are varied reports on the effectiveness of ultrasound-guided steroid injections. Most studies have shown that ultrasound-guided steroid injections are both effective and cost effective for short-term pain relief usually at 3 months, with the effect ranging between 4 weeks and 9 months and being more significant and long lasting if the injection is delivered early in the course of symptoms and for lesions <5 mm.75–79
Ultrasound-guided alcohol injections are an alternative effective treatment. Partial or total pain relief has been reported in up to 94% of patients at 6 months and in 74% of patients at 1 year after the injection, with 30% ultrasound-proven reduction in lesion size at 6 months.80–83 However, it does not lead to permanent resolution of symptoms, and although it is usually a safe procedure, it may be associated with complications.83 Other ultrasound-guided techniques for MNs include radiofrequency ablation under local anaesthetic with reported success in 85% of cases at 6 months84,85 and the injection of botulinum toxin A, reported by a single pilot study.86 Both of the above options lack strong evidence and need further research.
The technique for ultrasound-guided injections for MNs includes positioning the patient supine on the examination table with the knees extended and using a high-frequency linear array transducer to identify the MN.4,5 The solution is injected using a short (1.5-inch) 23- or 25-G needle, which is inserted through the respective interdigital fold parallel to the long axis of the transducer and is advanced under continuous guidance to the neuroma.5 Punctures can be performed either dorsal or plantar to the interdigital skin crease, with and without preliminary subcutaneous local anaesthesia. The dorsal approach should be favoured, because it is better tolerated and is associated to decreased risk of plantar fat pad atrophy, which may cause pain and gait disturbance.87,88 The use of preliminary local anaesthesia does not confer any benefit.87 Aseptic technique is used, including skin sterilization, probe cleaning and sterile gloves. A sterile probe cover may not be used, as usually the probe is held at a distance from the site of puncture.64 After the injection, patients are instructed to avoid excessive weight bearing for 1 week and to modify footwear on wider fitting and low heels or use orthotic insoles. Follow-up is performed using pain diaries and visual analogue scales. Multiple injections (usually a course up to three) may be needed.64
In case of alcohol injection, a solution of 0.1 ml of 100% ethyl alcohol diluted in 0.4 ml of 0.25% bupivacaine (total = 0.5 ml of 20% ethyl alcohol) is injected under direct ultrasound guidance into the neuroma.81–83 In case of steroid injection, 1 ml of methylprednisolone 40 mg ml−1 and 1.5 ml of bupivacaine hydrochloride 0.5% is mixed in the syringe and a total of 0.75–1.5 ml is injected.4 During real-time evaluation of the injection procedure, the echogenic needle tip can be seen entering the hypoechoic neuroma and the echogenic material can be seen filling the neuroma and increasing the echogenicity of the lesion. The volume injected depends on a variety of factors including lesion size and the presence of adventitial bursal formation that allows larger volumes to be injected. The use of a longer acting anaesthetic (bupivacaine) in the mixture helps to reduce the incidence of a painful flare response that occasionally accompanies percutaneous steroid injections, and the use of a highly soluble steroid (methylprednisolone) is preferred to other steroid agents with larger crystals (hydrocortisone), as it is associated with reduced risk of fat atrophy and skin discolouration.76–78,88
In conclusion, ultrasound is the imaging test of choice both for the diagnosis and for guided treatment of MNs. Ultrasound-guided techniques include localized injections of local anaesthetics, steroids or alcohol into the affected web space. Ultrasound can be used to accurately deliver the injectate in the MNs to increase the efficacy of the injection compared with blind injections.
PLANTAR FASCIITIS
Plantar fasciitis (plantar fasciopathy) is the commonest cause of heel pain in adults.89 It primarily affects the fascial enthesis at the medial tubercle of the calcaneus.89 It is estimated to be the cause of approximately 7% of all foot pain in adults aged 65 years and over and 24% of all foot pain in athletic individuals.89 The incidence of plantar fasciitis is equal in males and females and peaks between the ages of 40 and 60 years.89
The aetiology of the condition remains controversial and not fully understood. The causes are thought to be multifactorial, including repetitive microtrauma and microtearing of the plantar fascia at the calcaneal insertion. Biomechanical causes have been proposed as the origin of plantar fasciitis. These include pes planus, lowered medial longitudinal arches, over pronation, excessive lateral tibial torsion and excessive femoral anteversion.89–91 It is suggested that these cause excessive tensile strain within the plantar fascia resulting in microscopic tears and chronic inflammation. Overuse rather than anatomy is thought to be the commonest cause in athletes. Although plantar fasciitis presents with the typical clinical signs of inflammation such as pain and swelling in acute cases; in chronic cases, the histological findings do not show inflammation but are instead characterized by tissue destruction and tissue repair, neovascularization and fibrosis with infiltration of macrophages, lymphocytes and plasma cells.90 The histological findings in chronic plantar fasciitis therefore support a degenerative rather than an inflammatory process.90
Controversy with regard to the aetiology of plantar fasciitis has also generated controversy with regard to its treatment.91,92 This is particularly so with regard to the use of serial corticosteroid injections in chronic plantar fasciitis as the condition does not demonstrate inflammation histologically.90 Plantar fasciitis is normally regarded as a self-limiting condition and usually resolves within 6–18 months.91,92 Various treatments for plantar fasciitis have been proposed. These include rest, stretching and strengthening exercises, arch supports and orthotics, night splints, extracorporeal shock wave therapy, iontophoresis, non-steroidal anti-inflammatory medications and corticosteroid injections.
Corticosteroid injections are often the next treatment employed when conservative management fails.93–97 The effectiveness of corticosteroid injection therapy for plantar fasciitis remains a subject of debate, however. A Cochrane review about interventions for treating plantar heel pain has concluded that there was some evidence for the effectiveness of injected corticosteroid but only for short term and minor pain relief, and the quality of the trials found in the literature was poor.94 Since the Cochrane review, however, there remain very few published randomized trials also concluding that steroid injection may be more effective than placebo up to 4 weeks following injection, but the difference does not persist at 8 and 12 weeks.93–98
There remains a paucity of good, prospective, randomized controlled and blinded studies into the effectiveness of corticosteroid injections for plantar fasciitis. The shortcoming with many studies published so far includes the lack of a standardized technique for the injection of steroid. In some studies, the injections are performed blindly by palpation; in others, ultrasound guidance is used; some practitioners may carry out dry needling of the inflamed plantar fascia as well as injecting steroid around it. The site of steroid injections is also not standard. Some operators may inject superficial to the inflamed plantar fascia, others deep or both. Finally, few studies have conducted long-term follow-up beyond 4 weeks after injection.
PRP has also been suggested as a potential treatment for plantar fasciitis.99–110 The various studies were not consistent in the methods of PRP preparation and platelet count. Each used a different device to prepare PRP and different volumes and concentration of platelets. The randomized studies so far published demonstrated a significantly greater improvement in symptoms between baseline and last follow-up assessment.99,101,110 None of the studies recorded major complications. The evidence so far shows that PRP is promising and safe. Further research is required to determine the efficacy of PRP in particular prospective randomized placebo controlled trials with long-term follow-up. The efficacy of other blood-derived growth factors, such as autologous conditioned plasma, has also being evaluated, showing improvement using autologous conditioned plasma but no significant differences compared with extracorporeal shockwave therapy.110 Less evidence is available about the use of botulinum toxin Type A, showing promising results.111,112
Resistant heel pain may also be treated with percutaneous fenestration, but so far, the evidence for its effectiveness is poor.113 Many surgical techniques for the treatment of resistant plantar fasciopathy are reported in the literature with little agreement on a single curative method. These include fasciotomy using fluoroscopic guidance, denervation, drilling or osteotomy of the posterior calcaneus.114
CONCLUSION
There are a variety of techniques and injectable agents available in clinical practice to treat ankle and foot soft-tissue and joint disease. Image-guided interventional techniques together with physiotherapy may be helpful in Achilles tendinopathy, MNs and Plantar fasciitis, while image guidance allows accurate and safe intra-articular and peritendinous injections and soft-tissue interventions. A high level of training as well as thorough knowledge of the literature and the limitations of the above methods will allow the physician to decide the most appropriate way of managing the patient with a problem in the ankle and foot.
Contributor Information
Eleni E Drakonaki, Email: drakonaki@yahoo.gr.
Gina M Allen, Email: gina_m_allen@btinternet.com.
Roland Watura, Email: roland.watura@gmail.com.
REFERENCES
- 1.Frizziero A, Bonsangue V, Trevisan M, Ames PR, Masiero S. Foot tendinopathies in rheumatic diseases: etiopathogenesis, clinical manifestations and therapeutic options. Clin Rheumatol 2013; 32: 547–55. doi: 10.1007/s10067-012-2158-2 [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Jacobson JA, Kim SM, Fessell D, Jiang Y, Dong Q. US and MRI of the peroneal tendons and associated pathology. Skeletal Radiol 2013; 42: 1191–200. doi: 10.1007/s00256-013-1631-6 [DOI] [PubMed] [Google Scholar]
- 3.Allison SJ, Nazarian LN. Musculoskeletal US: evaluation of ankle tendons and ligaments. AJR Am J Roentgenol 2010; 194: W514. doi: 10.2214/AJR.09.4067 [DOI] [PubMed] [Google Scholar]
- 4.Bianchi S, Martinoli C. US of the musculoskeletal system. Berlin, Germany: Springer Verlag; 2007 [Google Scholar]
- 5.Allen GM, Drakonaki E, Maybury M, Wilson DJ. The US intervention technique digital book; 2015. Available from: http://www.stlukesradiology.org.uk/index.php/book [Google Scholar]
- 6.Morvan G, Vuillemin V, Guerini H. Interventional musculoskeletal ultrasonography of the lower limb. Diagn Interv Imaging 2012; 93: 652–64. doi: 10.1016/j.diii.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 7.Sofka CM, Adler RS. US-guided interventions in the foot and ankle. Semin Musculoskelet Radiol 2002; 6: 163–8. doi: 10.1055/s-2002-32362 [DOI] [PubMed] [Google Scholar]
- 8.Holmes GB, Jr, Mann RA. Possible epidemiological factors associated with rupture of the posterior tibial tendon. Foot Ankle 1992; 13: 70–9. doi: 10.1177/107110079201300204 [DOI] [PubMed] [Google Scholar]
- 9.Ozgenel GY, Etoz A. Effects of repetitive injections of hyaluronic acid on peritendinous adhesions after flexor tendon repair: a preliminary randomized, placebo-controlled clinical trial. Ulus Travma Acil Cerrahi Derg 2012; 18: 11–17. doi: 10.5505/tjtes.2012.95530 [DOI] [PubMed] [Google Scholar]
- 10.Orlandi D, Corazza A, Fabbro E, Ferrero G, Sabino G, Serafini G, et al. US-guided percutaneous injection to treat de Quervain's disease using three different techniques: a randomized controlled trial. Eur Radiol 2015; 25: 1512–9. doi: 10.1007/s00330-014-3515-0 [DOI] [PubMed] [Google Scholar]
- 11.Grasset W, Mercier N, Chaussard C, Carpentier E, Aldridge S, Saragaglia D. The surgical treatment of peroneal tendinopathy (excluding subluxations): a series of 17 patients. J Foot Ankle Surg 2012; 51: 13–19. doi: 10.1053/j.jfas.2011.10.010 [DOI] [PubMed] [Google Scholar]
- 12.Shrier I, Matheson GO, Kohl HW, 3rd. Achilles tendonitis: are corticosteroid injections useful or harmful? Clin J Sports Med 1996; 6: 245–50. doi: 10.1097/00042752-199610000-00007 [DOI] [PubMed] [Google Scholar]
- 13.Wong MW, Tang YN, Fu SC, Lee KM, Chan KM. Triamcinolone suppresses human tenocyte cellular activity and collagen synthesis. Clin Orthop Relat Res 2004; 421: 277–81. doi: 10.1097/01.blo.0000118184.83983.65 [DOI] [PubMed] [Google Scholar]
- 14.Wong MW, Lui WT, Fu SC, Lee KM. The effect of glucocorticoids on tendon cell viability in human tendon explants. Acta Orthop 2009; 80: 363–7. doi: 10.3109/17453670902988386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei AS, Callaci JJ, Juknelis D, Marra G, Tonino P, Freedman KB, et al. The effect of corticosteroid on collagen expression in injured rotator cuff tendon. J Bone Joint Surg Am 2006; 88: 1331–8. doi: 10.2106/JBJS.E.00806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet 2010; 376: 1751–67. doi: 10.1016/S0140-6736(10)61160-9 [DOI] [PubMed] [Google Scholar]
- 17.Hays JT, Ebbert JO. Bupropion sustained release for treatment of tobacco dependence. Mayo Clin Proc 2003; 78: 1020–4; quiz 1024. doi: 10.4065/78.8.1020 [DOI] [PubMed] [Google Scholar]
- 18.McCullagh KG, Goodship AE, Silver IA. Tendon injuries and their treatment in the horse. Vet Rec 1979; 105: 54–7. doi: 10.1136/vr.105.3.54 [DOI] [PubMed] [Google Scholar]
- 19.Taylor MA, Norman TL, Clovis NB, Blaha JD. The response of rabbit patellar tendons after autologous blood injection. Med Sci Sports Exerc 2002; 34: 70–3. doi: 10.1097/00005768-200201000-00012 [DOI] [PubMed] [Google Scholar]
- 20.James SL, Ali K, Pocock C, Robertson C, Walter J, Bell J, et al. US guided dry needling and autologous blood injection for patellar tendinosis. Br J Sports Med 2007; 41: 518–21; discussion 522. doi: 10.1136/bjsm.2006.034686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Virchenko O, Aspenberg P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop 2006; 77: 806–12. doi: 10.1080/17453670610013033 [DOI] [PubMed] [Google Scholar]
- 22.Anitua E, Andía I, Sanchez M, Azofra J, del Mar Zalduendo M, de la Fuente M, et al. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res 2005; 23: 281–6. doi: 10.1016/j.orthres.2004.08.015 [DOI] [PubMed] [Google Scholar]
- 23.Kajikawa Y, Morihara T, Sakamoto H, Matsuda K, Oshima Y, Yoshida A, et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J Cell Physiol 2008; 215: 837–45. doi: 10.1002/jcp.21368 [DOI] [PubMed] [Google Scholar]
- 24.Majewski M, Ochsner PE, Liu F, Flückiger R, Evans CH. Accelerated healing of the rat Achilles tendon in response to autologous conditioned serum. Am J Sports Med 2009; 37: 2117–25. doi: 10.1177/0363546509348047 [DOI] [PubMed] [Google Scholar]
- 25.Sadoghi P, Rosso C, Valderrabano V, Leithner A, Vavken P. The role of platelets in the treatment of Achilles tendon injuries. J Orthop Res 2013; 31: 111–18. doi: 10.1002/jor.22199 [DOI] [PubMed] [Google Scholar]
- 26.Kaux JF, Crielaard JM. Platelet-rich plasma application in the management of chronic tendinopathies. Acta Orthop Belg 2013; 79: 10–15. [PubMed] [Google Scholar]
- 27.Vannini F, Di Matteo B, Filardo G, Kon E, Marcacci M, Giannini S. Platelet-rich plasma for foot and ankle pathologies: a systematic review. Foot Ankle Surg 2014; 20: 2–9. doi: 10.1016/j.fas.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 28.Autologous blood injection for tendinopathy NICE Guidance. NICE interventional procedure guidance 438 guidance. 2013. Available from: https://www.nice.org.uk/guidance/ipg438
- 29.Halici M, Karaoglu S, Canoz O, Kabak S, Baktir A. Sodium hyaluronate regulating angiogenesis during Achilles tendon healing. Knee Surg Sports Traumatol Arthrosc 2004; 12: 562–7. doi: 10.1007/s00167-004-0536-2 [DOI] [PubMed] [Google Scholar]
- 30.Hart L. Corticosteroid and other injections in the management of tendinopathies: a review. Clin J Sport Med 2011; 21: 540–1. doi: 10.1097/01.jsm.0000407929.35973.b9 [DOI] [PubMed] [Google Scholar]
- 31.Clarke AW, Alyas F, Morris T, Robertson CJ, Bell J, Connell DA. Skin-derived tenocyte-like cells for the treatment of patellar tendinopathy. Am J Sports Med 2011; 39: 614–23. doi: 10.1177/0363546510387095 [DOI] [PubMed] [Google Scholar]
- 32.Asplund CA, Best TM. Achilles tendon disorders. BMJ 2013; 346: f1262. doi: 10.1136/bmj.f1262 [DOI] [PubMed] [Google Scholar]
- 33.Riley G. Tendinopathy–from basic science to treatment. Nat Clin Pract Rheumatol 2008; 4: 82–9. doi: 10.1038/ncprheum0700 [DOI] [PubMed] [Google Scholar]
- 34.Wijesekera NT, Chew NS, Lee JC, Mitchell AW, Calder JD, Healy JC. US-guided treatments for chronic Achilles tendinopathy: an update and current status. Skeletal Radiol 2010; 39: 425–34. doi: 10.1007/s00256-009-0873-9 [DOI] [PubMed] [Google Scholar]
- 35.Allison GT, Purdam C. Eccentric loading for Achilles tendinopathy–strengthening or stretching? Br J Sports Med 2009; 43: 276–9. doi: 10.1136/bjsm.2008.053546 [DOI] [PubMed] [Google Scholar]
- 36.McLean SM, Burton M, Bradley L, Littlewood C. Interventions for enhancing adherence with physiotherapy: a systematic review. Man Ther 2010; 15: 514–21. doi: 10.1016/j.math.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 37.Paoloni JA, Appleyard RC, Nelson J, Murrell GA. Topical glyceryl trinitrate treatment of chronic noninsertional achilles tendinopathy. A randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am 2004; 86-A: 916–22. [DOI] [PubMed] [Google Scholar]
- 38.Paoloni JA, Murrell GA. Three-year follow up study of topical glyceryl trinitrate treatment of chronic noninsertional Achilles tendinopathy. Foot Ankle Int 2007; 28: 1064–8. doi: 10.3113/FAI.2007.1064 [DOI] [PubMed] [Google Scholar]
- 39.Phizackerley D. Glyceryl trinitrate not licensed for Achilles tendinopathy in UK. BMJ 2013; 346: f2172. doi: 10.1136/bmj.f2172 [DOI] [PubMed] [Google Scholar]
- 40.van der Worp H, van den Akker-Scheek I, van Schie H, Zwerver J. ESWT for tendinopathy: technology and clinical implications. Knee Surg Sports Traumatol Arthrosc 2013; 21: 1451–8. doi: 10.1007/s00167-012-2009-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rompe JD, Nafe B, Furia JP, Maffulli N. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med 2007; 35: 374–83. doi: 10.1177/0363546506295940 [DOI] [PubMed] [Google Scholar]
- 42.Rompe JD, Furia J, Maffulli N. Eccentric loading versus eccentric loading plus shock-wave treatment for midportion Achilles tendinopathy: a randomized controlled trial. Am J Sports Med 2009; 37: 463–70. doi: 10.1177/0363546508326983 [DOI] [PubMed] [Google Scholar]
- 43.Rasmussen S, Christensen M, Mathiesen I, Simonson O. Shockwave therapy for chronic Achilles tendinopathy: a double-blind, randomized clinical trial of efficacy. Acta Orthop 2008; 79: 249–56. doi: 10.1080/17453670710015058 [DOI] [PubMed] [Google Scholar]
- 44.NICE. Interventional procedure overview of extracorporeal shockwave therapy for refractory Achilles tendinopathy. 2009. Available from: https://www.nice.org.uk/guidance/ipg312
- 45.NICE. Understanding NICE guidance Information for people who use NHS services. Treating refractory Achilles tendinopathy using shockwave therapy. 2009. Available from: https://www.nice.org.uk/guidance/ipg312/resources/treating-refractory-achilles-tendinopathy-using-shockwave-therapy-312736429
- 46.Chan O, O'Dowd D, Padhiar N, Morrissey D, King J, Jalan R, et al. High volume image guided injections in chronic Achilles tendinopathy. Disabil Rehabil 2008; 30: 1697–708. doi: 10.1080/09638280701788225 [DOI] [PubMed] [Google Scholar]
- 47.Maffulli N, Spiezia F, Longo UG, Denaro V, Maffulli GD. High volume image guided injections for the management of chronic tendinopathy of the main body of the Achilles tendon. Phys Ther Sport 2013; 14: 163–7. doi: 10.1016/j.ptsp.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 48.Ohberg L, Alfredson H. US guided sclerosis of neovessels in painful chronic Achilles tendinosis: pilot study of a new treatment. Br J Sports Med 2002; 36: 173–5; discussion 176–7. doi: 10.1136/bjsm.36.3.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alfredson H, Ohberg L. Sclerosing injections to areas of neo-vascularisation reduce pain in chronic Achilles tendinopathy: a double-blind randomised controlled trial. Knee Surg Sports Traumatol Arthrosc 2005; 13: 338–44. doi: 10.1007/s00167-004-0585-6 [DOI] [PubMed] [Google Scholar]
- 50.Boesen MI, Torp-Pedersen S, Koenig MJ, Christensen R, Langberg H, Hölmich P, et al. US guided electrocoagulation in patients with chronic non-insertional Achilles tendinopathy: a pilot study. Br J Sports Med 2006; 40: 761–6. doi: 10.1136/bjsm.2006.027334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yelland MJ, Del Mar C, Pirozzo S, Schoene ML. Prolotherapy injections for chronic low back pain: a systematic review. Spine (Phila Pa 1976) 2004; 29: 2126–33. doi: 10.1097/01.brs.0000141188.83178.b3 [DOI] [PubMed] [Google Scholar]
- 52.Maxwell NJ, Ryan MB, Taunton JE, Gillies JH, Wong AD. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. AJR Am J Roentgenol 2007; 189: W215–20. doi: 10.2214/AJR.06.1158 [DOI] [PubMed] [Google Scholar]
- 53.Ganguly A, Aniq H, Skiadas B. Lumps and bumps around the foot and ankle: an assessment of frequency with US and MRI. Skeletal Radiol 2013; 42: 1051–60. doi: 10.1007/s00256-013-1575-x [DOI] [PubMed] [Google Scholar]
- 54.Azevedo CP, Casanova JM, Guerra MG, Santos AL, Portela MI, Tavares PF. Tumors of the foot and ankle: a single-institution experience. J Foot Ankle Surg 2013; 52: 147–52. doi: 10.1053/j.jfas.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 55.Kliman ME, Freiberg A. Ganglia of the foot and ankle. Foot Ankle 1982; 3: 45–6. doi: 10.1177/107110078200300110 [DOI] [PubMed] [Google Scholar]
- 56.Saboeiro GR, Sofka CM. US-guided ganglion cyst aspiration. HSS J 2008; 4: 161–3. doi: 10.1007/s11420-008-9079-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Latif A, Ansar A, Butt MQ. Treatment of ganglions; a five year experience. J Pak Med Assoc 2014; 64: 1278–81. [PubMed] [Google Scholar]
- 58.Blankstein A, Cohen I, Heiman Z, Salai M, Heim M, Chechick A. Localization, detection and guided removal of soft tissue in the hands using sonography. Arch Orthop Trauma Surg 2000; 120: 514–17. doi: 10.1007/s004020000173 [DOI] [PubMed] [Google Scholar]
- 59.Fox MG, Wright PR, Alford B, Patrie JT, Anderson MW. Lateral mortise approach for therapeutic ankle injection: an alternative to the anteromedial approach. AJR Am J Roentgenol 2013; 200: 1096–100. doi: 10.2214/AJR.12.9227 [DOI] [PubMed] [Google Scholar]
- 60.Kirk KL, Campbell JT, Guyton GP, Schon LC. Accuracy of posterior subtalar joint injection without fluoroscopy. Clin Orthop Relat Res 2008; 466: 2856–60. doi: 10.1007/s11999-008-0236-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu KK. Morton's interdigital neuroma: a clinical review of its etiology, treatment, and results. J Foot Ankle Surg 1996; 35: 112–19; discussion 187–8. [DOI] [PubMed] [Google Scholar]
- 62.Hassouna H, Singh D. Morton's metatarsalgia: pathogenesis, aetiology and current management. Acta Orthop Belg 2005; 71: 646–55. [PubMed] [Google Scholar]
- 63.Valero J, Gallart J, González D, Deus J, Lahoz M. Multiple interdigital neuromas: a retrospective study of 279 feet with 462 neuromas. J Foot Ankle Surg 2015; 54: 320–2. doi: 10.1053/j.jfas.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 64.Park HJ, Kim SS, Rho MH, Hong HP, Lee SY. Sonographic appearances of Morton's neuroma: differences from other interdigital soft tissue masses. Ultrasound Med Biol 2011; 37: 1204–9. doi: 10.1016/j.ultrasmedbio.2011.05.008 [DOI] [PubMed] [Google Scholar]
- 65.Mahadevan D, Venkatesan M, Bhatt R, Bhatia M. Diagnostic accuracy of clinical tests for Morton's neuroma compared with ultrasonography. J Foot Ankle Surg 2015; 54: 549–53. doi: 10.1053/j.jfas.2014.09.021 [DOI] [PubMed] [Google Scholar]
- 66.Jain S, Mannan K. The diagnosis and management of Morton's neuroma: a literature review. Foot Ankle Spec 2013; 6: 307–17. doi: 10.1177/1938640013493464 [DOI] [PubMed] [Google Scholar]
- 67.Quinn TJ, Jacobson JA, Craig JG, van Holsbeeck MT. Sonography of Morton's neuromas. AJR Am J Roentgenol 2000; 174: 1723–8. doi: 10.2214/ajr.174.6.1741723 [DOI] [PubMed] [Google Scholar]
- 68.Bignotti B, Signori A, Sormani MP, Molfetta L, Martinoli C, Tagliafico A. US versus magnetic resonance imaging for Morton neuroma: systematic review and meta-analysis. Eur Radiol 2015; 25: 2254–62. doi: 10.1007/s00330-015-3633-3 [DOI] [PubMed] [Google Scholar]
- 69.Xu Z, Duan X, Yu X, Wang H, Dong X, Xiang Z. The accuracy of ultrasonography and magnetic resonance imaging for the diagnosis of Morton's neuroma: a systematic review. Clin Radiol 2015; 70: 351–8. doi: 10.1016/j.crad.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 70.Fazal MA, Khan I, Thomas C. Ultrasonography and magnetic resonance imaging in the diagnosis of Morton's neuroma. J Am Podiatr Med Assoc 2012; 102: 184–6. [DOI] [PubMed] [Google Scholar]
- 71.Read JW, Noakes JB, Kerr D, Crichton KJ, Slater HK, Bonar F. Morton's metatarsalgia: sonographic findings and correlated histopathology. Foot Ankle Int 1999; 20: 153–61. doi: 10.1177/107110079902000303 [DOI] [PubMed] [Google Scholar]
- 72.Symeonidis PD, Iselin LD, Simmons N, Fowler S, Dracopoulos G, Stavrou P. Prevalence of interdigital nerve enlargements in an asymptomatic population. Foot Ankle Int 2012; 33: 543–7. doi: 10.3113/FAI.2012.0543 [DOI] [PubMed] [Google Scholar]
- 73.Morgan P, Monaghan W, Richards S. A systematic review of US-guided and non-US-guided therapeutic injections to treat Morton's neuroma. J Am Podiatr Med Assoc 2014; 104: 337–48. doi: 10.7547/0003-0538-104.4.337 [DOI] [PubMed] [Google Scholar]
- 74.Younger AS, Claridge RJ. The role of diagnostic block in the management of Morton's neuroma. Can J Surg 1998; 41: 127–30. [PMC free article] [PubMed] [Google Scholar]
- 75.Edwards RT, Yeo ST, Russell D, Thomson CE, Beggs I, Gibson JN, et al. Cost-effectiveness of steroid (methylprednisolone) injections versus anaesthetic alone for the treatment of Morton's neuroma: economic evaluation alongside a randomised controlled trial (MortISE trial). J Foot Ankle Res 2015; 8: 6. doi: 10.1186/s13047-015-0064-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomson CE, Beggs I, Martin DJ, McMillan D, Edwards RT, Russell D, et al. , Methylprednisolone injections for the treatment of Morton neuroma: a patient-blinded randomized trial. J Bone Joint Surg Am 2013; 95: 790–8, S1. doi: 10.2106/JBJS.I.01780 [DOI] [PubMed] [Google Scholar]
- 77.Markovic M, Crichton K, Read JW, Lam P, Slater HK. Effectiveness of US-guided corticosteroid injection in the treatment of Morton's neuroma. Foot Ankle Int 2008; 29: 483–7. doi: 10.3113/FAI.2008.0483 [DOI] [PubMed] [Google Scholar]
- 78.Sofka CM, Adler RS, Ciavarra GA, Pavlov H. US-guided interdigital neuroma injections: short-term clinical outcomes after a single percutaneous injection–preliminary results. HSS J 2007; 3: 44–9. doi: 10.1007/s11420-006-9029-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Makki D, Haddad BZ, Mahmood Z, Shahid MS, Pathak S, Garnham I. Efficacy of corticosteroid injection versus size of plantar interdigital neuroma. Foot Ankle Int 2012; 33: 722–6. doi: 10.3113/FAI.2012.0722 [DOI] [PubMed] [Google Scholar]
- 80.Hughes RJ, Ali K, Jones H, Kendall S, Connell DA. Treatment of Morton's neuroma with alcohol injection under sonographic guidance: follow-up of 101 cases. AJR Am J Roentgenol 2007; 188: 1535–9. doi: 10.2214/AJR.06.1463 [DOI] [PubMed] [Google Scholar]
- 81.Morgan PA, Monaghan GA, Richards S. US-guided alcohol injection for Morton's neuroma. Foot Ankle Int 2015; 36: 55–9. [DOI] [PubMed] [Google Scholar]
- 82.Musson RE, Sawhney JS, Lamb L, Wilkinson A, Obaid H. US guided alcohol ablation of Morton's neuroma. Foot Ankle Int 2012; 33: 196–201. doi: 10.3113/FAI.2012.0196 [DOI] [PubMed] [Google Scholar]
- 83.Gurdezi S, White T, Ramesh P. Alcohol injection for Morton's neuroma: a five-year follow-up. Foot Ankle Int 2013; 34: 1064–7. doi: 10.1177/1071100713489555 [DOI] [PubMed] [Google Scholar]
- 84.Chuter GS, Chua YP, Connell DA, Blackney MC. US-guided radiofrequency ablation in the management of interdigital (Morton's) neuroma. Skeletal Radiol 2013; 42: 107–11. doi: 10.1007/s00256-012-1527-x [DOI] [PubMed] [Google Scholar]
- 85.Genon MP, Chin TY, Bedi HS, Blackney MC. Radio-frequency ablation for the treatment of Morton's neuroma. ANZ J Surg 2010; 80: 583–5. doi: 10.1111/j.1445-2197.2010.05401.x [DOI] [PubMed] [Google Scholar]
- 86.Climent JM, Mondéjar-Gómez F, Rodríguez-Ruiz C, Díaz-Llopis I, Gómez-Gallego D, Martín-Medina P. Treatment of Morton neuroma with botulinum toxin A: a pilot study. Clin Drug Investig 2013; 33: 497–503. doi: 10.1007/s40261-013-0090-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yap LP, McNally E. Patient's assessment of discomfort during US-guided injection of Morton's neuroma: selecting the optimal approach. J Clin Ultrasound 2012; 40: 330–4. [DOI] [PubMed] [Google Scholar]
- 88.Basadonna PT, Rucco V, Gasparini D, Onorato A. Plantar fat pad atrophy after corticosteroid injection for an interdigital neuroma: a case report. Am J Phys Med Rehabil 1999; 78: 283–5. doi: 10.1097/00002060-199905000-00021 [DOI] [PubMed] [Google Scholar]
- 89.Wearing SC, Smeathers JE, Urry SR, Hennig EM, Hills AP. The pathomechanics of plantar fasciitis. Sports Med 2006; 36: 585–611. doi: 10.2165/00007256-200636070-00004 [DOI] [PubMed] [Google Scholar]
- 90.Lemont H, Ammirati KM, Usen N. Plantar fasciitis: a degenerative process (fasciosis) without inflammation. J Am Podiatr Med Assoc 2003; 93: 234–7. doi: 10.7547/87507315-93-3-234 [DOI] [PubMed] [Google Scholar]
- 91.Goff JD, Crawford R. Diagnosis and treatment of plantar fasciitis. Am Fam Physician 2011; 84: 676–82. [PubMed] [Google Scholar]
- 92.Young CC, Rutherford DS, Niedfeldt MW. Treatment of plantar fasciitis. Am Fam Physician 2001; 63: 467–74, 477–8. [PubMed] [Google Scholar]
- 93.Ball EM, McKeeman HM, Patterson C, Burns J, Yau WH, Moore OA, et al. Steroid injection for inferior heel pain: a randomised controlled trial. Ann Rheum Dis 2013; 72: 996–1002. doi: 10.1136/annrheumdis-2012-201508 [DOI] [PubMed] [Google Scholar]
- 94.Crawford F, Thomson C. Interventions for treating plantar heel pain. Cochrane Database Syst Rev 2003; (3): CD000416. [DOI] [PubMed] [Google Scholar]
- 95.Blockey NJ. The painful heel; a controlled trial of the value of hydrocortisone. Br Med J 1956; 1: 1277–8. doi: 10.1136/bmj.1.4978.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crawford F, Atkins D, Young P, Edwards J. Steroid injection for heel pain: evidence of short-term effectiveness. A randomized controlled trial. Rheumatology (Oxford) 1999; 38: 974–7. doi: 10.1093/rheumatology/38.10.974 [DOI] [PubMed] [Google Scholar]
- 97.Schulhofer SD, Short-term benefits of US-guided corticosteroid injection in plantar fasciitis. Clin J Sport Med 2013; 23: 83–4. doi: 10.1097/JSM.0b013e31827e9ec9 [DOI] [PubMed] [Google Scholar]
- 98.Li S, Shen T, Liang Y, Zhang Y, Bai B. Miniscalpel-needle versus steroid injection for plantar fasciitis: a randomized controlled trial with a 12-month follow-up. Evid Based Complement Alternat Med 2014; 2014: 164714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peerbooms JC, van Laar W, Faber F, Schuller HM, van der Hoeven H, Gosens T. Use of platelet rich plasma to treat plantar fasciitis: design of a multi centre randomized controlled trial. BMC Musculoskelet Disord 2010; 11: 69. doi: 10.1186/1471-2474-11-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PM R 2014; 6: 152–8. doi: 10.1016/j.pmrj.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 101.Franceschi F, Papalia R, Franceschetti E, Paciotti M, Maffulli N, Denaro V. Platelet-rich plasma injections for chronic plantar fasciopathy: a systematic review. Br Med Bull 2014; 112: 83–95. doi: 10.1093/bmb/ldu025 [DOI] [PubMed] [Google Scholar]
- 102.Shetty VD, Dhillon M, Hegde C, Jagtap P, Shetty S. A study to compare the efficacy of corticosteroid therapy with platelet-rich plasma therapy in recalcitrant plantar fasciitis: a preliminary report. Foot Ankle Surg 2014; 20: 10–13. doi: 10.1016/j.fas.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 103.Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg 2009; 17: 602–8. [DOI] [PubMed] [Google Scholar]
- 104.Monto RR. Platelet-rich plasma efficacy versus corticosteroid injection treatment for chronic severe plantar fasciitis. Foot Ankle Int 2014; 35: 313–18. doi: 10.1177/1071100713519778 [DOI] [PubMed] [Google Scholar]
- 105.Akşahin E, Doğruyol D, Yüksel HY, Hapa O, Doğan O, Celebi L, et al. The comparison of the effect of corticosteroids and platelet-rich plasma (PRP) for the treatment of plantar fasciitis. Arch Orthop Trauma Surg 2012; 132: 781–5. [DOI] [PubMed] [Google Scholar]
- 106.Kumar V, Millar T, Murphy PN, Clough T. The treatment of intractable plantar fasciitis with platelet-rich plasma injection. Foot (Edinb) 2013; 23: 74–7. doi: 10.1016/j.foot.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 107.Martinelli N, Marinozzi A, Carnì S, Trovato U, Bianchi A, Denaro V. Platelet-rich plasma injections for chronic plantar fasciitis. Int Orthop 2013; 37: 839–42. doi: 10.1007/s00264-012-1741-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ragab EM, Othman AM. Platelets rich plasma for treatment of chronic plantar fasciitis. Arch Orthop Trauma Surg 2012; 132: 1065–70. doi: 10.1007/s00402-012-1505-8 [DOI] [PubMed] [Google Scholar]
- 109.Wilson JJ, Lee KS, Miller AT, Wang S. Platelet-rich plasma for the treatment of chronic plantar fasciopathy in adults: a case series. Foot Ankle Spec 2014; 7: 61–7. doi: 10.1177/1938640013509671 [DOI] [PubMed] [Google Scholar]
- 110.Chew KT, Leong D, Lin CY, Lim KK, Tan B. Comparison of autologous conditioned plasma injection, extracorporeal shockwave therapy, and conventional treatment for plantar fasciitis: a randomized trial. PM R 2013; 5: 1035–43. doi: 10.1016/j.pmrj.2013.08.590 [DOI] [PubMed] [Google Scholar]
- 111.Díaz-Llopis IV, Gómez-Gallego D, Mondéjar-Gómez FJ, López-García A, Climent-Barberá JM, Rodríguez-Ruiz CM. Botulinum toxin type A in chronic plantar fasciitis: clinical effects one year after injection. Clin Rehabil 2013; 27: 681–5. [DOI] [PubMed] [Google Scholar]
- 112.Díaz-Llopis IV, Rodríguez-Ruíz CM, Mulet-Perry S, Mondéjar-Gómez FJ, Climent-Barberá JM, Cholbi-Llobel F. Randomized controlled study of the efficacy of the injection of botulinum toxin type A versus corticosteroids in chronic plantar fasciitis: results at one and six months. Clin Rehabil 2012; 26: 594–606. [DOI] [PubMed] [Google Scholar]
- 113.Cotchett MP, Munteanu SE, Landorf KB. Effectiveness of trigger point dry needling for plantar heel pain: a randomized controlled trial. Phys Ther 2014; 94: 1083–94. doi: 10.2522/ptj.20130255 [DOI] [PubMed] [Google Scholar]
- 114.Hassan FO. Percutaneous fenestration of the anteromedial aspect of the calcaneus for resistant heel pain syndrome. Foot Ankle Surg 2009; 15: 90–5. doi: 10.1016/j.fas.2008.08.006 [DOI] [PubMed] [Google Scholar]