Abstract
Orbital myiasis is a potentially destructive infestation of the orbital tissues. It is uncommon in clinical practice and is seen in patients with poor hygiene with debilitated or immunocompromised state. We report a case of orbital myiasis in an empty socket of an immunocompetent individual. A 65-year-old immunocompetent patient was found to have orbital myiasis in an empty socket status postevisceration, for which he underwent treatment by manual removal of the larvae after application of a suffocating agent, turpentine oil. A total of 12 larvae were removed over the ensuing week. The tissues healed with secondary intention leaving an irregular healthy scar. It was noteworthy that once eviscerated the eye was neglected by the patient. Empty orbital sockets are potential sites for infestations.
Background
Orbital myiasis is a potentially destructive infestation which can be managed by early detection of larvae in the wound. Patients from rural background are at risk of possible parasitic infestations in an empty socket. Maintenance of personal hygiene and awareness among such population at risk should be emphasised.
Case presentation
Case
A 65-year-old male farmer presented with pain, redness, pruritis and swelling of right eye (OD) upper eyelid for 15 days. There was no history of recent trauma. The patient had been using over the countermedication (steroid–antibiotic combination drops) for about a week without any improvement. He had a history of loss of vision in OD after a trauma 10 years back. Evisceration was performed at that time, though medical records were not available. He denied history of diabetes, hypertension or any other chronic systemic disease or any prolonged use of medications. He did not report any progressive loss of weight or appetite.
Examination
The patient belonged to low socioeconomic strata with poor hygiene and residing in rural area. He reported no systemic diseases.
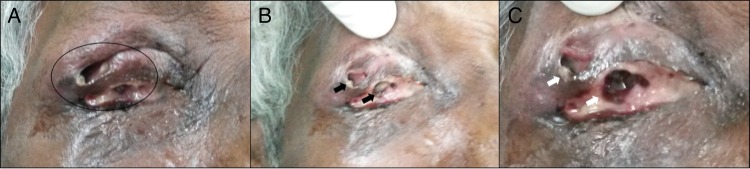
OD: Examination revealed eviscerated socket with severe conjunctival congestion and purulent discharge. There was severe periorbital puffiness. The upper lid was inflamed with a large defect filled with ulcerated necrotic tissue and discharge. Careful examination of the defect in upper eyelid, caused by ulcer, showed motile larva within the necrotic tissue. These maggots were photosensitive and retracted deeper into the tissues on examination (figure 1A–C).
Figure 1.
Ulcer crater and ‘burrows’ created by the larvae (A). Larvae in the superficial part of the wound (B). Larvae retracted deeper into the tissues on exposure to light (C).
Left eye (OS): Lids and conjunctiva of the left eye were normal. Best-corrected visual acuity was 6/6, N6. Anterior segment and posterior segment were normal on examination.
Nose and paranasal sinuses were uninvolved. Notably there was no central nervous system involvement on neuroimaging. Based on these findings a diagnosis of orbital myiasis was made. Blood sugar levels were found to be normal and HIV test was negative.
Investigations
Non-contrast CT of the head and orbit.
Blood investigations for sugar and HIV.
Treatment
Management
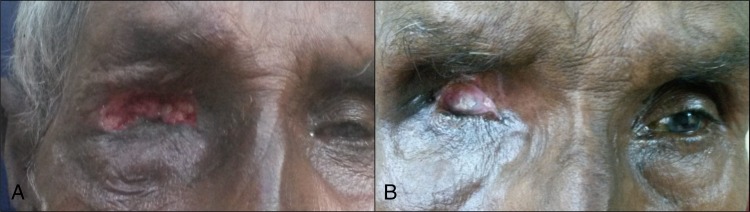
The patient's ulcerated wound was explored under anaesthesia. Topical 4% xylocaine and turpentine oil packing was used to immobilise the larvae. They were gently pulled out with forceps (figure 2A, B). In the first sitting eight larvae were removed from the orbit. The patient was started on oral amoxycillin 500 mg three times a day, topical gatifloxacin 0.3% antibiotic drops and ointment. Systemic and topical antibiotics were started, considering the exploratory procedure and the presence of mucopurulent discharge. The antibiotic–steroid combination drops advised earlier elsewhere were discontinued. Daily wound cleaning was carried out and over the next 3 days 4 more larvae were removed, taking the total number to 12. When subsequent explorations did not reveal any visible larvae, a single dose of 200 µg/kg of ivermectin was given. Ivermectin did not cause any serious adverse effects in this patient. The patient came for a follow-up after 7 days with improvement in symptoms. Examination of the affected area revealed significant resolution of inflammation (figure 3A).
Figure 2.
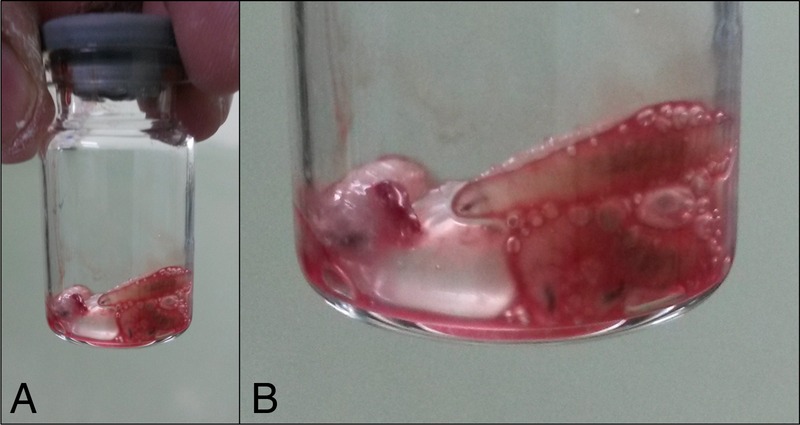
Extracted larvae (A). A magnified view (B).
Figure 3.
Two weeks after removal of all larvae a healing wound is noted (A). Healed healthy socket (B).
Entomological evaluation of the larvae revealed sizes ranging from 5 to 10 mm. They were whitish in colour. The specimen was sent to the department of entomology at the agriculture university and morphological assessment confirmed them to be Chrysoma bezziana.
Outcome and follow-up
On subsequent follow-up after 2 weeks, the patient was asymptomatic. Examination showed a markedly healthy wound with scarring. The socket and conjunctiva were healthy with no discharge (figure 3B).
Discussion
Ophthalmomyiasis is of three types: orbital myiasis, ophthalmomyiasis externa: involving the cornea or conjunctiva and ophthalmomyiasis interna: involving intraocular structures.1–3 This disease is due to infestation of the ocular structures by larvae of various flies most commonly being sheep botfly (Oestrus ovis), screw-worm fly (Phaenicia lucilia, C. bezziana), human botfly (Dermatobia hominis) and the cattle botfly (Hypoderma bovis).4 5 They are more common in tropical and subtropical areas where contact with sheep, goat or other animals is common and with warm weather favouring the larval growth. Ophthalmic involvement is uncommonly seen between 5% and 14% cases.6 7 Human beings are accidental hosts for dipterian flies while sheep and goats are definitive hosts. Adult flies lay eggs, which over a period of few days hatch into larvae. Larvae or maggots grow in size and form the main feeding stage. Larvae in the next 3–5 days undergo transformation into pupa which consists of a hard shell protecting the developing fly inside. In another 4–6 days adult flies come out of pupa. Apart from poor hygiene, local tissue necrosis, malignancies (basal cell carcinoma, squamous cell carcinoma) and ischaemia are risk factors for ectoparasitic infestations.8–10
Orbital myiasis is a destructive disease. Early identification of the disease is essential as these larvae can penetrate the orbital tissues, surrounding paranasal sinuses and the intracranial space. In the presence of an intact eyeball, there is risk of intraocular penetration leading to the condition of ophthalmomyiasis interna. In our patient the larvae had infiltrated the orbital tissues but the surrounding paranasal sinuses and intracranial space were still uninvolved.
Maggots show negative phototaxis by moving away from light stimulus. This response is due to photoreceptors on anterior end of the larvae which makes them bury deeper into the tissues on exposure to light. As the larvae are photosensitive and mobile, immobilisation with topical anaesthesia and various suffocating agents like paraffin and turpentine oil has been advocated.11 12 Meticulous mechanical removal of the larvae is performed under topical/local anaesthesia after exploration and this has to be followed by frequent examinations over extended periods the same day and daily thereafter for attempting the removal of residual larvae. We found four more larvae after the initial batch of eight which were removed on the first day. This highlights the importance of daily wound examination, dressing and cleaning till the area is maggot free. Surgical debridement of the wound and mechanical removal with plain forceps by pulling out the maggots is the standard practice, a disadvantage being the fact that the larvae crawl deep into the tissues on being exposed to light. Complete mechanical removal of live larvae may not be possible. Hence the need for suffocating formulations such as turpentine oil, liquid paraffin and petroleum jelly, which temporary immobilise the larvae making it easier to mechanically pull them out. Anaesthetic agents like xylocaine help in immobilising the crawling larvae and prevent their retreat into deeper tissues. Since larvae are only temporarily incapacitated, manual removal is required. Use of larvicidal drugs like hydrogen peroxide and isopropyl alcohol helps in killing maggots and facilitate their mechanical removal.
As the fly lays eggs in multiple batches, hatching of larvae (maggots) from these eggs also occurs continuously. As in our case, maggot removal needs to be carried out on multiple occasions. Also there is a chance of missing small-sized maggots. Ivermectin is a larvicidal drug and helps in complete clearance of all the maggots. In necrotic tissues as the sensory nerves have been damaged, the patient may not feel the crawling sensation in spite of the presence of maggots. Antihelminthics like ivermectin or benzimidazoles like albendazole are effective against adult as well as larval stages.
Puthran et al13 have described orbital myiasis in an anophthalmic socket of a patient from rural background who sustained lid injury. As in our case, the authors used a combination of treatment consisting of turpentine oil, mechanical removal and oral ivermectin and reported good results with complete healing of the wound.
There are case reports of orbital myiasis secondary to cutaneous malignancies like SCC and BCC.8 9 Khataminia et al11 have reported orbital myasis with orbital destruction by C. bezziana in an 85-year-old female which was treated by exenteration. Sachdev et al4 have described orbital myiasis in an immunocompetent patient with no obvious risk factors treated successfully with mechanical removal aided by turpentine oil.
Patients' neglect of the empty socket is a reminder that they need to be educated to take care about maintaining cleanliness of the empty socket as it is at risk of ectoparasitic infestations such as myiasis. To the best of our knowledge there is only one other case report by Puthran et al13 in PubMed where orbital myiasis was seen in an eviscerated socket. The presence of empty socket along with poor hygiene and rural backdrop were perhaps the predisposing elements despite being an immunocompetent individual and these need to be watched out for in similar individuals.
Learning points.
Orbital myiasis is a rare disease with potential to cause complications.
Early recognition and meticulous larvae removal is the key to management.
Maintenance of personal hygiene and awareness among population at risk should be emphasised.
We recommend daily cleaning of the socket for any accumulated secretions.
Patients from rural background should be educated about possible parasitic infestations in an empty socket.
Footnotes
Contributors: CK contributed the idea, design, analysis and literature review. NR contributed in the design, analysis, literature search and editing. AM contributed in the analysis, literature review and editing.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cameron JA, Shoukrey NM, al Garni AA. Conjunctival ophthalmomyiasis caused by sheep nasal botfly (Oestrus ovis). Ann J Opthalmol 1991;112:331–4. 10.1016/S0002-9394(14)76736-4 [DOI] [PubMed] [Google Scholar]
- 2.Risco JM, Al Dosari F, Millar L. Sheep nasal botfly (Oestrus ovis) larvae infestation of the conjunctiva. Arch Opthalmol 1995;113:529–30. 10.1001/archopht.1995.01100040151043 [DOI] [PubMed] [Google Scholar]
- 3.Sigauke E, Beebe WE, Gander RM et al. Case report: ophthalmomyiasis externa in Dallas County, Texas. Am J Trop Med Hyg 2003;68:46–7. [PubMed] [Google Scholar]
- 4.Sachdev MS, Kumar H, Roop et al. Destructive ocular myiasis in a non-compromised host. Indian J Opthalmol 1990;38:184–6. [PubMed] [Google Scholar]
- 5.Huynth N, Dolan B, Lee S et al. Management of phaeniciatic ophthalmomyiasis externa. Br J Opthalmol 2005;89:1377–8. 10.1136/bjo.2005.071597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilhelmus KR. Myiasis palpebrarum. Am J Ophthalmol 1986;101:496–8. 10.1016/0002-9394(86)90661-6 [DOI] [PubMed] [Google Scholar]
- 7.Scott HG. Human myiasis in North America (1952–1962 Inclusive). Fla Entamol 1964;47:255–61. 10.2307/3493743 [DOI] [Google Scholar]
- 8.Raina UK, Gupta M, Kumar V et al. Orbital myiasis in a case of invasive basal cell carcinoma. Oman J Ophthalmol 2009;2:41–2. 10.4103/0974-620X.48422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamal S, Bodh SA, Kumar S et al. Orbital myiasis complicating squamous cell carcinoma in xeroderma pigmentosum. Orbit 2012;31:137–9. 10.3109/01676830.2011.638103 [DOI] [PubMed] [Google Scholar]
- 10.Balasubramanya R, Pushker N, Bajaj MS et al. Massive orbital and ocular invasion in ophthalmomyiasis. Can J Ophthalmol 2003;38:297–8. 10.1016/S0008-4182(03)80096-0 [DOI] [PubMed] [Google Scholar]
- 11.Khataminia G, Aghajanzadeh R, Vazirianzadeh B et al. Orbital myiasis. J Ophthalmic Vis Res 2011;6(3):199–203. [PMC free article] [PubMed] [Google Scholar]
- 12.Prasanna Kumar S, Ravikumar A, Somu L et al. Tracheostomal myiasis: a case report and review of the literature. Case Rep Otolaryngol 2011;2011:303510 10.1155/2011/303510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puthran N, Hedge V, Anupama B et al. Ivermectin treatment for massive orbital myiasis in an empty socket with concomitant scalp pediculosis. Indian J Ophthalmol 2012;60:225–7. 10.4103/0301-4738.95880 [DOI] [PMC free article] [PubMed] [Google Scholar]